Abstract
Sustainable chemistry is a relatively new field that aims to achieve both economic and environmental goals simultaneously. This paper discusses a cleaner and more sustainable method for dyeing cotton fabric using Direct Violet 09 and Reactive Violet 05 dye. It has been noticed that good color characteristics were obtained when cotton fabric was dyed with 35 mL of microwave-treated Reactive Violet 05 dye solution of 7 pH and 2 g Aluminum sulfate for 30 min at 60 °C. When dyeing cotton fabric with Direct Violet 09 dye, it has been observed that cotton fabric, when microwave treated for 6 min and dyed at 80 °C for 50 min using 35 mL of non-treated dye solution of 7 pH with 2 g Potassium sulfate, produces good results. The colorfastness of the dyed fabrics using ISO standards was also evaluated. Overall, it was found that the use of microwave radiation improves the sustainability of the dyeing process.
Keywords:
cotton; Direct Violet 9 dye; inorganic salts; Reactive Violet 05 Dye; silk; sustainability 1. Introduction
The importance of eco-friendly techniques in textile industries to meet society’s demand is growing day by day. The textile sector contributes significantly to global economic growth by providing direct employment, as well as increasing foreign exchange earnings. Clothing is a basic need for survival as it is used to cover the body and protect it from extreme weather conditions. After 1960, the introduction of fashion trends in youth began swiftly. The invention of synthetic dye revolutionized the textile industry. Synthetic dyes are coloring compounds with complex chemical structures that are used to color various objects such as foodstuffs, textiles, paper, etc. [1]. Now, in modern times, due to rapid progress in the fashion and textile industries, the use of synthetic dyes has been expanding. However, besides influencing the textile industry positively, synthetic dyes also affect the environment and human beings deleteriously, which is against the basic principles of green chemistry. Green chemistry demands a process that is adequate for nature, reduces environmental impacts, and promotes human health [2,3]. However, the effluent from textile industries contains electrolytes, surfactants, heavy metals, and unfixed dye molecules that have an adverse effect on human health and the environment [4,5]. Under this scenario, global environmental agencies such as the Environmental Protection Agency (EPA), the Ecological and Toxicological Association of Dyers (ETAD), and Environmental Impact Assessment (EIA) are forcing industrialists and researchers to introduce methods that result in zero discharge emissions by introducing methods and techniques that are safe, powerful, and sustainable. For this purpose, some advanced and innovative techniques such as ultraviolet, ultrasonic, plasma, and microwaves are being introduced to fulfill the criteria of environmental agencies [6,7,8]. Among these, the microwave technique is becoming a dominant one because of its cost-effectiveness, speed, and efficiency of heating [9,10,11]. Microwave radiation is capable of transferring electromagnetic energy with smaller wavelengths. It has a wavelength range from 1 mm to 1 m with a frequency range from 300 GHz to 300 MHz. Heating by microwave radiation has the advantage that it distributes uniform heat throughout the material, as compared to the conventional heating method [12].
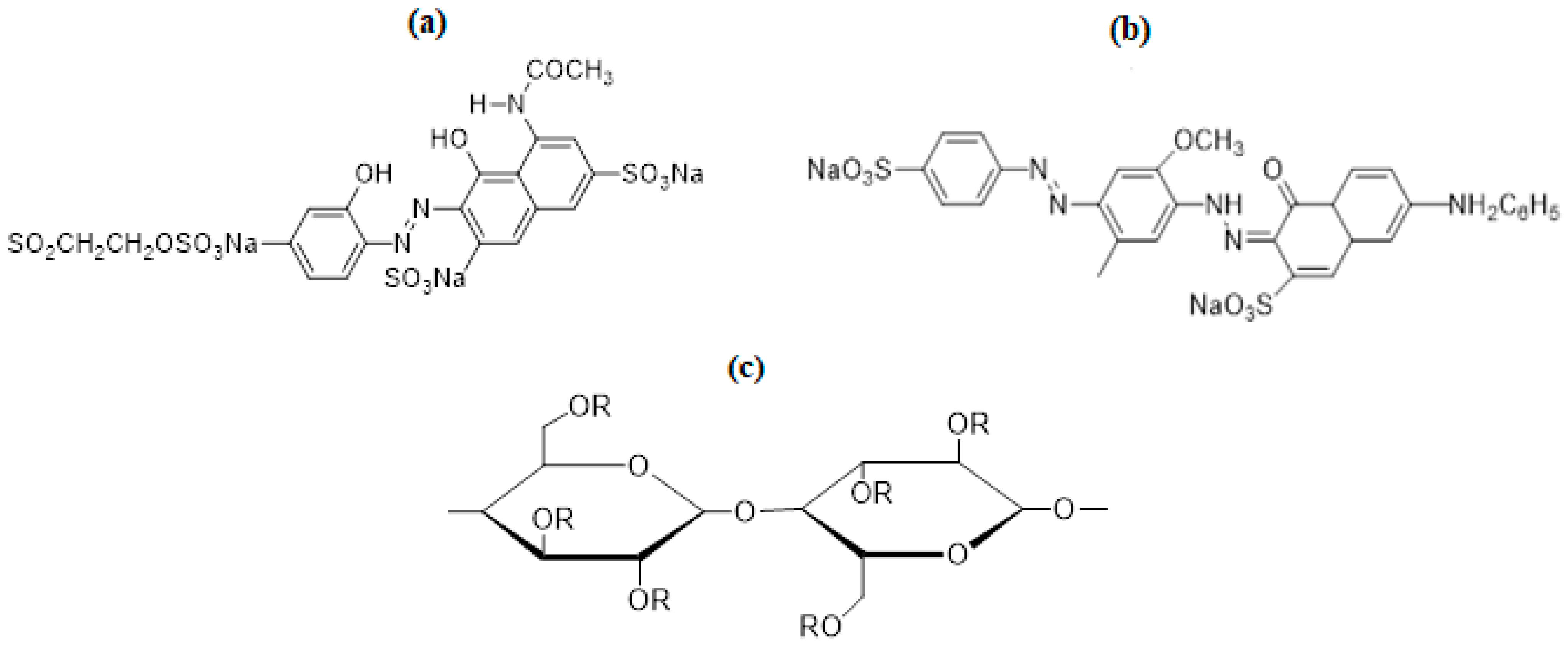
In industries, many classes of dyes are used depending upon the nature of work and field, as per global demand. Among these dyes, direct and reactive dyes have a great share in the applied fields [13]. Direct dye is the sodium salt of an aromatic compound, with an anionic nature and solubility in water. These dyes are very popular due to their low cost, wide color range, and ease of use [14,15]. Direct dyes are widely used in printing and dyeing silk, cotton, wool, leather, etc. For the current study, Direct Violet 09 (DV 09 dye = CI 27885) was selected for improving the dyeing behavior of cotton fabric. Direct Violet 09 dye (Figure 1b) is a bis-azo class dye with a bluish-purple color. Another important class of dye that is famous because of its permanent bonding with the fiber is the reactive dye. Reactive Violet 05 (RV 05 dye = CI 18097) (Figure 1a) is a single azo complex dye that is purple in color, easily soluble in water, and mostly used for the dyeing of cotton fabrics [16]. Cotton (Figure 1b) is a soft, natural, and staple fiber of plant origin with glycosidic linkages [17,18]. The main constituent of cotton fiber is cellulose. Hydroxyl groups present in the cellulosic unit are responsible for interacting with moisture, colorants, and finishing chemicals.
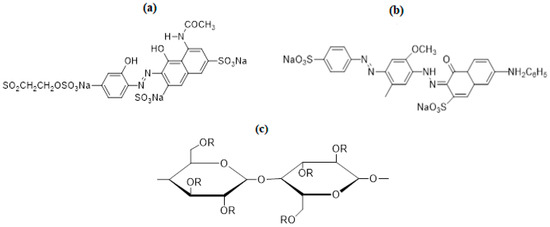
Figure 1.
Structure of (a) Reactive Violet 5, (b) Direct Violet 9, and (c) functional unit of cotton.
Keeping in view the advantages of M.W. radiation, the aim of the study was:
- To improve the color characteristics of cellulosic fabric dyed with Reactive Violet 5 and Direct Violet 9 dye by using microwave radiation;
- To study the physical and chemical characteristics of fabrics before and after treatment;
- To analyze the dyeing conditions using central composite design through response surface methodology
- To assess the color fastness properties of cotton fabric dyed with Reactive Violet 5 and Direct Violet 9 dye.
2. Experimental Section
2.1. Materials
Cotton fabric (GSM = 70) was bought from the regional textile market in Faisalabad, Pakistan. Reactive Violet 05 (RV05 = CI 18097) and Direct Violet 09 (DV09 = CI 27885) dyes were purchased from Kukdacolor, Karachi, Pakistan. All the chemicals utilized in the dyeing process, such as neutral soap, sodium chloride (NaCl), aluminum sulfate (Al2SO4), iron sulfate (FeSO4), potassium sulfate (K2SO4), sodium sulfate (Na2SO4), zinc sulfate (ZnSO4), and sodium hydroxide (NaOH), were of commercial grade.
2.2. Irradiation Process and Dyeing
Dye solutions were made by adding 0.5 g of DV 09 and RV 05 dyes into 100 mL of water separately. Dye solutions and cotton fabric were treated for 2–10 min using a microwave oven (Dawlance DW-220 S) at high power (2450 MHz, 700 W). The dyeing process was performed under various conditions, as presented in Table 1.

Table 1.
Irradiation and dyeing condition for cotton fabric using Reactive Violet 05 and Direct Violet 09 dye.
2.3. Selection of Dyeing Conditions
A comprehensive set of 32 experiments was devised using central composite design (CCD) and RSM as a statistical method after determining the behavior of M.W. radiation on cotton fabric. In the set of experiments, irradiated cotton fabrics (RF) were dyed using an irradiated dye solution (RS) of 25, 35, 45, 55, and 65 mL with 6, 7, 8, 9, and 10 pH and 0.5, 1, 1.5, 2, and 2.5 g/100 mL of NaCl for 20, 30, 40, 50, and 60 min at 50, 60, 70, 80, and 90 °C.
2.4. Effect of Different Inorganic Salts
In textile dyeing, salts are used as exhausting agents for achieving even dyeing. In this study, different inorganic salts such as sodium chloride (NaCl), iron sulfate (FeSO4), sodium sulfate (Na2SO4), potassium sulfate (K2SO4), aluminum sulfate (Al2SO4), and zinc sulfate (ZnSO4) were used to observe the effect of these salts on the color strength of the cotton fabrics dyed with Direct Violet 09 and Reactive Violet 05 dyes. A set of 25 experiments was performed by using different concentrations of these inorganic salts (0.5–2.5 g/100 mL) for dyeing cotton fabric with Direct Violet 09. Another set of 25 experiments was performed by using different concentrations of these inorganic salts (0.5–2.5 g/100 mL) for dyeing cotton fabric with Reactive Violet 05 dyes. Dyed fabrics were subjected to the CIE Lab system for analysis.
2.5. FTIR and SEM Analysis
FTIR analysis was used to examine the chemical change that occurred after treating cotton fabrics with microwave radiation. To observe any physical change, SEM analysis with a magnification power of 1000× was performed before and after the irradiation of fabrics at given conditions.
2.6. Determination of Maximum Wavelength
Direct Violet 09 dye and Reactive Violet 05 dyes were dissolved separately in a sufficient amount of distilled water to prepare stock solutions. By diluting the stock solutions, 20 ppm dye solutions were prepared and λmax of both dyes was determined using a UV-visible spectrophotometer
2.7. Evaluation of Quality of Dyed Fabrics
The fastness of the colored fabrics was evaluated using ISO standards for washing (ISO 105 C03), light (ISO 105 B02), and rubbing (ISO 105-X12) using the CIELAB color space.
3. Results and Discussion
Sustainable treatment tools such as cost and energy-effective microwave treatment have given new dimensions to the field of fabric processing because they have the ability to improve the substantivity of the fabric via pretreatment on the fabric surface [19,20,21]. Microwave treatment is gaining worldwide fame because it improves the dyeing process through its unique mode of action, thus providing promising results by changing the fabric behavior towards synthetic dye. In this study, M.W. treatment has been used to improve the dyeing behavior of cotton fabric with two different classes of dye.
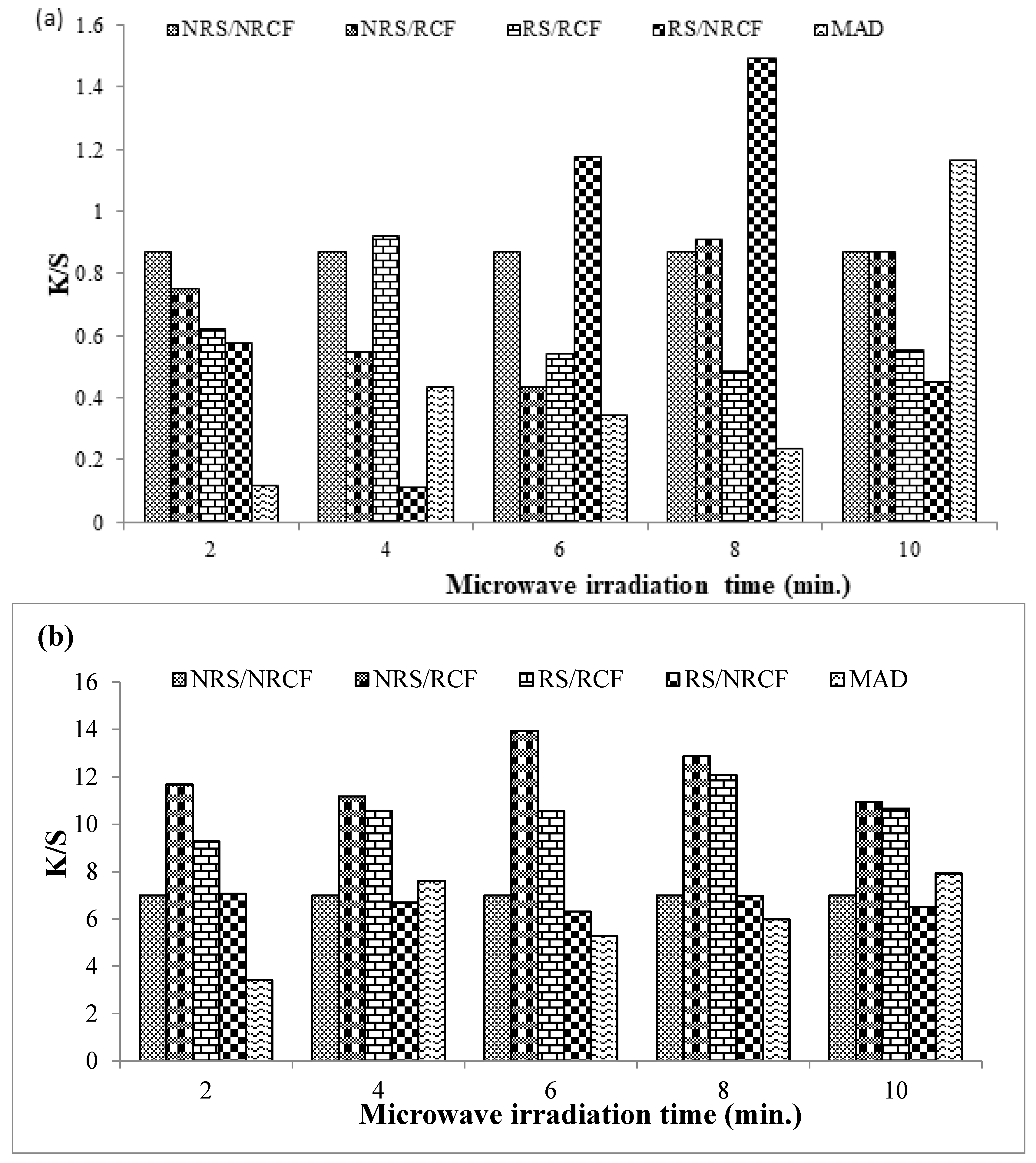
For cotton fabric dyed with Reactive Violet 05 dye, a significant change in color strength was observed when fabrics were irradiated with microwave radiation, as shown in Figure 2a. A higher color strength (K/S = 1.4974) was noticed by using the radiated dye solution (RS) and non-radiated cotton fabric (NRCF) as compared to non-treated cotton fabric (NRCF) and non-treated dye solution (NRS) (K/S = 0.8709). The shades obtained under controlled conditions (NRS/NRCF) were brighter (L* = 65.97), with a reddish-yellow hue (a* = 12,44, b* = 15.11), as presented in Table 2. However, shades obtained by dyeing fabric under optimal conditions (NRS/RCF) were darker (L* = 62.00) with a more reddish-yellow tone (a* = 18.71, b* = 20.43). The reason may lie in the fact that the RV 05 dye molecules were already smaller in size, and further microwave radiation helps to break the cluster of reactive dye molecules and thus helps to absorb the small-sized pores or capillary spaces present in the cotton fabrics more effectively.
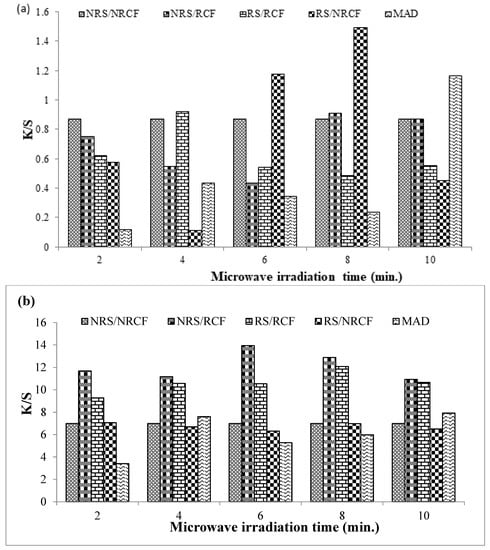
Figure 2.
Dyeing of microwave-treated and un-treated cotton fabric with Reactive Violet 05 (a) and Direct Violet 09 (b) dye.

Table 2.
Tonal variation cotton fabric dyed with Reactive Violet 05 and Direct Violet 09 dye at selected irradiation conditions.
For cotton fabric dyed with Direct Violet 09 dye, a good color strength (K/S = 13.938) was obtained when the 6 min irradiated cotton fabric (RCF) was dyed with non-radiated dye solution (NRS), as compared to the dyeing of non-treated fabric (NRCF) with non-treated solution (NRS) (K/S = 6.9945). Mostly, fabric dyed under optimum conditions, i.e., by using a non-radiated solution (NRS) and irradiated cotton fabric (RCF), was darker (L* = 25.61) and reddish bluish in hue (a* = 18.75, b* = −24.65) as given in Table 2. In this case, microwave treatment modified the fabric surface, which in turn was able to absorb large-sized Direct Violet 09 particles significantly.
3.1. FTIR and SEM Analysis
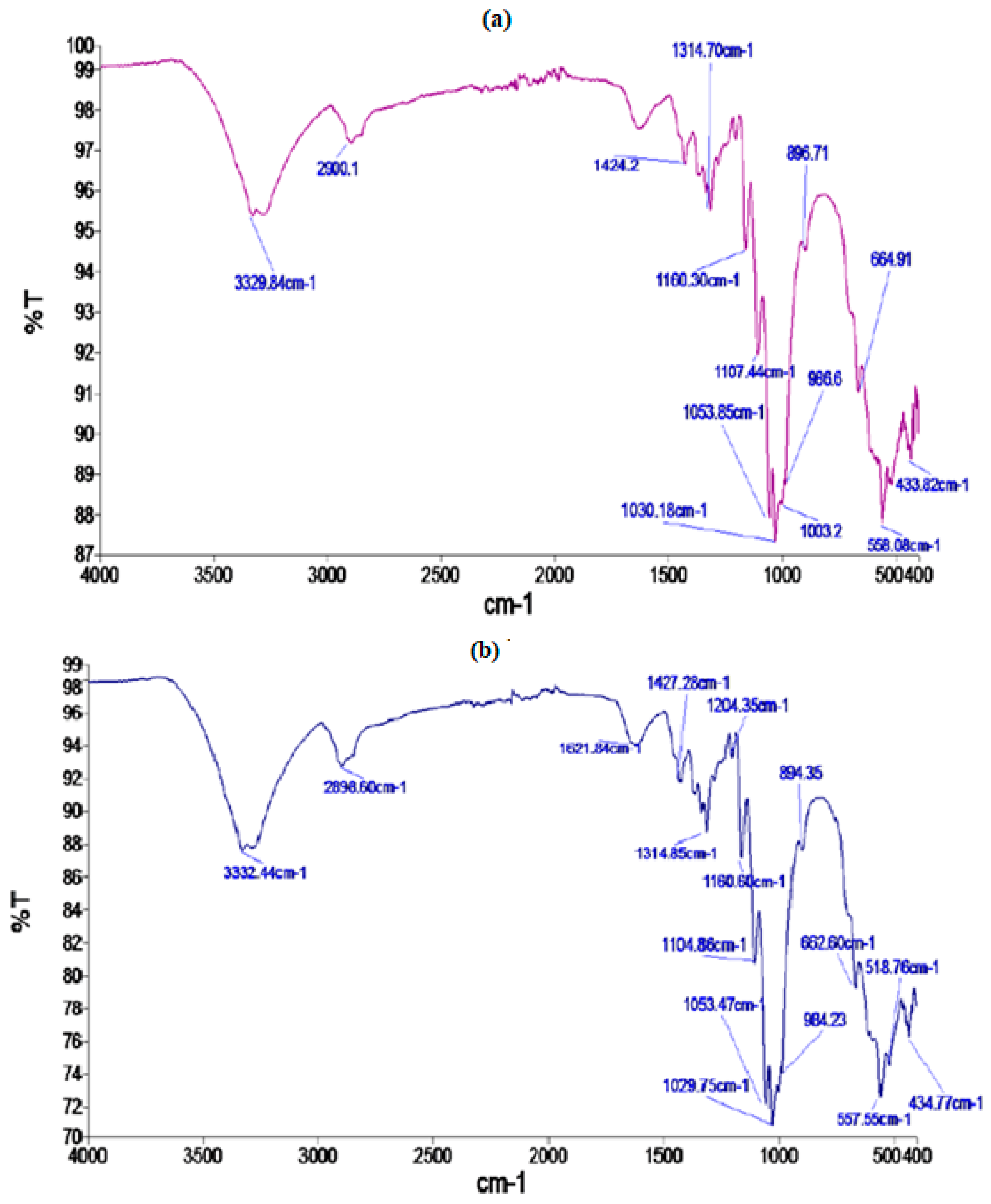
The presence of different functional groups and any change in the position of functional groups after microwave treatment in cotton fabric was confirmed by using FTIR analysis. FTIR peaks at 3329.84 cm−1 and at 2900.1 cm−1 are due to the presence of O-H and C-H bonds present in polysaccharides, respectively (Figure 3a). The characteristic peaks at 1424, 1314, 1030 cm−1 and 896.71 indicate stretching and bending vibrations of -CH2 and -CH, -OH, and C-O bonds in the cellulosic units. The FTIR spectra of cotton fabric after M.W. treatment displayed in Figure 3b indicates that there is a negligible difference in the peaks of the functional groups, indicating that M.W. irradiation treatment has no effect on the position of the characteristic peaks of cellulose units, i.e., no chemical alteration was observed.
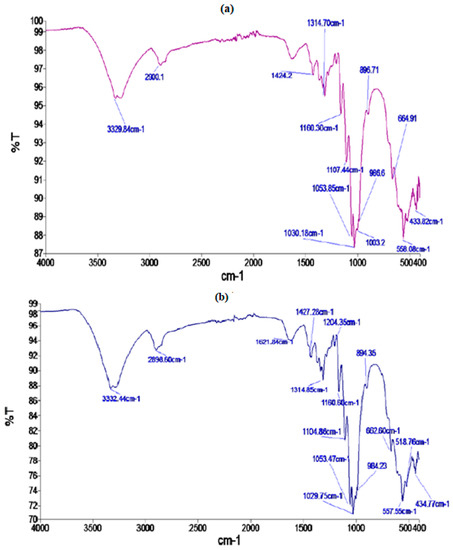
Figure 3.
FTIR spectra of control (a) and microwave treated (b) cotton fabric.
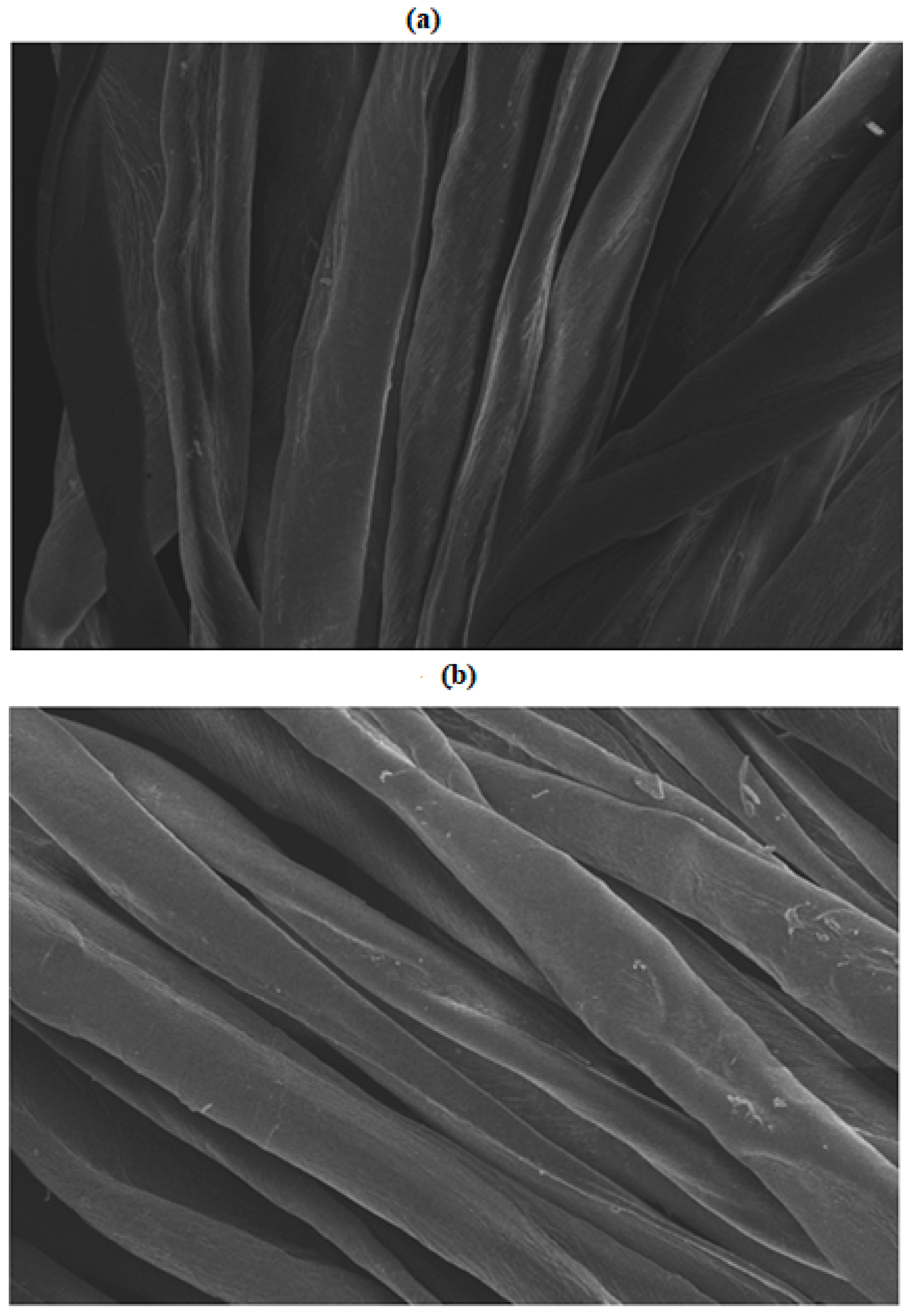
SEM images of control and microwave-treated cotton fabrics for 6 min given in Figure 4a,b show that M.W. radiation is responsible for creating scratches on the surface of cotton fabric, which helps to absorb more dye particles [22]. Another reason lies in the fact that M.W. treatment may activate the functional sites available on the surface of cotton fabric, providing adsorption and the migration of dye to the fabric more easily. Hence, it has been found that M.W. rays do not alter the chemistry of fabric but only physically improve its nature. It is obvious that 8 min of microwave treatment of cotton fabric during dyeing with Reactive Violet dye also modified the surface of the fabric, but in this case the role of the Reactive Violet 05 dye molecules’ smaller size and higher adsorption rate was more significant, as described earlier.
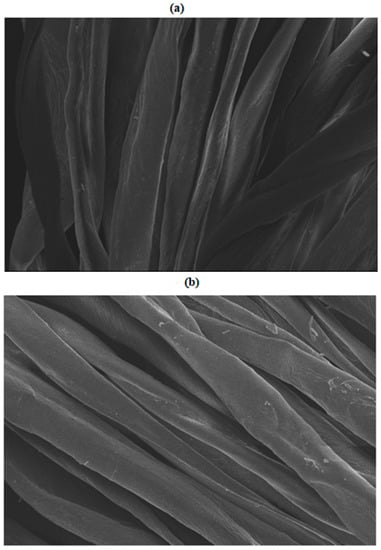
Figure 4.
SEM analysis of (a) non-treated and (b) microwave treated cotton fabric.
3.2. Determination of λmax of Reactive Violet 05 and Direct Violet 09
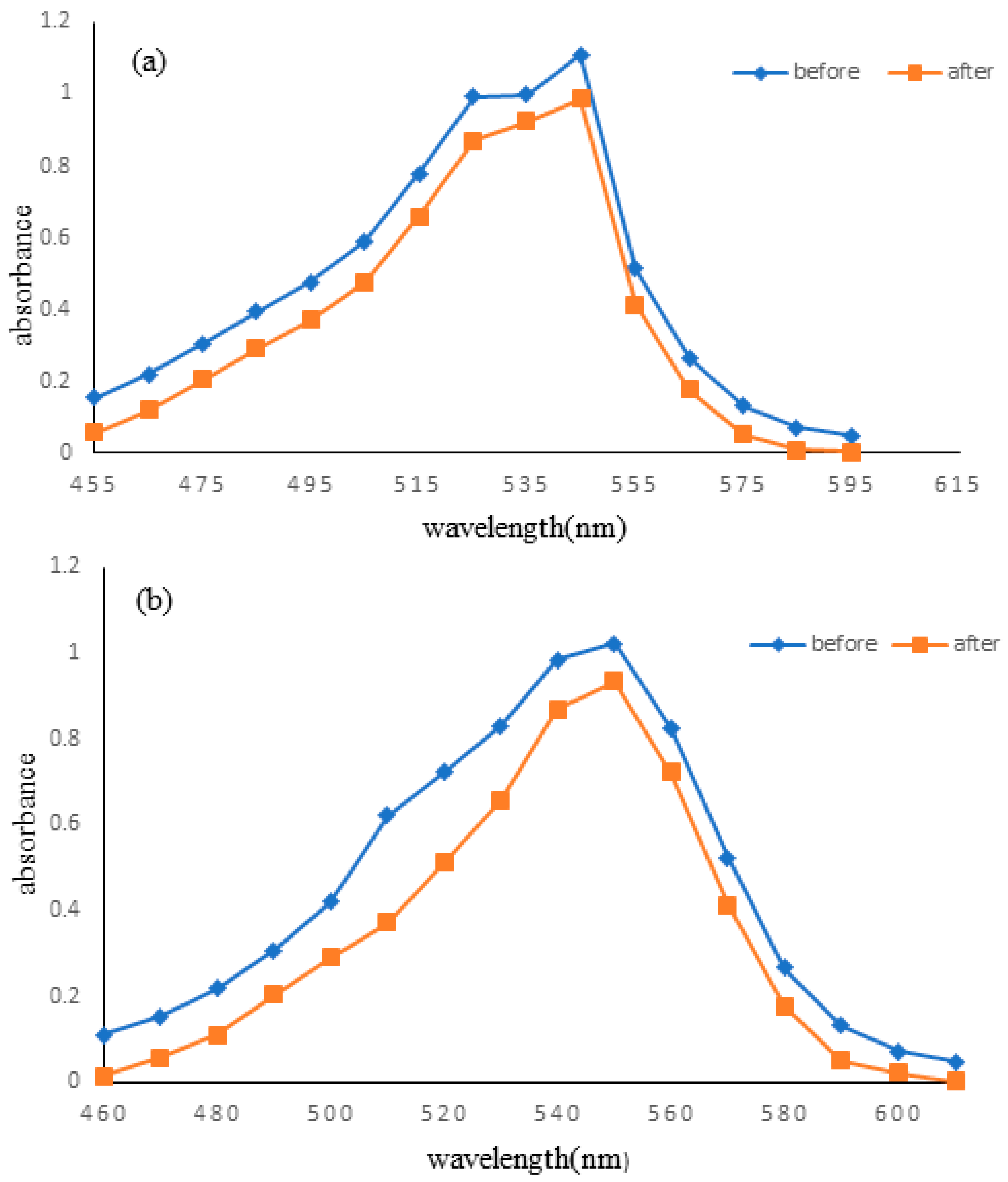
The standard solutions of RV 05 and DV 09 dye solutions were scanned using a UV-visible spectrophotometer to determine any changes in wavelength. It was observed that there were no significant changes in the maximum wavelengths of RV 05 (546 nm) and DV 09 (550 nm) dyes (Figure 5a,b). However, absorption values change significantly because of the sorption of dye into the fabric.
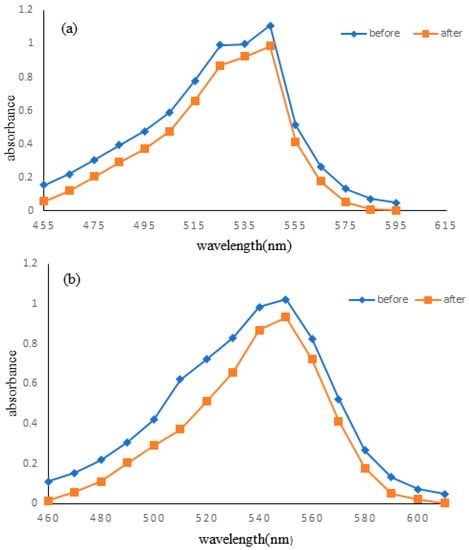
Figure 5.
Maximum wavelengths of (a) Reactive Violet and (b) Direct Violet.
3.3. Effect of Inorganic Salts on the Dyeing of Cotton Fabric
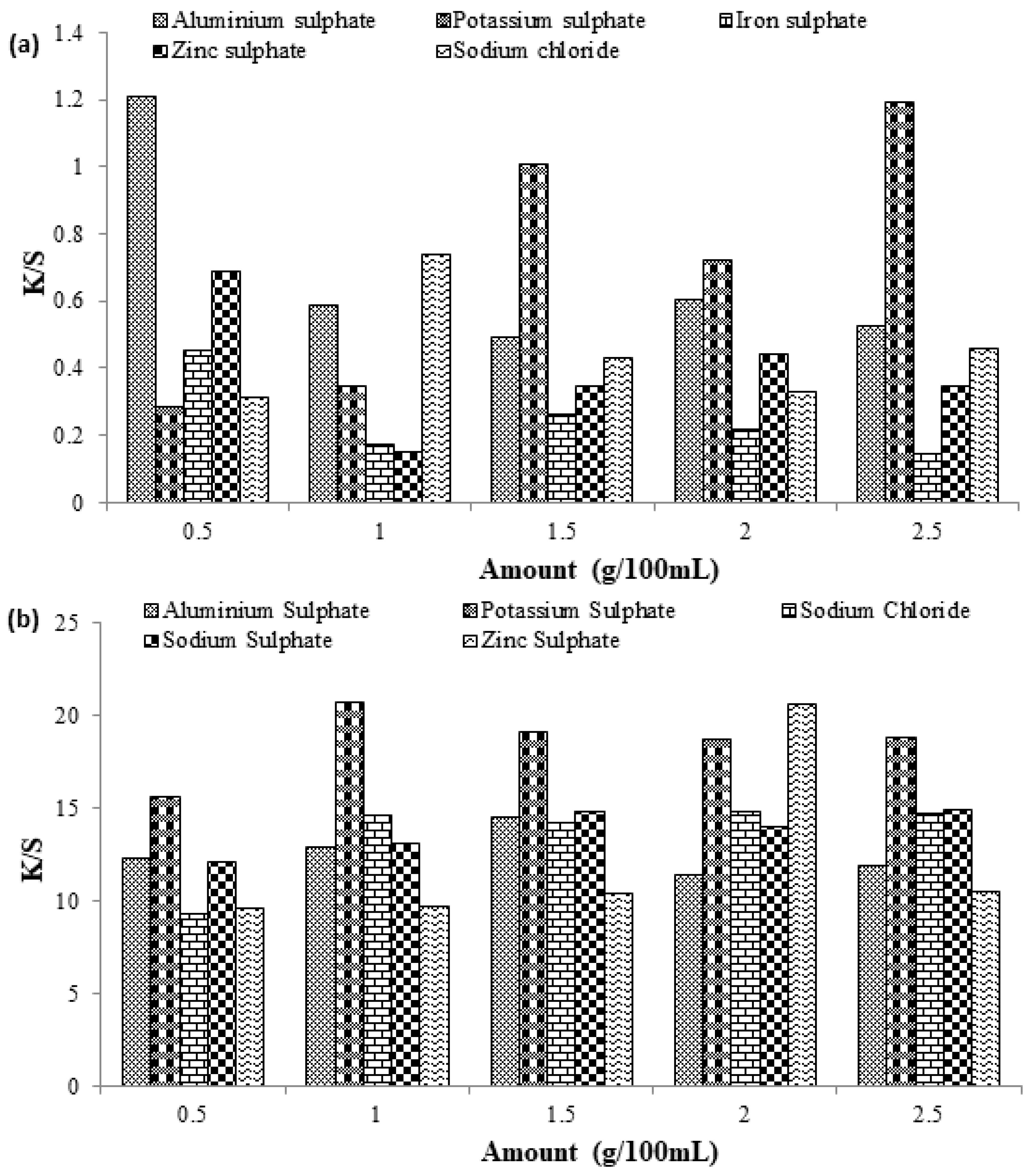
In the dyeing of cellulosic fibers, salts such as sodium sulfate, sodium chloride, etc., are used as the dyebath exhausting agents to increase the dye uptake ability onto the surface of the fabric by reducing negative charge build-up on the surface of the fabric to overcome the repulsion between fiber and dye [23]. In this part, different sulfates of metals, such as aluminum, potassium, iron, and zinc sulfates, were used to study the effect of these exhausting agents on the dyeing behavior of cotton fabric dyed with direct and reactive dye. For Reactive Violet 05 dye, good color characteristics (K/S = 1.2041) were observed by using only 0.5 g/100 mL of aluminum sulfate, while during the dyeing of cotton fabric with Direct Violet 09 dye, the use of 1 g/100 mL of potassium sulfate provides promising results. By using 1 g of potassium sulfate, the highest color strength of (K/S = 20.62) was observed as compared to other salts, even at higher concentrations (Figure 6a,b). This was because, at higher concentrations, added salt may act as a retarding agent and favor a stripping process that is reversible in nature and acts as a barrier during the process.
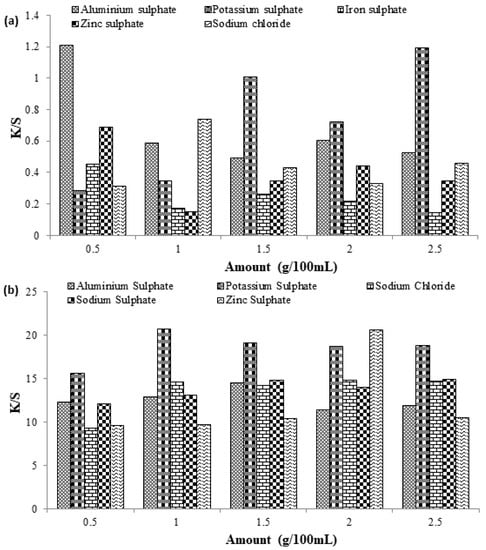
Figure 6.
Effect of different salt concentrations on the dyeing behavior of cotton fabric dyed with (a) Reactive Violet 05 and (b) Direct Violet 09 dye.
3.4. Statistical Selection of Dyeing Parameters
The results regarding the dyeing of cotton fabric with Reactive Violet 05 dye and Direct Violet 09 dye (Table 3, Table 4 and Table 5)demonstrate that the model is fit and linear (p = 0.000). The significance of the individual dyeing variables such as time (p = 0.000), pH (p = 0.009), and salt (p = 0.006) roles have been observed. Additionally, the significant interaction variables, including pH with pH (p = 0.001), volume with volume (p = 0.001), salt with salt (p = 0.003), pH with time (p = 0.084), volume with salt (p = 0.018), volume with time (p = 0.000) and salt with time (p = 0.000), have been noted. Hence, the dyeing of cotton fabrics with RV5 dye for 30 min at 60 °C using 2 g/100 mL of salt solution and 35 mL of dye bath of 7 pH has been recommended (Table 3). Similarly, for DV 09, the results presented in Table 5 indicate that, individually, the roles of volume (p = 0.002), salt (p = 0.001), time (p = 0.018), and pH (p = 0.018) are significant. Similarly, the interaction variables such as pH with volume (p = 0.023), volume with salt (p = 0.029), and volume with time (p = 0.005) have been observed to be highly significant. Thus, 35 mL of a dye solution of 7 pH containing 2 g/100 mL solution for 50 min at 80 °C has given excellent results

Table 3.
Selection of dyeing parameters for cellulosic fabrics using Reactive Violet 05 and Direct Violet 09 dye through RSM.

Table 4.
Analysis of variance of cotton fabric for RV 05 dye.

Table 5.
Analysis of variance of cotton fabric for DV 09 dye.
3.5. Colorfastness Rating
In the dyeing method, the fastness ratings are important characteristics of dyed fabrics. The shades made at optimum conditions show that M.W. treatment enhances the color characteristics of fabrics. The results narrated in Table 6 show the light, washing, dry, and wet rubbing fastness qualities. It was discovered that dyeing cotton fabrics using RV5 and DV9 dye improves colorfastness ratings.

Table 6.
Rating of fastness properties of fabric dyed at optimal conditions using RV5 and DV 9 dye.
4. Conclusions
This research explored the use of microwave radiation for the dyeing of cotton fabric as a way to introduce environmentally friendly technologies. The results show that this process is more efficient and cheaper compared to traditional methods, as it uses fewer chemicals and enhances the color characteristics of the fabric. It was noticed that good color characteristics were obtained when the cotton fabric was dyed with 35 mL of microwave-treated Reactive Violet 05 dye solution of 7 pH with 2 g aluminum sulfate for 30 min at 60 °C. When dyeing cotton fabric with Direct Violet 09 dye, it was observed that cotton fabric when microwave treated for 6 min, dyed at 80 °C for 50 min, and using 35 mL of the non-treated dye solution of 7 pH with 2 g potassium sulfate produced good results. The results suggest that the M.W. treatment has a significant impact on enhancing the dyeing characteristics of fabrics, as well as reducing time and energy usage and making the process more environmentally friendly.
Author Contributions
T.H.B. supervised and S.A. co-supervised the work, whereas S.B. conducted experiments, F.-u.-R., M.A.Q. and S.P.K. helped in formal analysis of data. B.A. and M.A.A.M. helped in draft writing and scientific guidance of smooth running of work. All authors have read and agreed to the published version of the manuscript.
Funding
Authors are grateful to the Department of Chemistry, Government College Faisalabad, Pakistan and School of Renewable Natural Resources, Louisiana State University, Baton Rouge, LA 70803, USA for joint funding this research work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golinska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Abdussalam-Mohammed, W.; Ali, A.Q.; Errayes, A.O. Green chemistry: Principles, applications, and disadvantages. Chem. Methodol. 2020, 4, 408–423. [Google Scholar]
- Gulzar, T.; Farooq, T.; Kiran, S.; Ahmad, I.; Hameed, A. The Impact and Prospects of Green Chemistry for Textile Technology; Elsevier: Alpharetta, GA, USA, 2019. [Google Scholar]
- Adane, T.; Adugna, A.T.; Alemayehu, E. Textile industry effluent treatment techniques. J. Chem. 2021, 2021, 5314404. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, S. A Critical Review on PLA-Algae Composite: Chemistry, Mechanical, and Thermal Properties. J. Text. Sci. Eng. 2020, 10. [Google Scholar]
- Adeel, S.; Saeed, M.; Abdullah, A.; Khan, S.G. Ultrasonic assisted improved dyeing of cellulosic fabric using Vat Blue 4. J. Nat. Fibers 2020, 17, 745–758. [Google Scholar] [CrossRef]
- Shabbir, M.U.; Adeel, S.; Bokhari, T.H.; Usman, M.; Khosa, M.K.; Ahmad, T.; Inayat, A. Eco-friendly acid dyeing of silk and wool fabrics using acid violet 49 dye. Environ. Sci. Pollut. 2022, 30, 9808–9819. [Google Scholar] [CrossRef]
- Haji, A.; Naebe, M. Cleaner dyeing of textiles using plasma treatment and natural dyes: A review. J. Clean. Prod. 2020, 265, 121866. [Google Scholar] [CrossRef]
- Ahmed, B.; Gwon, J.; Thapaliya, M.; Adhikari, A.; Ren, S.; Wu, Q. Combined effects of deep eutectic solvent and microwave energy treatments on cellulose fiber extraction from hemp bast. Cellulose 2023, 30, 2895–2911. [Google Scholar] [CrossRef]
- Ahmed, B.; Wu, Q.; Lin, H.; Gwon, J.; Negulescu, I.; Cameron, B. Degumming of hemp fibers using combined microwave energy and deep eutectic solvent treatment. Ind. Crops Prod. 2022, 184, 115046. [Google Scholar] [CrossRef]
- Kiran, S.; Adeel, S.; Rehman, F.U.; Gulzar, T. Ecofriendly dyeing of microwave treated cotton fabric using reactive violet H3R. Glob. Nest J. 2019, 21, 43–47. [Google Scholar]
- Sundhu, M.; Khosa, M.K.; Adeel, S.; Ahmad, T. Microwave-assisted eco-friendly acid dyeing of proteinous fabrics using acid violet 3b dye. J. Nat. Fibers 2022, 19, 8065–8074. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El-Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Zolriasatein, A.A. Thermodynamics, Kinetics and Isotherms Studies for Sorption of Direct Dye onto the Pectinase Pre-treated Jute Yarn. Recent Innov. Chem. Eng. 2019, 12, 160–171. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Salihu, G. The role of auxiliaries in the immersion dyeing of textile fibres: Part 5 practical aspects of the role of inorganic electrolytes in dyeing cellulosic fibres with direct dyes. Dye. Pigment. 2019, 161, 581–594. [Google Scholar] [CrossRef]
- Irfan, M.; Zhang, H.; Syed, U.; Hou, A. Low liquor dyeing of cotton fabric with reactive dye by an eco-friendly technique. J. Clean. Prod. 2018, 197, 1480–1487. [Google Scholar] [CrossRef]
- Gao, D.; Li, X.; Li, Y.; Lyu, B.; Ren, J.; Ma, J. Long-acting antibacterial activity on the cotton fabric. Cellulose 2021, 28, 1221–1240. [Google Scholar] [CrossRef]
- El-Sayed, E.; Othman, H.; Hassabo, A.G. A short observation on the printing cotton fabric using some technique. J. Text. Color. Polym. 2022, 19, 17–24. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating wetlands: A sustainable tool for wastewater treatment. Clean–Soil Air Water 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Glavič, P. Evolution and current challenges of sustainable consumption and production. Sustainability 2021, 13, 9379. [Google Scholar] [CrossRef]
- Gbolarumi, F.T.; Wong, K.Y.; Olohunde, S.T. Sustainability assessment in the textile and apparel industry: A review of recent studies. IOP Conf. Ser. Mater. Sci. Eng. 2021, 151, 012099. [Google Scholar] [CrossRef]
- Adeel, S.; Liaqat, S.; Hussaan, M.; Mia, R.; Ahmed, B.; Wafa, H. Environmental friendly bio-dyeing of silk using Alkanna tinctoria based Alkannin natural dye. Ind. Crops Prod. 2020, 186, 115301. [Google Scholar]
- Burkinshaw, S.M. The role of inorganic electrolyte (salt) in cellulosic fibre dyeing: Part 2 theories of how inorganic electrolyte promotes dye uptake. Color. Technol. 2021, 137, 547–586. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).