Abstract
Slippery liquid-infused porous surfaces (SLIPS) have received growing attention as promising icephobic materials. In this study, SLIPS were prepared on aluminum alloys by combining anodization and infusion of common silicone oil. An SLIPS with low ice-adhesion strength (6 kPa) was obtained by optimizing the anodizing time parameters (10 min). In addition, the frosting process and freezing of water droplets on the as-prepared SLIPS at −10 °C were delayed for 2000 s and 4800 s, respectively. Simultaneously, the as-prepared SLIPS also exhibited excellent anti-icing performance in glaze ice, since the supercooled water drips/ice slipped from the surface. The ice weight of the as-prepared SLIPS was significantly lower than that of the bare aluminum surface and the anti-icing-fluid-coated aluminum surface, which was reduced by 38.2%–63.6% compared with the bare aluminum surface. The ice weight increased with decreased temperature and inclination angle. This work proposes a method suitable for large-area preparation of SLIPS that achieves excellent anti-icing performance and significantly reduces the weight of glaze ice.
1. Introduction
Icing on outdoor infrastructure during extreme cold waves can result in equipment malfunctions and serious security accidents [1,2,3,4]. The breakdown caused by the icing of transmission lines is a serious disaster in power systems. Ice accretion on transmission lines causes electric accidents, such as short circuits, line breaking and tower collapse, causing prolonged power outages [1,2,5,6]. Ice accretion may occur on exposed aircraft surfaces because of the impingement of supercooled droplets in clouds [3,7]. Icing poses a seriously threat to flight safety and leads to large economic losses. According to statistics, about 41% of weather-related plane accidents were caused by icing over the past 30 years [8,9].
In recent years, many anti-icing strategies have been studied and reported, consisting of active and passive methods. Active approaches include overcurrent ice melting, vapor heating, the use of anti-icing liquid and mechanical deicing [1,4,8,10]. However, these active anti-icing strategies have many disadvantages, such as high energy consumption, the requirement for complex equipment an environmentally unfriendly nature, etc. [11,12]. A passive anti-icing method involves constructing a surface to prevent icing or reduce ice-adhesion strength [13]. Inspired by the “lotus effect”, superhydrophobic surfaces (SHSs) with extreme water repellency have attracted much attention [12,14,15]. SHSs have been proven to reduced ice formation and adhesion in the laboratory. However, it also been shown that the water repellency is reduced if water vapor condenses on the rough structure of SHSs [15]. Furthermore, SHSs increase ice adhesion due to the mechanical interlocking effect of ice with the rough structure in low-temperature and high-humidity environments [16,17]. This problem can be solved by slippery liquid-infused porous surfaces (SLIPS). Inspired by nepenthes, SLIPS create a smooth liquid surface with impregnated lubricant in porous nanostructures and have been considered promising icephobic surfaces [18,19,20]. SLIPS can reduce the nucleation of supercooled water to achieve a chemically homogeneous and molecularly smooth liquid interface [21,22,23]. Additionally, supercooled water drips and ice can easily slip from SLIPS, owing to low adhesion strength [18]. Three conditions must be met to construct SLIPS: (1) The chemical affinity between the lubricant and substrate must be greater than that between the water and the substrate. (2) The lubricant must be immiscible with water. (3) The lubricant must be easily penetrated and locked into the porous substrate. The characteristics of both the microstructure and lubricant are critical factors to consider when preparing SLIPS for anti-icing. Studies have shown that the microstructure of SLIPS is an important factor for anti-icing [21]. Uniform nanostructures are beneficial to retain lubricant because of increased Laplace pressure and capillarity. Varanasi et al. [24] reported that ice-adhesion strength on a slippery surface with a stable lubricant layer depends on texture and that this ice adhesion decreases with increased textural density. Liu et al. [25] prepared a network structure with interconnected microchannels and crosslinked nanosheets, which presenting excellent oil retention performance, while a hierarchical structure with larger features results in lubricant exposed at the interface, which leads to increased pinning [15]. For the lubricants used in SLIPS, parameters such as viscosity and the type of lubricant affect anti-icing performance. The lubricants used in SLIPS are mainly organic fluids, such as perfluoropolyether or silicon oils. Research conducted by Yuan et al. [19] suggests that the ice-adhesion strength of SLIPS decreases with decreasing silicone oil viscosity, while the effect on durability it the opposite. Many methods to prepare the porous structure of SLIPS have been developed, such as electrochemical deposition [15], chemical etching [26], flame plating [27] and self-assembly [28]. However, most methods are not suitable for large-area preparation of SLIPS on aluminum alloys with complex shapes, such as aluminum conductors and aluminum alloy parts of aircraft. Anodic oxidation is the most convenient and low-cost method for preparing porous structures, which is suitable for large-area preparation of SLIPS on aluminum alloys. The parameters (pore size, porosity and roughness) of porous surfaces are critical for the anti-icing performance of SLIPS. Furthermore, recent research on SLIPS has mainly focused on reducing the ice-adhesion strength [29,30]. The actual icing conditions are extremely abominable. There are three forms of atmospheric icing: glaze ice, rime, and frost and snow. Glaze ice is the most serious threat to outdoor equipment, owing to its high density and strong adhesion [31,32]. Consequently, the anti-icing properties of SLIPS need to be investigated comprehensively by ice-adhesion strength tests, anti-freezing tests and artificial glaze icing simulations. This work aims to obtain SLIPS with excellent anti-icing performance on aluminum alloys and investigate their anti-icing performance under static freezing and glaze ice conditions.
In this work, we fabricated SLIPS on aluminum alloys by combining anodization and infusion of silicone oil. The optimal porous surface was obtained on the substrate by changing the anodizing time. The as-prepared SLIPS exhibited low ice-adhesion strength (6 kPa). The frosting and freezing of water drops on SLIPS was effectively delayed. The as-prepared SLIPS has an excellent low ice-adhesion strength, which caused ice automatic slipping from the SLIPS. The as-prepared SLIPS can effectively delay the icing process and reduces the weight of ice under glaze conditions. In general, this paper proposes a rapidly prepared, economical and effective SLIPS, which is of great significance to the practical application of SLIPS in anti-icing.
2. Materials and Methods
2.1. Materials
Commercial aluminum alloy plates (7075-T651) were purchased from Dongguan Chaomei Aluminum Product Co., Ltd. (Dongguan, China), and cut into 300 mm × 200 mm × 1.5 mm pieces. Sodium hydroxide (NaOH), phosphoric acid (H3PO4), pure water and ethanol were purchased from Chongqing Huadong Chemical Co., Ltd., China. Hexadecyltrimethoxysilane (HDTMS) and silicone oil (50 mPa·s) were supplied by Aladdin Reagent Co., Ltd., Shanghai, China. Anti-icing fluid was purchased from Tianjin Haoming Chemical Co., Ltd., China.
2.2. Sample Preparation
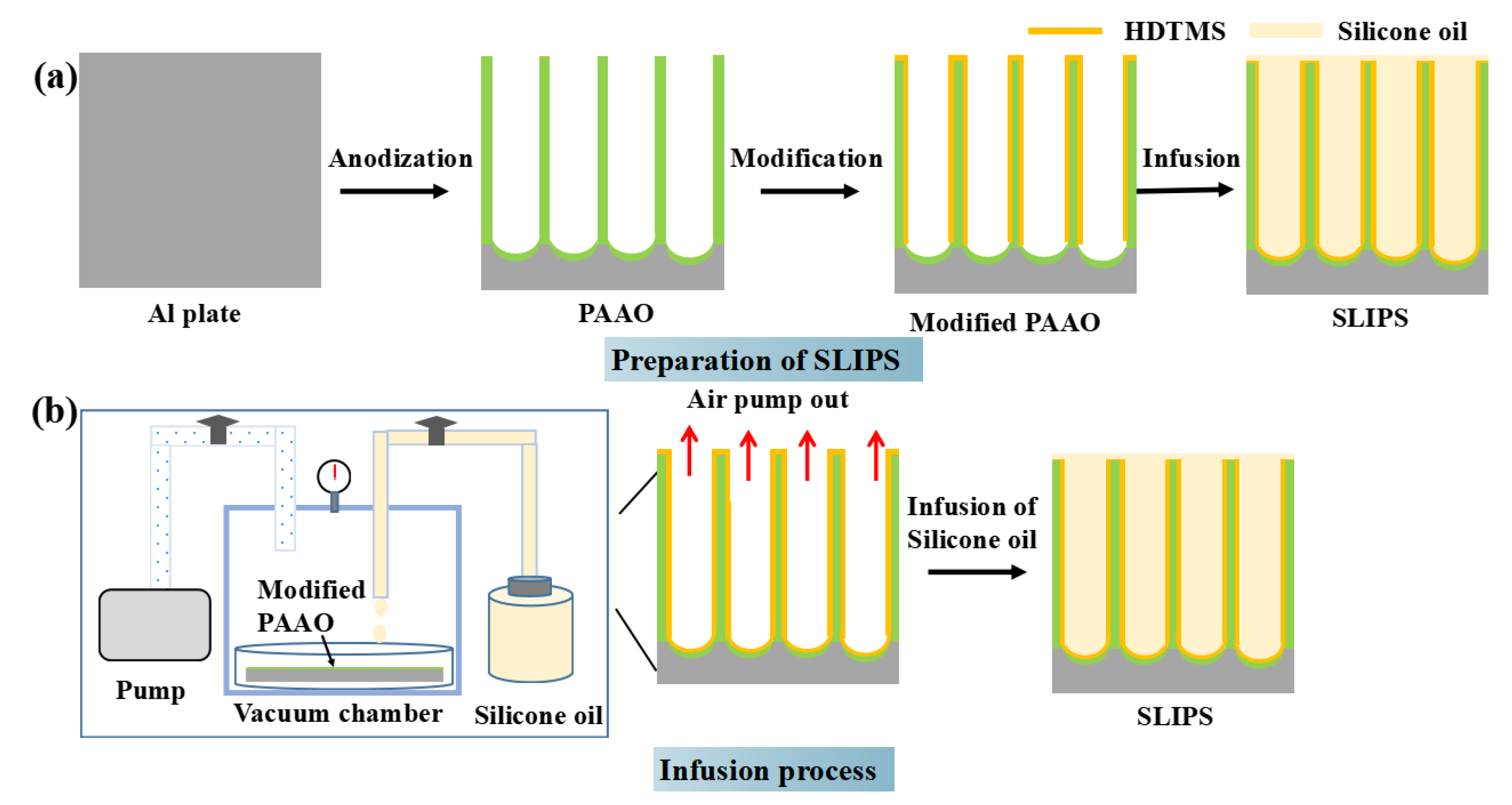
The preparation of SLIPS is illustrated in Figure 1. First, aluminum plates were washed ultrasonically with ethanol for 10 min. Then, the samples were dipped in 1 mol/L NaOH solution at 25 °C for 2 min and washed ultrasonically with pure water to remove the natural oxide film. Secondly, the porous anodic aluminum oxide (PAAO) layers were prepared by anodization at a constant current density of 0.025 A/cm2 in 0.3 M phosphoric acid solution at 20 °C for 5~20 min powered by a direct-current power supply. The anodization voltage was 98 V~120 V. Then, the PAAO layers were washed ultrasonically with pure water for 10 min. Thirdly, the PAAO layers were modified by an ethanol solution of 2 wt% HDTMS for 30 min and cured at 90 °C for 10 min to improve the hydrophobicity and compatibility [21]. Fourth, a SLIPS was obtained by the vacuum impregnation method, as shown in Figure 1. In detail, the sample was placed in a vacuum chamber for 30 min and subsequently evacuated to remove the air from the nanopores. Then, the liquid valve was opened, and the silicone oil was pumped into a container containing the modified PAAO. The sample was immersed in silicone oil under a pressure of −0.1 MPa for 4 h. The sample was placed vertically to drain the excess silicone oil on the sample surface. Finally, the SLIPS were successfully prepared. The three different SLIPS were named SLIPS-5, SLIPS-10 and SLIPS-20, with anodizing times of 5 min, 10 min and 20 min, respectively. The anti-icing-fluid-coated sample was prepared by immersing the aluminum plates in aviation anti-icing fluid whose the main component was propylene glycol.
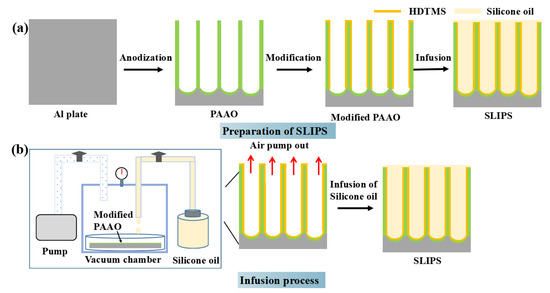
Figure 1.
Process diagram for the fabrication of slippery liquid-infused porous surfaces (SLIPS) (a), the infusion device and the complete infusion process of silane oil (b).
2.3. Characterization
The surface morphology was determined and PAAO elemental analysis was conducted using a scanning electron microscope (SEM, Zeiss Auriga, Oberkochen, Germany) equipped with an EDS. The crystal structure of PAAO was characterized by X-ray diffraction (XRD, Panalytical Empyrea, Almelo, The Netherlands). The topography and roughness of PAAO were investigated by a laser scanning confocal microscope (LSCM, LEXT OLS4000, Olympus, Tokyo, Japan). The element composition of the SLIPS was studied using a Fourier transform infrared spectroscope (FTIR, Nicolet iS50, MA, USA). The wettability of SLIPS at low temperatures was measured using a CA meter (SDC-100, Sindin, Dongguan, China) and a semiconductor refrigerator Peltier-based cooling stage with the temperature of the cooling stage set at −10 °C, the temperature of the water droplets set a about 2 °C and a 10 μL volume of water droplets.
The ice-adhesion strength of SLIPS was tested by a horizontal shear test. The ice-adhesion test platform comprised a semiconductor refrigerator Peltier-based cooling stage (temperature control range: −25~80 °C; control accuracy: ±0.1 °C), an XY motion stage, a force transducer (Handpi, HP-200, Tianjin, China) and a plastic cylinder (inner diameter = 14 mm) filled with DI-water (2 mL). All ice-adhesion strength tests were carried out at −20 °C, with icing for 90 min. After the water in the plastic cylinder was frozen, the XY motion stage was moved at a speed of 1 mm/s until the cylinder shifted on the surface, at which point we recorded the value of the force transducer. The ice-adhesion strength was calculated by dividing the measured maximum force by the cross-section area of the ice–sample interface. The average ice-adhesion strength was acquired by measuring the sample 5 times.
The antifrosting and antifreezing performances of SLIPS were investigated on a Peltier-based cooling platform. The temperature of the cooling plate was set to −10 °C. The relative environmental humidity was about 85%. Images of the frost formation and freezing of water drops were recorded by a camera. The volume of water droplets on the cooling platform was 10 μL. The anti-icing performance of SLIPS in glaze ice was studied using a multifunctional artificial climate chamber (1 m × 1 m × 1 m; ambient temperature control range: 80~−40 °C; wind speed: 0–8 m/s). Water at a temperature of 1–2 °C was sprayed onto the sample surface. The diameter of sprayed water was about 100 μm, and the relative humidity was 85 ± 5%. The spraying density was 60 Lh−1m−2.
3. Results and Discussion
3.1. Surface Morphology and Chemical Composition
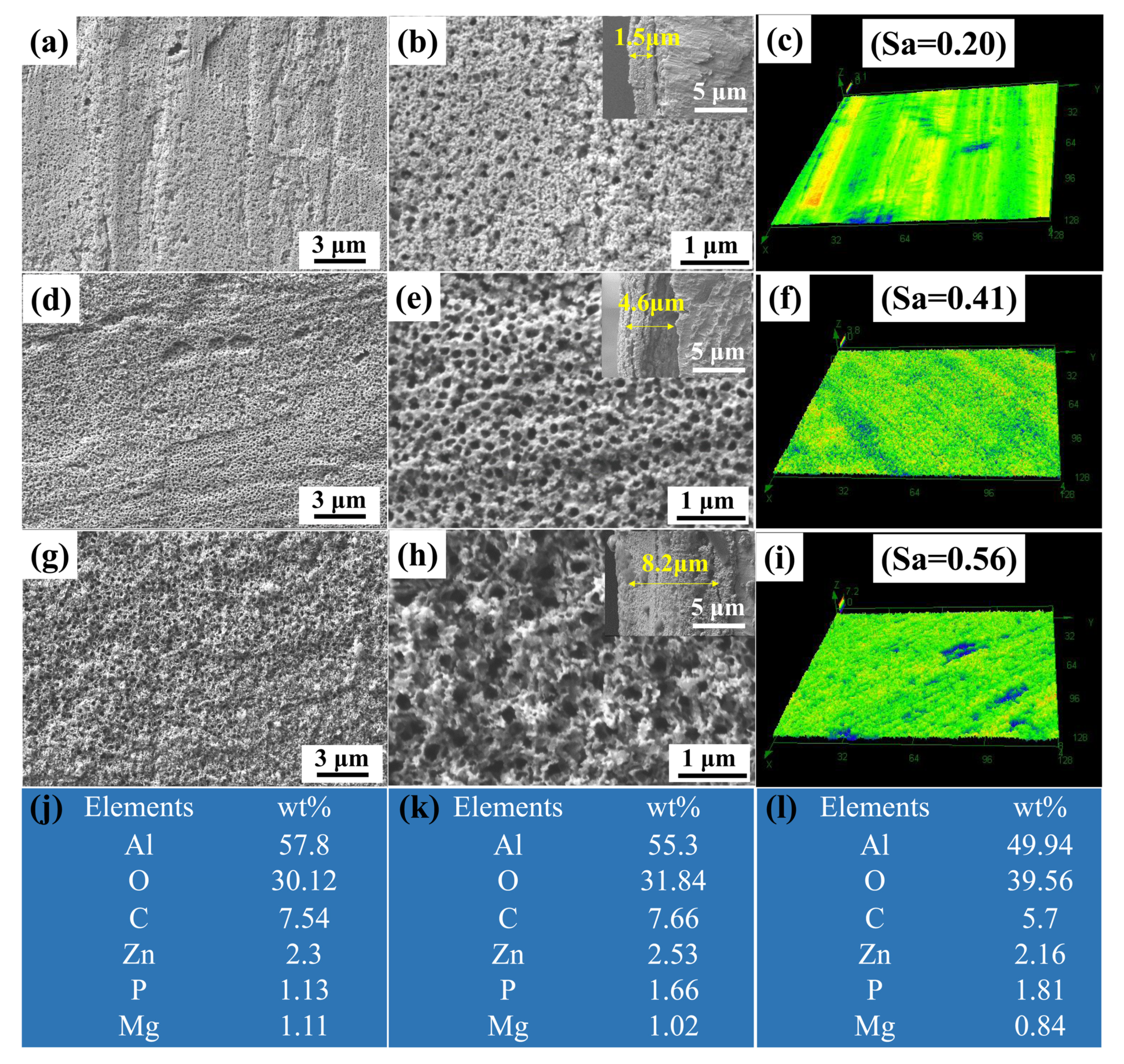
The anodizing time has a great impact on the surface morphology and the pore structure of PAAO film, such as pore size, porosity and film thickness. Phosphoric acid was used as the electrolyte of anodization. The main reason for the selection of phosphoric acid as the electrolyte is that PAAO prepared by phosphoric acids has characteristics of high porosity and large size, which are favorable for storing more lubricant. The morphologies of the as-prepared anodic oxide film with different anodizing times are shown in Figure 2. The porous anodic aluminum oxide (PAAO) surface with an anodizing time of 5 min was composed of sparse pores, as shown in Figure 2a,b. The average pore size and pore depth were 78 nm and 1.5 μm, respectively. The pore size of the surface was increased with an increase in anodizing time. As shown in Figure 2d,e, a compact and uniform pore structure was obtained after 10 min of anodizing. The average pore size increased to 127 nm. The pore depth was 4.6 μm. As the anodizing time was increased to 20 min, the surfaces become rough, and the pore size significantly increased to 226 nm. The pore depth was increased to 8.2 μm when the anodizing time increased to 20 min. The topography and surface roughness of PAAO were investigated by a laser scanning confocal microscope. As shown in Figure 2c, a flat structure with an average roughness (Sa) of 0.2 μm was obtained after 5 min of anodizing. There was a dense and uniform nanostructure with an Sa of 0.41 μm after 10 min of anodizing, as shown in Figure 2f. The surface become rougher, and the surface roughness was increased to 0.56 um within 20 min of anodization, as show in Figure 2i. Energy-dispersive spectrometric (EDS) analysis was conducted to confirm the components of PAAO, the results of which are shown in Figure 2j–l. EDS spectra of the PAAO surface mainly contained Al, O, C, Zn, P and Mg elements. The O content of the PAAO surface increased with anodizing time, indicating an increase in the content of alumina.
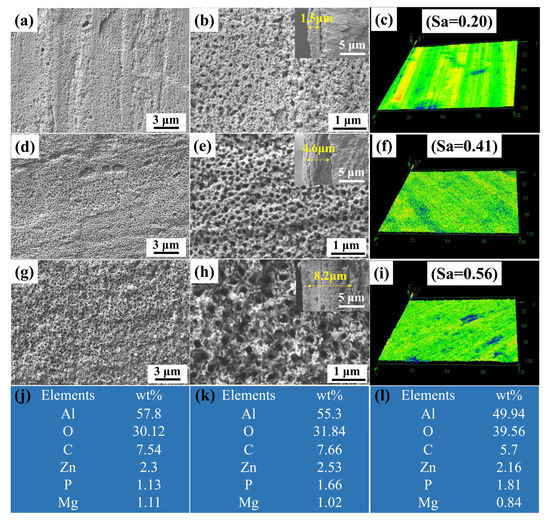
Figure 2.
SEM images of anodic oxide film: (a,b) 5 min, (d,e) 10 min and (g,h) 20 min; the insets show cross-section SEM images of anodic oxide films. Laser scanning confocal microscopic images of anodic oxide films: (c) 5 min, (f) 10 min and (i) 20 min. EDS spectra of anodic oxide films: (j) 5 min, (k) 10 min and (l) 20 min.
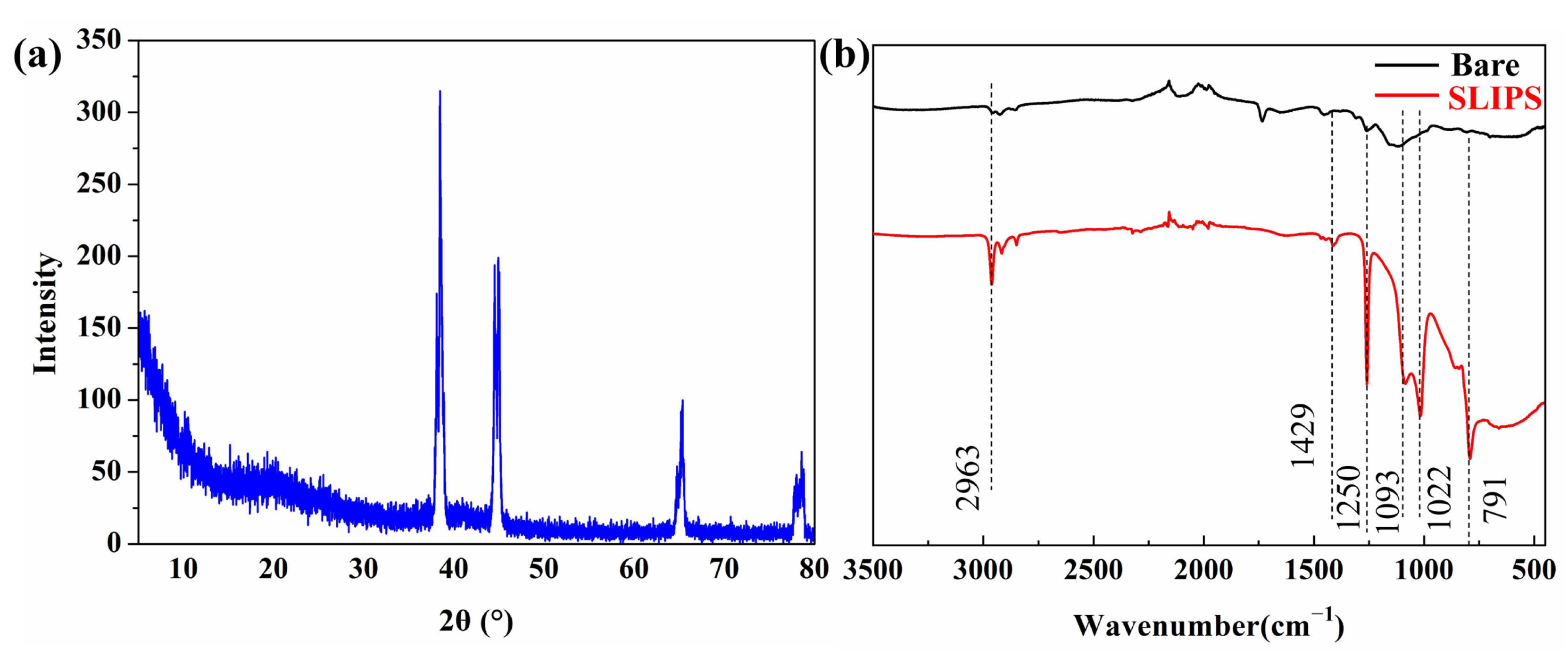
XRD was used to analyze the phase of PAAO. Figure 3a shows the XRD patterns of PAAO with an anodizing time of 10 min. The peaks centered at 2θ = 38.5°, 2θ = 44.6°, 2θ = 65.4° and 2θ = 78.5° correspond to the (111), (200), (220) and (311) planes of Al (JCPDS Card No.04-Z0787), respectively. The halo peaks at 2θ = 20°~38° were caused by the amorphous-phase aluminum oxide generated by anodic oxidation. XRD results suggest that a PAAO film was successfully formed on the aluminum substrate. FTIR was employed to analyze the functional groups of the bare aluminum and SLIPS, the result of which is exhibited in Figure 3. There was no obvious peak in spectroscopic images of the bare aluminum surface. For SLIPS, the absorption peaks at 2963 and 1250 cm−1 are related to the stretching vibration of —CH3 [33]. The peaks at 1093 and 1022 cm−1 were assigned to the stretching vibration of Si–O [34]. The vibration signals of the [-O-Si-(CH3)2-]n structure appearing at 791 and 1429 cm−1 could be related to the -CH2- bending of silicone oil [33]. The FTIR result suggests the presence of silicone oil in SLIPS.
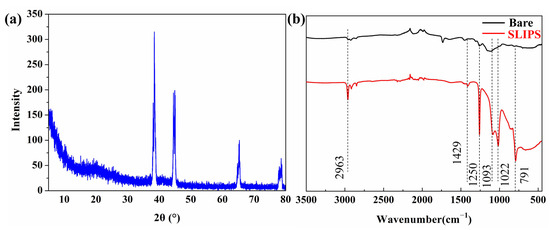
Figure 3.
XRD spectrum of porous anodic aluminum oxide (PAAO) (a) and the FTIR spectrum of a bare aluminum alloy surface and SLIPS (b).
3.2. Ice Adhesion and Antifreezing
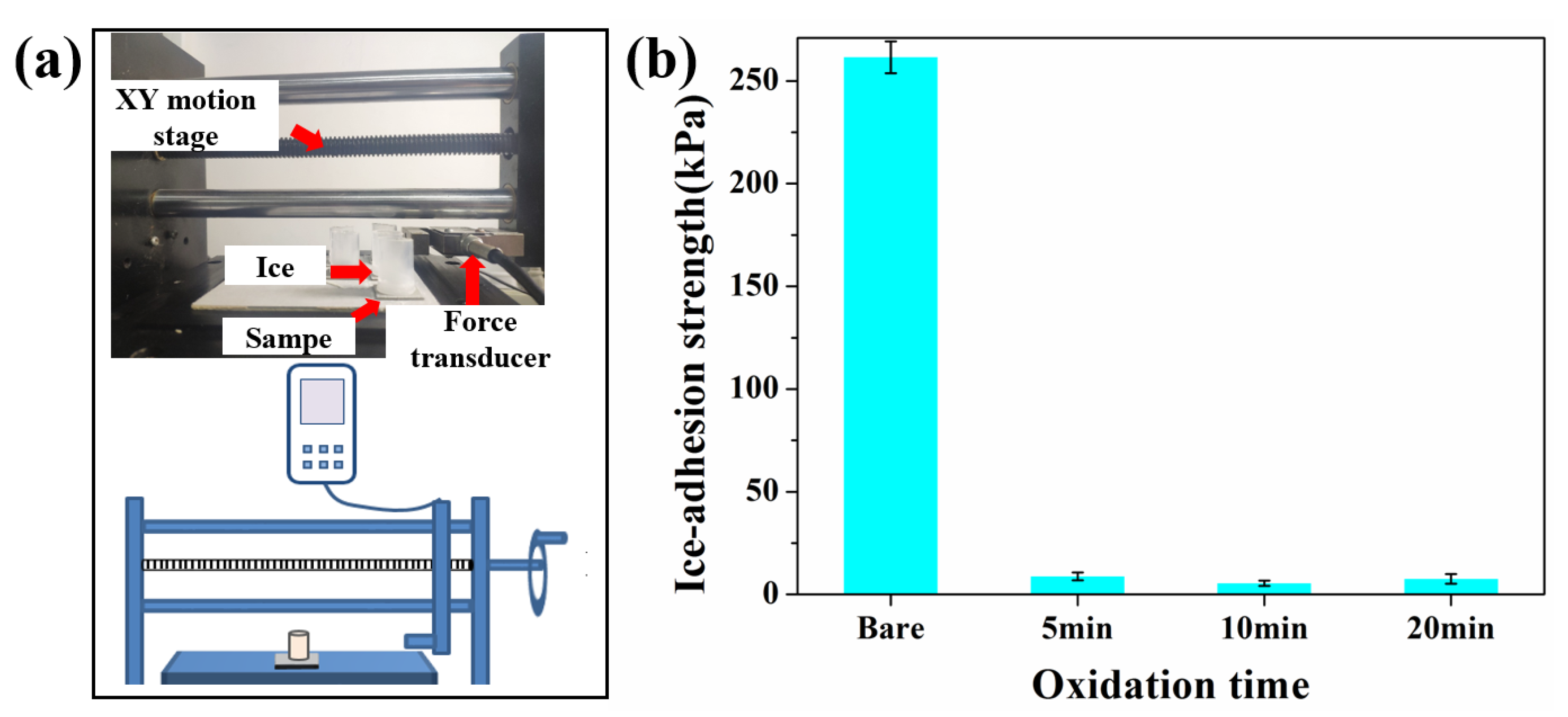
Supercooled raindrops inevitably freeze when they come in contact with a sub-zero-temperature surface. Ice-adhesion strength (τice) is a vital parameter for an anti-icing surface, which estimates how easily ice can be detached from the surface. Research suggests that icephobia is generally used to describe surfaces for which τice < 100 kPa. Ice can drop automatically under its own weight or other natural factors without human intervention when τice < 20 kPa [11]. The τice was measured by an ice-adhesion test platform, as shown in Figure 4a. The ice-adhesion strength of bare aluminum was 261 kPa, while the ice-adhesion strength of SLIPS was significantly decreased, as shown in Figure 4b. The ice-adhesion strength of SLIPS-5, SLIPS-10 and SLIPS-20 was 9.2 kPa, 6 kPa and 7.1 kPa, respectively. SLIPS-10 exhibited the lowest ice-adhesion strength, which was reduced by 97.7% compared with the bare aluminum surface, showing that the compact and uniform pore structure was conducive to the formation of a uniform lubricating oil layer on the surface, which reduces the interlock between ice and SLIPS [35]. Table 1 shows a comparison of ice-adhesion strength between this study and the results reported in [36,37,38,39]. Therefore, the SLIPS prepared in this study had lower ice-adhesion strength. We attribute such low ice-adhesion strength to the ultrasmooth solid-liquid interface present on the SLIPS, with significantly fewer heterogeneities and pinning points than the solid–solid interface found on bare aluminum surfaces [18,19,40]. In summary, according to the results of SEM and ice-adhesion strength, 10 min of anodizing time is regarded as an appropriate parameter for fabricating SLIPS. Consequently, SLIPS-10 was used as a typical sample for the following experiments.
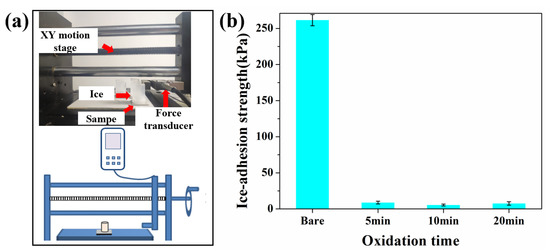
Figure 4.
(a) Ice-adhesion strength test device. (b) Ice-adhesion strength of SLIPS with different anodizing times.

Table 1.
Comparison of the ice-adhesion strength of SLIPS prepared by different methods.
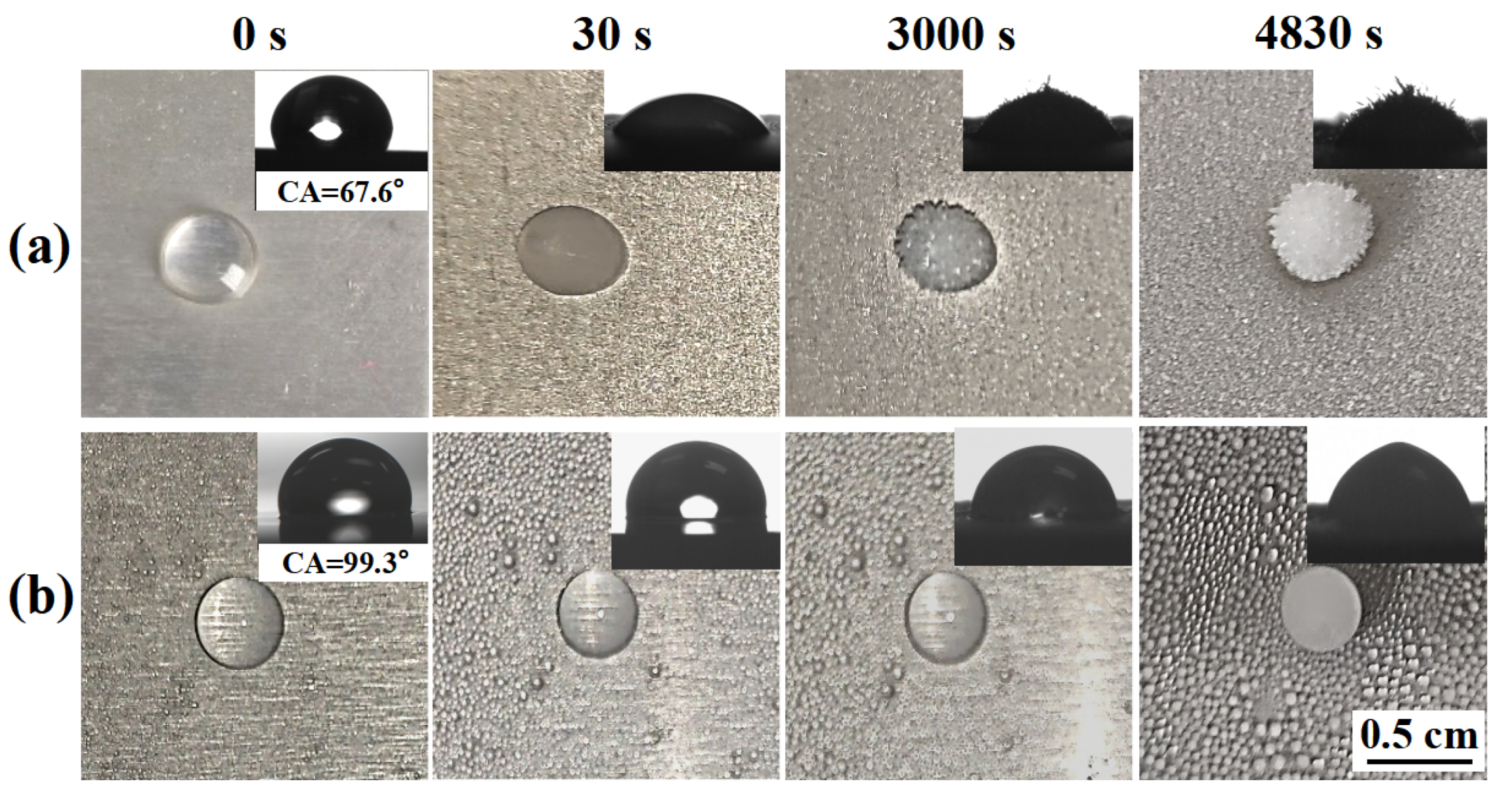
The freezing delay time of water droplets on the anti-icing surface is an important parameter to determine the anti-icing performance. Figure 5 shows the freezing process of water drops on a bare aluminum surface and SLIPS at −10 °C (relative humidity of 85%). It only took about 30 s for the nucleus of a water drop to form on the bare aluminum surface, as shown in Figure 5a. In contrast, the water drops frozen on SLIPS at 4830 s took much longer to form than those on the bare aluminum surface, as shown in Figure 5b. The reasons for the difference in time between the bare aluminum surface and SLIPS are as follows: the silicone oil film on SLIPS remains liquid at subzero temperatures, thereby maintaining slippery performance. Moreover, SLIPS have a high contact angle (CA = 99.3°) at −10 °C compared to the bare aluminum surface (67.6°) due to the low surface energy of silicone oil. According to Fourier’s law [32], the heat transfer (Q) between surface and water droplets can be expressed as , where T1 and T2 are the temperatures of water droplets and the surface, respectively; K is the heat transfer coefficient; and S is the solid–liquid contact area. Therefore, Q is proportional to S. The solid–liquid contact area was calculated by , where r is the radius of the droplet, and θ is the CA of the surface. Therefore, thermal transfer efficiency is inversely proportional to θ. Thus, SLIPS reduce the contact area and thermal transmission between the ice and the surface [30,32]. In addition, SLIPS with a molecularly smooth liquid interface pose a higher free energy barrier, which effectively inhibits ice nucleation [18,23]. A long delay time is conducive to the supercooled droplets leaving the surface before nucleation and icing, so an anti-icing effect was achieved.
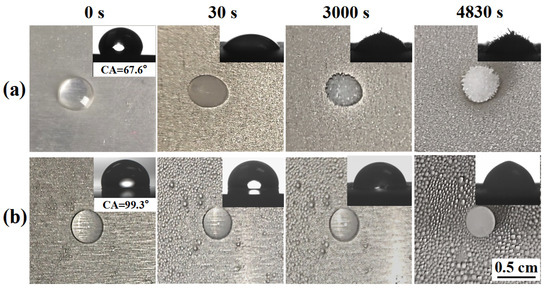
Figure 5.
The freezing process of water drops on a bare aluminum surface (a) and SLIPS (b) at −10 °C; the insets show the contact angle of bare aluminum surfaces and SLIPS.
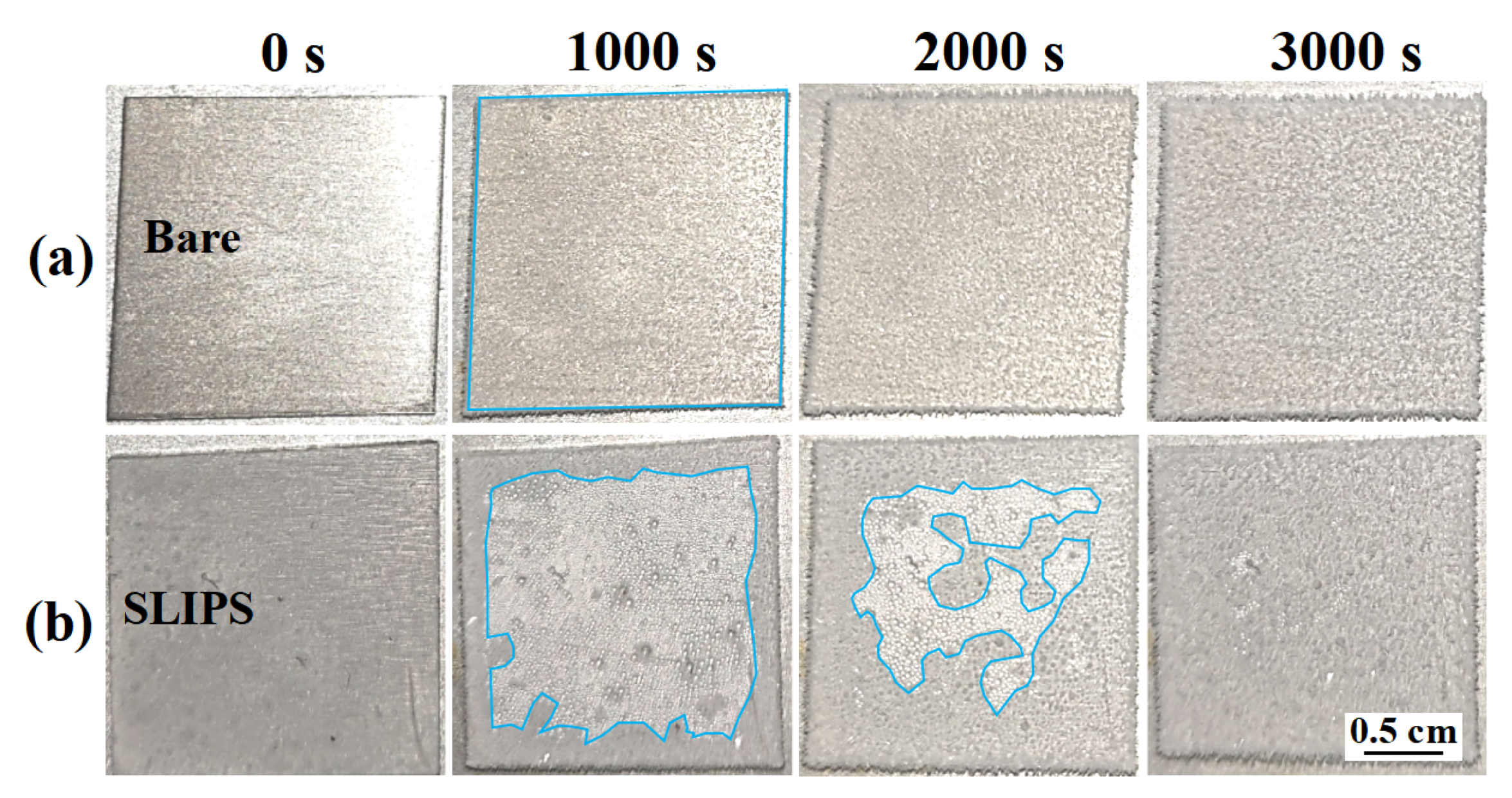
Frost is a common phenomenon that can cause serious harm to outdoor equipment; frost formation on icephobic materials may cause anti-icing performance degradation [16]. Thus, the antifrosting performance is a critical factor influencing the anti-icing performance of SLIPS. A condensation test was carried out to determine the distinction between SLIPS and bare aluminum surfaces. Figure 6 shows the frosting process of a bare aluminum surface and SLIPS at −10 °C (85% relative humidity). The whole bare aluminum surface was eventually covered with frost after 1000 s, as shown in Figure 6a. On the contrary, only some frost formed on the edge of the SLIPS due to the edge effect after 1000 s, and there were many tiny droplets in other areas, as shown in Figure 6b. As the condensation process went on, the ice grew gradually toward to the central area of SLIPS after 2000 s. The SLIPS was totally covered by frost after 3000 s, with a delay time three times as long as that for bare aluminum. SLIPS present a chemically homogeneous and molecularly smooth liquid interface, which reduces the number of potential nucleation sites [26]. Therefore, SLIPS can reduce the nucleation temperature of supercooled water by reducing the formation of ice nuclei and the formation rate of ice in high-humidity environments [21]. Additionally, the uniform silicone oil film on SLIPS increases the thermal resistance between the condensate droplets and SLIPS [21].
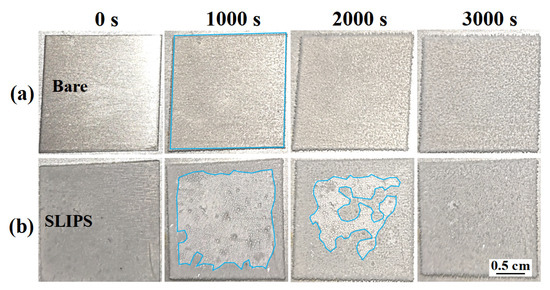
Figure 6.
The frosting process of a bare aluminum surface (a) and SLIPS (b) at −10 °C. The areas within the blue markings in the figure are the frosted areas of samples.
3.3. Anti-Icing Performance in Glaze Ice
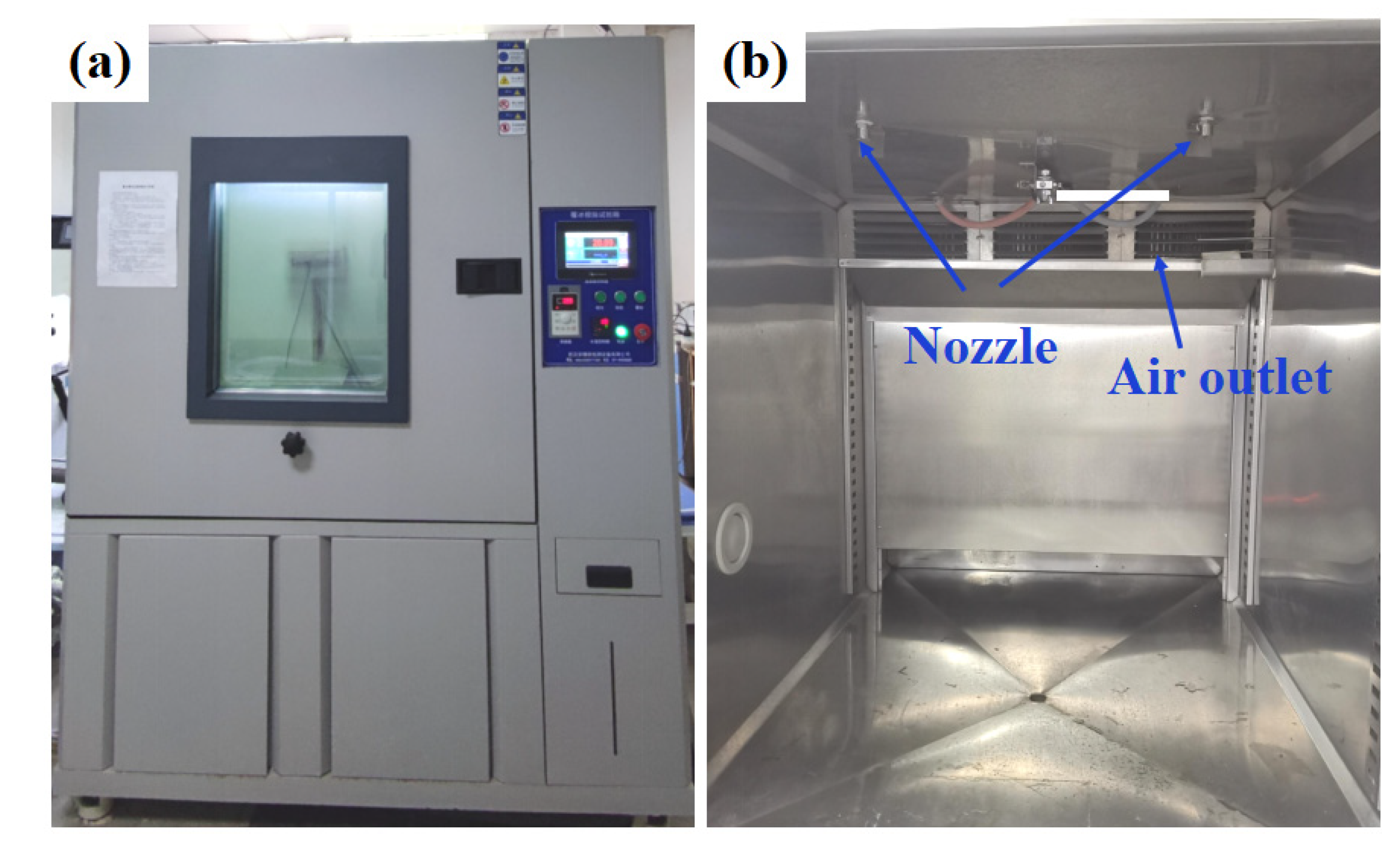
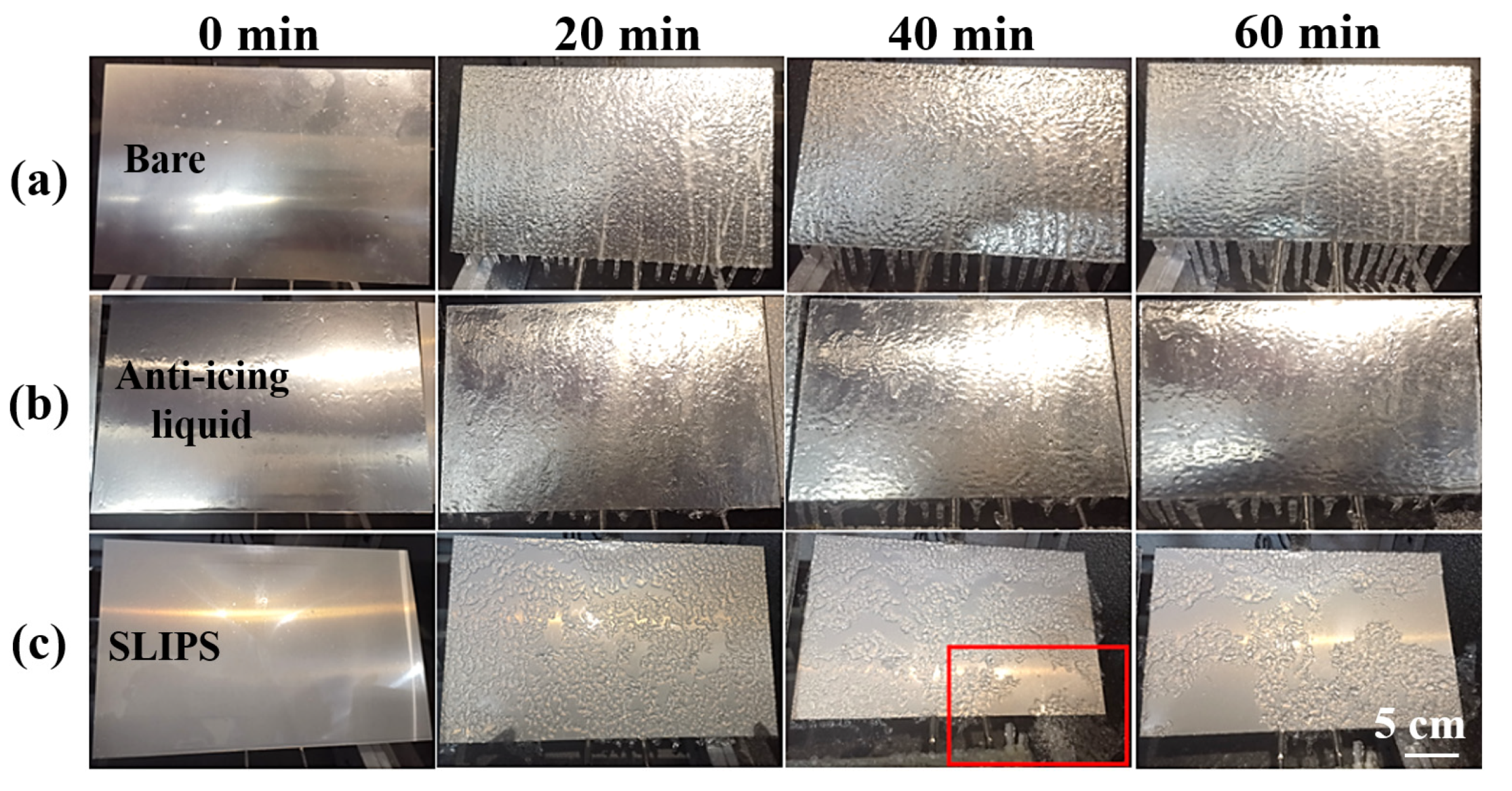
The glaze ice was the most hazardous type of icing due to its high ice-adhesion strength and high density [41]. In this research, glaze ice was simulated by an artificial climate chamber (Figure 7) to study the anti-icing performance of SLIPS under harsh glaze ice conditions. The inner size of the artificial climate chamber was 1 m × 1 m × 1 m. A standard nozzle was installed on the top of the icing simulation test chamber, which can produce water drops of about 100 μm. The water was cooled to about 1 °C before the icing test. The wind was provided by an adjustable fan, which can generate 0–8 m/s wind speeds. The scale of samples was 200 mm × 300 mm × 1.5 mm. The temperature and flow rate of freezing rain were kept at −6 °C and 60 L/h·m2, respectively. The ambient wind speed was 3 m/s. The samples were placed on an adjustable-angle inclined sample holder which was placed in the artificial climate chamber. The horizontal inclination angle (θ) of the sample was 60°. The icing test was continued with spraying for 60 min. For comparison with the traditional anti-icing strategy, an aluminum plate coated commercial anti-icing fluid was used as a control sample. The main component of anti-icing fluid is propylene glycol. The icing morphologies of the bare aluminum surface, anti-icing fluid sample and SLIPS at −6 °C are shown in Figure 8. The sprayed water drops were attached to a bare aluminum sample whose surface was covered by an ice layer after 20 min, as shown in Figure 8a. Some icicles formed on the bare sample. The thickness of the ice and the icicle length on the bare aluminum increased with the extension of time. Similar results were observed on the anti-icing-fluid-coated sample, but the length of the icicles was shorter than on the bare sample, as shown in Figure 8b. In contrast, most of the SLIPS maintained liquid water droplets after 20 min, as shown in Figure 8c. The falling droplets maintained a droplet style rather than that of water film on the SLIPS. Moreover, small droplets continuously gathered into large droplets and quickly slid off the SLIPS under the effect of gravity before nucleation. After 40 min, the SLIPS was sparsely covered with ice. Interestingly, some ice slipped from SLIPS under the force of gravity, as shown in the red box in Figure 8c. After 60 min, more ice slipped from the SLIPS and formed a large, clean area, indicating that the SLIPS has an excellent low ice-adhesion strength.
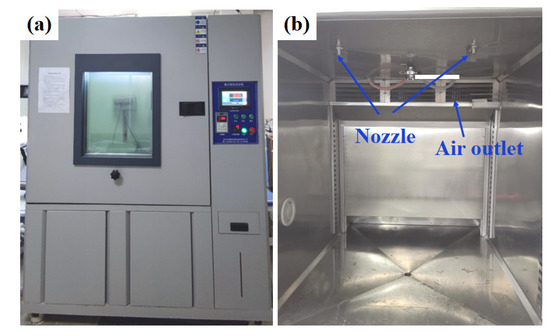
Figure 7.
The appearance (a) and internal structure (b) of the artificial icing climate chamber.
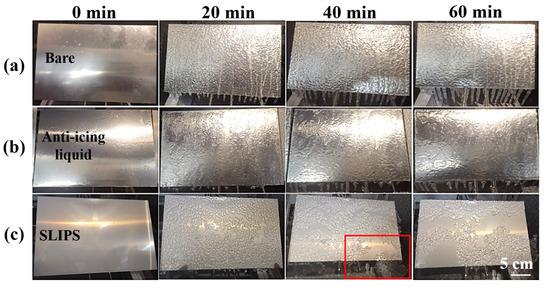
Figure 8.
Icing process of a bare aluminum surface (a), anti-icing-fluid-coated sample (b) and SLIPS (c) with a 60° tilting angle at −6 °C for 60 min.
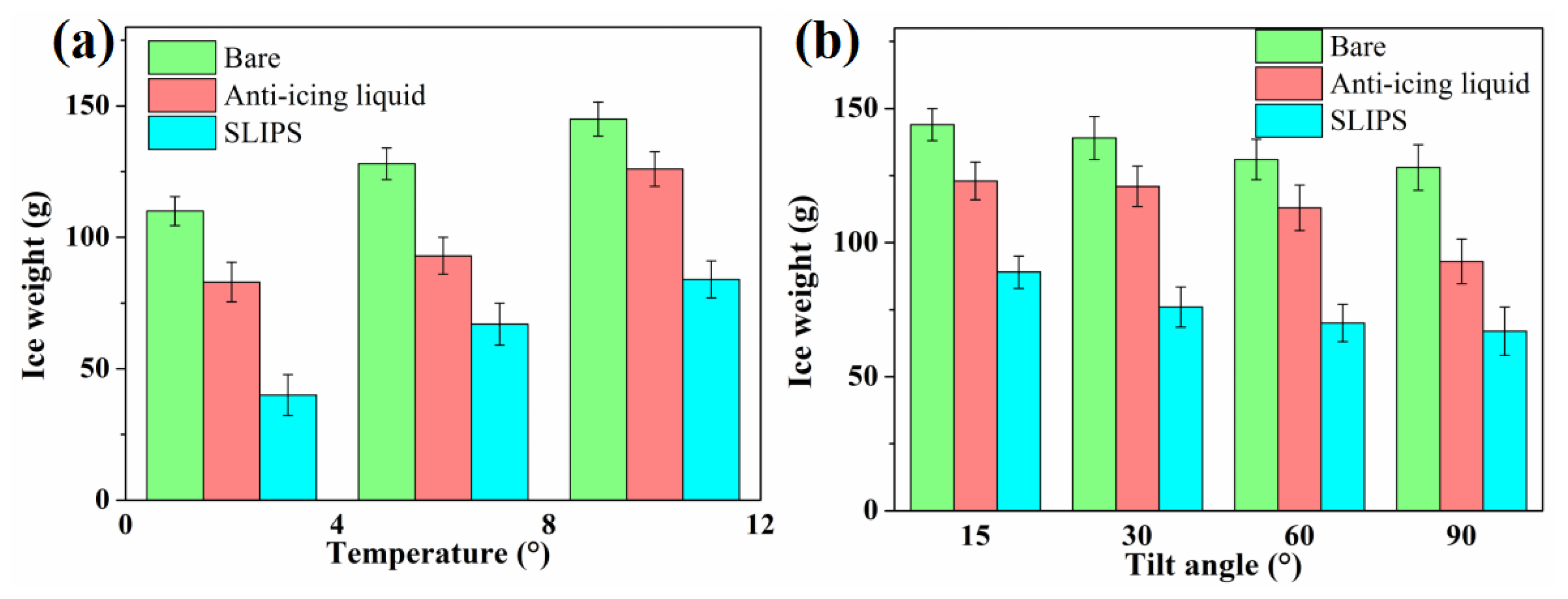
The anti-icing performance was further evaluated by ice weight, which was calculated according to the weight of samples before and after icing tests, as shown in Figure 9. The effects of chamber temperature (−2 °C, −6 °C or −10 °C) and horizontal inclination angle (15°, 30°, 60° or 90°) on the ice weight of SLIPS were studied. The icing test was continued with spraying for 60 min. It can be seen that the weight of the bare aluminum surface was as high as 110 g after 60 min of the icing test at −2 °C. The ice weight of the anti-icing-liquid-coated sample was 83 g, which is less than that of the bare aluminum surface. This is because the mixture of water drops and anti-icing fluid on the surface of the aluminum plate can reduce the freezing point of the mixture, thereby delaying icing, while the ice weight of the SLIPS was only 40 g, which is only about 36% of the weight of the bare aluminum surface. With a decrease in the temperature of the chamber, the ice weight of the three samples tends to increase, as shown in Figure 9a. When the temperature was −10 °C, the ice weight of the bare aluminum surface, anti-icing-liquid-coated sample and SLIPS was 145 g, 126 g and 84 g, respectively. The ice weight on the SLIPS was markedly lower than the ice weight on the bare aluminum surface and the anti-icing fluid sample under varying temperature conditions. According to Fourier’s law [32], the heat transfer () is proportional to T1–T2. A lower ambient temperature results in a higher heat transfer and higher cooling speed, thereby promoting the freezing process of water drops. The droplets impacted on the surface froze into ice for a short time at a lower temperature. This could lead to less water slipping from the samples. However, the long freeze delay time of water droplets on SLIPS was conducive to the supercooled droplets leaving the surface before nucleation. However, the ice weight of the SLIPS increased with the reduction in temperature, which was lower than that of bare the aluminum surface and the anti-icing fluid sample. Figure 9b shows the ice weight of the three sample with different tilting angles. When the tilting angle was 15°, the ice weight of the bare aluminum surface, anti-icing-liquid-coated sample and SLIPS was 144 g, 123 g and 89 g, respectively. The ice weight of the three sample was decreased with the increase in the tilting angle. When the tilting angle increased to 90°, the ice weight of the bare aluminum surface, the anti-icing-liquid-coated sample and the SLIPS was 128 g, 93 g and 67 g, respectively. To sum up, the ice weight of the SLIPS was reduced by 38.2%–63.6% compared with the bare aluminum surface.
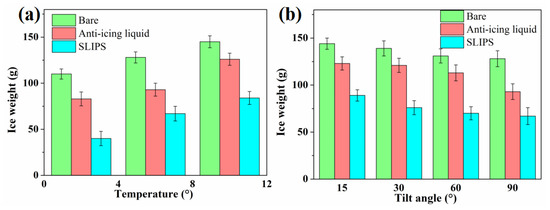
Figure 9.
Ice weight of the bare aluminum surface and anti-icing-fluid-coated sample at different temperatures (a) and tilting angles (b).
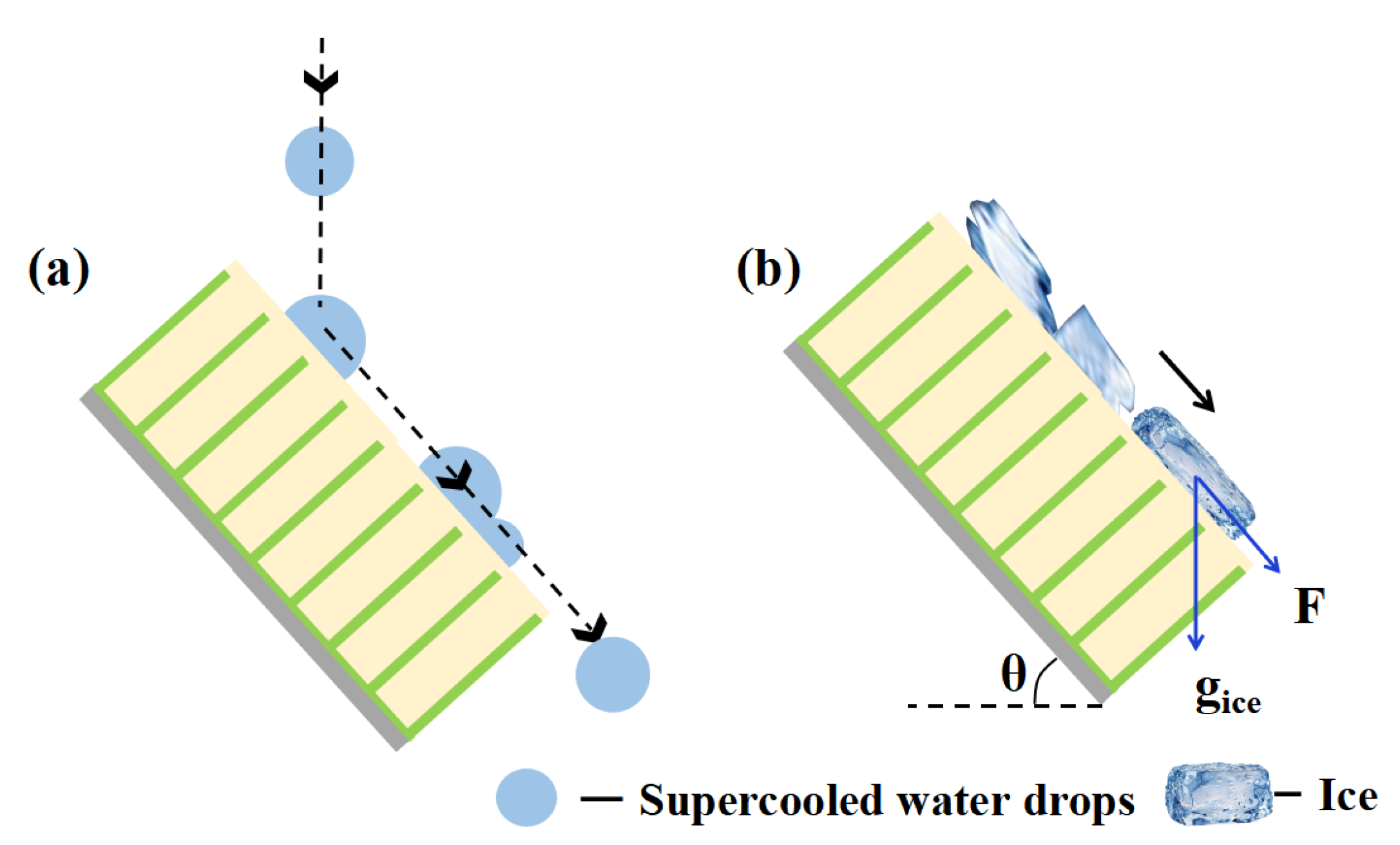
We proposed a mechanism of excellent anti-icing performance in glaze ice, as illustrated in Figure 10. The SLIPS has good water droplet mobility and anti-freezing performance, as supercooled water can slip away from the surface under the effect of gravity before ice nucleation [19]. Furthermore, the supercooled water droplets were constantly impacted and merged with the remaining water droplets [15,21]. The coalesced large droplets rolled off and swept the SLIPS powered by the kinetic energy of falling droplets, as shown in Figure 10a. Consequently, the SLIPS could prevent ice accumulation by preventing the accumulation of supercooled water droplets in the earlier stage of the test. Once the water droplets on the SLIPS freeze into ice, the ice layer can slide away easily under the action of gravity due to the low ice adhesion of the lubricating surface, as shown in Figure 10b. The gravity component of raindrops (F = gice sinθ) on the samples increased with increased tilting angle (θ), which led to water slipping from the samples more easily.
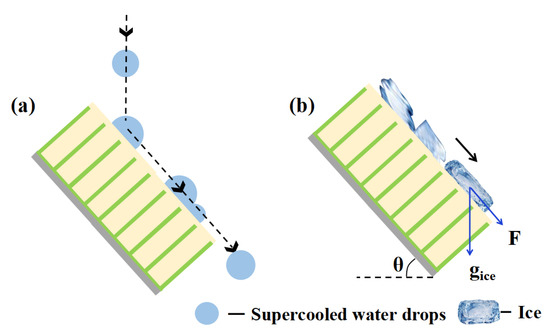
Figure 10.
Schematic diagram of the anti-icing mechanism of the SLIPS (a) before icing and (b) after icing.
Icing/deicing cycles were carried out to research the oil losses of SLIPS by measuring the variation of the Si element content. Like the ice-adhesion strength measurement, both the sample and a cylindrical mold with water were placed on a Peltier cooler. The temperature of the Peltier cooler was set to −20 °C and maintained for 90 min to make the water completely frozen. Then, the temperature was increased to 25 °C to melt the ice, which is called an icing/deicing cycle. Table 2 displays the elemental contents of SLIPS during icing/deicing cycles. The content of Si element on original SLIPS was 29.0%. The content of Si element on the SLIPS decreased slowly to 24.2% in the first 15 cycles. The Si elemental content of SLIPS decreased rapidly and reached 17.4% at the 30th cycle. Then, it showed an obvious upward trend and reached 6.3% at the 45th cycle. The above results show that silanized AAO can reduce the loss of lubricant during icing/deicing cycles because the lubricant was locked in the porous silanized AAO. However, a large number of icing/deicing cycles also led to the consumption of the silicone oil from the surface. Therefore, it is important to take appropriate measures to prevent the loss of lubricant for actual anti-icing application of SLIPS.

Table 2.
Variation in the elemental contents of SLIPS after different icing/deicing cycles.
4. Conclusions
In summary, SLIPS were fabricated on aluminum alloy by combining anodization and infusion of common silicone oil. A SLIPS with excellent anti-icing performance was fabricated by optimizing the anodizing time parameters (10 min). The ice-adhesion strength of the as-prepared SLIPS was as low as 6 kPa, which is 97.7% lower than that of the bare aluminum surface. The as-prepared SLIPS could efficiently delay the freezing process of water drops and the frosting process. It took 3000 s for condensate droplets on SLIPS to freeze into frost at −10 °C, which is a delay time three times as long as that on bare aluminum. The freezing processes of water drops on SLIPS were effectively delayed for 4800 s at −10 °C. Simultaneously, the as-prepared SLIPS exhibited excellent anti-icing performance in glaze ice due to the ability of the supercooled water drops and ice to slip from SLIPS. The ice weight increased with decreasing temperatures and tilting angles. Additionally, the SLIPS significantly decreased the ice weight compared with bare aluminum and the anti-icing-fluid-coated aluminum surface, which was reduced by 38.2%–63.6% compared with bare aluminum surface.
This study proposes a method suitable for large-area preparation of SLIPS on aluminum surfaces that significantly reduces the weight of glaze ice. The effects of ambient temperature and tilt angle on the anti-icing performance and ice weight of SLIPS in glaze ice were studied. This work provides some guidance for the preparation of anti-icing surfaces destroyed by glaze ice. Further research can focus on improving the compatibility between the lubricant and porous structure to improve the durability of SLIPS.
Author Contributions
Conceptualization, B.L. and Y.Y.; methodology, J.B. and L.F.; software, Z.D. and X.M.; validation, J.B.; formal analysis, G.L.; investigation, J.B.; resources, B.L.; data curation, L.F.; writing—original draft preparation, B.L.; writing—review and editing, G.L. and H.M.; visualization, Z.D. and X.M.; supervision, Y.Y.; project administration, B.L.; funding acquisition, B.L. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Electric Power Research Institute of Guizhou Power Grid Co., Ltd., China (Contract No. 0666002022030101HX00001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to legal or ethical reasons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huo, B.; Liu, X.J.; Yang, S. Galloping of Iced Transmission Lines Considering Multi-Torsional Modes and Experimental Validation on a Continuous Model. IEEE Trans. Power Deliv. 2022, 37, 3016–3026. [Google Scholar] [CrossRef]
- Cai, D.D.; Yan, B.; Gao, Y.B.; Zhu, Y.Q.; Wu, C.A.; Ye, Z.F.; Zhang, B. Stability Behavior and Optimization of Tension Plates in Transmission Lines in Heavy Ice Zones. IEEE Trans. Power Deliv. 2022, 37, 3641–3648. [Google Scholar] [CrossRef]
- Tarquini, S.; Antonini, C.; Amirfazli, A.; Marengo, M.; Palacios, J. Investigation of ice shedding properties of superhydrophobic coatings on helicopter blades. Cold Reg. Sci. Technol. 2014, 100, 50–58. [Google Scholar] [CrossRef]
- Homola, M.C.; Virk, M.S.; Nicklasson, P.J.; Sundsbo, P.A. Performance losses due to ice accretion for a 5 mw wind turbine. Wind Energy 2012, 15, 379–389. [Google Scholar] [CrossRef]
- Wei, M.; Hu, J.L.; Wang, X.F.; Jiang, X.L.; Zhang, R.H.; Shu, L.C. Experimental Study on Thermal Characteristics of DC Arc Formation between Ice-Electrode Gap. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1497–1505. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, R.P.; Ma, J.Q.; Wang, X.F. Effects of Static Icing on Flashover Characteristics of High-Speed Train Roof Insulators. Coatings 2022, 12, 950. [Google Scholar] [CrossRef]
- Alamri, S.; Vercillo, V.; Aguilar-Morales, A.I.; Schell, F. Self-limited ice formation and efficient de-icing on superhydrophobic micro-structured airfoils through direct laser interference patterning. Adv. Mater. Interfaces 2020, 7, 2001231. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Lian, C.X.; Emersic, C.; Rajab, F.H.; Cotton, I.; Zhang, X.; Lowndes, R.; Li, L. Assessing the Superhydrophobic Performance of Laser Micropatterned Aluminium Overhead Line Conductor Material. IEEE Trans. Power Deliv. 2022, 37, 972–979. [Google Scholar] [CrossRef]
- Lu, J.Z.; Wu, C.P.; Tan, Y.J.; Zhu, S.G.; Sun, Y.C. Research of Large-Capacity Low-Cost DC Deicer with Reactive Power Compensation. IEEE Trans. Power Deliv. 2018, 33, 3036–3044. [Google Scholar] [CrossRef]
- Golovin, K.; Dhyani, A.; Thouless, M.D.; Tuteja, A. Low–interfacial toughness materials for effective large-scale deicing. Science 2019, 364, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Song, X.; Liao, R.; Zhao, X.; Yuan, Y. Understanding the anti-icing property of nanostructured superhydrophobic aluminum surface during glaze ice accretion. Int. J. Heat Mass Transfer 2019, 133, 119–128. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, H.; Liu, G.; Liao, R. Fabrication of phase change microcapsules and their applications to anti-icing coating. Surf. Interfaces 2021, 27, 101516. [Google Scholar] [CrossRef]
- Liu, G.Y.; Yuan, Y.; Liao, R.J.; Xiang, H.Y.; Wang, L.; Yu, Q.; Zhang, C. Robust and self-healing superhydrophobic aluminum surface with excellent anti-icing performance. Surf. Interfaces 2022, 28, 101588. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery? Nat. Rev. Mater. 2016, 1, 15003. [Google Scholar] [CrossRef]
- Jing, C.; Jie, L.; Min, H. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 2012, 101, 41–932. [Google Scholar]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired surfaces with superwettability: New insight on theory, design, and applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.; Tang, S.K.Y.; Smythe, E.; Hatton, B.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Liu, G.Y.; Yuan, Y.; Liao, R.J.; Wang, L.; Gao, X. Fabrication of a porous slippery icephobic surface and effect of lubricant viscosity on anti-icing properties and durability. Coatings 2020, 10, 896. [Google Scholar] [CrossRef]
- Cheng, S.; Guo, P.; Wang, X.; Che, P.; Han, X.; Jin, R.; Heng, L.; Jiang, L. Photothermal slippery surface showing rapid self-repairing and exceptional anti-icing/deicing property. Chem. Eng. J. 2021, 431, 133411. [Google Scholar]
- Xiang, H.; Yuan, Y.; Zhang, C.; Dai, X.; Zhu, T.; Song, L.; Gai, Y.; Liao, R. Key factors affecting durable anti-icing of slippery surfaces: Pore size and porosity. ACS Appl. Mater. Interfaces 2022, 15, 3599–3612. [Google Scholar] [CrossRef] [PubMed]
- Baumli, P.; Teisala, H.; Bauer, H.; Garcia-Gonzalez, D.; Damle, V.; Geyer, F.; D’Acunzi, M.; Kaltbeitzel, A.; Butt, H.J.; Vollmer, D. Flow-Induced Long-Term Stable Slippery Surfaces. Adv. Sci. 2019, 6, 1900019. [Google Scholar] [CrossRef] [PubMed]
- Nguyenpark, T.B. Effects of hydrophobicity and lubricant characteristics on anti-icing performance of slippery lubricant-infused porous surfaces. J. Ind. Eng. Chem. 2019, 69, 99–105. [Google Scholar]
- Heydarian, S.; Jafari, R.; Momen, G. Recent progress in the anti-icing performance of slippery liquid-infused surfaces. Prog. Org. Coat. 2021, 151, 106096. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Huang, M.; Zhou, Y.X.; Liu, Y.Y.; Liang, X.D. Durability of a lubricant-infused electrospray silicon rubber surface as an anti-icing coating. Appl. Surf. Sci. 2015, 346, 68–76. [Google Scholar] [CrossRef]
- Luo, H.; Yin, S.; Huang, S.; Chen, F.; Tang, Q.; Li, X. Fabrication of slippery zn surface with improved water-impellent, condensation and anti-icing properties. Appl. Surf. Sci. 2019, 470, 1139–1147. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Zhang, Y.; And, Y.R.; Yang, S. Transparent and superamphiphobic surfaces from one-step spray coating of stringed silica nanoparticle/sol solutions. Part. Part. Syst. Charact. 2014, 31, 763–770. [Google Scholar] [CrossRef]
- Manabe, K.; Nishizawa, S.; Kyung, K.H.; Shiratori, S. Optical phenomena and antifrosting property on biomimetics slippery fluid-infused antireflective films via layer-by-layer comparison with superhydrophobic and antireflective films. ACS Appl. Mater. Interfaces 2014, 6, 13985. [Google Scholar] [CrossRef]
- Zhuo, Y.; Feng, W.; Xiao, S.; He, J.; Zhang, Z. One-step fabrication of bioinspired lubricant-regenerable icephobic slippery liquid-infused porous surfaces. ACS Omega 2018, 3, 10139–10144. [Google Scholar] [CrossRef]
- Coady, M.J.; Wood, M.; Wallace, G.Q.; Nielsen, K.E.; Kietzig, A.M.; Lagugné-Labarthet, F.; Ragogna, P.J. Icephobic behavior of uv-cured polymer networks incorporated into slippery lubricant-infused porous surfaces: Improving slips durability. ACS Appl. Mater. Interfaces 2018, 10, 2890–2896. [Google Scholar] [CrossRef]
- Wang, J.H.; Long, M.J.; He, J.G.; Ma, Y.Y.; Liu, K.P. Experimental Study on Ice-covered Samples of Composite Material Tower. IEEE Trans. Dielectr. Electr. Insul 2017, 24, 2937–2944. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Y.; Jiang, Z.; Youdong, J.; Liang, W. Anti-frosting/anti-icing property of nano-zno superhydrophobic surface on al alloy prepared by radio frequency magnetron sputtering. Mater. Res. Express 2020, 7, 026401. [Google Scholar] [CrossRef]
- Peralta-Gonzalez, C.; Ramirez-Hernandez, A.; Rangel-Porras, G.; Aparicio-Saguilan, A.; Aguirre-Cruz, A.; Gonzalez-Garcia, G.; Baez-Garcia, J.E.; Paramo-Calderon, D.E. Synthesis and characterization of the starch/silicone oil composite and elaboration of its films. Silicon 2022, 14, 4157–4167. [Google Scholar] [CrossRef]
- Kim, C.H.; Joo, C.K.; Chun, H.J.; Yoo, B.R.; Noh, D.I.; Shim, Y.B. Instrumental studies on silicone oil adsorption to the surface of intraocular lenses. Appl. Surf. Sci. 2012, 262, 146–152. [Google Scholar] [CrossRef]
- Jin, H.; Mi, J.; Bl, B.; Ji, M.; Vp, A.; Ysk, A. Durable ice-lubricating surfaces based on polydimethylsiloxane embedded silicone oil infused silica aerogel-sciencedirect. Appl. Surf. Sci. 2020, 512, 145728. [Google Scholar]
- Heu, C.S.; Kim, S.W.; Kim, J.; Lee, S.; Kim, J.M.; Lee, K.S.; Kim, D.R. Frosting and defrosting behavior of slippery surfaces and utilization of mechanical vibration to enhance defrosting performance. Int. J. Heat Mass. Tran. 2018, 125, 858–865. [Google Scholar] [CrossRef]
- Barthwal, S.; Lee, B.; Lim, S.H. Fabrication of robust and durable slippery anti-icing coating on textured superhydrophobic aluminum surfaces with infused silicone oil. Appl. Surf. Sci. 2019, 496, 143677. [Google Scholar] [CrossRef]
- Chen, G.M.; Liu, S.C.; Sun, Z.Y.; Wen, S.F.; Feng, T.; Yue, Z.F. Intrinsic self-healing organogels based on dynamic polymer network with self-regulated secretion of liquid for anti-icing. Prog. Org. Coat. 2020, 144, 105641. [Google Scholar] [CrossRef]
- Khammas, R.; Koivuluoto, H. Durable Icephobic Slippery Liquid-Infused Porous Surfaces (SLIPS) Using Flame- and Cold-Spraying. Sustainability 2022, 148, 422. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Lu, C.; Liu, Y.; Liu, Y. Robust slippery liquid-infused porous network surfaces for enhanced anti-/de-icing performance. ACS Appl. Mater. Interfaces 2020, 12, 25471–25477. [Google Scholar] [CrossRef]
- He, G.; Hu, Q.; Shu, L. Impact of icing severity on corona performance of glaze ice-covered conductor. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2952–2959. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).