Fabrication of Slippery Surfaces on Aluminum Alloy and Its Anti-Icing Performance in Glaze Ice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
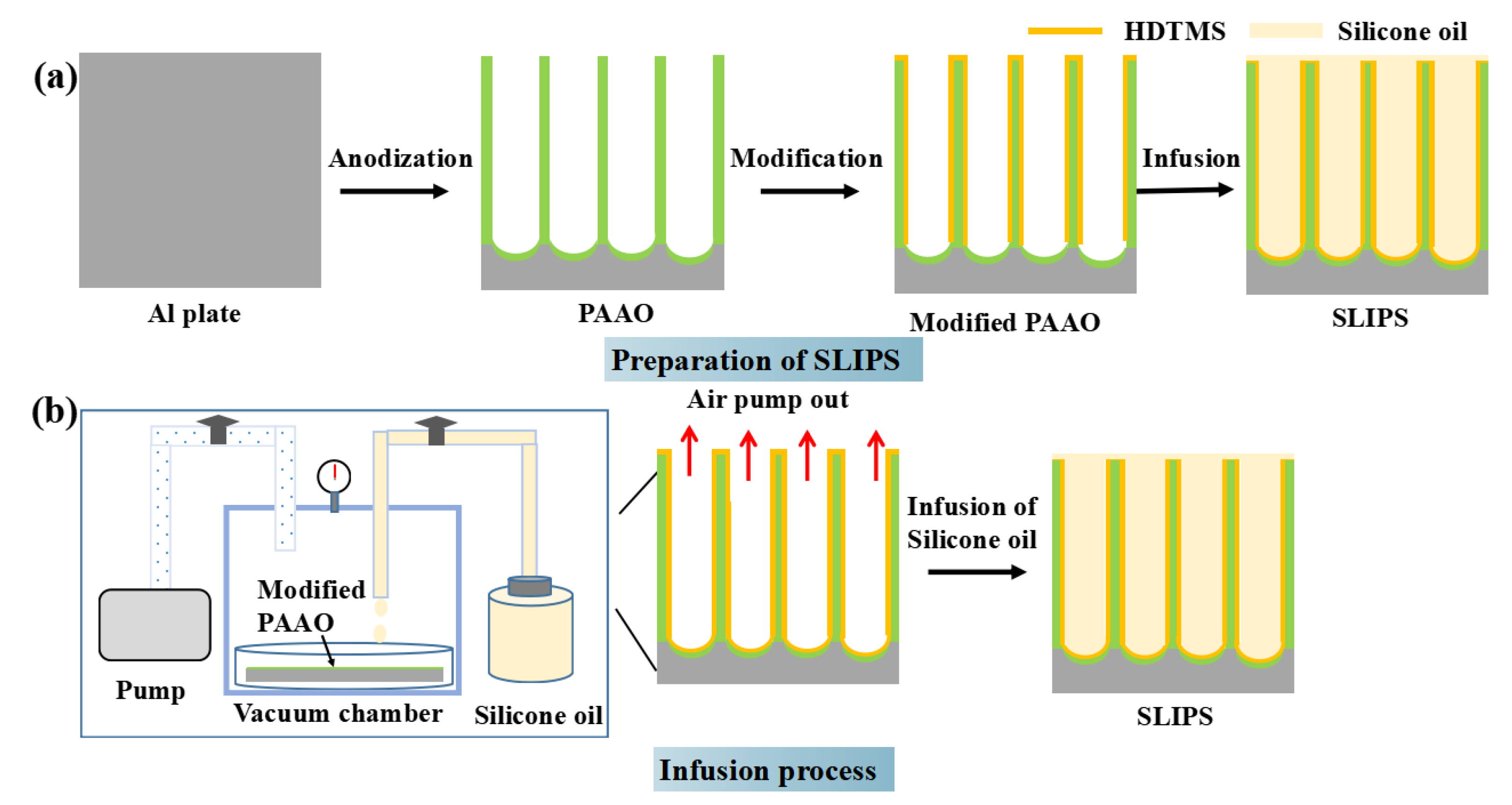
2.2. Sample Preparation
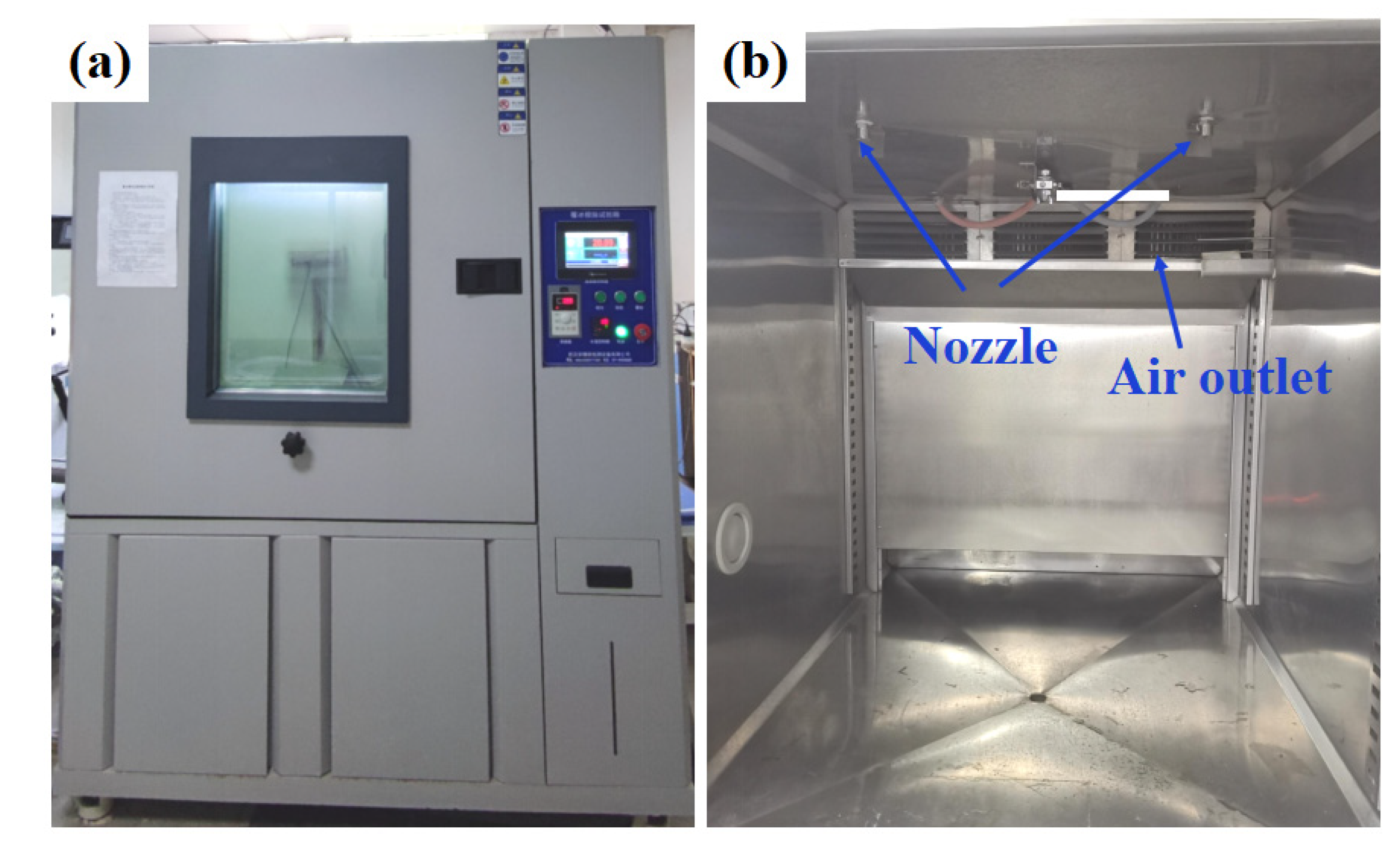
2.3. Characterization
3. Results and Discussion
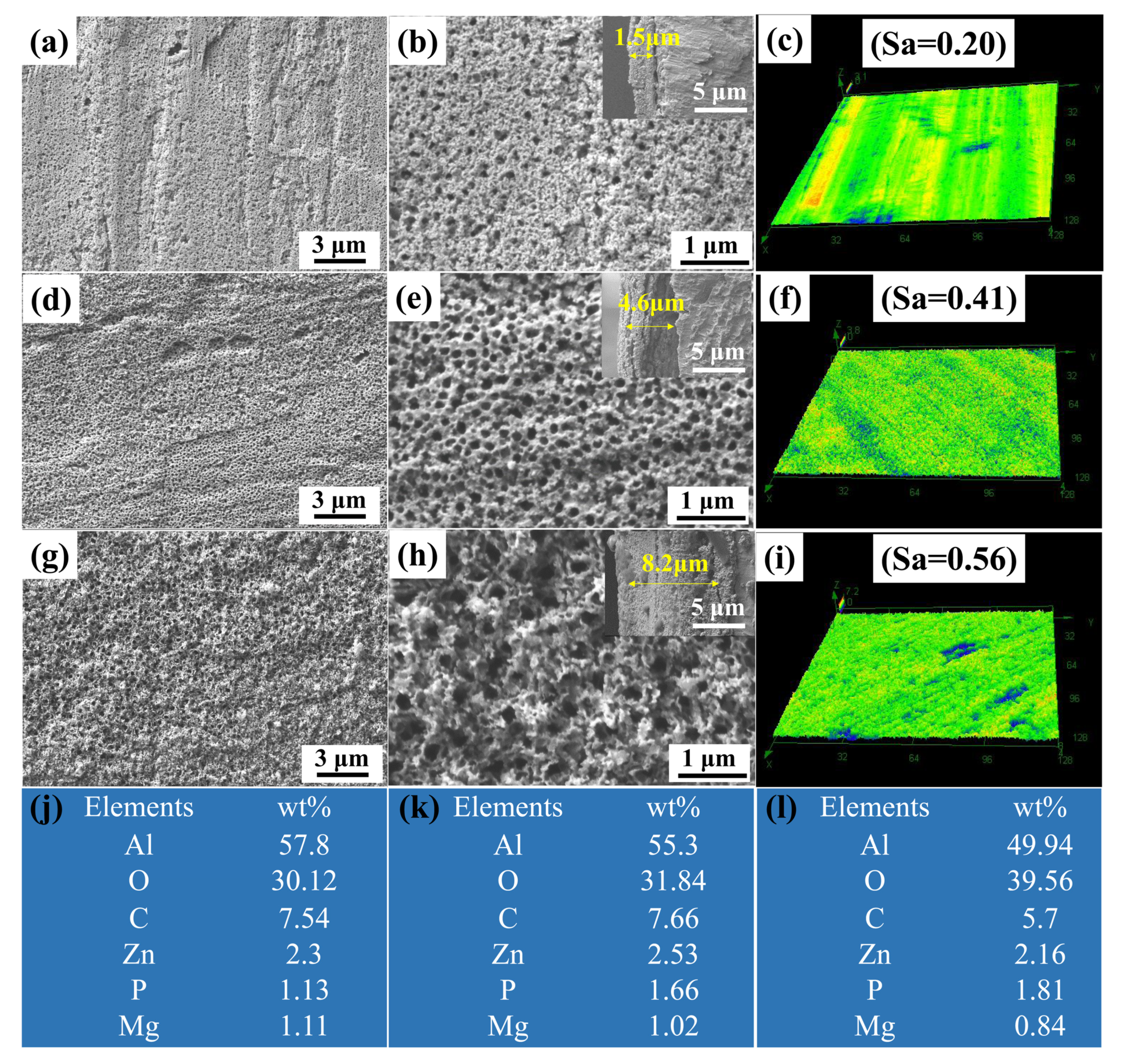
3.1. Surface Morphology and Chemical Composition
3.2. Ice Adhesion and Antifreezing
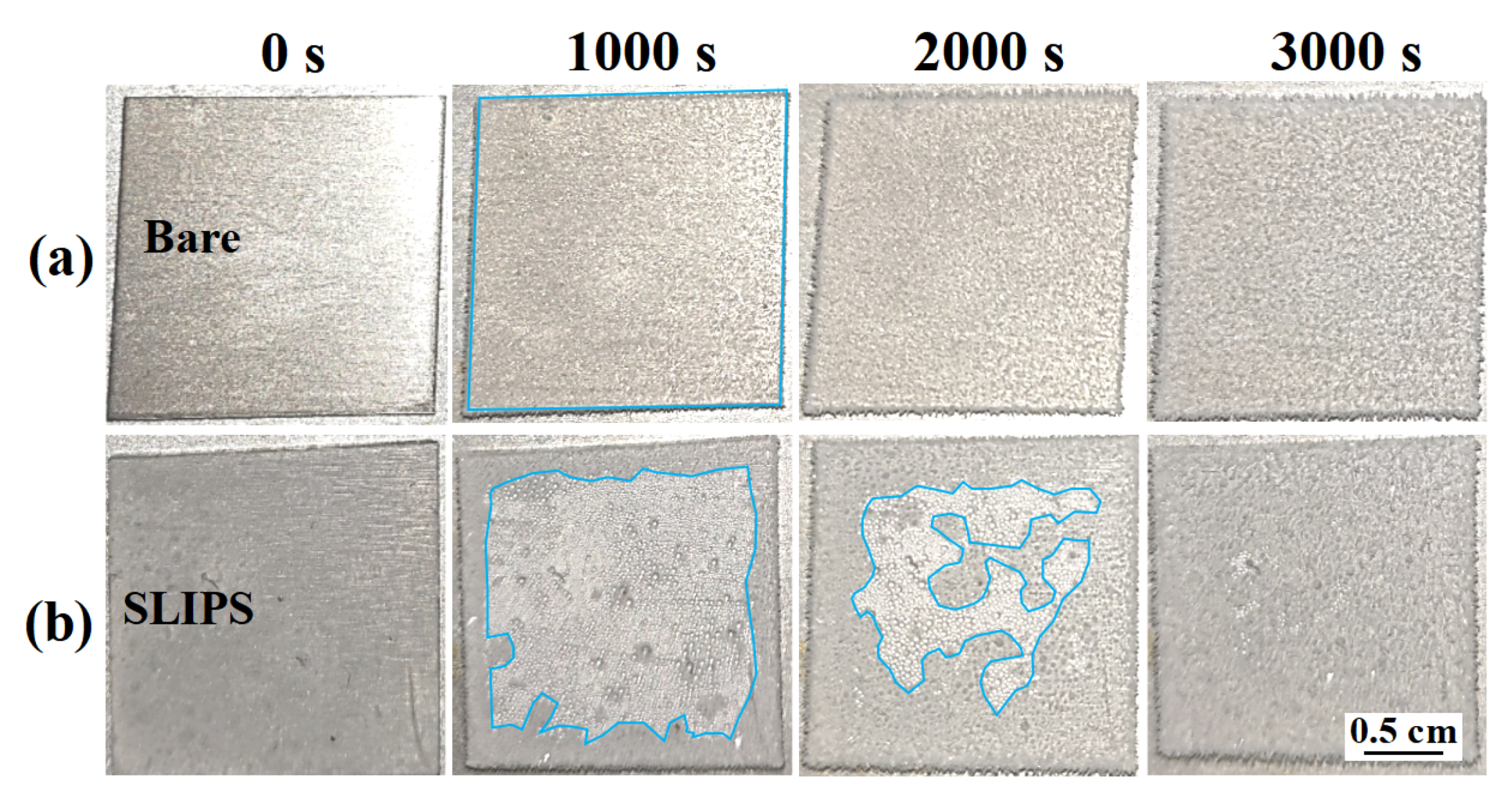
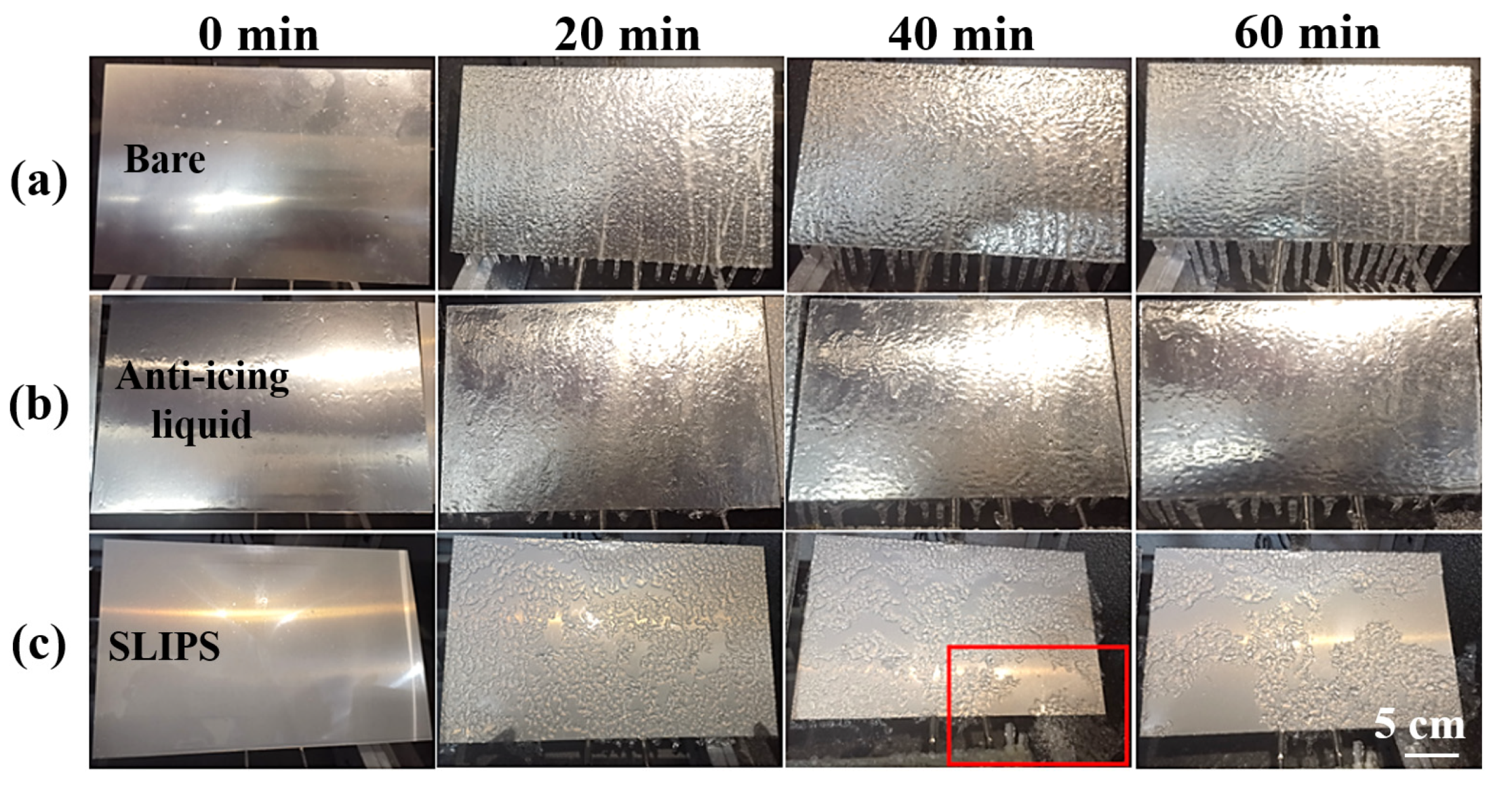
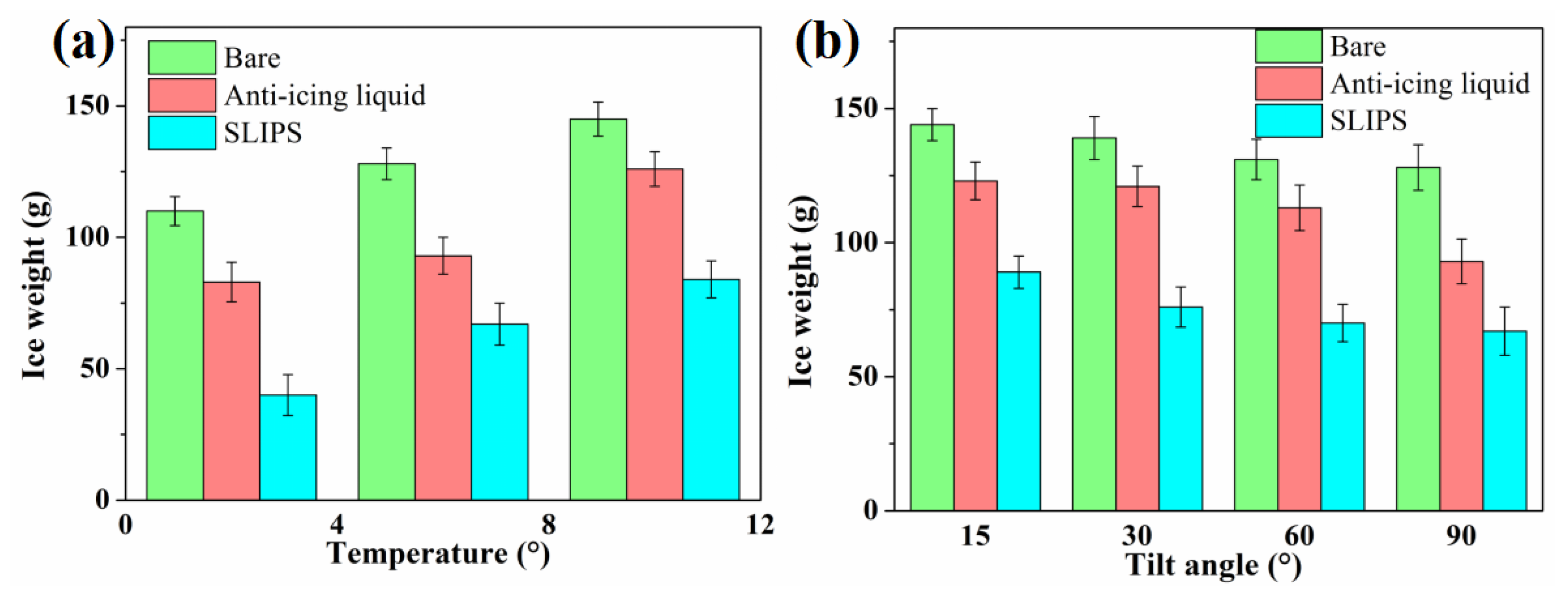
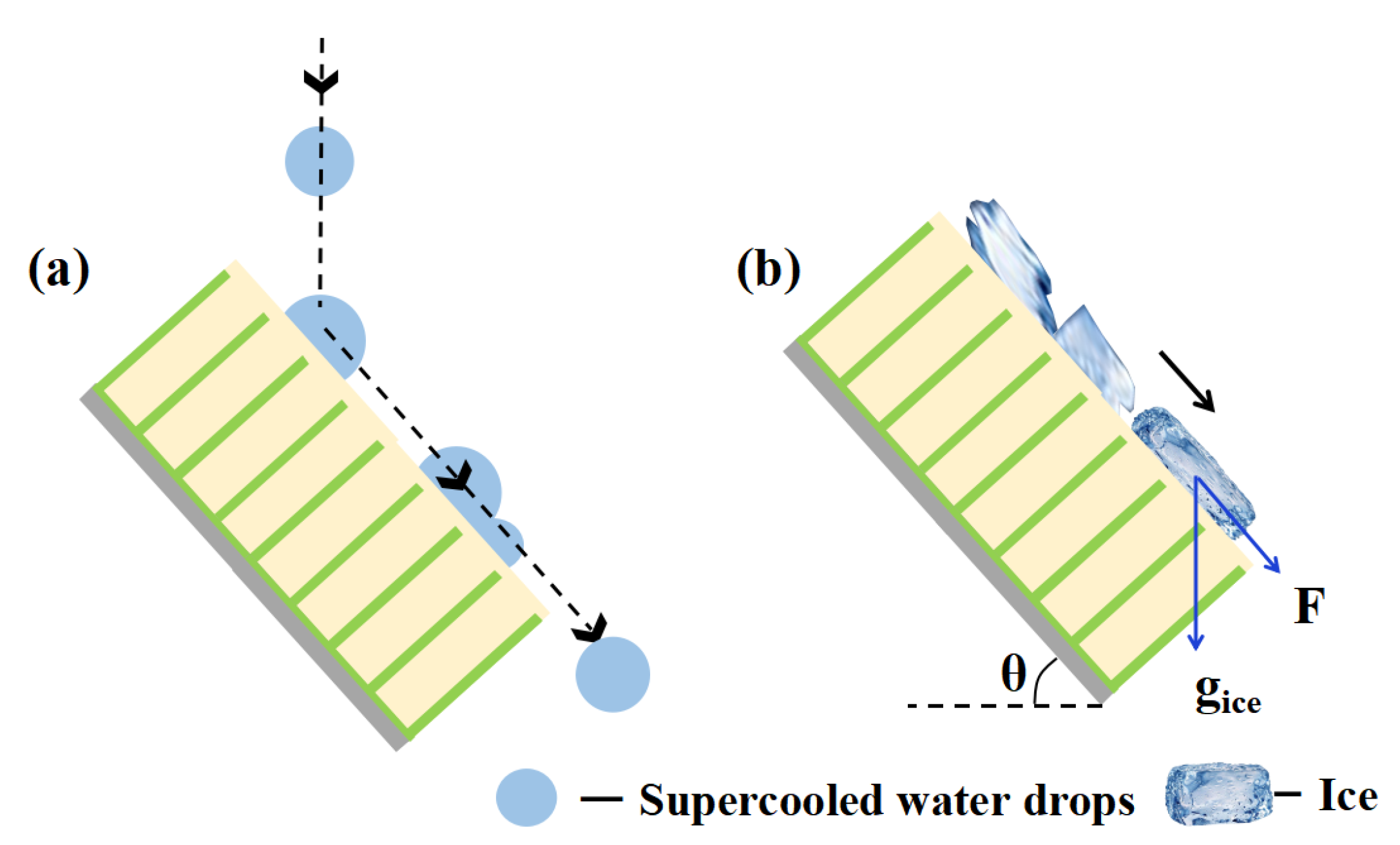
3.3. Anti-Icing Performance in Glaze Ice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huo, B.; Liu, X.J.; Yang, S. Galloping of Iced Transmission Lines Considering Multi-Torsional Modes and Experimental Validation on a Continuous Model. IEEE Trans. Power Deliv. 2022, 37, 3016–3026. [Google Scholar] [CrossRef]
- Cai, D.D.; Yan, B.; Gao, Y.B.; Zhu, Y.Q.; Wu, C.A.; Ye, Z.F.; Zhang, B. Stability Behavior and Optimization of Tension Plates in Transmission Lines in Heavy Ice Zones. IEEE Trans. Power Deliv. 2022, 37, 3641–3648. [Google Scholar] [CrossRef]
- Tarquini, S.; Antonini, C.; Amirfazli, A.; Marengo, M.; Palacios, J. Investigation of ice shedding properties of superhydrophobic coatings on helicopter blades. Cold Reg. Sci. Technol. 2014, 100, 50–58. [Google Scholar] [CrossRef]
- Homola, M.C.; Virk, M.S.; Nicklasson, P.J.; Sundsbo, P.A. Performance losses due to ice accretion for a 5 mw wind turbine. Wind Energy 2012, 15, 379–389. [Google Scholar] [CrossRef]
- Wei, M.; Hu, J.L.; Wang, X.F.; Jiang, X.L.; Zhang, R.H.; Shu, L.C. Experimental Study on Thermal Characteristics of DC Arc Formation between Ice-Electrode Gap. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1497–1505. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, R.P.; Ma, J.Q.; Wang, X.F. Effects of Static Icing on Flashover Characteristics of High-Speed Train Roof Insulators. Coatings 2022, 12, 950. [Google Scholar] [CrossRef]
- Alamri, S.; Vercillo, V.; Aguilar-Morales, A.I.; Schell, F. Self-limited ice formation and efficient de-icing on superhydrophobic micro-structured airfoils through direct laser interference patterning. Adv. Mater. Interfaces 2020, 7, 2001231. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Lian, C.X.; Emersic, C.; Rajab, F.H.; Cotton, I.; Zhang, X.; Lowndes, R.; Li, L. Assessing the Superhydrophobic Performance of Laser Micropatterned Aluminium Overhead Line Conductor Material. IEEE Trans. Power Deliv. 2022, 37, 972–979. [Google Scholar] [CrossRef]
- Lu, J.Z.; Wu, C.P.; Tan, Y.J.; Zhu, S.G.; Sun, Y.C. Research of Large-Capacity Low-Cost DC Deicer with Reactive Power Compensation. IEEE Trans. Power Deliv. 2018, 33, 3036–3044. [Google Scholar] [CrossRef]
- Golovin, K.; Dhyani, A.; Thouless, M.D.; Tuteja, A. Low–interfacial toughness materials for effective large-scale deicing. Science 2019, 364, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Song, X.; Liao, R.; Zhao, X.; Yuan, Y. Understanding the anti-icing property of nanostructured superhydrophobic aluminum surface during glaze ice accretion. Int. J. Heat Mass Transfer 2019, 133, 119–128. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, H.; Liu, G.; Liao, R. Fabrication of phase change microcapsules and their applications to anti-icing coating. Surf. Interfaces 2021, 27, 101516. [Google Scholar] [CrossRef]
- Liu, G.Y.; Yuan, Y.; Liao, R.J.; Xiang, H.Y.; Wang, L.; Yu, Q.; Zhang, C. Robust and self-healing superhydrophobic aluminum surface with excellent anti-icing performance. Surf. Interfaces 2022, 28, 101588. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery? Nat. Rev. Mater. 2016, 1, 15003. [Google Scholar] [CrossRef] [Green Version]
- Jing, C.; Jie, L.; Min, H. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 2012, 101, 41–932. [Google Scholar]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired surfaces with superwettability: New insight on theory, design, and applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.; Tang, S.K.Y.; Smythe, E.; Hatton, B.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Liu, G.Y.; Yuan, Y.; Liao, R.J.; Wang, L.; Gao, X. Fabrication of a porous slippery icephobic surface and effect of lubricant viscosity on anti-icing properties and durability. Coatings 2020, 10, 896. [Google Scholar] [CrossRef]
- Cheng, S.; Guo, P.; Wang, X.; Che, P.; Han, X.; Jin, R.; Heng, L.; Jiang, L. Photothermal slippery surface showing rapid self-repairing and exceptional anti-icing/deicing property. Chem. Eng. J. 2021, 431, 133411. [Google Scholar]
- Xiang, H.; Yuan, Y.; Zhang, C.; Dai, X.; Zhu, T.; Song, L.; Gai, Y.; Liao, R. Key factors affecting durable anti-icing of slippery surfaces: Pore size and porosity. ACS Appl. Mater. Interfaces 2022, 15, 3599–3612. [Google Scholar] [CrossRef] [PubMed]
- Baumli, P.; Teisala, H.; Bauer, H.; Garcia-Gonzalez, D.; Damle, V.; Geyer, F.; D’Acunzi, M.; Kaltbeitzel, A.; Butt, H.J.; Vollmer, D. Flow-Induced Long-Term Stable Slippery Surfaces. Adv. Sci. 2019, 6, 1900019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyenpark, T.B. Effects of hydrophobicity and lubricant characteristics on anti-icing performance of slippery lubricant-infused porous surfaces. J. Ind. Eng. Chem. 2019, 69, 99–105. [Google Scholar]
- Heydarian, S.; Jafari, R.; Momen, G. Recent progress in the anti-icing performance of slippery liquid-infused surfaces. Prog. Org. Coat. 2021, 151, 106096. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Huang, M.; Zhou, Y.X.; Liu, Y.Y.; Liang, X.D. Durability of a lubricant-infused electrospray silicon rubber surface as an anti-icing coating. Appl. Surf. Sci. 2015, 346, 68–76. [Google Scholar] [CrossRef]
- Luo, H.; Yin, S.; Huang, S.; Chen, F.; Tang, Q.; Li, X. Fabrication of slippery zn surface with improved water-impellent, condensation and anti-icing properties. Appl. Surf. Sci. 2019, 470, 1139–1147. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Zhang, Y.; And, Y.R.; Yang, S. Transparent and superamphiphobic surfaces from one-step spray coating of stringed silica nanoparticle/sol solutions. Part. Part. Syst. Charact. 2014, 31, 763–770. [Google Scholar] [CrossRef]
- Manabe, K.; Nishizawa, S.; Kyung, K.H.; Shiratori, S. Optical phenomena and antifrosting property on biomimetics slippery fluid-infused antireflective films via layer-by-layer comparison with superhydrophobic and antireflective films. ACS Appl. Mater. Interfaces 2014, 6, 13985. [Google Scholar] [CrossRef]
- Zhuo, Y.; Feng, W.; Xiao, S.; He, J.; Zhang, Z. One-step fabrication of bioinspired lubricant-regenerable icephobic slippery liquid-infused porous surfaces. ACS Omega 2018, 3, 10139–10144. [Google Scholar] [CrossRef]
- Coady, M.J.; Wood, M.; Wallace, G.Q.; Nielsen, K.E.; Kietzig, A.M.; Lagugné-Labarthet, F.; Ragogna, P.J. Icephobic behavior of uv-cured polymer networks incorporated into slippery lubricant-infused porous surfaces: Improving slips durability. ACS Appl. Mater. Interfaces 2018, 10, 2890–2896. [Google Scholar] [CrossRef]
- Wang, J.H.; Long, M.J.; He, J.G.; Ma, Y.Y.; Liu, K.P. Experimental Study on Ice-covered Samples of Composite Material Tower. IEEE Trans. Dielectr. Electr. Insul 2017, 24, 2937–2944. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Y.; Jiang, Z.; Youdong, J.; Liang, W. Anti-frosting/anti-icing property of nano-zno superhydrophobic surface on al alloy prepared by radio frequency magnetron sputtering. Mater. Res. Express 2020, 7, 026401. [Google Scholar] [CrossRef] [Green Version]
- Peralta-Gonzalez, C.; Ramirez-Hernandez, A.; Rangel-Porras, G.; Aparicio-Saguilan, A.; Aguirre-Cruz, A.; Gonzalez-Garcia, G.; Baez-Garcia, J.E.; Paramo-Calderon, D.E. Synthesis and characterization of the starch/silicone oil composite and elaboration of its films. Silicon 2022, 14, 4157–4167. [Google Scholar] [CrossRef]
- Kim, C.H.; Joo, C.K.; Chun, H.J.; Yoo, B.R.; Noh, D.I.; Shim, Y.B. Instrumental studies on silicone oil adsorption to the surface of intraocular lenses. Appl. Surf. Sci. 2012, 262, 146–152. [Google Scholar] [CrossRef]
- Jin, H.; Mi, J.; Bl, B.; Ji, M.; Vp, A.; Ysk, A. Durable ice-lubricating surfaces based on polydimethylsiloxane embedded silicone oil infused silica aerogel-sciencedirect. Appl. Surf. Sci. 2020, 512, 145728. [Google Scholar]
- Heu, C.S.; Kim, S.W.; Kim, J.; Lee, S.; Kim, J.M.; Lee, K.S.; Kim, D.R. Frosting and defrosting behavior of slippery surfaces and utilization of mechanical vibration to enhance defrosting performance. Int. J. Heat Mass. Tran. 2018, 125, 858–865. [Google Scholar] [CrossRef]
- Barthwal, S.; Lee, B.; Lim, S.H. Fabrication of robust and durable slippery anti-icing coating on textured superhydrophobic aluminum surfaces with infused silicone oil. Appl. Surf. Sci. 2019, 496, 143677. [Google Scholar] [CrossRef]
- Chen, G.M.; Liu, S.C.; Sun, Z.Y.; Wen, S.F.; Feng, T.; Yue, Z.F. Intrinsic self-healing organogels based on dynamic polymer network with self-regulated secretion of liquid for anti-icing. Prog. Org. Coat. 2020, 144, 105641. [Google Scholar] [CrossRef]
- Khammas, R.; Koivuluoto, H. Durable Icephobic Slippery Liquid-Infused Porous Surfaces (SLIPS) Using Flame- and Cold-Spraying. Sustainability 2022, 148, 422. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Lu, C.; Liu, Y.; Liu, Y. Robust slippery liquid-infused porous network surfaces for enhanced anti-/de-icing performance. ACS Appl. Mater. Interfaces 2020, 12, 25471–25477. [Google Scholar] [CrossRef]
- He, G.; Hu, Q.; Shu, L. Impact of icing severity on corona performance of glaze ice-covered conductor. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2952–2959. [Google Scholar] [CrossRef]



| Reference | Ice-Adhesion Strength (kPa) | Porous Structure | Lubricant |
|---|---|---|---|
| [36] | 68 | Chemical etching | Krytox 103 |
| [37] | 22 | Chemical etching + anodization | Silicone oil (0.00035 m2/s) |
| [38] | 14.3 | Crosslinked PDMS network | Silicone oil (20 mPa.s) |
| [39] | 22 | flame-spraying polymer coating | Silicone oil (25 cst) |
| SLIPS-10 | 6 | Anodization | Silicone oil (50 mPa.s) |
| Elements | C | Si | O | Al | Zn | Residue | |
|---|---|---|---|---|---|---|---|
| Times | |||||||
| 0 | 42.8 | 29.0 | 25.0 | 2.9 | 0.4 | 0 | |
| 15 | 46.5 | 24.2 | 20.9 | 7.4 | 0.5 | 0.4 | |
| 30 | 37.3 | 17.4 | 23.2 | 20.3 | 0.8 | 1.1 | |
| 45 | 27.6 | 6.3 | 28.6 | 33.8 | 1.6 | 2.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Bai, J.; Fan, L.; Mao, X.; Ding, Z.; Mu, H.; Liu, G.; Yuan, Y. Fabrication of Slippery Surfaces on Aluminum Alloy and Its Anti-Icing Performance in Glaze Ice. Coatings 2023, 13, 732. https://doi.org/10.3390/coatings13040732
Li B, Bai J, Fan L, Mao X, Ding Z, Mu H, Liu G, Yuan Y. Fabrication of Slippery Surfaces on Aluminum Alloy and Its Anti-Icing Performance in Glaze Ice. Coatings. 2023; 13(4):732. https://doi.org/10.3390/coatings13040732
Chicago/Turabian StyleLi, Bo, Jie Bai, Lei Fan, Xianyin Mao, Zhimin Ding, Hao Mu, Guoyong Liu, and Yuan Yuan. 2023. "Fabrication of Slippery Surfaces on Aluminum Alloy and Its Anti-Icing Performance in Glaze Ice" Coatings 13, no. 4: 732. https://doi.org/10.3390/coatings13040732
APA StyleLi, B., Bai, J., Fan, L., Mao, X., Ding, Z., Mu, H., Liu, G., & Yuan, Y. (2023). Fabrication of Slippery Surfaces on Aluminum Alloy and Its Anti-Icing Performance in Glaze Ice. Coatings, 13(4), 732. https://doi.org/10.3390/coatings13040732








