A Wind Tunnel Test of the Anti-Icing Properties of MoS2/ZnO Hydrophobic Nano-Coatings for Wind Turbine Blades
Abstract
:1. Introduction
2. Test Materials and Methods
2.1. Materials and Preparation
2.2. Preparation of Coating
2.3. Testing and Characterization
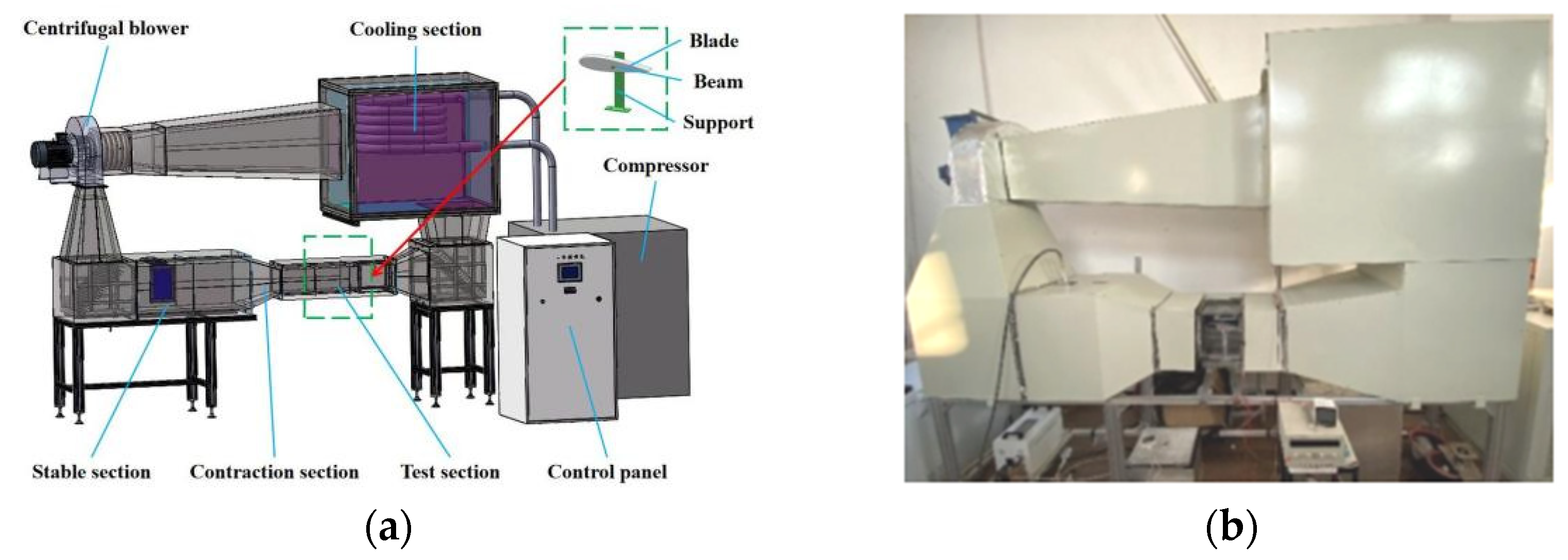
2.4. Anti-Icing Test in the Icing Wind Tunnel
2.5. Test Scheme
3. Results and Discussion
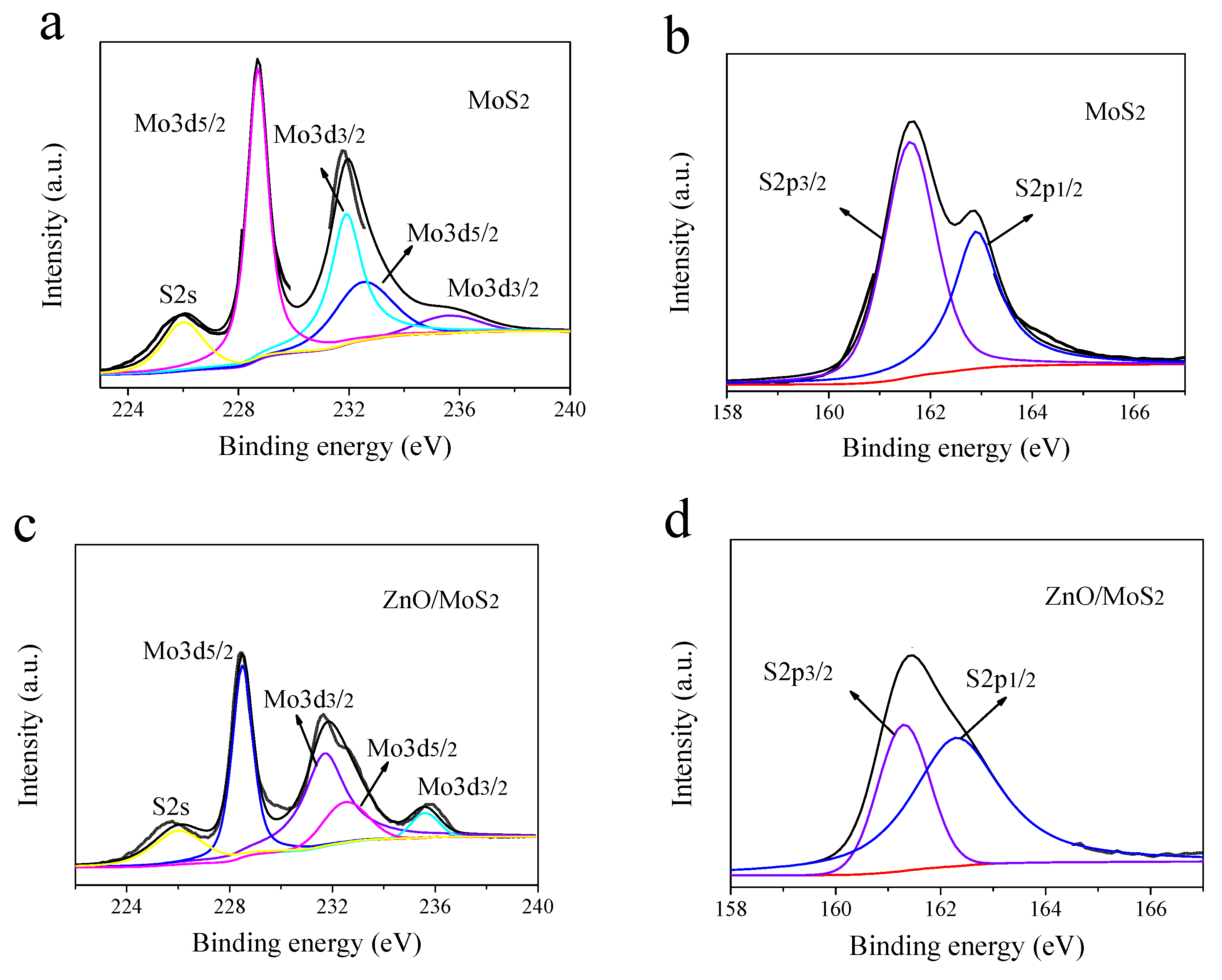
3.1. X-ray Photoelectron Spectroscopy Analysis
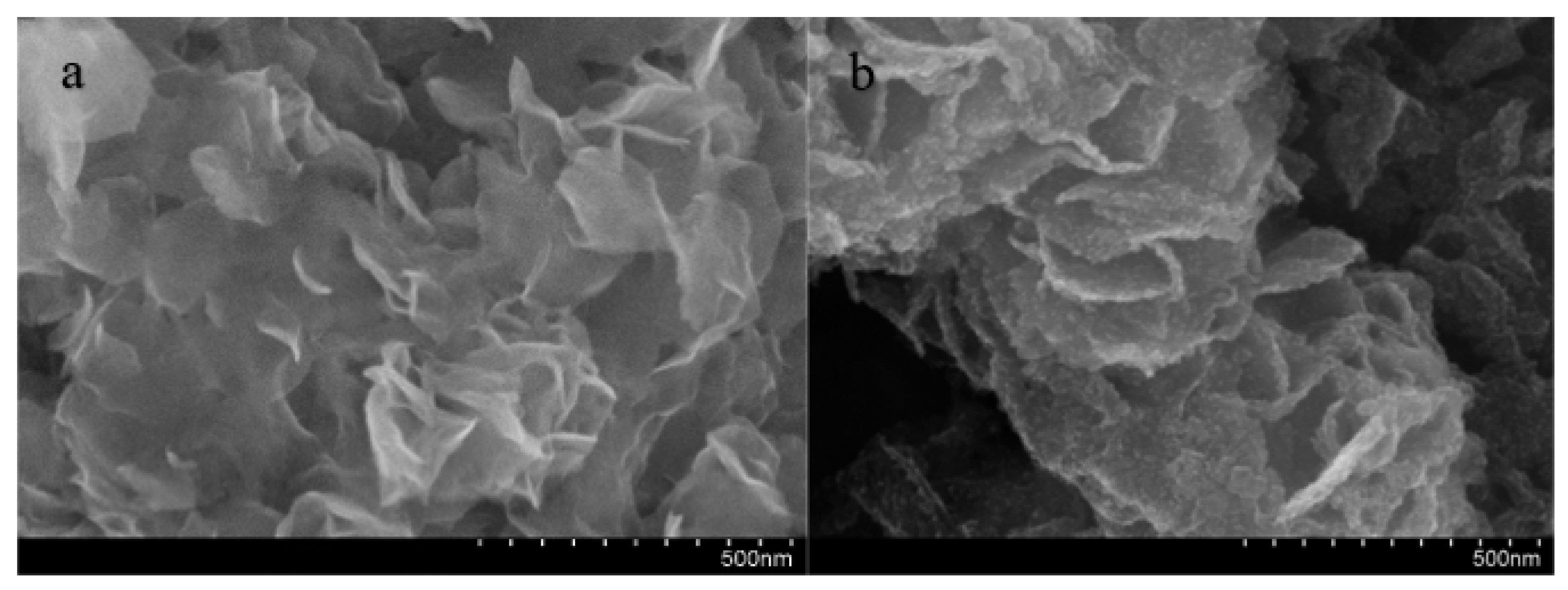
3.2. Microstructure Characterization of the Coating Surface
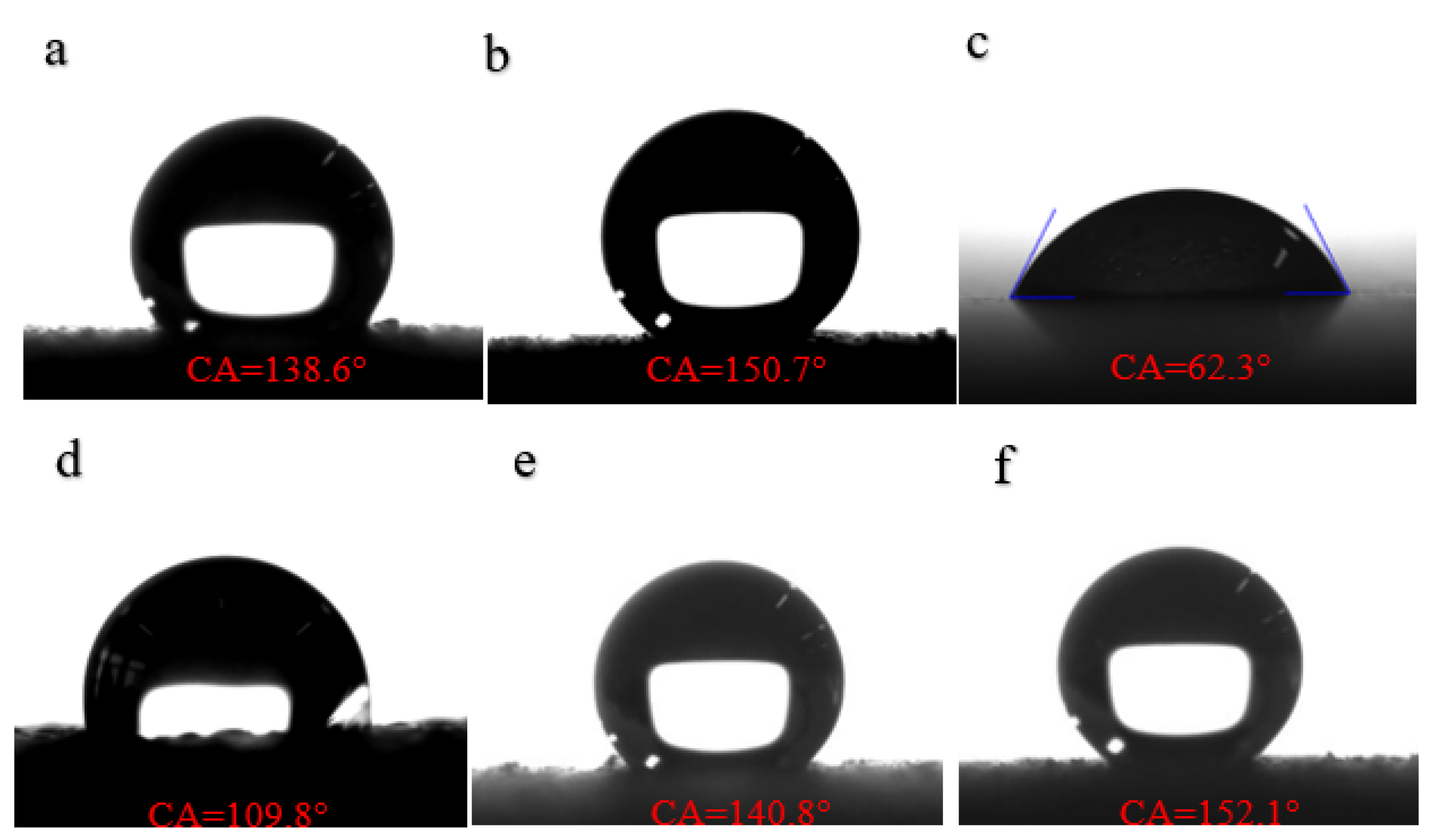
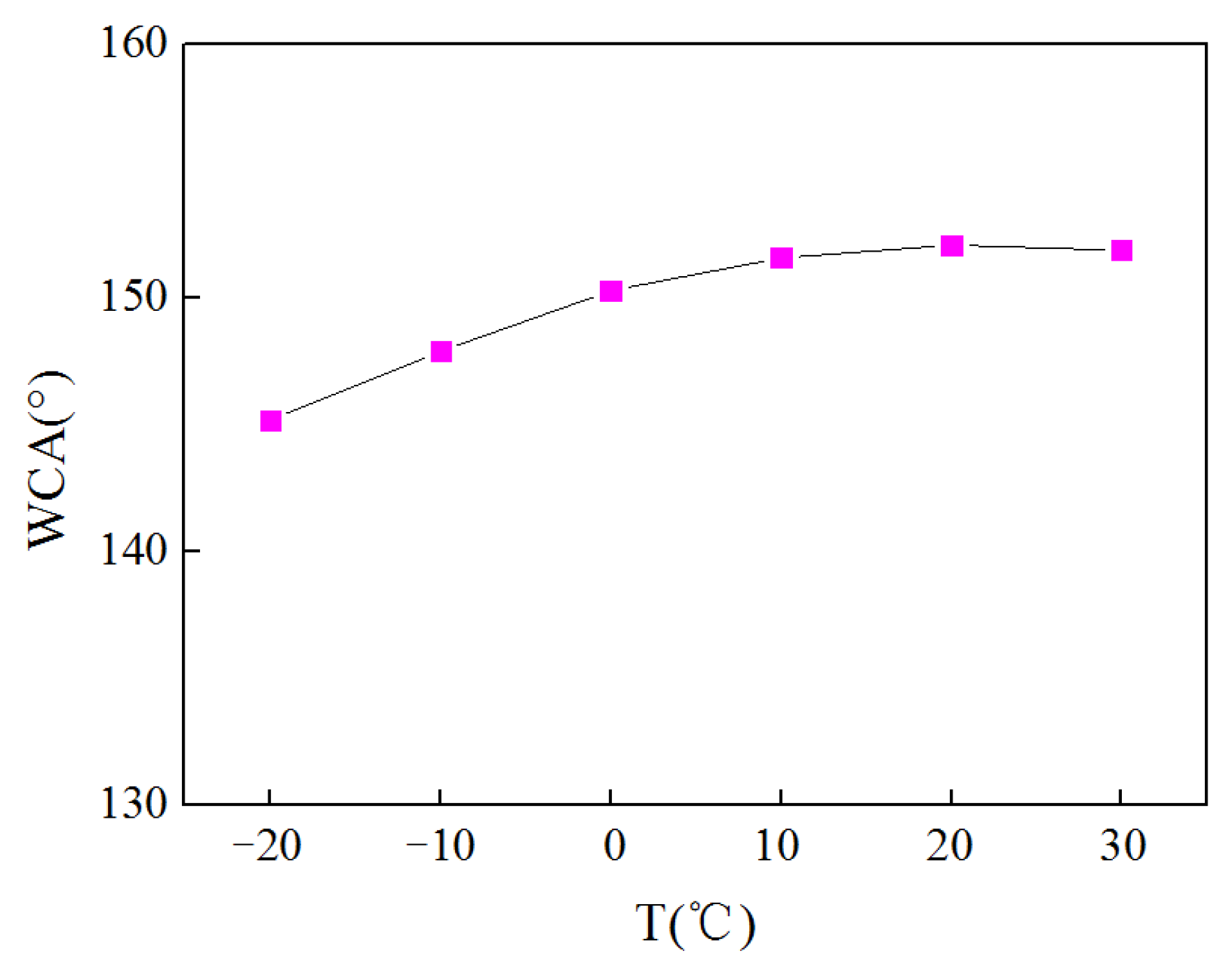
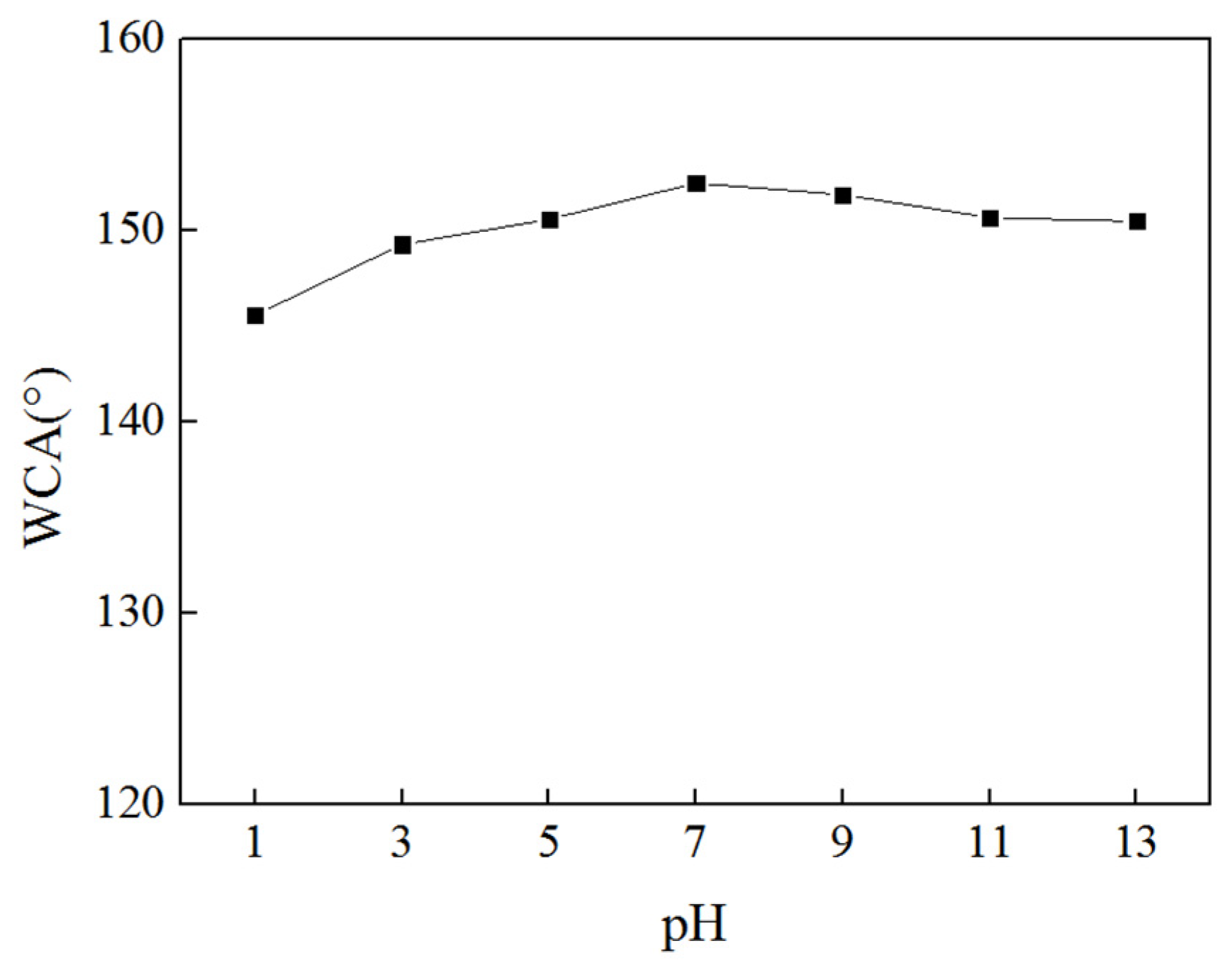
3.3. Coating Hydrophobicity Detection
3.4. Abrasion Resistance Test of Coating
3.5. Icing Adhesion Strength of the Coating
3.6. Chemical Reagent Stability
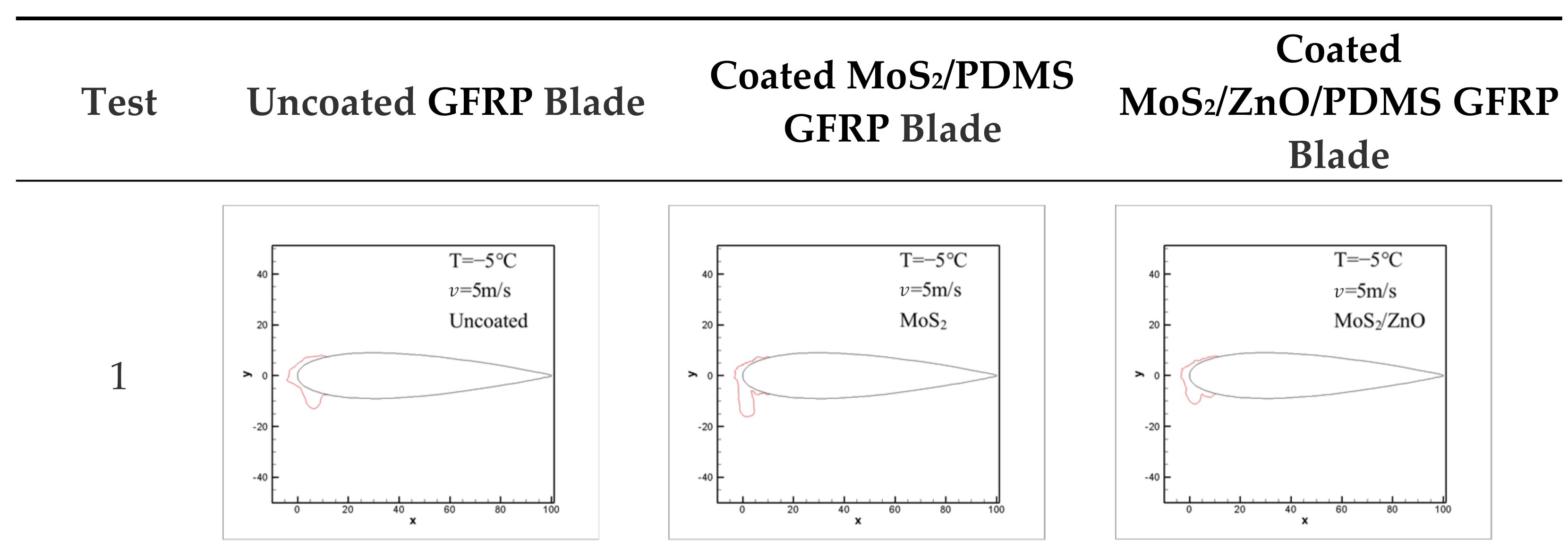
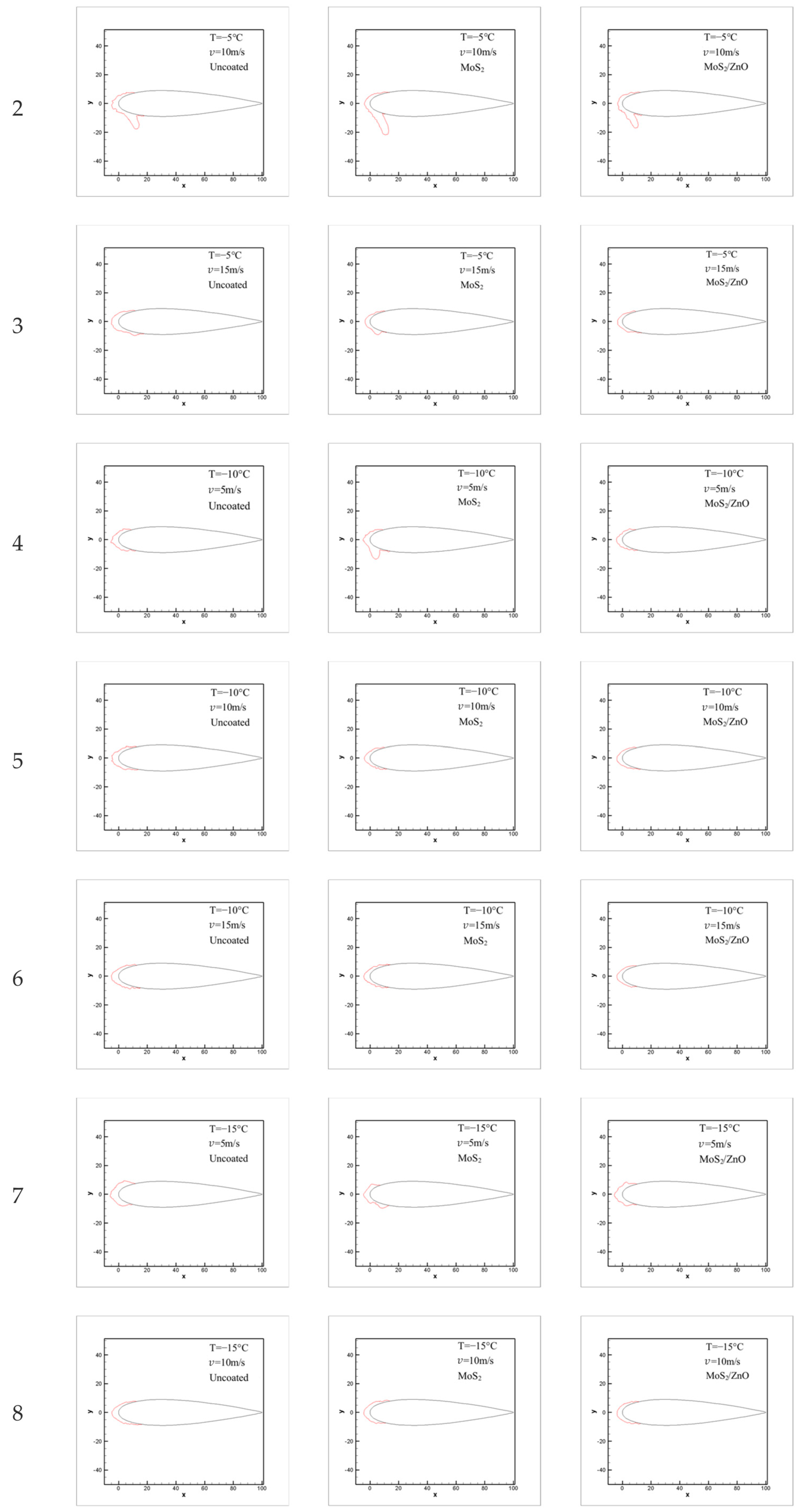
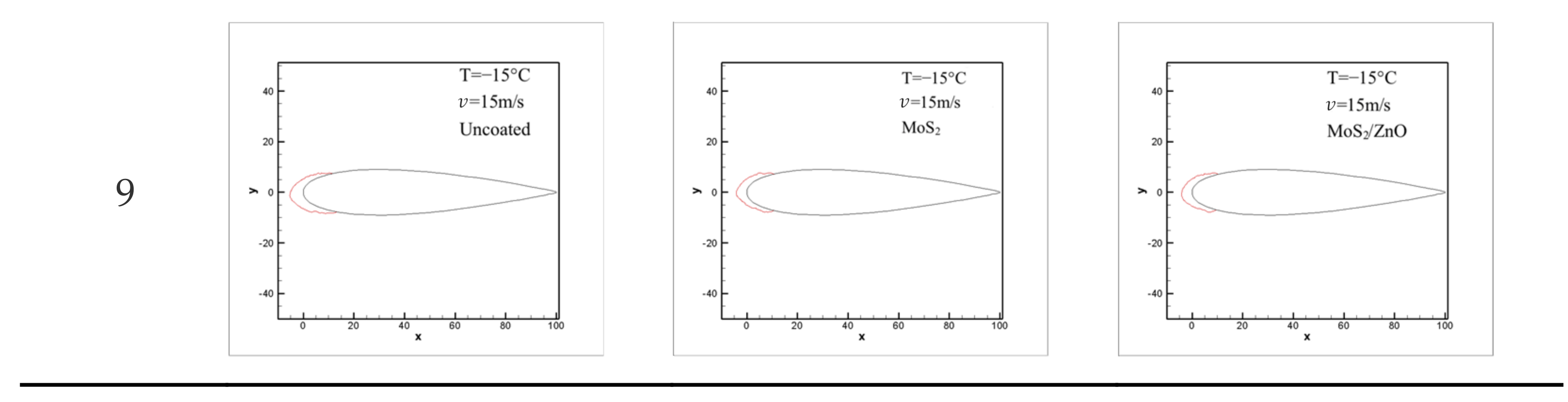
3.7. Icing Distribution on Blade Surface
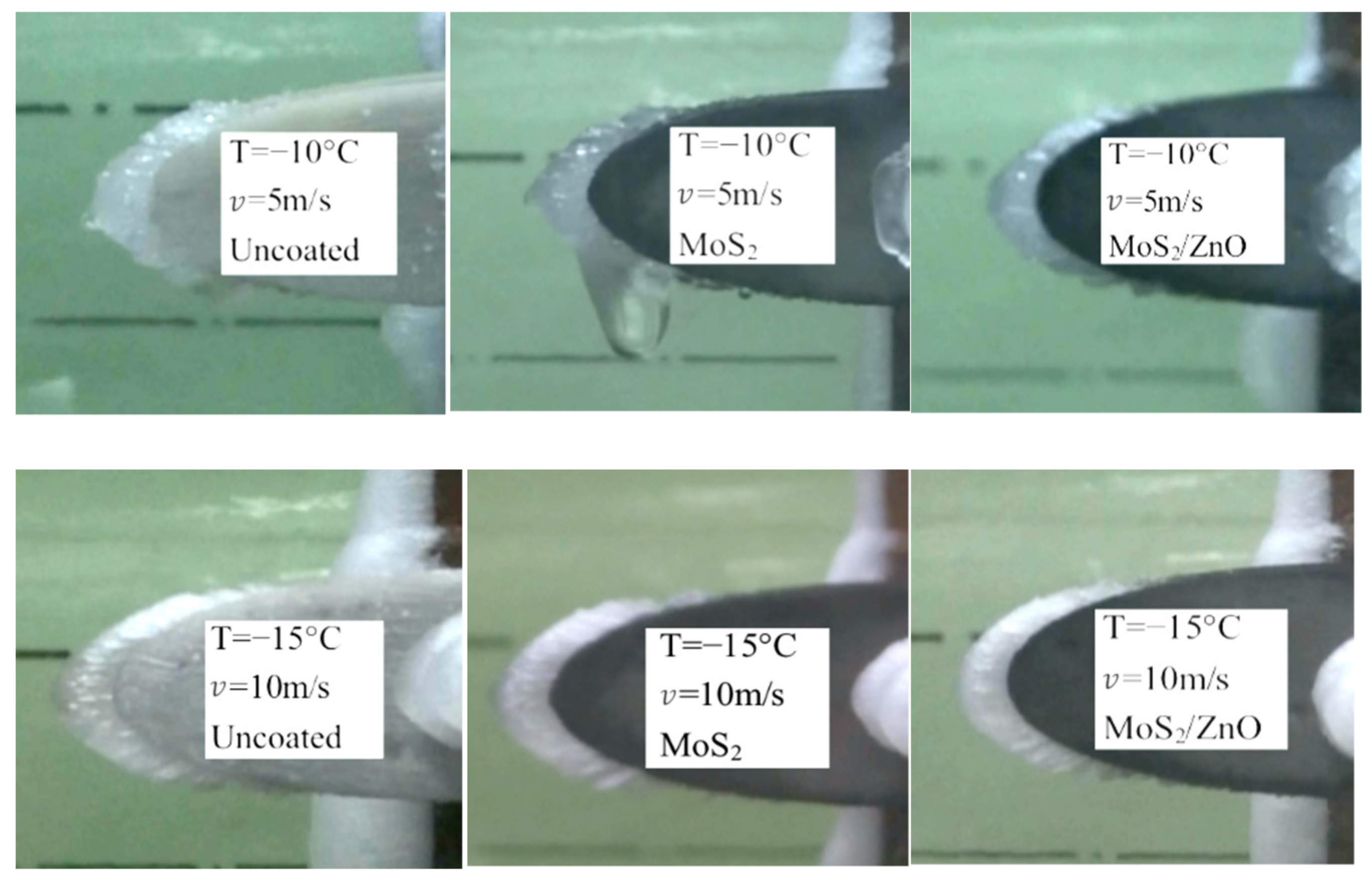
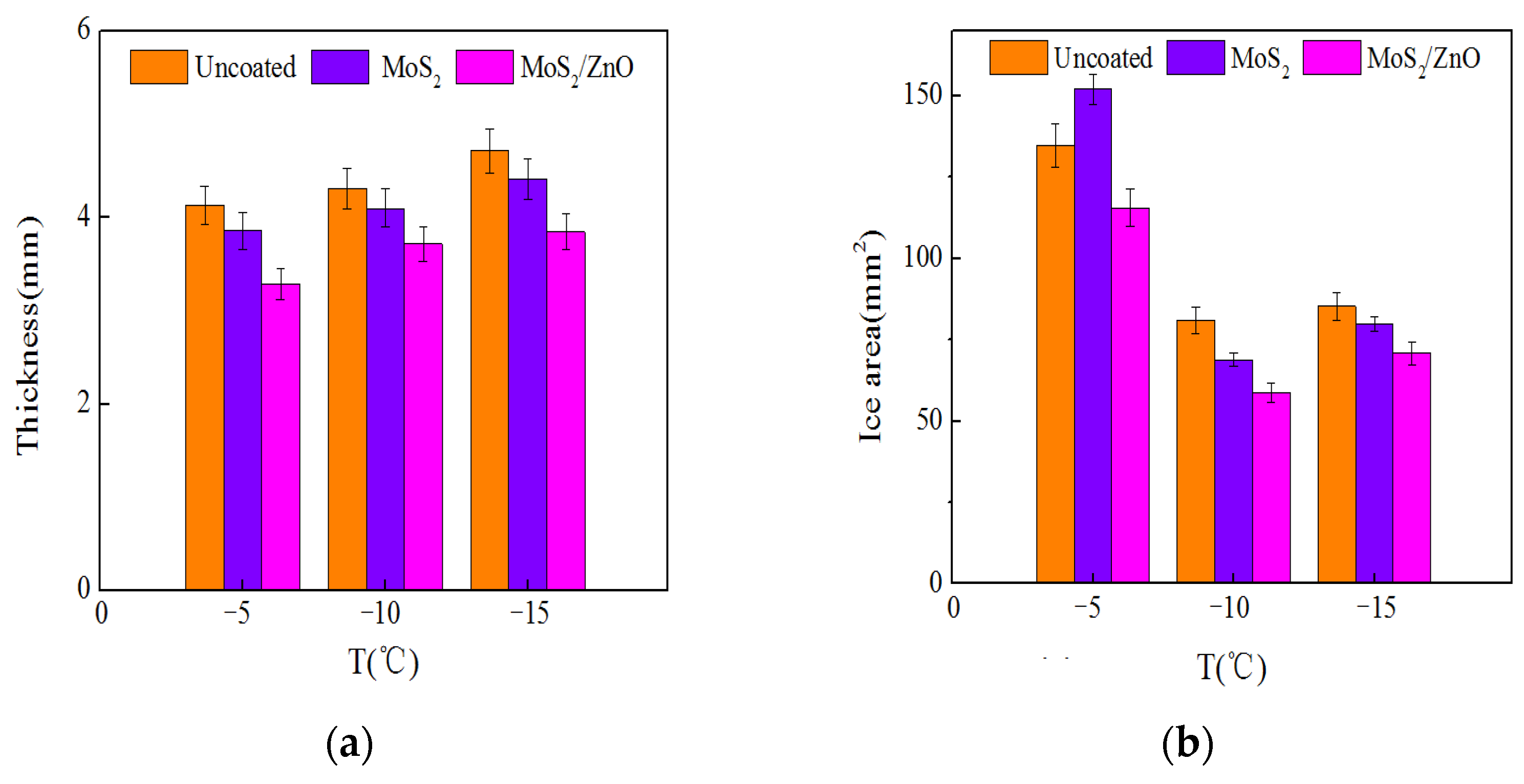
3.8. The Effect of Ambient Temperature on Icing Distribution
3.9. Influence of Wind Speed on Icing Distribution
4. Conclusions
- (1)
- In this study, MoS2/ZnO nano-superhydrophobic material with nano-rough structure on the surface was prepared using the hydrothermal method and the liquid phase method. The surface formed a micro-nano rough structure which was used to construct a superhydrophobic surface. The CA was 152.1°, and the SA was 4.7°.
- (2)
- The surface of the MoS2/ZnO/PDMS nano-superhydrophobic coating was porous and rough and formed a layer of air cushion in contact with liquid, which reduced the adhesion between liquids and the coating surface, thereby reducing the adhesion strength between the coating and ice.
- (3)
- The icing wind tunnel test showed that the MoS2/ZnO/PDMS nanomaterial-coated blade had lower amounts of icing than the uncoated blade under the same conditions. The icing thickness of the leading edge of the blade was decreased by up to 20.4%, and the icing area was reduced by about 28.3%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Shen, H.; Guo, W. Simulation and experimental study on the ultrasonic micro-vibration Deicing method for wind turbine blades. Energies 2021, 14, 8246. [Google Scholar] [CrossRef]
- Guo, W.; Shen, H.; Li, Y.; Feng, F.; Tagawa, K. Wind tunnel tests of the rime icing characteristics of a straight-bladed vertical axis wind turbine. Renew. Energy 2021, 179, 116–132. [Google Scholar] [CrossRef]
- Tong, G.; Li, Y.; Tagawa, K.; Feng, F. Effects of blade airfoil chord length and rotor diameter on aerodynamic performance of straight-bladed vertical axis wind turbines by numerical simulation. Energy 2023, 265, 126325. [Google Scholar] [CrossRef]
- Rashidi, M.M.; Mahariq, I.; Murshid, N.; Wongwises, S.; Mahian, O.; Nazari, M.A. Applying wind energy as a clean source for reverse osmosis desalination: A comprehensive review. Alex. Eng. J. 2022, 61, 12977–12989. [Google Scholar] [CrossRef]
- Zheng, C.W.; Li, C.Y.; Pan, J.; Liu, M.Y.; Xia, L.L. An overview of global ocean wind energy resource evaluations. Renew. Sustain. Energy Rev. 2016, 53, 1240–1251. [Google Scholar] [CrossRef]
- Caccia, F.; Guardone, A. Numerical simulation of ice accretion on wind turbine blades. Wind Energy Sci. Discuss. 2023, 8, 341–362. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Y.; Li, Y.; Tagawa, K.; Zhao, B. A Wind Tunnel Experimental Study on the Icing Characteristics of a Cylinder Rotating around a Vertical Axis. Appl. Sci. 2021, 11, 10383. [Google Scholar] [CrossRef]
- Jiang, F.; Qiu, T. Research on the effect of icing on aerodynamic performance of airfoil and power generation performance of wind turbine. J. Phys. Conf. Ser. 2020, 1684, 012141. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Z.; An, R.; Marklund, P.; Björling, M.; Shi, Y. Novel Intrinsic Self-Healing Poly-Silicone-Urea with Super-Low Ice Adhesion Strength. Small 2022, 18, 2200532. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, F.; Li, Y.; Sun, Y.; Tagawa, K. A corncob biochar-based superhydrophobic photothermal coating with micro-nano-porous rough-structure for ice-phobic properties. Surf. Coat. Technol. 2023, 457, 129299. [Google Scholar] [CrossRef]
- Liao, C.; Yi, Y.; Chen, T.; Cai, C.; Deng, Z.; Song, X.; Lv, C. Detecting Broken Strands in Transmission Lines Based on Pulsed Eddy Current. Metals 2022, 12, 1014. [Google Scholar] [CrossRef]
- Mu, Z.; Li, Y.; Guo, W.; Shen, H.; Tagawa, K. An Experimental Study on Adhesion Strength of Offshore Atmospheric Icing on a Wind Turbine Blade Airfoil. Coatings 2023, 13, 164. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Tian, L.; Hu, H.; Hu, H.; Liu, X.; Hogate, I.; Kohli, A. An experimental study on a hot-air-based anti-/de-icing system for aero-engine inlet guide vanes. Appl. Therm. Eng. 2020, 167, 114778. [Google Scholar] [CrossRef]
- Li, Y.; Shen, H.; Guo, W. Effect of ultrasonic vibration on the surface adhesive characteristic of iced Aluminum alloy plate. Appl. Sci. 2022, 12, 2357. [Google Scholar] [CrossRef]
- He, Z.; Xie, H.; Jamil, M.I.; Li, T.; Zhang, Q. Electro-/Photo-Thermal Promoted Anti-Icing Materials: A New Strategy Combined with Passive Anti-Icing and Active De-Icing. Adv. Mater. Interfaces 2022, 9, 2200275. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Shao, Y.; Wang, Y.; Liu, B. Preparation of a superhydrophobic coating based on polysiloxane modified SiO2 and study on its anti-icing performance. Surf. Coat. Technol. 2022, 437, 128359. [Google Scholar] [CrossRef]
- Rivero, P.J.; Rodriguez, R.J.; Larumbe, S.; Monteserín, M.; Martín, F.; García, A.; Acosta, C.; Clemente, M.J.; García, P.; Mora, J.; et al. Evaluation of functionalized coatings for the prevention of ice accretion by using icing wind tunnel tests. Coatings 2020, 10, 636. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Zhou, J.; Yang, G.; Wang, J.; Liu, D.; Chen, Z.; Lei, W. Solid phase exfoliation for producing dispersible transition metal dichalcogenides nanosheets. Adv. Funct. Mater. 2020, 30, 2004139. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Q.; Lin, Z.; Li, Y.; Jia, X.; Song, H. Growth of ultra-dense MoS2 nanosheets on carbon fibers to improve the mechanical and tribological properties of polyimide composites. Friction 2021, 9, 1150–1162. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Zhang, X.; Zhao, Z.; Zhu, Y. A multifunctional super-hydrophobic coating based on PDA modified MoS2 with anti-corrosion and wear resistance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 239–247. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, physics and applications. ChemPhysChem 2007, 8, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Saiduzzaman, M.; Takei, T.; Kumada, N. Hydrothermal magic for the synthesis of new bismuth oxides. Inorg. Chem. Front. 2021, 8, 2918–2938. [Google Scholar] [CrossRef]
- Ma, X.; Zhuang, J.; Zhao, P.; Zhang, H.; Li, H.; Xie, J.; Rong, J.; Ding, Y.; Wang, G. Hydrothermal synthesis and electrical properties of Co–Mn–Fe–Zn–O NTC nanopowder materials. J. Mater. Sci. Mater. Electron. 2021, 32, 25201–25213. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L.; Ling, Y.; Ge, Y.; Huang, C.; Zhou, S.; Xia, S.; Liang, M.; Zou, H. Enhanced mechanical and adhesive properties of PDMS coatings via in-situ formation of uniformly dispersed epoxy reinforcing phase. Prog. Org. Coat. 2023, 174, 107319. [Google Scholar] [CrossRef]
- Hong, S.; Wang, R.; Huang, X.; Liu, H. Facile one-step fabrication of PHC/PDMS anti-icing coatings with mechanical properties and good durability. Prog. Org. Coat. 2019, 135, 263–269. [Google Scholar] [CrossRef]
- Dhiman, V.; Kondal, N. MoS2–ZnO nanocomposites for photocatalytic energy conversion and solar applications. Phys. B Condens. Matter 2022, 628, 413569. [Google Scholar] [CrossRef]
- Shi, L.; Feng, F.; Guo, W.; Li, Y. Research and development of a small-scale icing wind tunnel test system for blade airfoil icing characteristics. Int. J. Rotating Mach. 2021, 2021, 5598859. [Google Scholar] [CrossRef]
- Selvaraj, R.; Kalimuthu, K.R.; Kalimuthu, V. A type-II MoS2/ZnO heterostructure with enhanced photocatalytic activity. Mater. Lett. 2019, 243, 183–186. [Google Scholar] [CrossRef]
- Krishnan, U.; Kaur, M.; Kaur, G.; Singh, K.; Dogra, A.R.; Kumar, M.; Kumar, A. MoS2/ZnO nanocomposites for efficient photocatalytic degradation of industrial pollutants. Mater. Res. Bull. 2019, 111, 212–221. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Liu, W.; Chen, Y.; Ma, S.; Yun, P. An excellent triethylamine (TEA) sensor based on unique hierarchical MoS2/ZnO composites composed of porous microspheres and nanosheets. Sens. Actuators B Chem. 2021, 333, 129616. [Google Scholar] [CrossRef]
- Liu, H.Q.; Yao, C.B.; Jiang, C.H.; Wang, X. Preparation, modification and nonlinear optical properties of semiconducting MoS2 and MoS2/ZnO composite film. Opt. Laser Technol. 2021, 138, 106905. [Google Scholar] [CrossRef]
- Yan, W.; Sun, J.; Golsanami, N.; Li, M.; Cui, L.; Dong, H.; Sun, Y. Evaluation of wettabilities and pores in tight oil reservoirs by a new experimental design. Fuel 2019, 252, 272–280. [Google Scholar] [CrossRef]







| Reagent Name | Grade | Manufacturer |
|---|---|---|
| Ammonium molybdate ((NH4)6Mo7O24·4H2O) | Analytical reagent (AR) | Tianjin Hengxin Chemical Co., Ltd., Tianjin, China |
| Thiourea (H2NCSNH2) | AR | Tianjin Tianli Chemical Reagent Co., Ltd., Tianjin, China |
| Zinc acetate (Zn(CHCOO)2·2H2O) | AR | Tianjin Tianli Chemical Reagent Co., Ltd. |
| Lithium hydroxide (LiOH·H2O) | AR | Tianjin Fuchen Chemical Reagent Factory, Tianjin, China |
| Absolute alcohol (CH3CH2OH) | AR | Tianjin Fuyu Fine Chemical Co., Ltd., Tianjin, China |
| Polydimethylsiloxane (PDMS, Sylgard 184 base and curing agent) | AR | American Dow Corning Company, Midland, MI, USA |
| n-hexane (C6H14) | AR | Tianjin Fuyu Fine Chemical Co., Ltd., Tianjin, China |
| Test Conditions | Ambient Temperature (°C) | Liquid Water Content (g/m3) | Water Droplet Diameter (μm) | Wind Velocity (m/s) | Time (min) |
|---|---|---|---|---|---|
| 1 | −5 | 0.5~1 | 50~70 | 5 | 3 |
| 2 | 10 | ||||
| 3 | 15 | ||||
| 4 | −10 | 5 | |||
| 5 | 10 | ||||
| 6 | 15 | ||||
| 7 | −15 | 5 | |||
| 8 | 10 | ||||
| 9 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Liu, Z.; Li, Y.; Feng, F. A Wind Tunnel Test of the Anti-Icing Properties of MoS2/ZnO Hydrophobic Nano-Coatings for Wind Turbine Blades. Coatings 2023, 13, 686. https://doi.org/10.3390/coatings13040686
Liu B, Liu Z, Li Y, Feng F. A Wind Tunnel Test of the Anti-Icing Properties of MoS2/ZnO Hydrophobic Nano-Coatings for Wind Turbine Blades. Coatings. 2023; 13(4):686. https://doi.org/10.3390/coatings13040686
Chicago/Turabian StyleLiu, Bo, Zhiyuan Liu, Yan Li, and Fang Feng. 2023. "A Wind Tunnel Test of the Anti-Icing Properties of MoS2/ZnO Hydrophobic Nano-Coatings for Wind Turbine Blades" Coatings 13, no. 4: 686. https://doi.org/10.3390/coatings13040686
APA StyleLiu, B., Liu, Z., Li, Y., & Feng, F. (2023). A Wind Tunnel Test of the Anti-Icing Properties of MoS2/ZnO Hydrophobic Nano-Coatings for Wind Turbine Blades. Coatings, 13(4), 686. https://doi.org/10.3390/coatings13040686







