Thermoelectric and Structural Properties of Transparent Sb-Doped ZnO Thin Films Sputtered in a Confocal Geometry
Abstract
1. Introduction
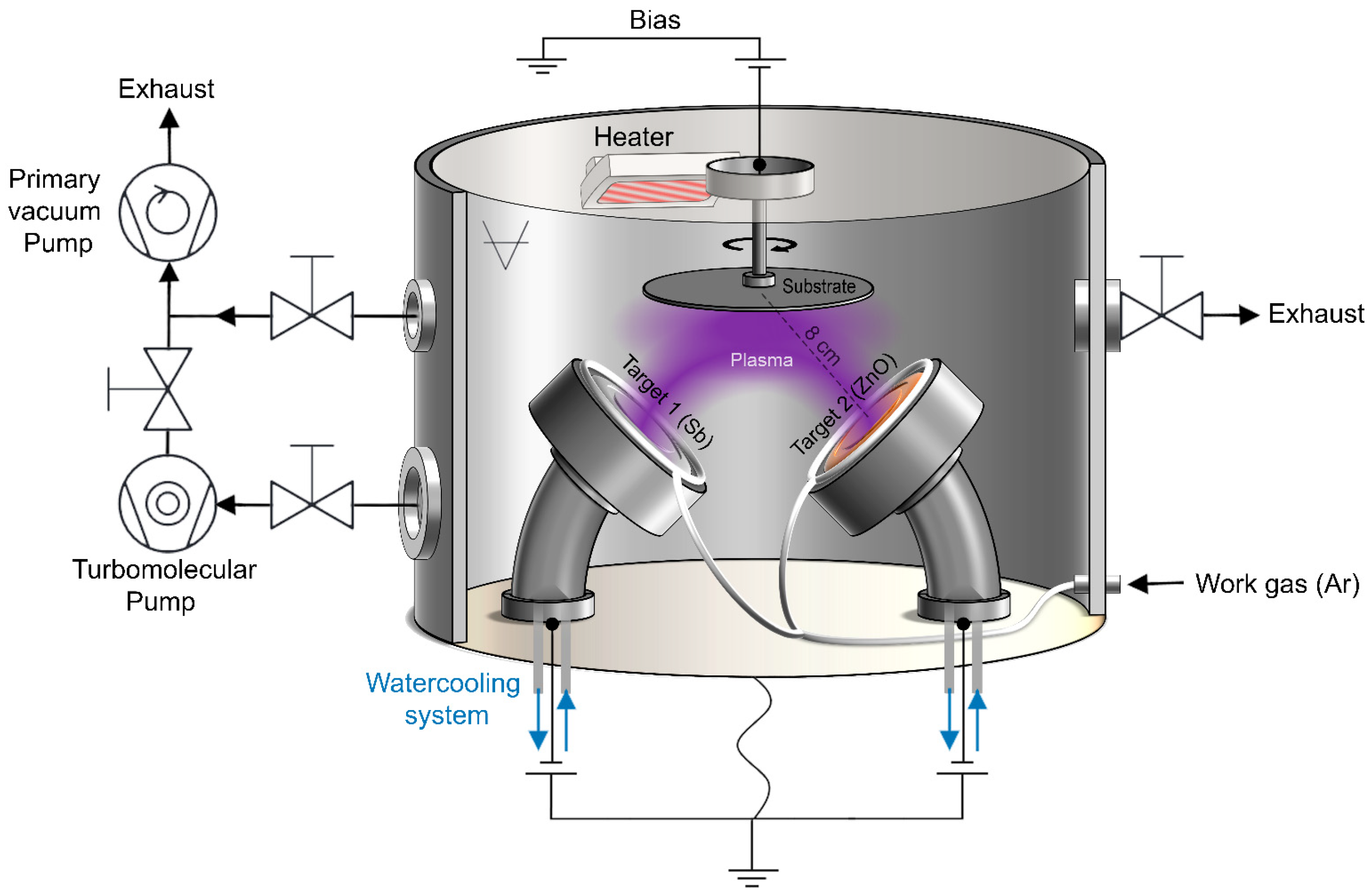
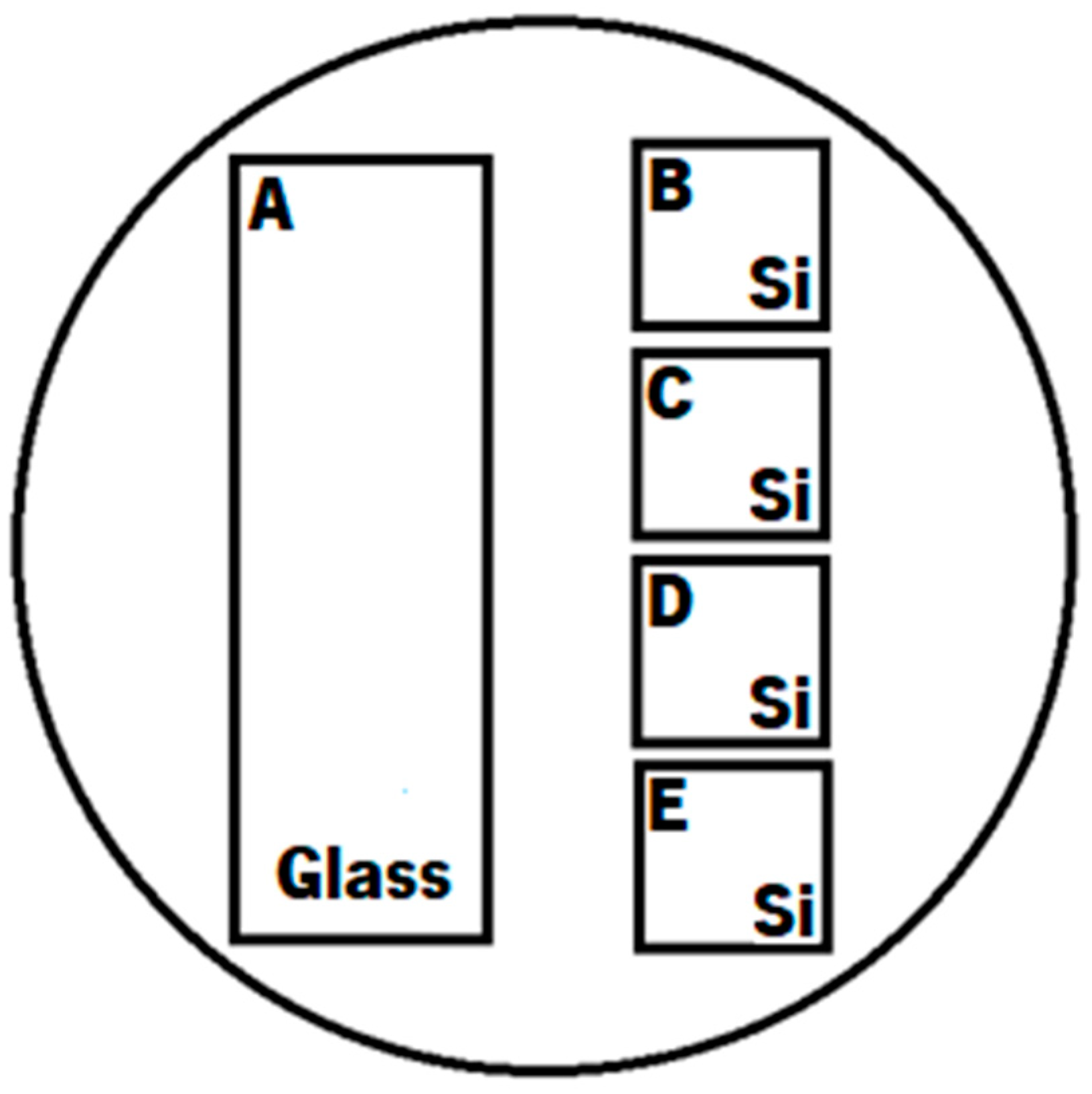
2. Materials and Methods
3. Results and Discussion
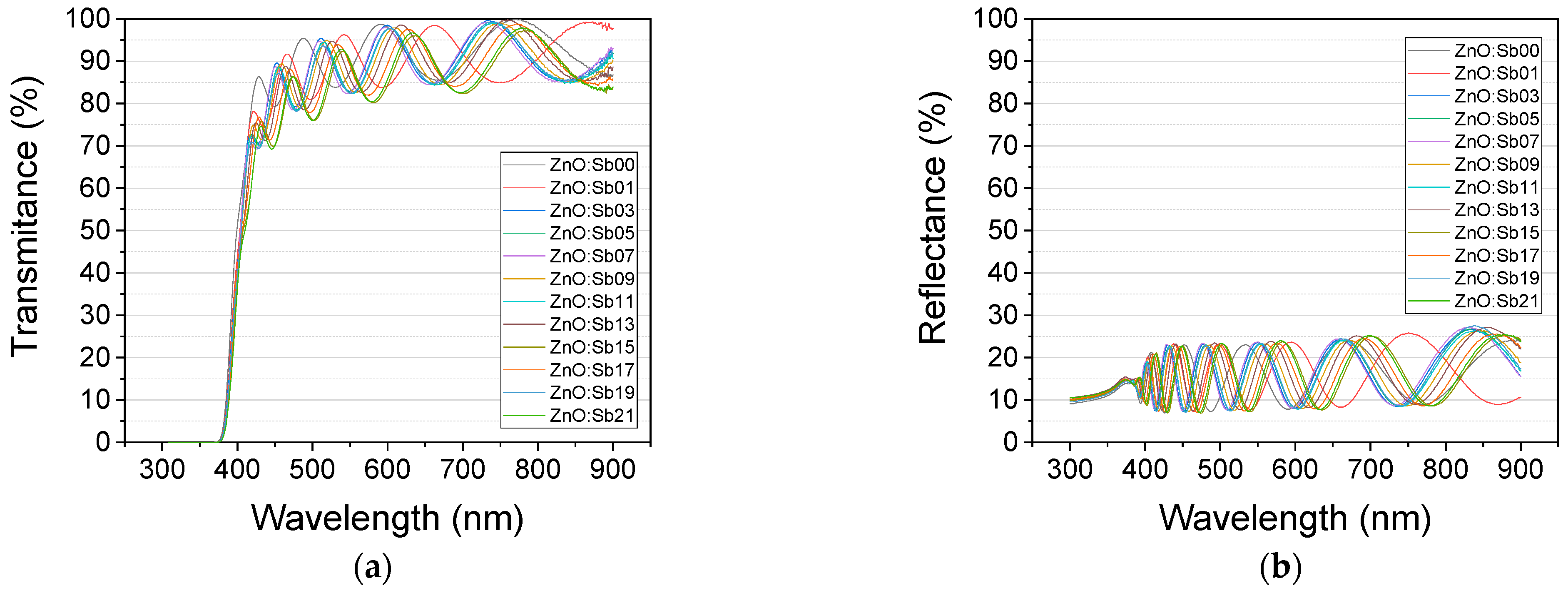
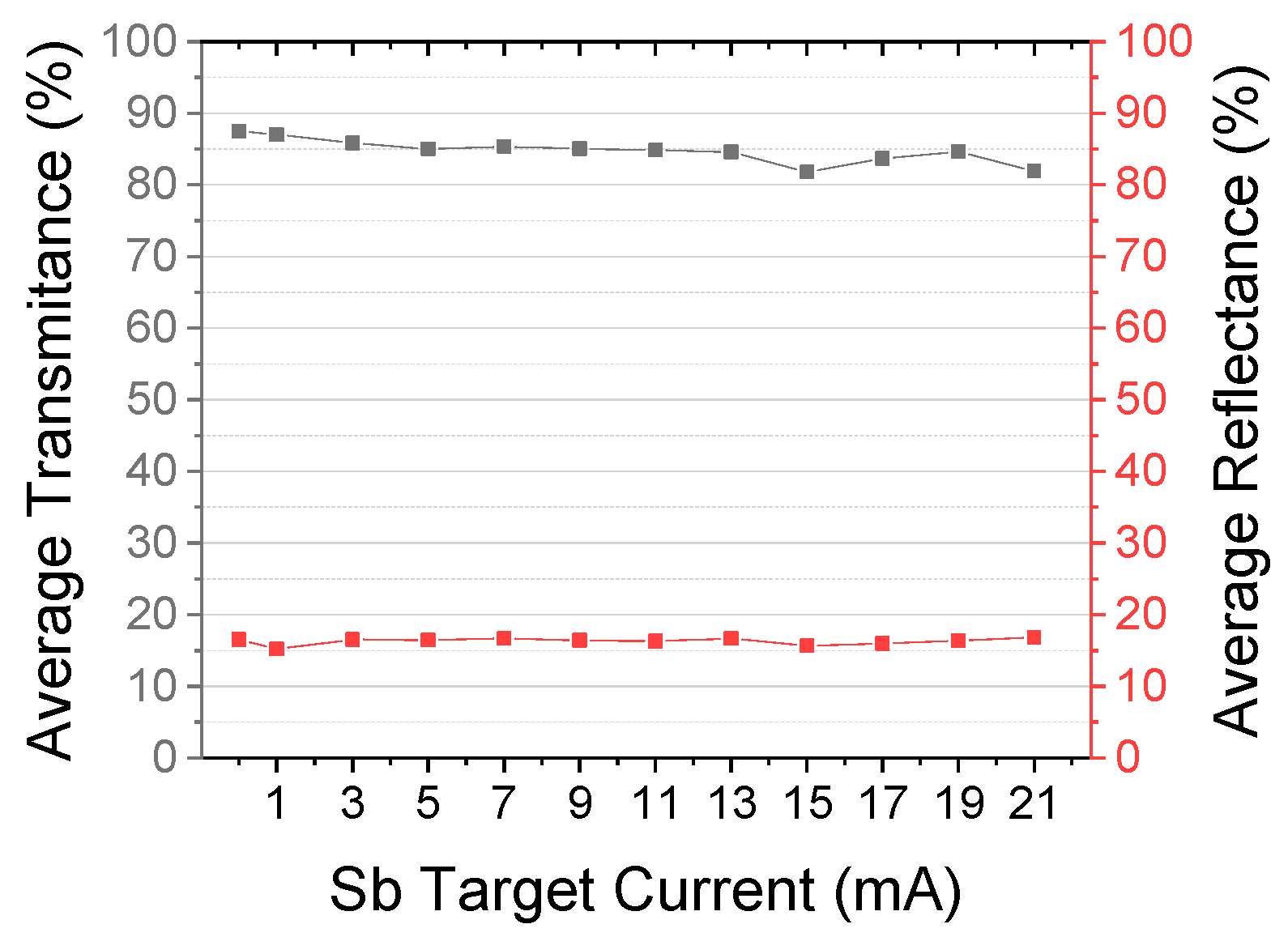
3.1. Optical Properties
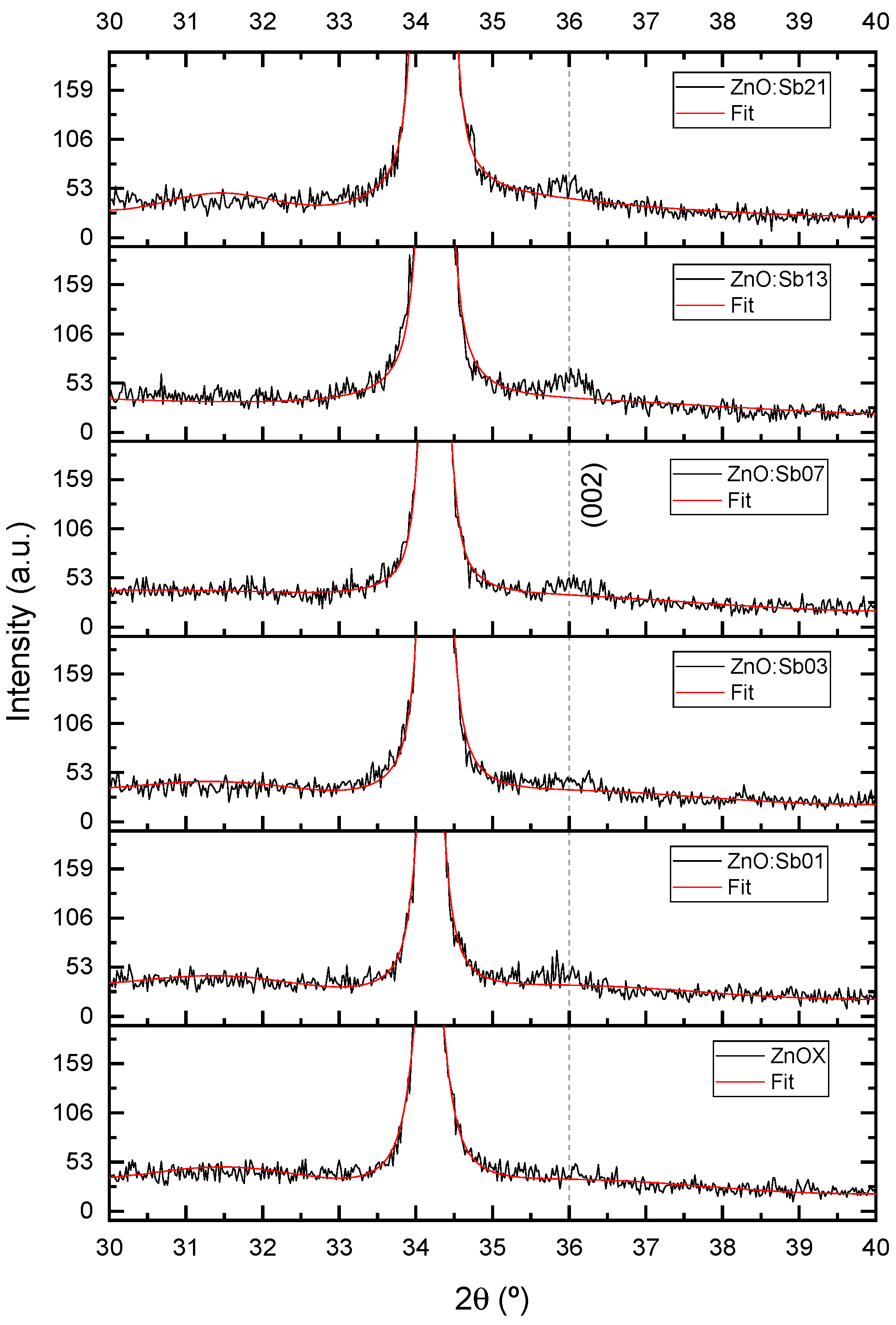
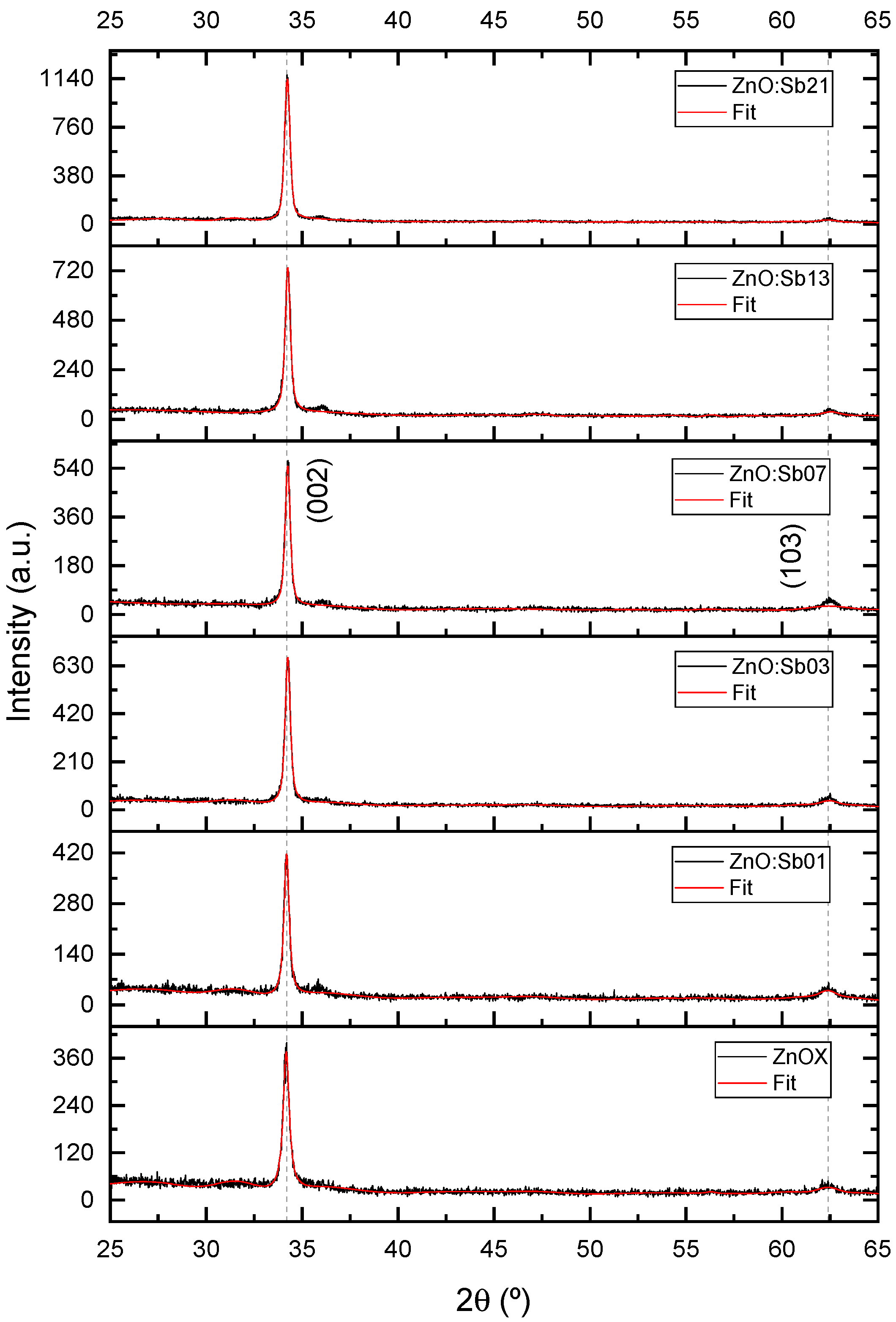
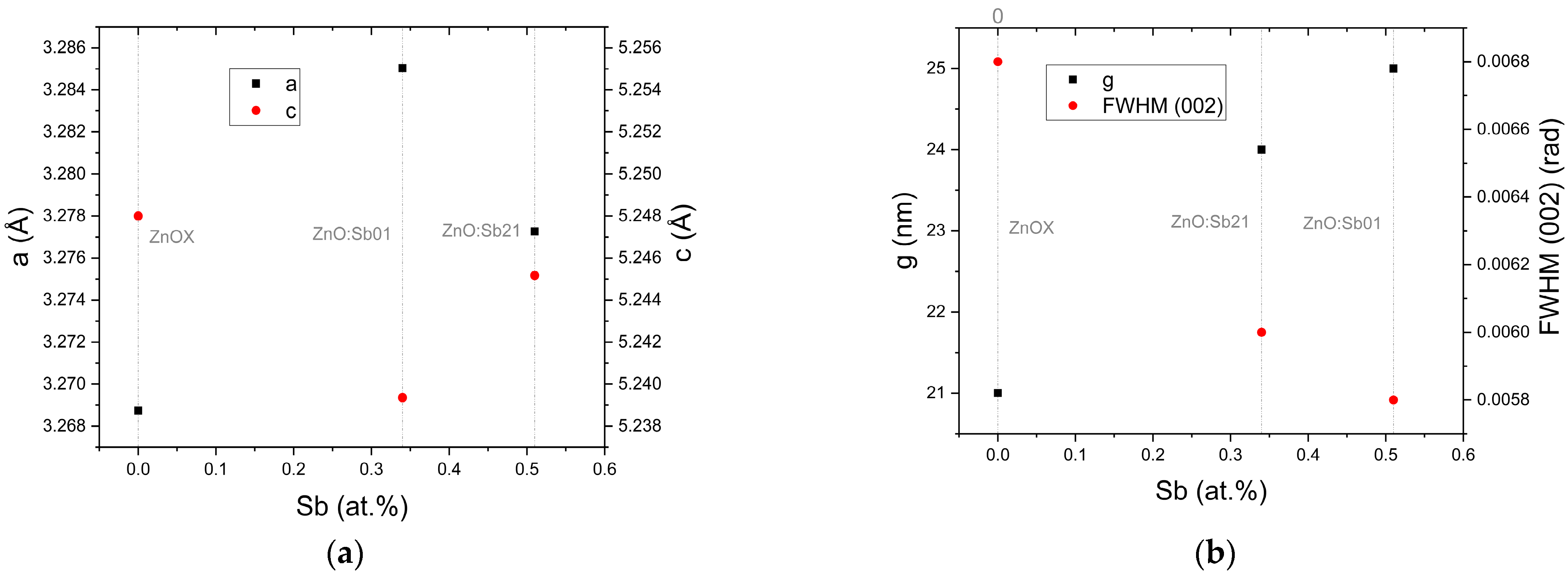
3.2. Compositional and Structural Analysis
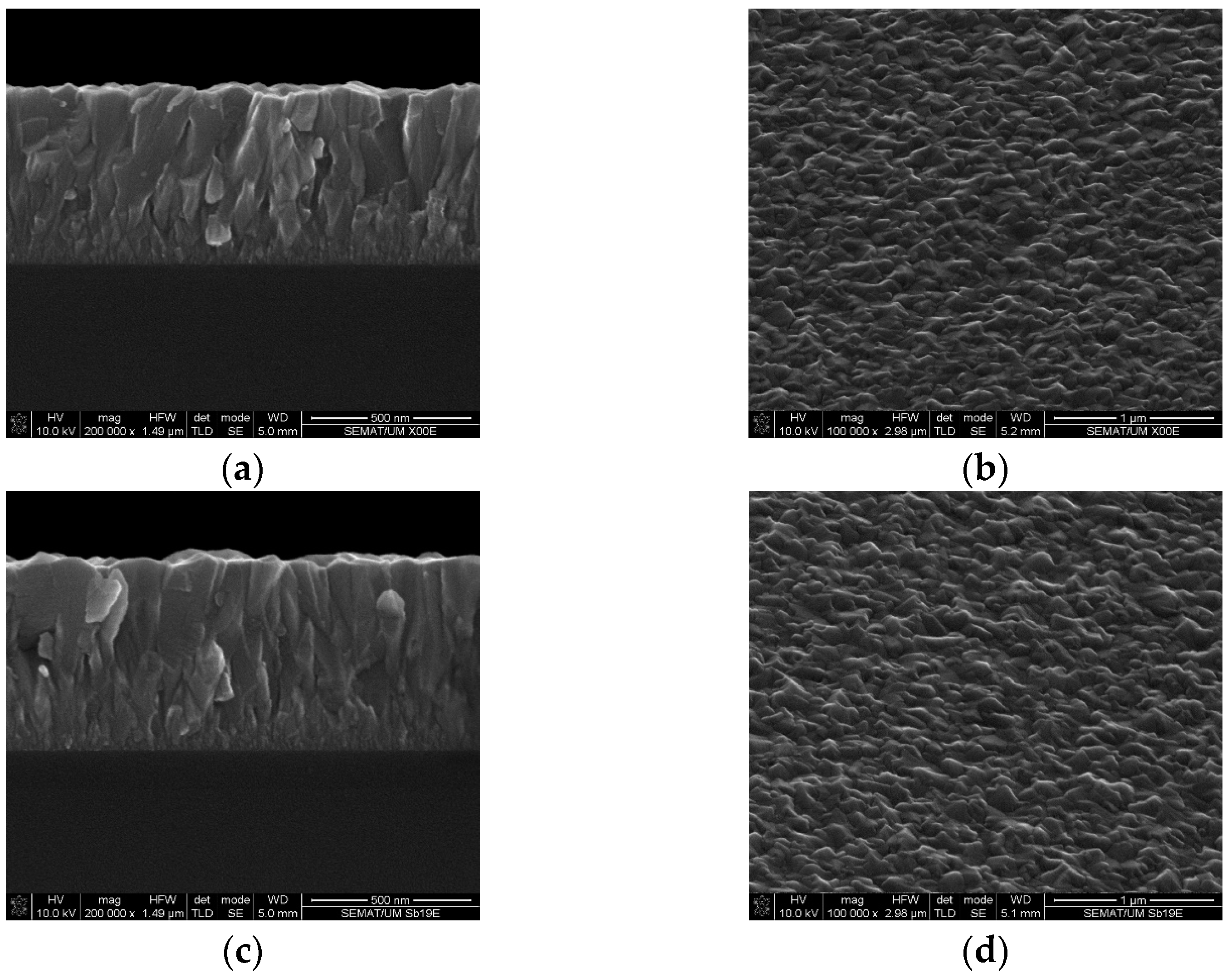
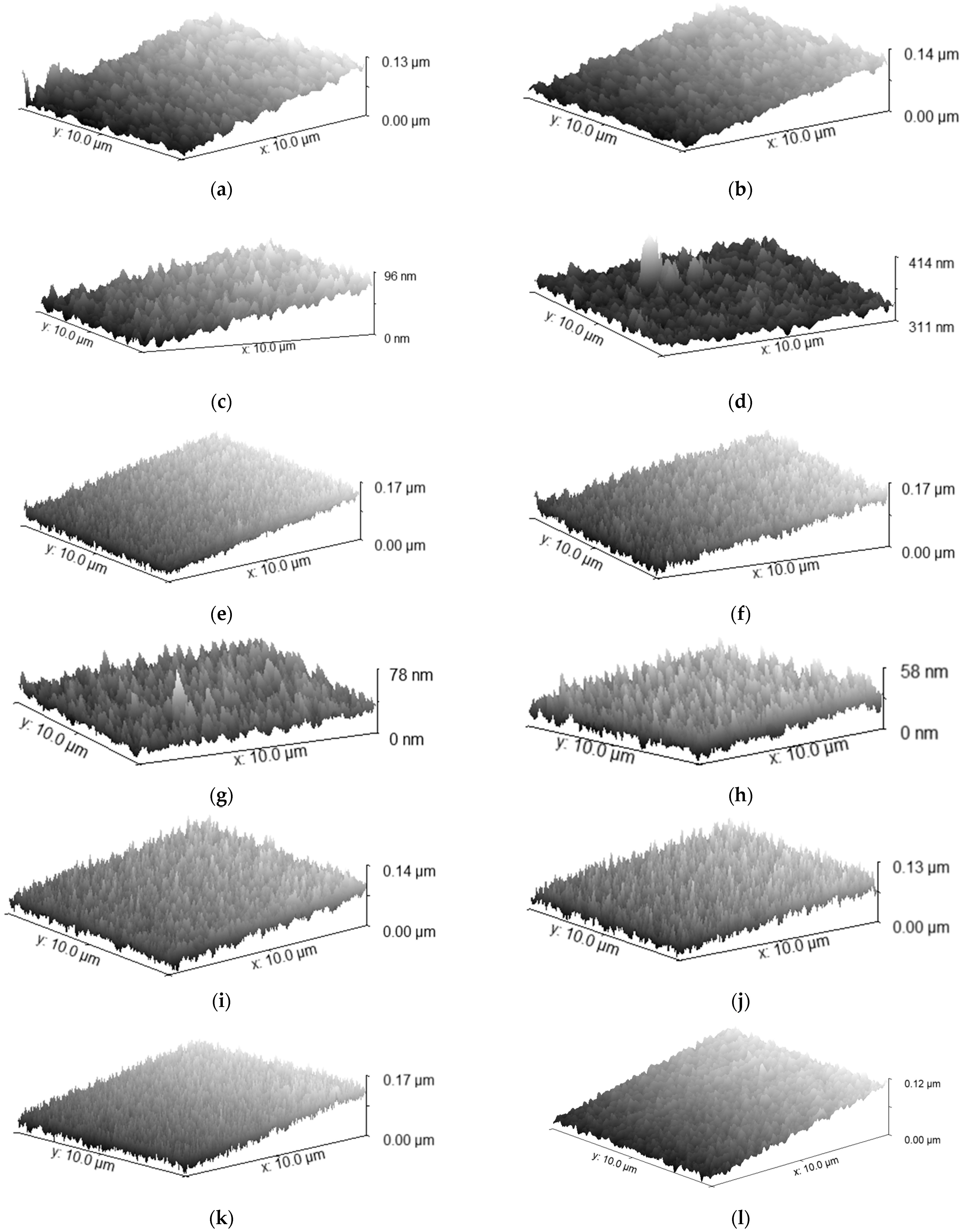
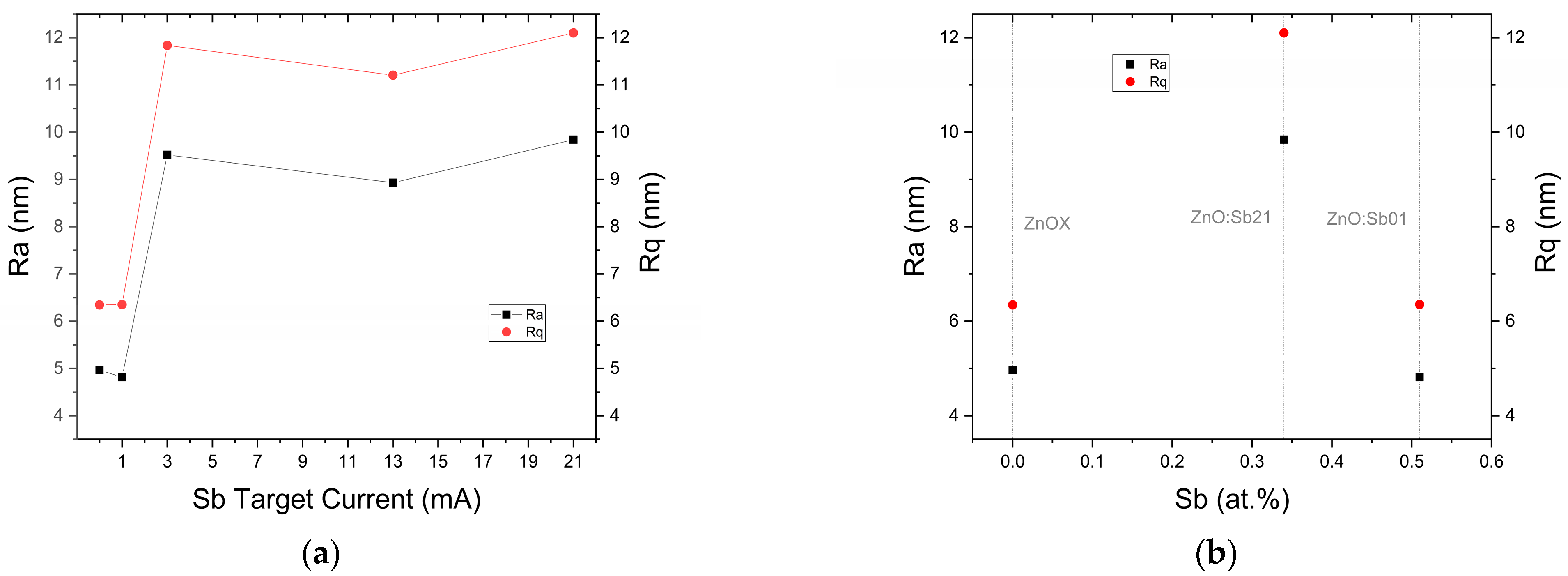
3.3. Grain Boundary Morphology
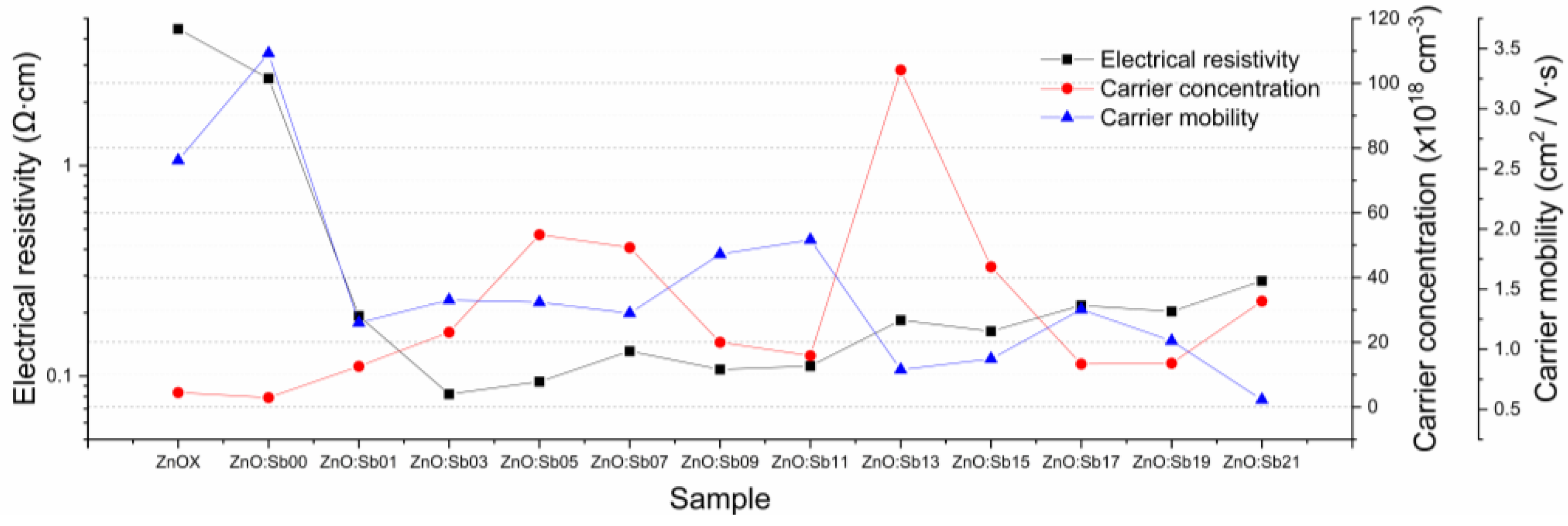
3.4. Electrical Properties
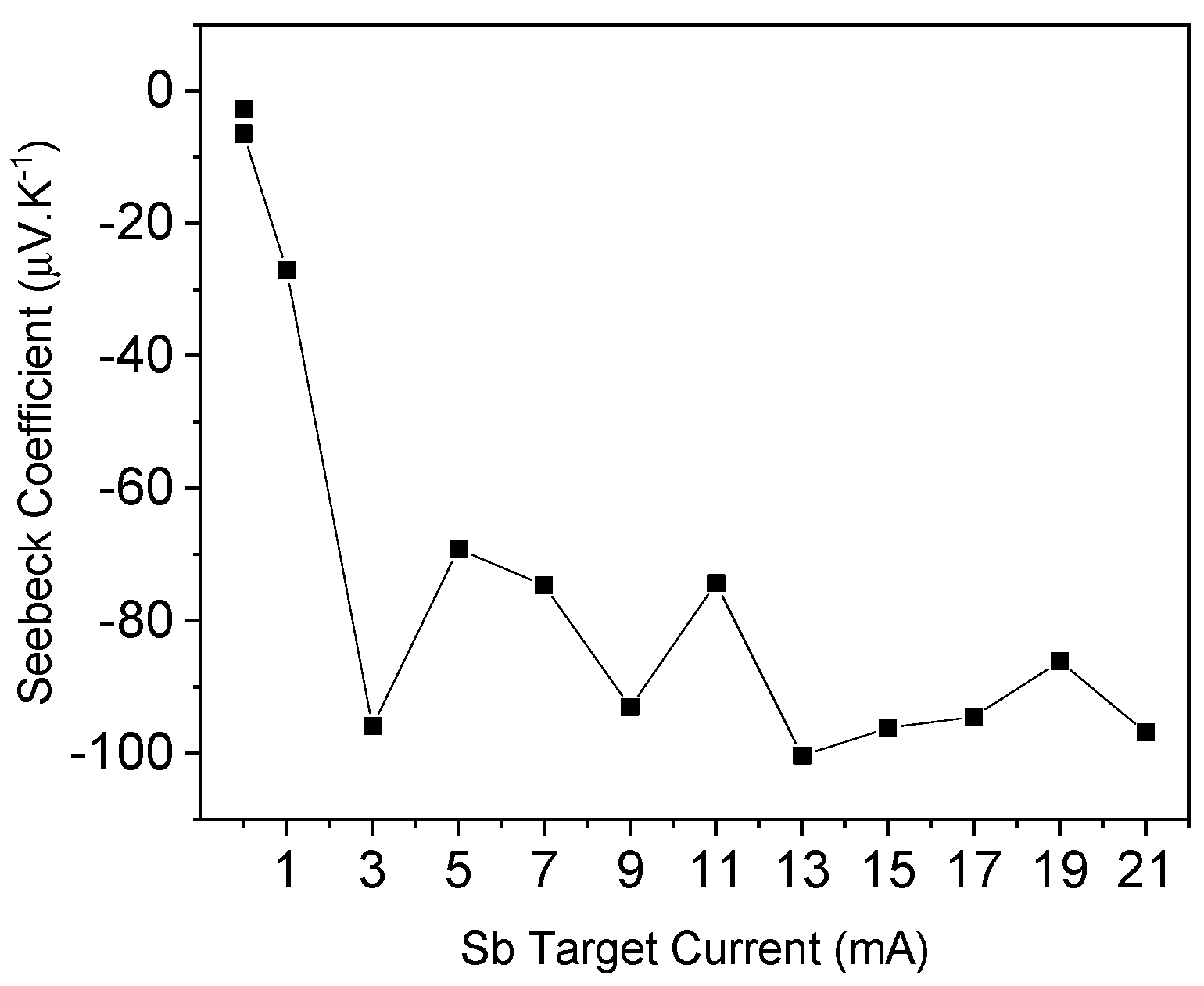
3.5. Thermoelectricity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
Appendix C
| Sample | |S| (μV/K) | PF (mW∙m−1∙K−2) | ρ (Ω∙cm) | n (×1018cm−3) | μ (cm2/V∙s) | a (Å) | c (Å) | g (nm) | Ra (nm) | Rq (nm) | t(SEM) (nm) | Sb(EDX) (at.%) | * (%) | * (%) | Eg (eV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZnOX | 2.8 | 0.2 × 10−4 | 4.46 | −4.5 | 2.6 | 3.27 | 5.25 | 21 | 4.97 | 6.35 | 578.47 | - | 87.41 | 16.74 | 3.26 |
| Zno:Sb00 | 6.5 | 0.2 × 10−3 | 2.60 | −2.9 | 3.46 | - | - | - | - | - | 627.03 | - | 87.50 | 16.54 | 3.26 |
| ZnoZnO:Sb01 | 27.1 | 0.4 × 10−2 | 0.19 | −12.6 | 1.22 | 3.28 | 5.25 | 25 | 4.82 | 6.35 | 702.30 | 0.51 | 87.01 | 15.21 | 3.25 |
| ZnO:Sb03 | 95.9 | 1.1 | 0.08 | −23.1 | 1.4 | 3.28 | 5.24 | 25 | 9.52 | 11.84 | 719.01 | - | 85.83 | 16.54 | 3.25 |
| ZnO:Sb05 | 69.2 | 0.5 | 0.09 | −53.3 | 1.4 | - | - | - | - | - | 740.74 | - | 85.00 | 16.45 | 3.25 |
| ZnO:Sb07 | 74.7 | 0.4 | 0.13 | −49.3 | 1.3 | 3.29 | 5.24 | 26 | 5.23 | 6.59 | 757.60 | 0.50 | 85.35 | 16.69 | 3.24 |
| ZnO:Sb09 | 93.0 | 0.8 | 0.11 | −20.0 | 1.8 | - | - | - | - | - | 771.38 | - | 85.03 | 16.42 | 3.26 |
| ZnO:Sb11 | 74.4 | 0.5 | 0.11 | −15.9 | 1.9 | - | - | - | - | - | 778.07 | 0.12 | 84.83 | 16.33 | 3.25 |
| ZnO:Sb13 | 100.4 | 0.5 | 0.18 | −104.1 | 0.8 | 3.27 | 5.24 | 25 | 8.93 | 11.21 | 782.57 | - | 84.59 | 16.69 | 3.25 |
| ZnO:Sb15 | 96.2 | 0.6 | 0.16 | −43.3 | 0.9 | - | - | - | - | - | 785.20 | - | 81.80 | 15.65 | 3.24 |
| ZnO:Sb17 | 94.5 | 0.4 | 0.22 | −13.3 | 1.3 | - | - | - | - | - | 787.07 | - | 83.69 | 15.99 | 3.25 |
| ZnO:Sb19 | 86.1 | 0.4 | 0.20 | −13.5 | 1.1 | - | - | - | - | - | 788.52 | - | 84.66 | 16.39 | 3.25 |
| ZnO:Sb21 | 96.9 | 0.3 | 0.28 | −32.7 | 0.6 | 3.29 | 5.24 | 24 | 9.84 | 12.10 | 789.70 | 0.34 | 81.91 | 15.93 | 3.24 |
References
- US Energy Information Administration, IEO 2021 Narrative, n.d. Available online: www.eia.gov (accessed on 19 January 2023).
- International Energy Agency. World Energy Outlook 2021. 2021. Available online: www.iea.org/weo (accessed on 19 January 2023).
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste heat recovery technologies and applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Beretta, D.; Neophytou, N.; Hodges, J.M.; Kanatzidis, M.G.; Narducci, D.; Gonzalez, M.M.; Beekman, M.; Balke, B.; Cerretti, G.; Tremel, W.; et al. Thermoelectrics: From history, a window to the future. Mater. Sci. Eng. R Rep. 2018, 138, 100501. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.; Gogna, P. New Directions for Low–Dimensional Thermoelectric Materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Tan, Q.; Li, J.-F. Thermoelectric properties of Pb-doped bismuth telluride thin films deposited by magnetron sputtering. J. Alloy. Compd. 2014, 590, 362–367. [Google Scholar] [CrossRef]
- Nuthongkum, P.; Sakdanuphab, R.; Horprathum, M.; Sakulkalavek, A. [Bi]:[Te] Control, Structural and Thermoelectric Properties of Flexible Bi x Te y Thin Films Prepared by RF Magnetron Sputtering at Different Sputtering Pressures. J. Electron. Mater. 2017, 46, 6444–6450. [Google Scholar] [CrossRef]
- Ribeiro, J.; Correia, F.; Kuzmin, A.; Jonane, I.; Kong, M.; Goñi, A.; Reparaz, J.; Kalinko, A.; Welter, E.; Tavares, C. Influence of Nb-doping on the local structure and thermoelectric properties of transparent TiO2:Nb thin films. J. Alloy. Compd. 2020, 838, 155561. [Google Scholar] [CrossRef]
- Correia, F.; Salvador, P.; Ribeiro, J.; Mendes, A.; Tavares, C. Effect on the electrical and morphological properties of Bi incorporation into ZnO:Ga and ZnO:Al thin films deposited by confocal magnetron sputtering. Vacuum 2018, 152, 252–260. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Lan, M.; Piao, Y.; Miyazaki, K.; Wang, Q. Effect of growth modes on electrical and thermal transport of thermoelectric ZnO:Al films. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 259–266. [Google Scholar] [CrossRef]
- Chao-Moo, W.; Vora-Ud, A.; Thaowankaew, S.; Muthitamongkol, P.; Seetawan, T. Effect of annealing treatment on thermoelectric properties of Ti-doped ZnO thin film. AIP Conf. Proc. 2018, 2010, 020013. [Google Scholar] [CrossRef]
- Gather, F.; Kronenberger, A.; Hartung, D.; Becker, M.; Polity, A.; Klar, P.J.; Meyer, B.K. Possibility of enhancing the thermoelectric figure of merit of ZnO by sulfur incorporation. Appl. Phys. Lett. 2013, 103, 082115. [Google Scholar] [CrossRef]
- Pham, A.T.T.; Le, O.K.T.; Phan, T.T.T.; Van Hoang, D.; Nguyen, T.H.; Le, N.D.; Phan, T.B.; Tran, V.C. Enhancing transparent thermoelectric properties of Sb-doped ZnO thin films via controlled deposition temperature. Vacuum 2022, 202, 111137. [Google Scholar] [CrossRef]
- Safeen, K.; Micheli, V.; Bartali, R.; Gottardi, G.; Safeen, A.; Ullah, H.; Laidani, N. Influence of intrinsic defects on the electrical and optical properties of TiO2:Nb films sputtered at room temperature. Thin Solid Films 2018, 645, 173–179. [Google Scholar] [CrossRef]
- Tao, J.; Pan, H.; Wong, L.M.; Wong, T.I.; Chai, J.W.; Pan, J.; Wang, S.J. Mechanism of insulator-to-metal transition in heavily Nb doped anatase TiO2. Mater. Res. Express 2014, 1, 015911. [Google Scholar] [CrossRef]
- Ding, L.; Akbarzadeh, A.; Tan, L. A review of power generation with thermoelectric system and its alternative with solar ponds. Renew. Sustain. Energy Rev. 2018, 81, 799–812. [Google Scholar] [CrossRef]
- Faustino, B.M.M.; Gomes, D.; Faria, J.; Juntunen, T.; Gaspar, G.; Bianchi, C.; Almeida, A.; Marques, A.; Tittonen, I.; Ferreira, I. CuI p-type thin films for highly transparent thermoelectric p-n modules. Sci. Rep. 2018, 8, 6867. [Google Scholar] [CrossRef] [PubMed]
- Mang, A.; Reimann, K.; Rübenacke, S. Band gaps, crystal-field splitting, spin-orbit coupling, and exciton binding energies in ZnO under hydrostatic pressure. Solid State Commun. 1995, 94, 251–254. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Ozgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Castro, M.; Cerqueira, M.; Rebouta, L.; Alpuim, P.; Garcia, C.; Júnior, G.; Tavares, C. Influence of hydrogen plasma thermal treatment on the properties of ZnO:Al thin films prepared by dc magnetron sputtering. Vacuum 2014, 107, 145–154. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Wong, Z.M.; Suwardi, A.; Zheng, Y.; Wei, F.; Wang, S.; Tan, T.L.; Wu, G.; Zhu, Q.; et al. Gallium-Doped Zinc Oxide Nanostructures for Tunable Transparent Thermoelectric Films. ACS Appl. Nano Mater. 2022, 5, 8631–8639. [Google Scholar] [CrossRef]
- Singh, G.; Shrivastava, S.B.; Jain, D.; Pandya, S.; Shripathi, T.; Ganesan, V. Effect of indium doping on zinc oxide films prepared by chemical spray pyrolysis technique. Bull. Mater. Sci. 2010, 33, 581–587. [Google Scholar] [CrossRef]
- Xiu, F.X.; Yang, Z.; Mandalapu, L.J.; Zhao, D.T.; Liu, J.L.; Beyermann, W.P. High-mobility Sb-doped p-type ZnO by molecular-beam epitaxy. Appl. Phys. Lett. 2005, 87, 152101. [Google Scholar] [CrossRef]
- Pan, X.; Ye, Z.; Li, J.; Gu, X.; Zeng, Y.; He, H.; Zhu, L.; Che, Y. Fabrication of Sb-doped p-type ZnO thin films by pulsed laser deposition. Appl. Surf. Sci. 2007, 253, 5067–5069. [Google Scholar] [CrossRef]
- De Campos, J.A.; Viseu, T.M.; Rolo, A.; Barradas, N.P.; Alves, E.; de Lacerda-Arôso, T.; Cerqueira, M.F. Electrical and Raman Scattering Studies of ZnO:P and ZnO:Sb Thin Films. J. Nanosci. Nanotechnol. 2010, 10, 2620–2623. [Google Scholar] [CrossRef]
- Kumar, N.S.; Bangera, K.V.; Shivakumar, G.K. Properties of antimony doped ZnO thin films deposited by spray pyrolysis technique. Semiconductors 2015, 49, 899–904. [Google Scholar] [CrossRef]
- Li, G.; Lin, X.; Liu, S.; Jia, B.; Wang, Q. Sb content dependent thermoelectric properties of the p-type ZnO:Sb films fabricated by oxidation method. Appl. Surf. Sci. 2018, 439, 82–87. [Google Scholar] [CrossRef]
- Fathima, N.; Pradeep, N.; Balakrishnan, J. Investigations of the effects of electrode geometry and mechanical stress on Antimony doped Zinc Oxide nanostructures based MSM UV photodetectors fabricated on flexible substrates. Sol. Energy Mater. Sol. Cells 2019, 194, 207–214. [Google Scholar] [CrossRef]
- van der Pauw, L.J. A method of measuring specific resistivity and hall effect of discs of arbitrary shape. In Semiconductor Devices Pioneering Papers; World Scientific Publishing Co Pte Ltd.: Singapore, 1991; pp. 174–182. [Google Scholar] [CrossRef]
- Sánchez-González, J.; Díaz-Parralejo, A.; Ortiz, A.; Guiberteau, F. Determination of optical properties in nanostructured thin films using the Swanepoel method. Appl. Surf. Sci. 2006, 252, 6013–6017. [Google Scholar] [CrossRef]
- Martin, J.; Wong-Ng, W.; Green, M.L. Seebeck Coefficient Metrology: Do Contemporary Protocols Measure Up? J. Electron. Mater. 2015, 44, 1998–2006. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy (XPS) Database, Version 3.5, (n.d.). Available online: http://srdata.nist.gov/xps/ (accessed on 22 March 2003).
- Correia, F.C.; Ribeiro, J.M.; Ferreira, A.; Reparaz, J.S.; Goñi, A.R.; Boll, T.; Mendes, A.; Tavares, C.J. The effect of Bi doping on the thermal conductivity of ZnO and ZnO:Al thin films. Vacuum 2023, 207, 111572. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Rodrigues, F.J.; Correia, F.C.; Pudza, I.; Kuzmin, A.; Kalinko, A.; Welter, E.; Barradas, N.P.; Alves, E.; LaGrow, A.P.; et al. The influence of Sb doping on the local structure and disorder in thermoelectric ZnO:Sb thin films. J. Alloy. Compd. 2023, 939, 168751. [Google Scholar] [CrossRef]

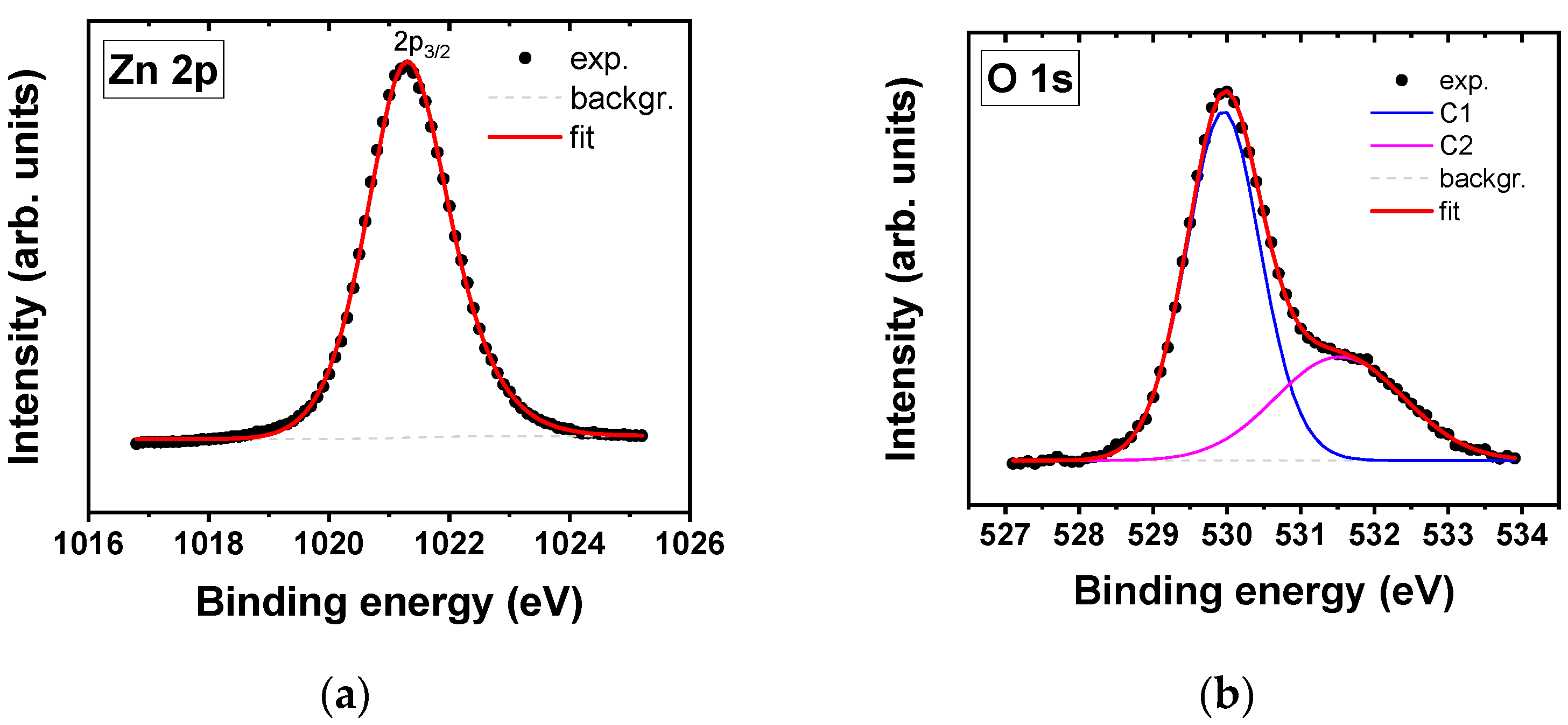
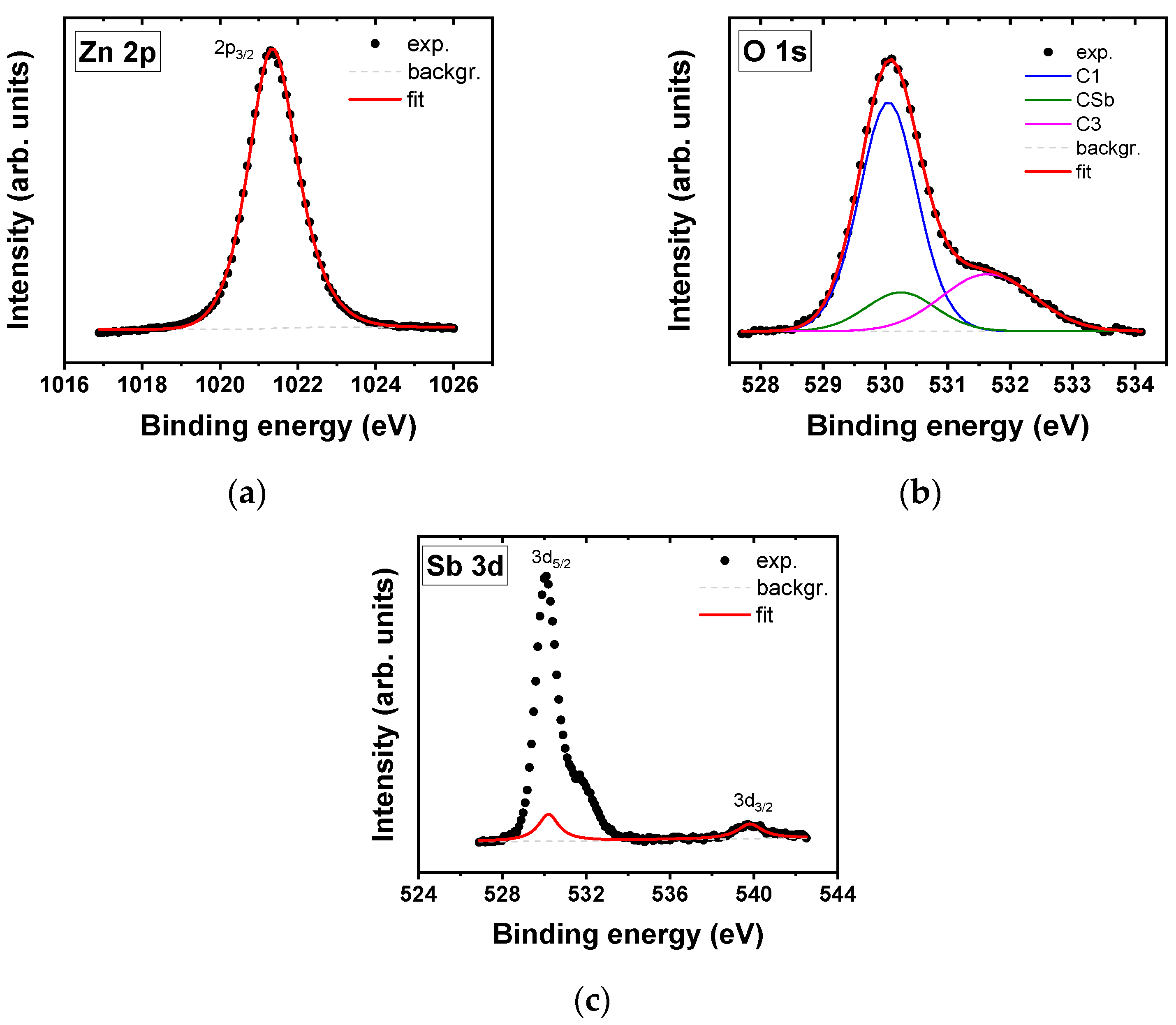
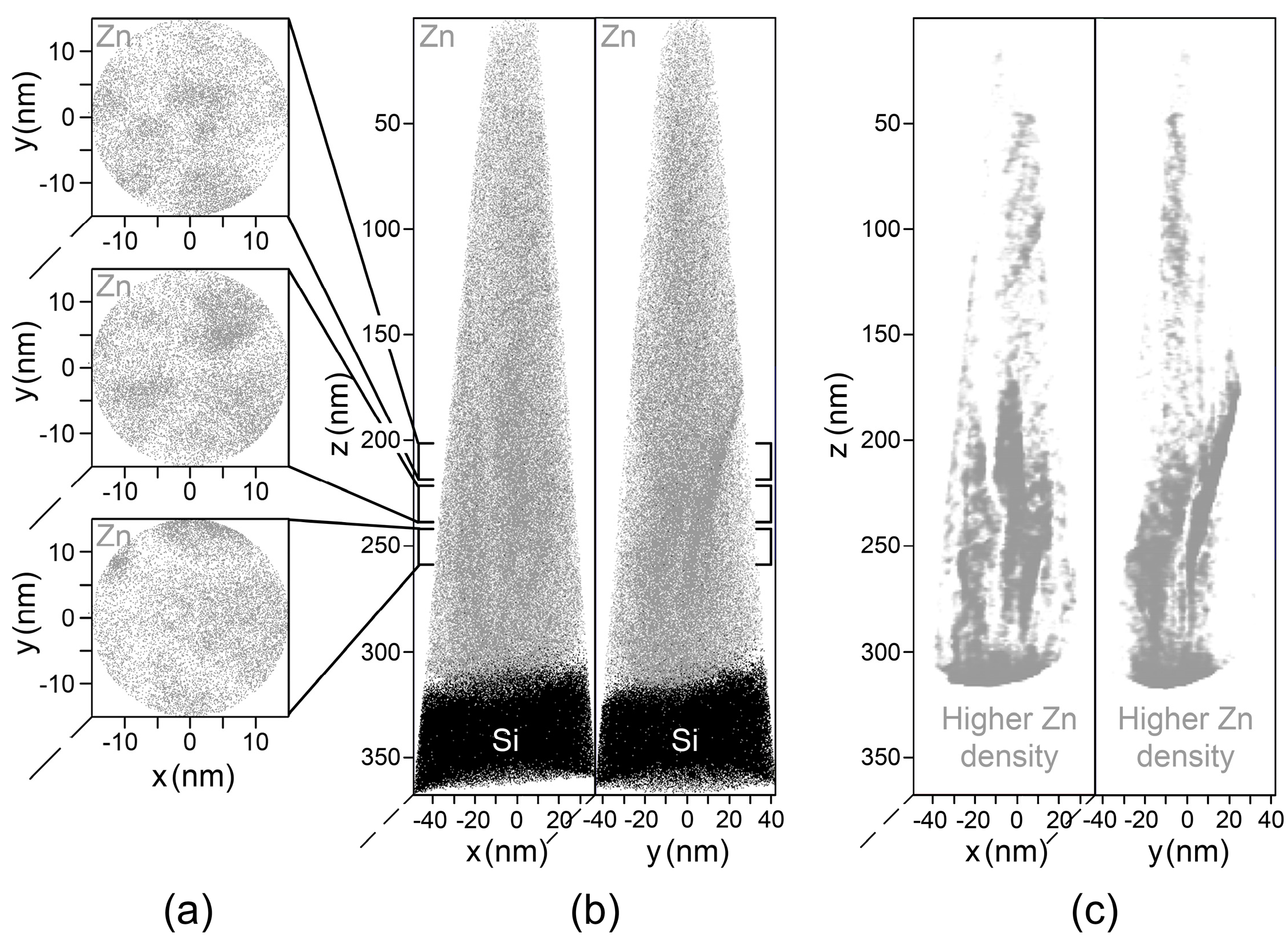
| Sample | Composition (at.%) | Zn 2p | O 1s | Sb 3d5/2 | |
|---|---|---|---|---|---|
| Position | C1 (β1) % | Position | |||
| /ΔE/ | CSb (β) % | /ΔE/ | |||
| (β) (eV) | C2 (β2) % | (β) (eV) | |||
| (eV) | |||||
| ZnOX | Zn | 49.7 | 529.9 | - | |
| (1.1) | |||||
| 66% | |||||
| O | 50.3 | 1021.3 23.1 (1.7) | |||
| - | |||||
| Sb | - | 531.6 | |||
| (1.7) | |||||
| 34% | |||||
| ZnO:Sb21 | Zn | 50.2 | 530.0 (1.1) 63% | ||
| O | 48.8 | 1021.3 23.1 (1.5) | 530.2 (1.2) 13% | 530.2 9.6 (1.2) | |
| Sb | 1.0 | 532.0 (1.5) 24% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, H.F.; Ribeiro, J.M.; Boll, T.; Tavares, C.J. Thermoelectric and Structural Properties of Transparent Sb-Doped ZnO Thin Films Sputtered in a Confocal Geometry. Coatings 2023, 13, 735. https://doi.org/10.3390/coatings13040735
Faria HF, Ribeiro JM, Boll T, Tavares CJ. Thermoelectric and Structural Properties of Transparent Sb-Doped ZnO Thin Films Sputtered in a Confocal Geometry. Coatings. 2023; 13(4):735. https://doi.org/10.3390/coatings13040735
Chicago/Turabian StyleFaria, Helder Filipe, Joana Margarida Ribeiro, Torben Boll, and Carlos José Tavares. 2023. "Thermoelectric and Structural Properties of Transparent Sb-Doped ZnO Thin Films Sputtered in a Confocal Geometry" Coatings 13, no. 4: 735. https://doi.org/10.3390/coatings13040735
APA StyleFaria, H. F., Ribeiro, J. M., Boll, T., & Tavares, C. J. (2023). Thermoelectric and Structural Properties of Transparent Sb-Doped ZnO Thin Films Sputtered in a Confocal Geometry. Coatings, 13(4), 735. https://doi.org/10.3390/coatings13040735








