Study on Anti-Icing Performance of Biogas-Residue Nano-Carbon Coating for Wind-Turbine Blade
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
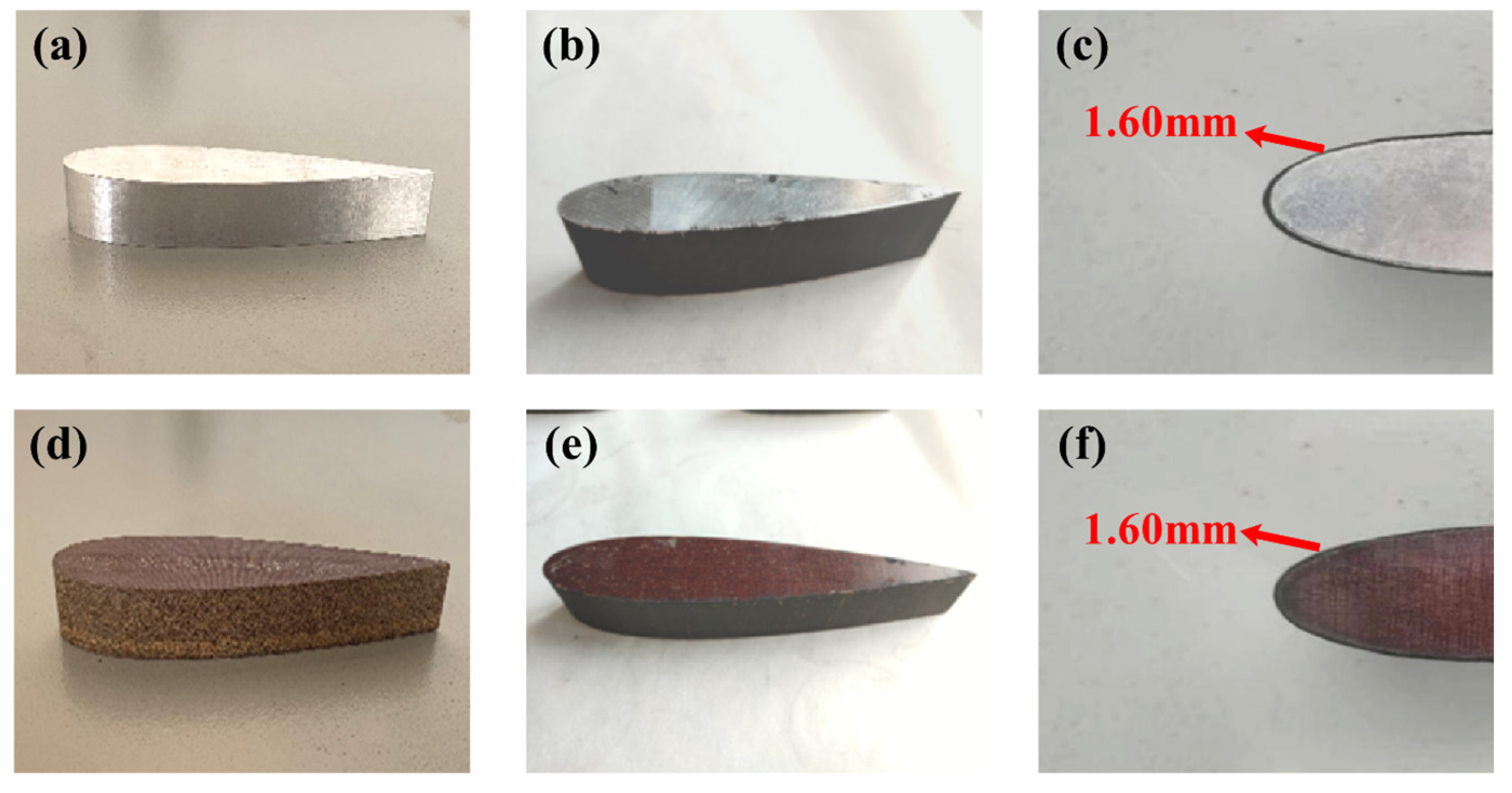
2.2. Fabrication of Biomas-Based Carbon Nano-Coating Blades
2.3. Characterizations
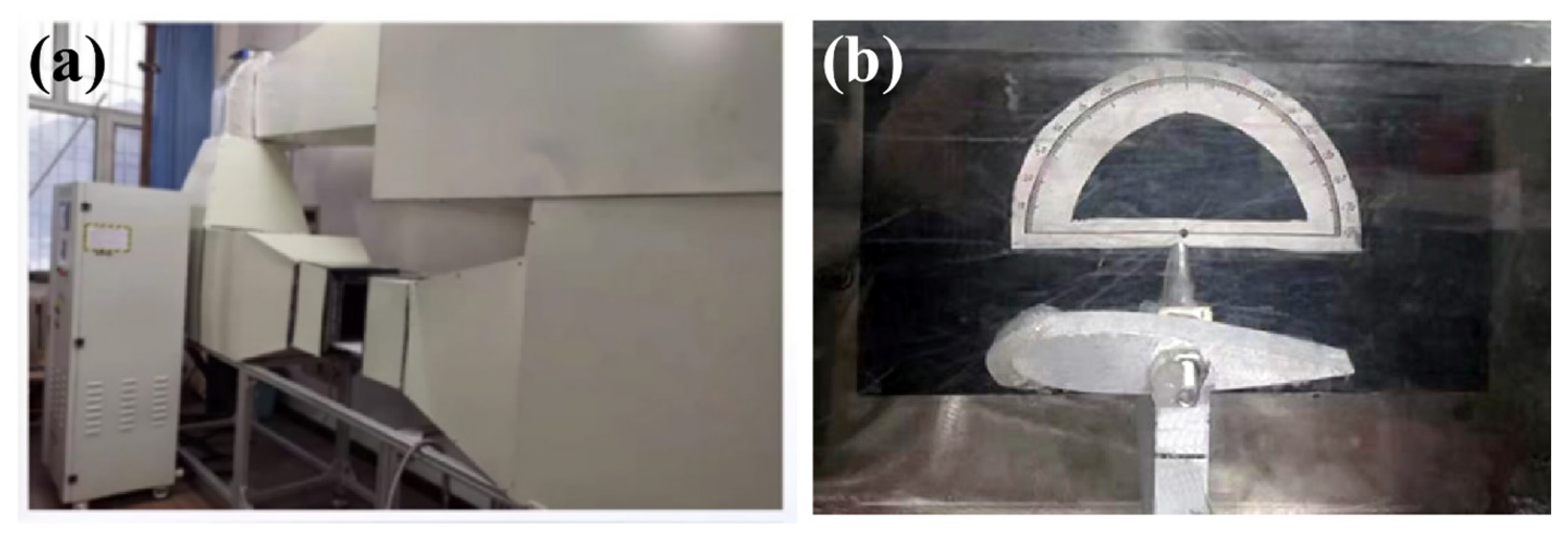
2.4. Anti-Icing Test
3. Results and Discussion
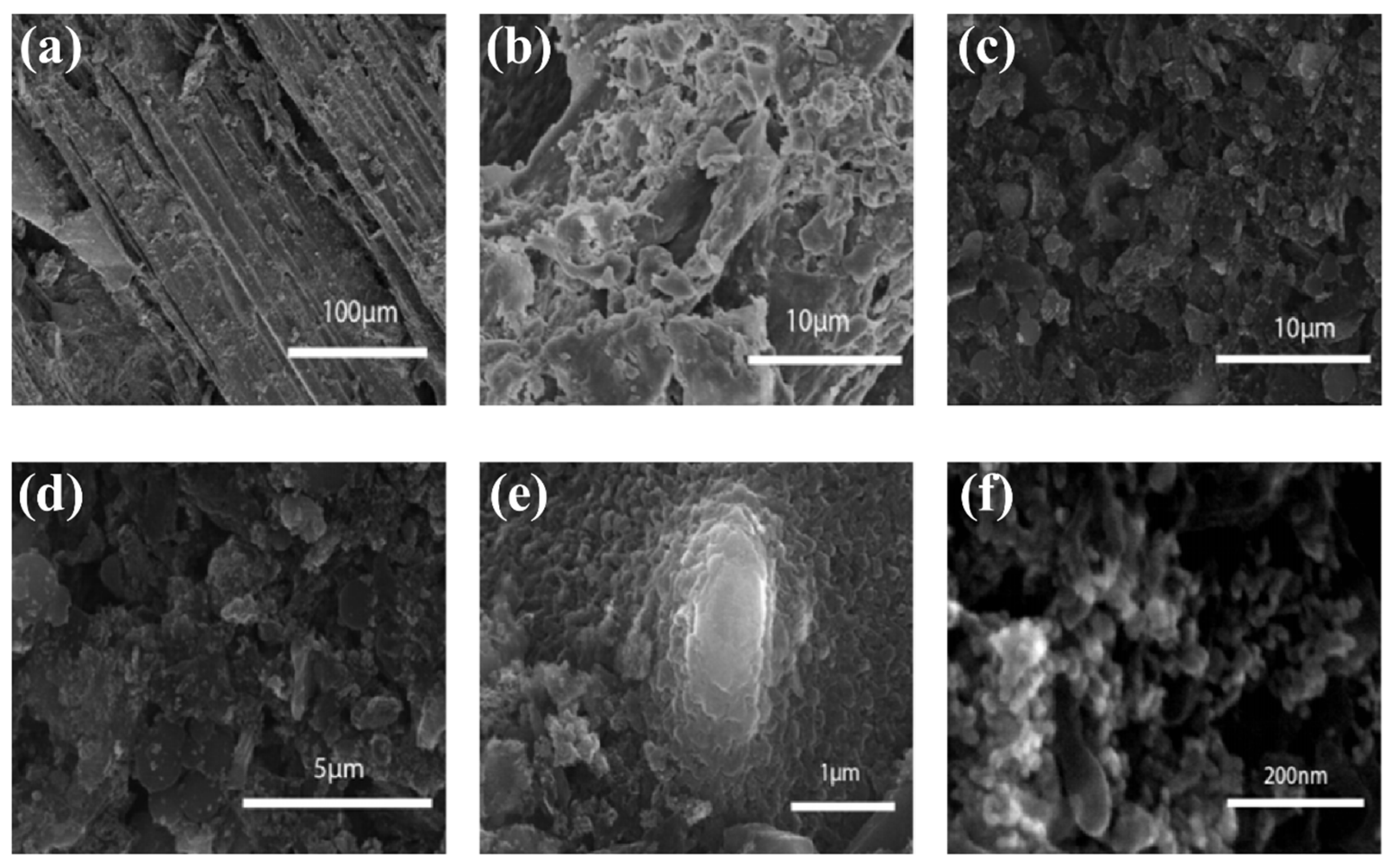
3.1. SEM Morphology
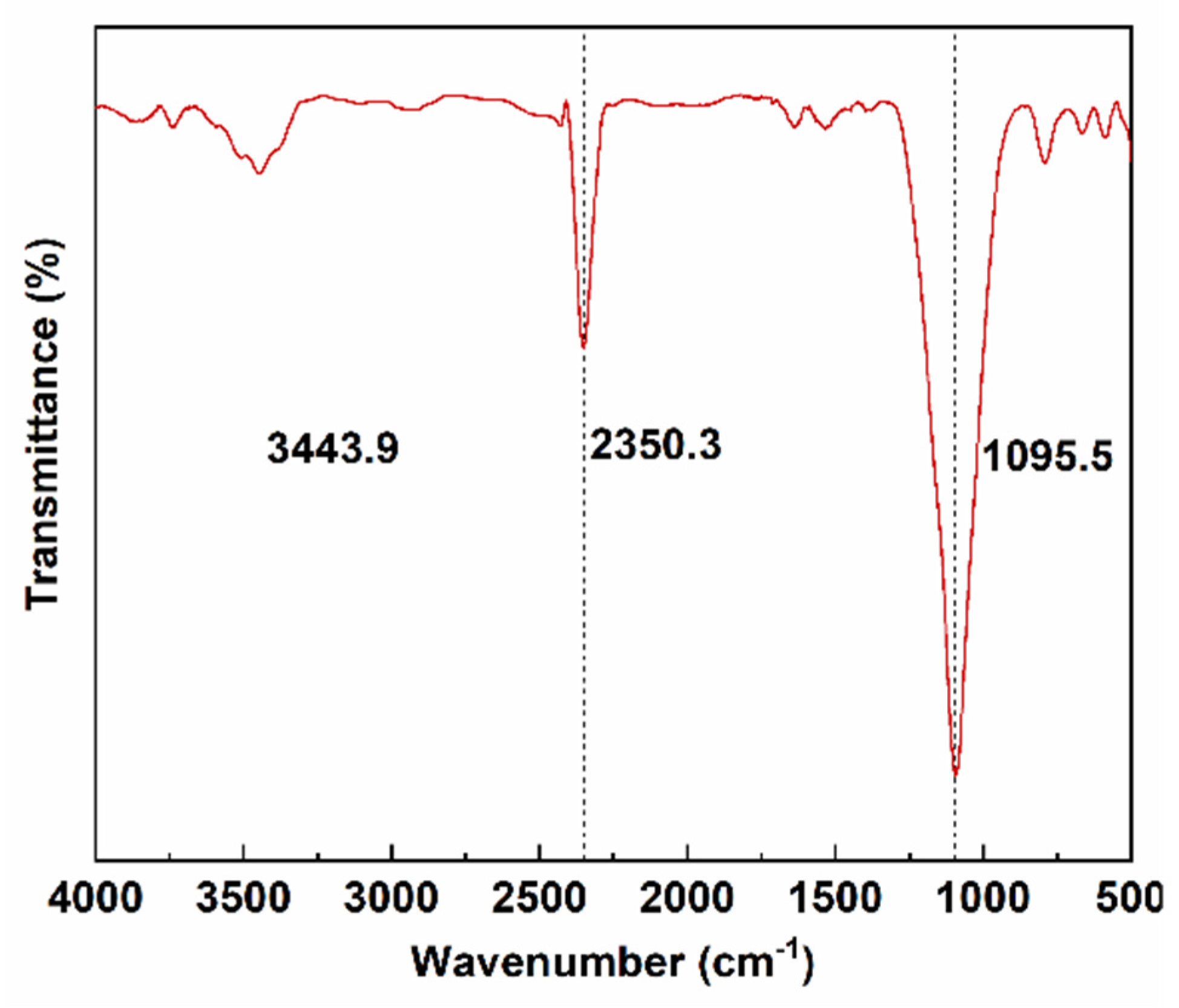
3.2. FTIR Analysis
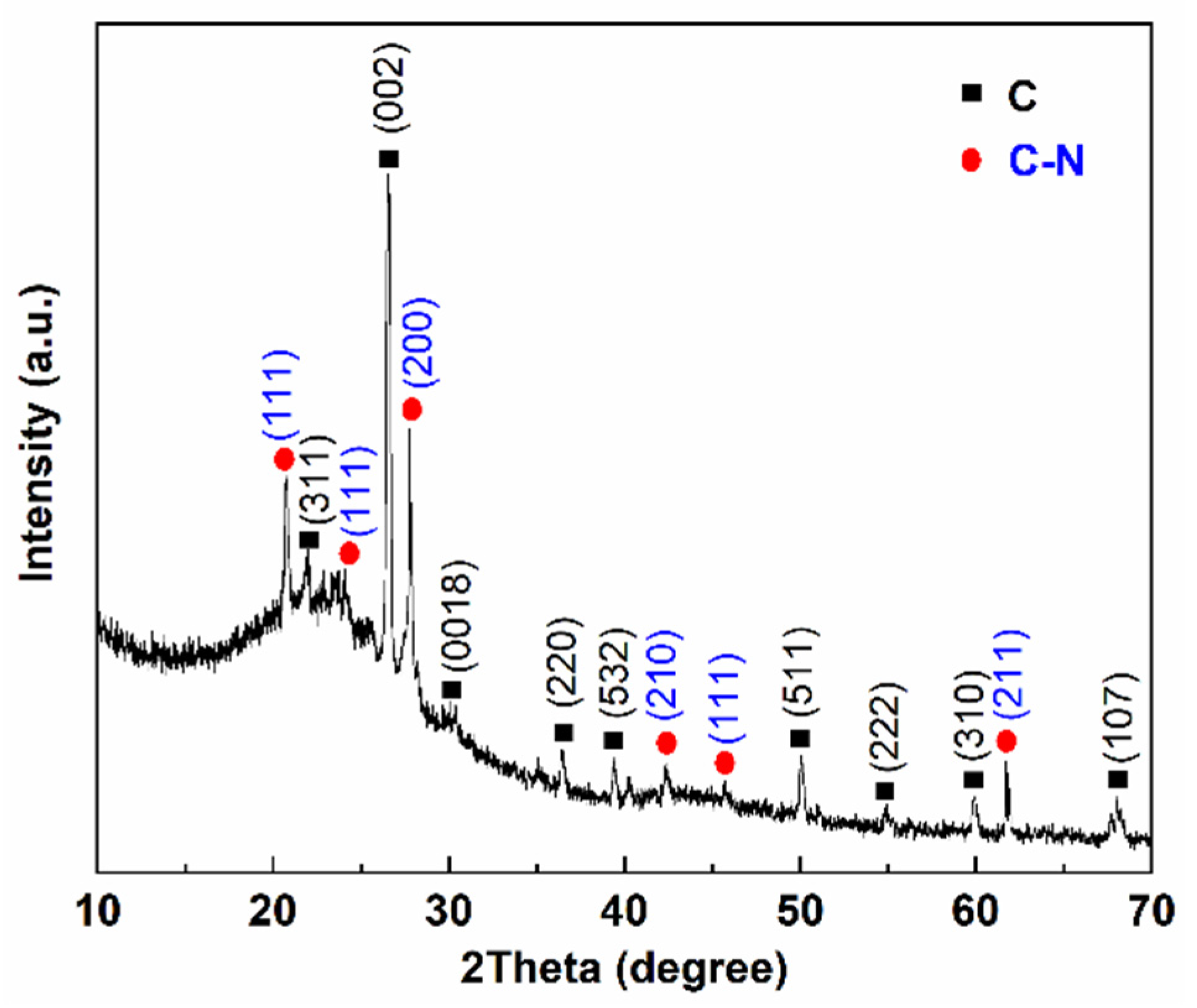
3.3. XRD Analysis
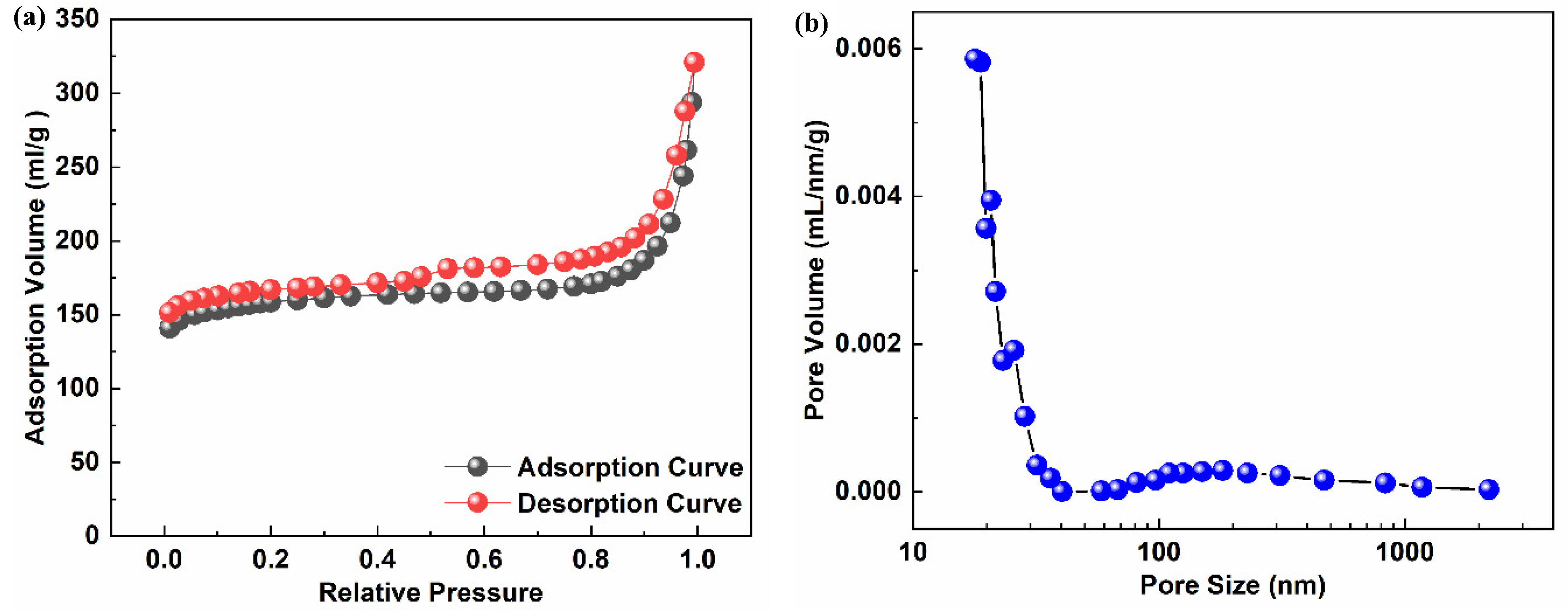
3.4. BET Analysis
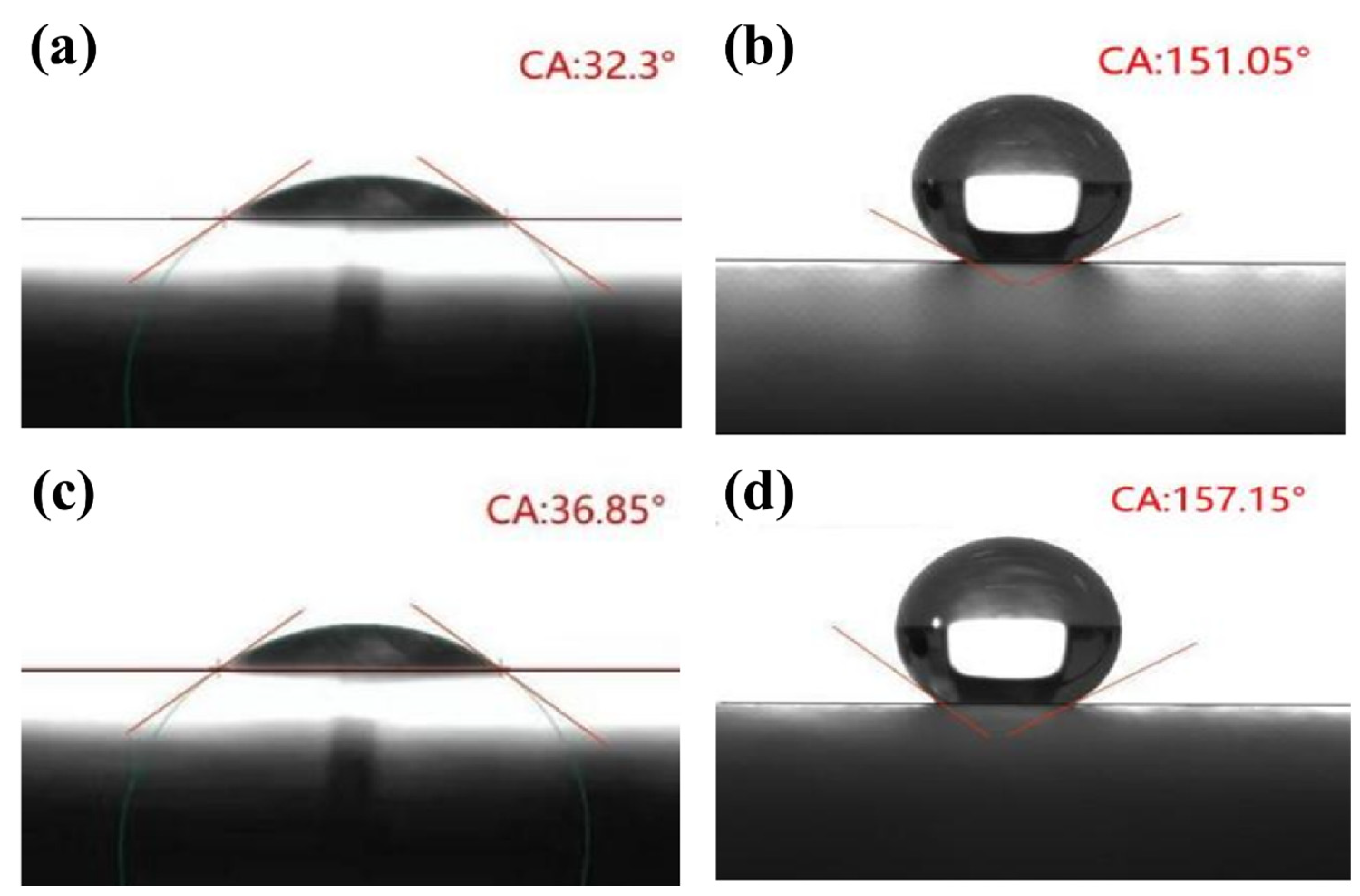
3.5. The Static Contact Angle Analysis
3.6. Wind-Tunnel Dynamic Icing Test
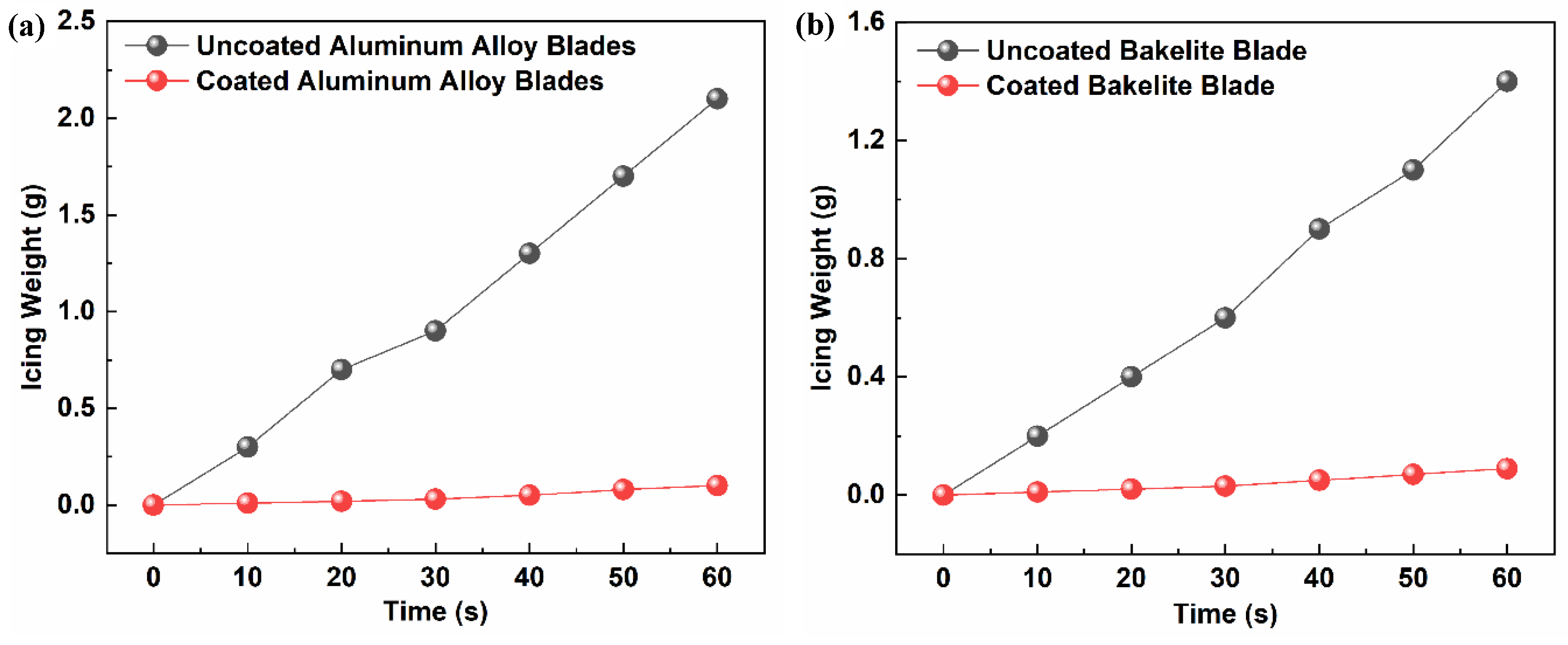
3.6.1. Comparison of Ice-Weight Differences
3.6.2. Comparison of Maximum Icing Thicknesses
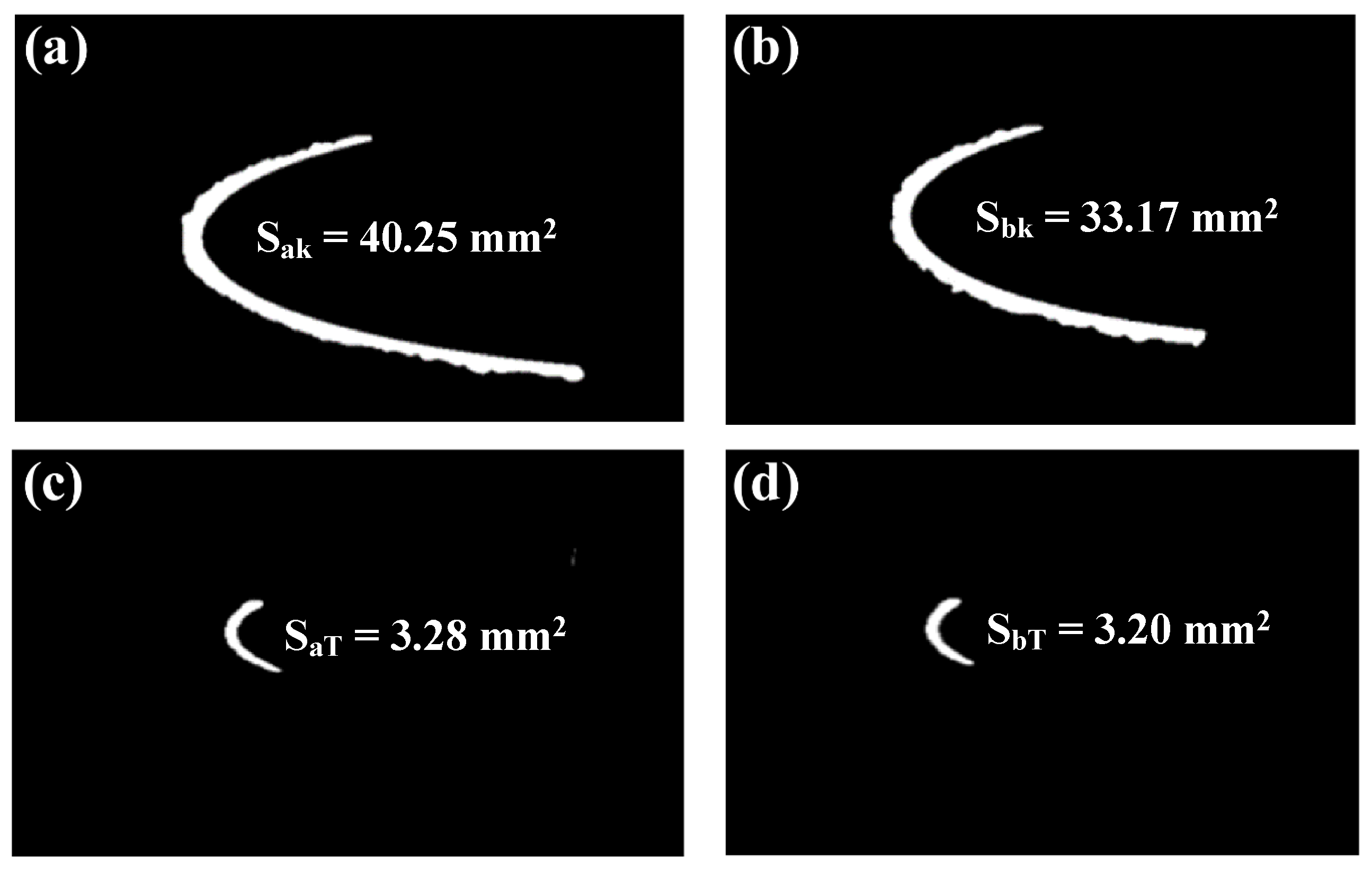
3.6.3. Comparison of Ice-Area Ratio
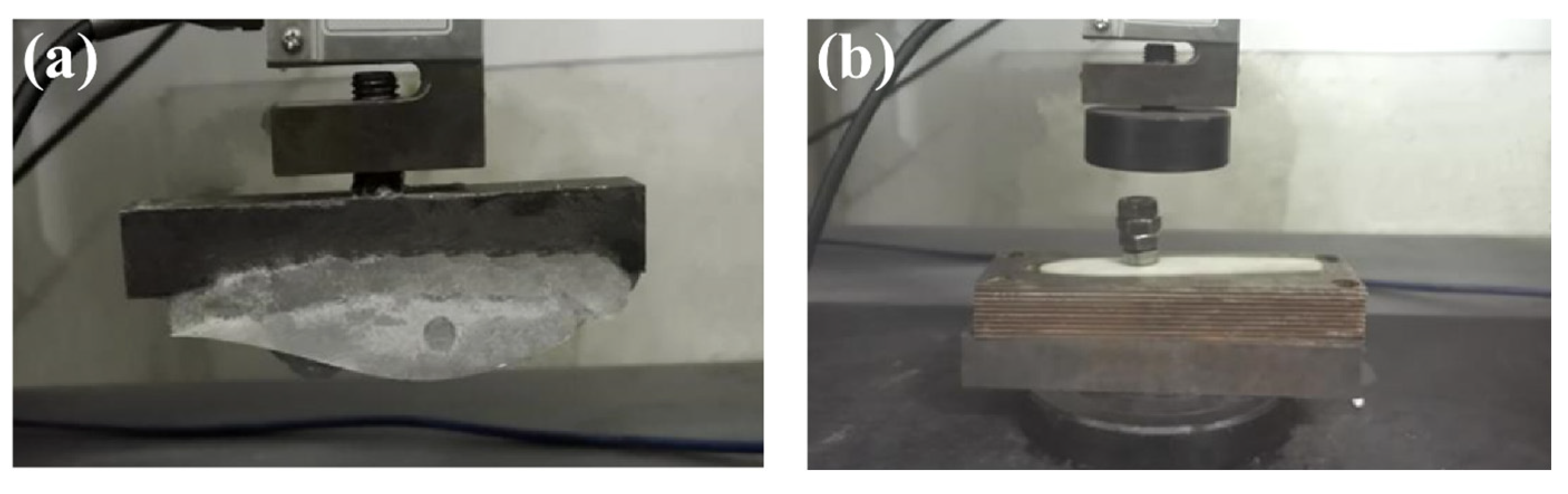
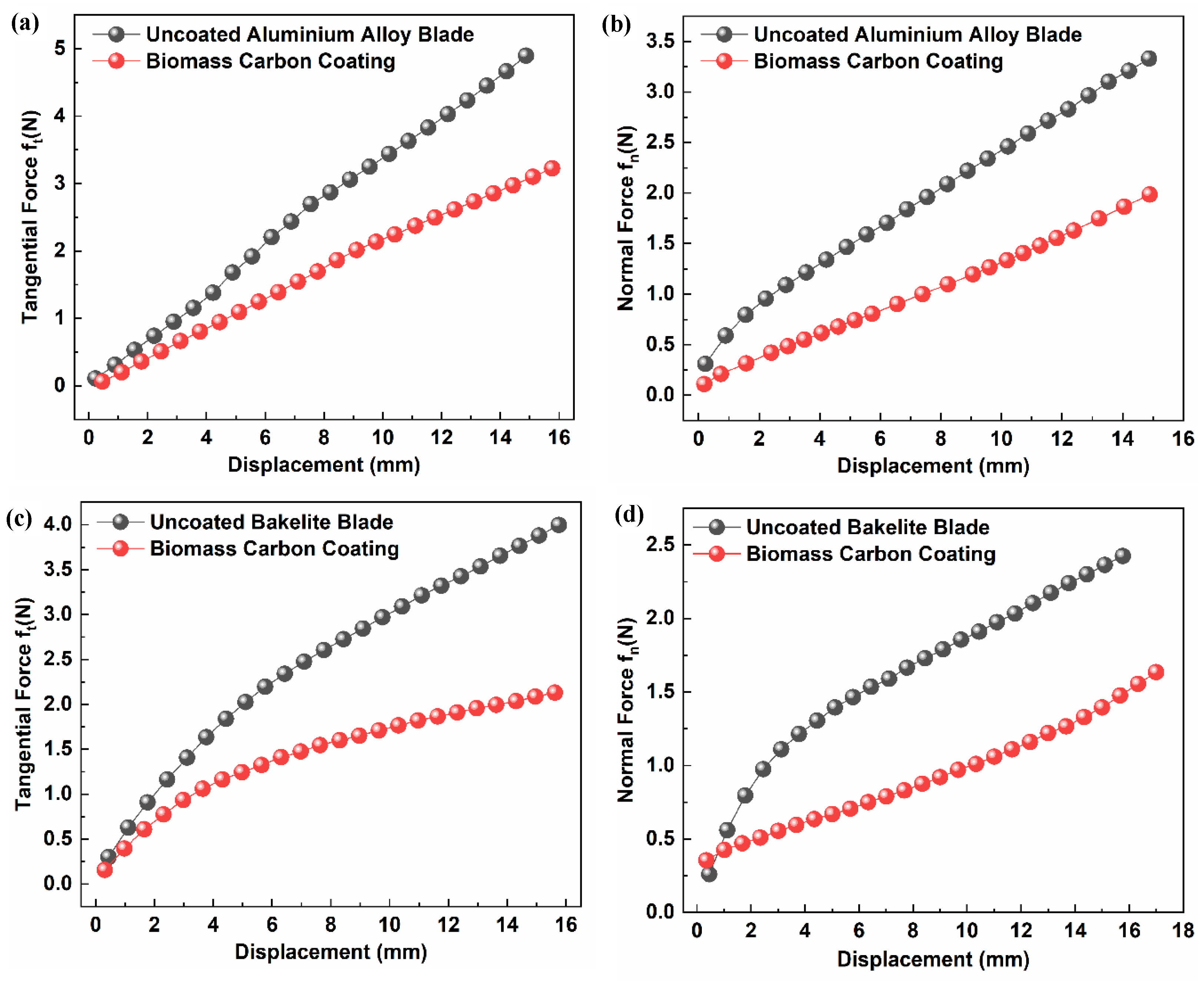
3.7. Test for Comparison of Blades’ Icing Adhesion
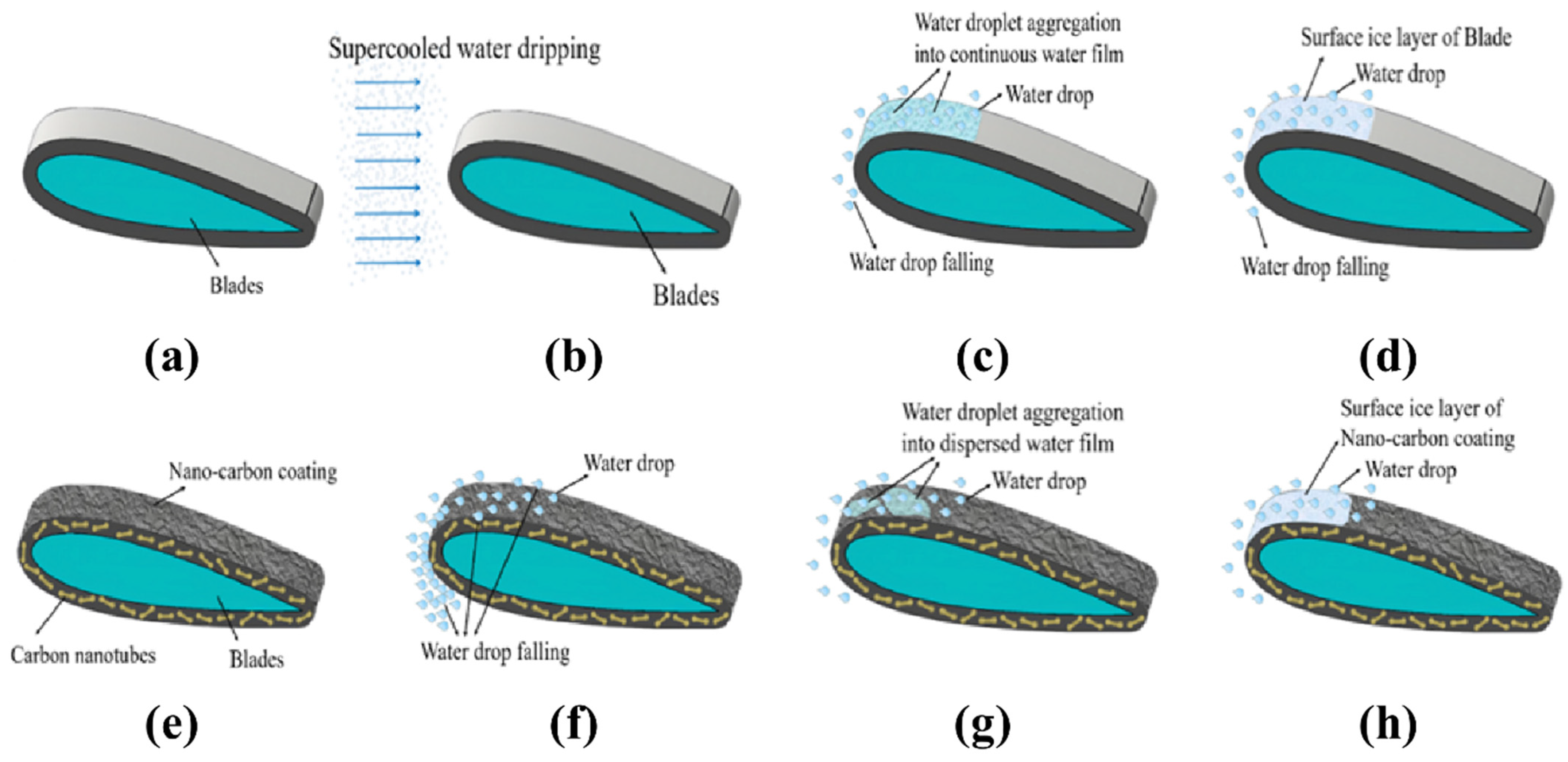
3.8. Anti-Icing Mechanism of Blade with Nano-Carbon Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalili, N.; Edrisy, A.; Carriveau, R. A review of surface engineering issues critical to wind turbine performance. Renew. Sustain. Energy Rev. 2009, 13, 428–438. [Google Scholar] [CrossRef]
- Zeng, J.; Song, B. Research on experiment and numerical simulation of ultrasonic de-icing for wind turbine blades. Renew. Energy 2017, 113, 706–712. [Google Scholar] [CrossRef]
- Mu, Z.Q.; Li, Y.; Guo, W.F.; Shen, H.; Tagawa, K. An experimental study on adhesion strength of offshore atmospheric icing on a wind turbine blade airfoil. Coatings 2023, 13, 164. [Google Scholar] [CrossRef]
- Jolin, N.; Bolduc, D.; Swytink-Binnema, N.; Rosso, G.; Godreau, C. Wind turbine blade ice accretion: A correlation with nacelle ice accretion. Cold Reg. Sci. Technol. 2019, 157, 235–241. [Google Scholar] [CrossRef]
- Ji, S.; Yang, D. Ice loads and ice-induced vibrations of offshore wind turbine based on coupled DEM-FEM simulations. Ocean Eng. 2022, 243, 110197. [Google Scholar] [CrossRef]
- Song, Z.; Liu, J.; Hu, Y.; Fang, F.; Yu, Z. Modeling and analysis of bottom fixed platform offshore wind turbine under ice loads. Proc. Chin. Soc. Electron. Eng. 2021, 41, 4144–4152. [Google Scholar]
- Ye, K.H.; Li, C.; Chen, F.D.; Xu, Z.F.; Zhang, W.F.; Zhang, J.W. Floating ice load reduction of offshore wind turbines by two approaches. Int. J. Struct. Stab. Dyn. 2018, 18, 31. [Google Scholar] [CrossRef]
- Xu, K.; Hu, J.L.; Jiang, X.L.; Meng, W.; Lan, B.H.; Shu, L.C. Anti-icing performance of hydrophobic silicone-acrylate resin coatings on wind blades. Coatings 2018, 8, 12. [Google Scholar] [CrossRef]
- Peng, C.; Xing, S.; Yuan, Z.; Xiao, J.; Wang, C.; Zeng, J. Preparation and anti-icing of superhydrophobic PVDF coating on a wind turbine blade. Appl. Surf. Sci. 2012, 259, 764–768. [Google Scholar] [CrossRef]
- Wei, K.X.; Yang, Y.; Zuo, H.Y.; Zhong, D.Q. A review on ice detection technology and ice elimination technology for wind turbine. Wind Energy 2020, 23, 433–457. [Google Scholar] [CrossRef]
- Li, C.; Li, X.H.; Tao, C.; Ren, L.X.; Zhao, Y.H.; Bai, S.; Yuan, X.Y. Amphiphilic antifogging/anti-icing coatings containing POSS-PDMAEMA-b-PSBMA. ACA Appl. Mater. Interfaces 2017, 9, 22959–22969. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.-V.N.; Nanda, S.; Mishra, A.; Chao, H.-P.; Bajpai, A.K. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Sun, Y.; Li, Y. Bioaugmentation improves batch psychrophilic anaerobic co-digestion of cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126118. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, M.; Guo, Z. Robust, heat-resistant and multifunctional superhydrophobic coating of carbon microflowers with molybdenum trioxide nanoparticles. J. Colloid Interface Sci. 2017, 506, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; Li, W.; Wang, B.S.; Deng, B.W.; Ma, F.M.; Yu, Z.L. Preparation and anti-icing behavior of superhydrophobic surfaces on aluminum alloy substrates. Langmuir 2013, 29, 8482–8491. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.K.; Liu, S.; Xing, R. Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog. Org. Coat. 2019, 137, 105373. [Google Scholar] [CrossRef]
- Cui, H.; Hu, M.; Yu, Z.; Xiao, J. Preparation and characterization of a durable superhydrophobic hyperbranched poly(dimethylolbutanoic acid-glycidyl ester of versatic acid)/nano-SiO2 coating. Appl. Surf. Sci. 2019, 466, 171–178. [Google Scholar] [CrossRef]
- Liu, J.P.; Janjua, Z.A.; Roe, M.; Xu, F.; Turnbull, B.; Choi, K.S.; Hou, X.H. Super-hydrophobic/icephobic coatings based on silica nanoparticles modified by self-assembled monolayers. Nanomaterials 2016, 6, 232. [Google Scholar] [CrossRef]
- Montano-Figueroa, A.G.; Alcantar-Pena, J.J.; Tirado, P.; Abraham, A.; de Obaldia, E.; Auciello, O. Tailoring of polycrystalline diamond surfaces from hydrophilic to superhydrophobic via synergistic chemical plus micro-structuring processes. Carbon 2018, 139, 361–368. [Google Scholar] [CrossRef]
- Ding, S.; Xiang, T.; Li, C.; Zheng, S.; Wang, J.; Zhang, M.; Dong, C.; Chan, W. Fabrication of self-cleaning super-hydrophobic nickel/graphene hybrid film with improved corrosion resistance on mild steel. Mater. Des. 2017, 117, 280–288. [Google Scholar] [CrossRef]
- Stevens, K.A.; Esplin, C.D.; Davis, T.M.; Butterfield, D.J.; Ng, P.S.; Bowden, A.E.; Jensen, B.D.; Iverson, B.D. Superhydrophobic, carbon-infiltrated carbon nanotubes on Si and 316L stainless steel with tunable geometry. Appl. Phys. Lett. 2018, 112, 5. [Google Scholar] [CrossRef]
- Eseev, M.K.; Kapustin, S.N.; Lugvishchuk, D.S.; Mordkovich, V.Z.; Lyakh, N.L. A superhydrophobic coating based on onion-like carbon nanoparticles. Tech. Phys. Lett. 2020, 46, 1120–1123. [Google Scholar] [CrossRef]
- Zhang, X.X.; Chen, M. Icephobicity of functionalized graphene surfaces. J. Nanomater. 2016, 2016, 6731840. [Google Scholar] [CrossRef]
- Valentini, L.; Bittolo Bon, S.; Pugno, N.M.; Hernandez Santana, M.; Lopez-Manchado, M.A.; Giorgi, G. Synergistic icephobic behaviour of swollen nitrile butadiene rubber graphene and/or carbon nanotube composites. Compos. Part B Eng. 2019, 166, 352–360. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Xu, X.; Meng, X.; Qu, J.; Wang, Z.; Liu, C.; Qu, B. Preparation, characterization and application of activated carbon from corn cob by KOH activation for removal of Hg(II) from aqueous solution. Bioresour. Technol. 2020, 306, 123154. [Google Scholar] [CrossRef]
- Liu, C.; Li, N.; Peng, L.; Zhong, W.Z.; Mao, L.Q.; Yin, D.L. Hydrothermal carbonization of renewable natural plants as superior metal-free catalysts for aerobic oxidative coupling of amines to imines. ACS Sustain. Chem. Eng. 2020, 8, 11404–11412. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, Z.D. Comparison of hydrochar- and pyrochar-based solid acid catalysts from cornstalk: Physiochemical properties, catalytic activity and deactivation behavior. Bioresour. Technol. 2020, 297, 122477. [Google Scholar] [CrossRef]
- Xing, X.; Jiang, W.; Li, S.; Zhang, X.; Wang, W. Preparation and analysis of straw activated carbon synergetic catalyzed by ZnCl2-H3PO4 through hydrothermal carbonization combined with ultrasonic assisted immersion pyrolysis. Waste Manag. 2019, 89, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.P.; Fan, L.L.; Yu, T.; Tan, X.; Shi, Z.Q. Hydrothermal synthesis of lignin-based carbon microspheres as anode material for lithium-ion batteries. Int. J. Electrochem. Sci. 2020, 15, 1035–1043. [Google Scholar] [CrossRef]
- Ma, H.F.; Chen, Z.H.; Wang, X.D.; Liu, Z.B.; Liu, X.X. A simple route for hierarchically porous carbon derived from corn straw for supercapacitor application. J. Renew. Sustain. Energy 2019, 11, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Xie, W.; Wang, Y.; Kang, J. Effects of temperature, time and acidity of hydrothermal carbonization on the hydrochar properties and nitrogen recovery from corn stover. Biomass Bioenergy 2019, 122, 175–182. [Google Scholar] [CrossRef]
- Xie, X.M.; Wang, Y.; Li, X.F.; Wei, X.S.; Yang, S. Pickering emulsions stabilized by amphiphilic carbonaceous materials derived from wheat straw. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 558, 65–72. [Google Scholar] [CrossRef]
- Tong, G.; Li, Y.; Tagawa, K.; Feng, F. Effects of blade airfoil chord length and rotor diameter on aerodynamic performance of straight-bladed vertical axis wind turbines by numerical simulation. Energy 2023, 265, 126325. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef]
- Gao, X.; Su, L.; Jiang, G.Q.; Pang, J.Y.; Lin, L. Dimensional stability of lotus leaf-like nanostructure superhydrophobic bamboo by modification using xylan. BioResources 2020, 15, 3443–3457. [Google Scholar] [CrossRef]
- Lu, L.; Huggins, T.; Jin, S.; Zuo, Y.; Ren, Z.J. Microbial metabolism and community structure in response to bioelectrochemically enhanced remediation of petroleum hydrocarbon-contaminated soil. Environ. Sci. Technol. 2014, 48, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Feng, F.; Li, Y.; Sun, Y.; Tagawa, K. A corncob biochar-based superhydrophobic photothermal coating with micro-nano-porous rough-structure for ice-phobic properties. Surf. Coat. Technol. 2023, 457, 129299. [Google Scholar] [CrossRef]

| Raw Materials | Pretreatment | Autoclave Conditions | Ref. | ||
|---|---|---|---|---|---|
| Activator | T (°C) | Time (h) | |||
| Corn-straw biogas residue | - | 5% H2SO4 | 130 | 12 | This study |
| Rice husk | Ethanol | 95%–98% H2SO4 | 170 | 48 | [25] |
| Corn straw | - | - | 150–190 | 24 | [26] |
| Corn straw | - | >98% H2SO4 | 180 | 12 | [27] |
| Wheat straw | - | 85% H3PO4 | 175 | 24 | [28] |
| 200 | |||||
| 225 | |||||
| Corn straw | 4% H2SO4 | 50% NaOH | 200 | 24 | [29] |
| 36 | |||||
| 48 | |||||
| 60 | |||||
| Corn straw | - | - | 220 | 12 | [30] |
| Corn straw | - | 5% HCl | 220 | 12 | [31] |
| Wheat straw | - | 12% HCl | 180 | 12 | [32] |
| 36% HCl | 210 | ||||
| Biomass Species | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Corn straw | 39.29 | 30.21 | 14.42 |
| Corn straw biogas residue | 21.53 | 18.59 | 9.59 |
| Blade Type | Experimental Temperature (°C) | LWC (g/m3) | MVD (µm) | Atmospheric Pressure (kPa) | Wind Speed (m/s) | Time Interval of Icing Shape Collection (s) | Total Icing Time (min) |
|---|---|---|---|---|---|---|---|
| AAB | 0 | 0.48 | 50 | 99.20 | 5 | 10 | 1 |
| −5 | |||||||
| −10 | |||||||
| BB | 0 | ||||||
| −5 | |||||||
| −10 |
| Blade Name | Contact Angle (°) | f1 | f2 |
|---|---|---|---|
| uncoated AAB | 32.30 ± 0.16 | 6.78 | 93.22 |
| coated AAB | 151.05 ± 0.03 | ||
| uncoated BB | 36.85 ± 0.08 | 4.33 | 95.67 |
| coated BB | 157.15 ± 0.08 |
| Blade Type | Wind Speed (m/s) | Experimental Temperature (°C) | Total Icing Weight (g) | |
|---|---|---|---|---|
| Uncoated | Uncoated | |||
| AAB | 5 | 0 | 2.10 | 0.10 |
| −5 | 3.50 | 0.14 | ||
| −10 | 5.20 | 0.18 | ||
| BB | 0 | 1.40 | 0.09 | |
| −5 | 3.20 | 0.11 | ||
| −10 | 4.80 | 0.15 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, F.; Wang, R.; Yuan, W.; Li, Y. Study on Anti-Icing Performance of Biogas-Residue Nano-Carbon Coating for Wind-Turbine Blade. Coatings 2023, 13, 814. https://doi.org/10.3390/coatings13050814
Feng F, Wang R, Yuan W, Li Y. Study on Anti-Icing Performance of Biogas-Residue Nano-Carbon Coating for Wind-Turbine Blade. Coatings. 2023; 13(5):814. https://doi.org/10.3390/coatings13050814
Chicago/Turabian StyleFeng, Fang, Ruixue Wang, Wei Yuan, and Yang Li. 2023. "Study on Anti-Icing Performance of Biogas-Residue Nano-Carbon Coating for Wind-Turbine Blade" Coatings 13, no. 5: 814. https://doi.org/10.3390/coatings13050814
APA StyleFeng, F., Wang, R., Yuan, W., & Li, Y. (2023). Study on Anti-Icing Performance of Biogas-Residue Nano-Carbon Coating for Wind-Turbine Blade. Coatings, 13(5), 814. https://doi.org/10.3390/coatings13050814







