Fabrication of a Flower-like Copper Oxide Film-Coated Nanoporous Stainless Steel Using Anodization-Assisted Electrodeposition as a Novel Antibacterial Material
Abstract
1. Introduction
2. Experimental Methods
2.1. NPSS and Cu/NPSS Samples Preparation
2.2. Sample Characterization
2.3. In Vitro Bactericidal Activity
3. Results and Discussions
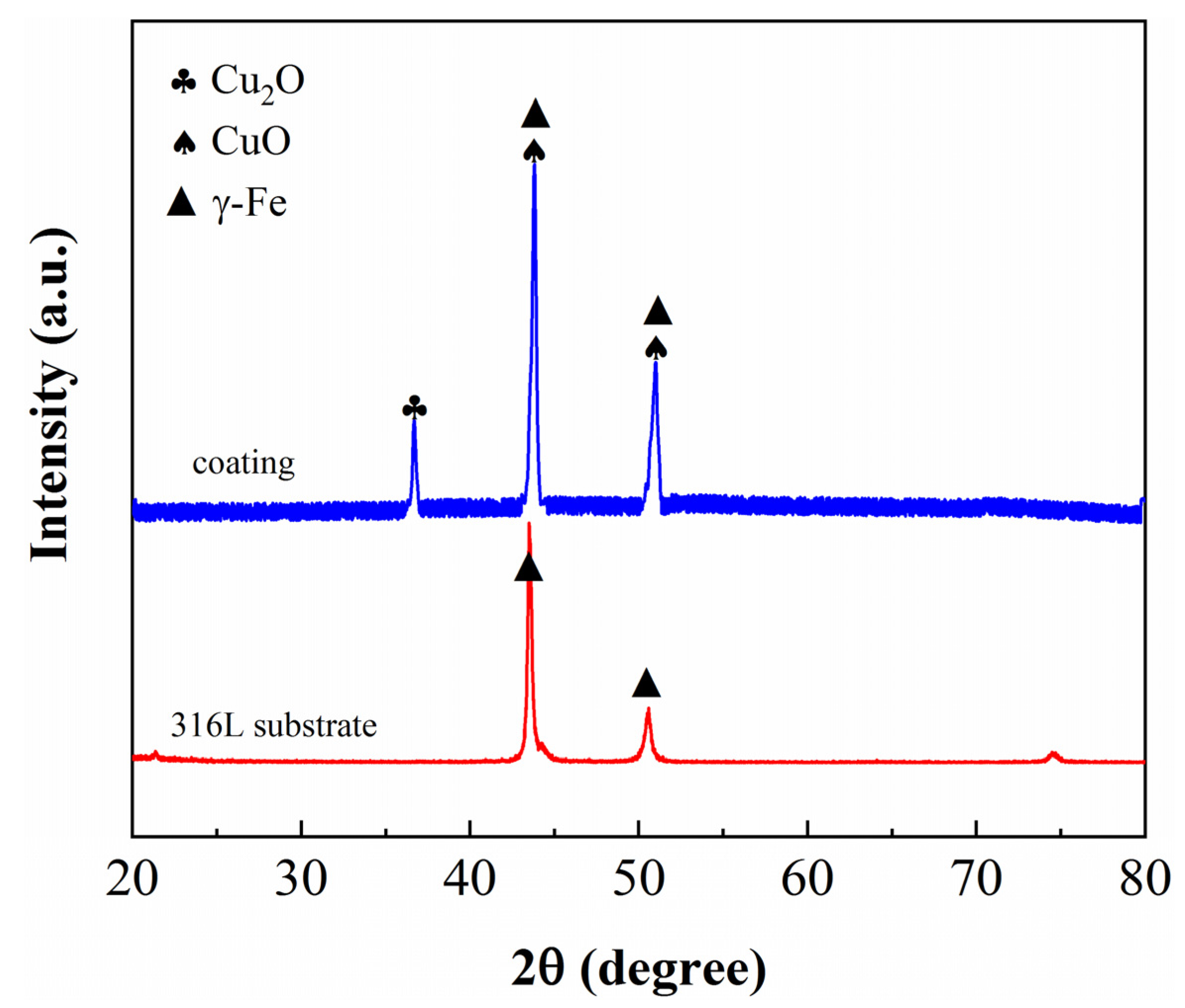
3.1. Composition and Phase Characterization of NPSS and Cu/NPSS Samples
3.2. Microstructural of NPSS and Cu/NPSS Samples
3.3. Antimicrobial Activities of NPSS and Cu/NPSS Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muthupandi, V.; Srinivasan, P.B.; Seshadri, S.; Sundaresan, S. Effect of weld metal chemistry and heat input on the structure and properties of duplex stainless steel welds. Mater. Sci. Eng. A 2003, 358, 9–16. [Google Scholar] [CrossRef]
- Hong, I.T.; Koo, C.H. Antibacterial properties, corrosion resistance and mechanical properties of Cu-modified SUS 304 stainless steel. Mater. Sci. Eng. A 2005, 393, 213–222. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Ding, D.; Jiang, Z.; Xia, C. Development of three-layer intermediate temperature solid oxide fuel cells with direct stainless steel based anodes. Int. J. Hydrogen Energy 2012, 37, 4401–4405. [Google Scholar] [CrossRef]
- Wyrwa, D.W.; Schmid, G. Metal Nanoparticles on Stainless Steel Surfaces as Novel Heterogeneous Catalysts. J. Clust. Sci. 2007, 18, 476–493. [Google Scholar] [CrossRef]
- Wu, H.-B.; Niu, G.; Wu, F.-J.; Tang, D. Reverse-transformation austenite structure control with micro/nanometer size. Int. J. Miner. Met. Mater. 2017, 24, 530–537. [Google Scholar] [CrossRef]
- Rezaei, B.; Havakeshian, E.; Ensafi, A.A. Fabrication of a porous Pd film on nanoporous stainless steel using galvanic replacement as a novel electrocatalyst/electrode design for glycerol oxidation. Electrochim. Acta 2014, 136, 89–96. [Google Scholar] [CrossRef]
- Zhan, W.; Ni, H.; Chen, R.; Song, X.; Huo, K.; Fu, J. Formation of nanopore arrays on stainless steel surface by anodization for visible-light photocatalytic degradation of organic pollutants. J. Mater. Res. 2012, 27, 2417–2424. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Nah, Y.-C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 Nanotubes: Synthesis and Applications. Chem. Phys. Chem. 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Cui, Z.D.; Zhu, S.L.; Liu, Y.; Yang, X.J. Silver nanoparticles supported on TiO2 nanotubes as active catalysts for ethanol oxidation. J. Catal. 2011, 278, 276–287. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Lin, N.; Huang, X.; Hang, R.; Fan, A.; Tang, B. Microstructure, antibacterial properties and wear resistance of plasma Cu–Ni surface modified titanium. Surf. Coat. Technol. 2013, 232, 515–520. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf. Coat. Technol. 2010, 205, 219–223. [Google Scholar] [CrossRef]
- Chan, Y.H.; Huang, C.F.; Ou, K.L.; Peng, P.W. Mechanical properties and antibacterial activity of copper doped diamond-like carbon films. Surf. Coat. Technol. 2011, 206, 1037–1040. [Google Scholar] [CrossRef]
- Rezaei, B.; Havakeshian, E.; Ensafi, A.A. Decoration of nanoporous stainless steel with nanostructured gold via galvanic replacement reaction and its application for electrochemical determination of dopamine. Sens. Actuators B 2015, 213, 484–492. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, J.; Yuan, F.; Hang, R.; Huang, X.; Tang, B. In situ growth of self-organized Cu-containing nano-tubes and nano-pores on Ti90−xCu10Alx (x = 0, 45) alloys by one-pot anodization and evaluation of their antimicrobial activity and cytotoxicity. Surf. Coat. Technol. 2014, 240, 167–178. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Du, K. A simple and efficient combined AC–DC electrodeposition method for fabrication of highly ordered Au nanowires in AAO template. Appl. Surf. Sci. 2013, 265, 149–156. [Google Scholar] [CrossRef]
- Ali, G.; Maqbool, M. Fabrication of cobalt-nickel binary nanowires in a highly ordered alumina template via AC electrodeposition. Nanoscale Res. Lett. 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Zhu, S.L.; He, J.L.; Yang, X.J.; Cui, Z.D.; Pi, L.L. Ti oxide nano-porous surface structure prepared by dealloying of Ti-Cu amorphous alloy. Electrochem. Commun. 2011, 13, 250–253. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Popov, K.I.; Pavlović, L.J.; Pavlović, M.G. Morphologies of copper deposits obtained by the electrodeposition at high overpotentials. Surf. Coat. Technol. 2006, 201, 560–566. [Google Scholar] [CrossRef]
- Popov, K.I.; Nikolic, N.D.; Rakočević, Z.L. An estimation of the interfacial energy of the copper–copper sulphate solution interface and of the specific surface of cop- per powder. J. Serb. Chem. Soc. 2002, 67, 635–638. [Google Scholar] [CrossRef]
- Popov, K.I.; Nikolic, N.D.; Rakočević, Z.L. The estimation of solid copper surface tension in copper sulfate solutions. J. Serb. Chem. Soc. 2002, 67, 769–775. [Google Scholar] [CrossRef]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Wang, F.; Lou, M.-M.; Xie, G.-L.; Li, B.; Bo, Z.; Zhang, G.-Q.; Liu, H.; Wareth, A. Copper as an antibacterial agent for human pathogenic multidrug resistant Burkholderia cepacia complex bacteria. J. Biosci. Bioeng. 2011, 112, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.E.; Vidic, R.D.; Stout, J.E.; Mccartney, C.A.; Yu, V.L. Inactivation of mycobacterium avium by copper and silver ions. Water Res. 1998, 32, 1997–2000. [Google Scholar] [CrossRef]
- Nan, L.; Liu, Y.; Lü, M.; Yang, K. Study on antibacterial mechanism of copper–bearing austenitic antibacterial stainless steel by atomic force microscopy. J. Mater. Sci. Mater. Med. 2008, 19, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium–copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.G.; Peretiatko, T.; Budkowski, A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef]
- Wen, C.E.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153. [Google Scholar] [CrossRef]
- Oh, S.; Daraio, C.; Chen, L.H.; Pisanic, T.R.; Fiñones, R.R.; Jin, S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res. 2006, 78, 97–103. [Google Scholar] [CrossRef]





| Strain | Bacterial Reduction Rate |
|---|---|
| E. coli | 99.6% |
| S. Aureus | 97.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Lin, N.; Zhang, J.; Jia, Y.; Zhao, H. Fabrication of a Flower-like Copper Oxide Film-Coated Nanoporous Stainless Steel Using Anodization-Assisted Electrodeposition as a Novel Antibacterial Material. Coatings 2023, 13, 782. https://doi.org/10.3390/coatings13040782
Wang H, Lin N, Zhang J, Jia Y, Zhao H. Fabrication of a Flower-like Copper Oxide Film-Coated Nanoporous Stainless Steel Using Anodization-Assisted Electrodeposition as a Novel Antibacterial Material. Coatings. 2023; 13(4):782. https://doi.org/10.3390/coatings13040782
Chicago/Turabian StyleWang, Hefeng, Naiming Lin, Jiaojiao Zhang, Yiwei Jia, and Hongting Zhao. 2023. "Fabrication of a Flower-like Copper Oxide Film-Coated Nanoporous Stainless Steel Using Anodization-Assisted Electrodeposition as a Novel Antibacterial Material" Coatings 13, no. 4: 782. https://doi.org/10.3390/coatings13040782
APA StyleWang, H., Lin, N., Zhang, J., Jia, Y., & Zhao, H. (2023). Fabrication of a Flower-like Copper Oxide Film-Coated Nanoporous Stainless Steel Using Anodization-Assisted Electrodeposition as a Novel Antibacterial Material. Coatings, 13(4), 782. https://doi.org/10.3390/coatings13040782






