Corrosion Evolution of Nickel Aluminum Bronze in Clean and Sulfide-Polluted Solutions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Solution Preparation
2.2. SVET Measurement
2.3. Electrochemical Measurements
2.4. Microstructure and Chemical Analyses
3. Results and Discussion
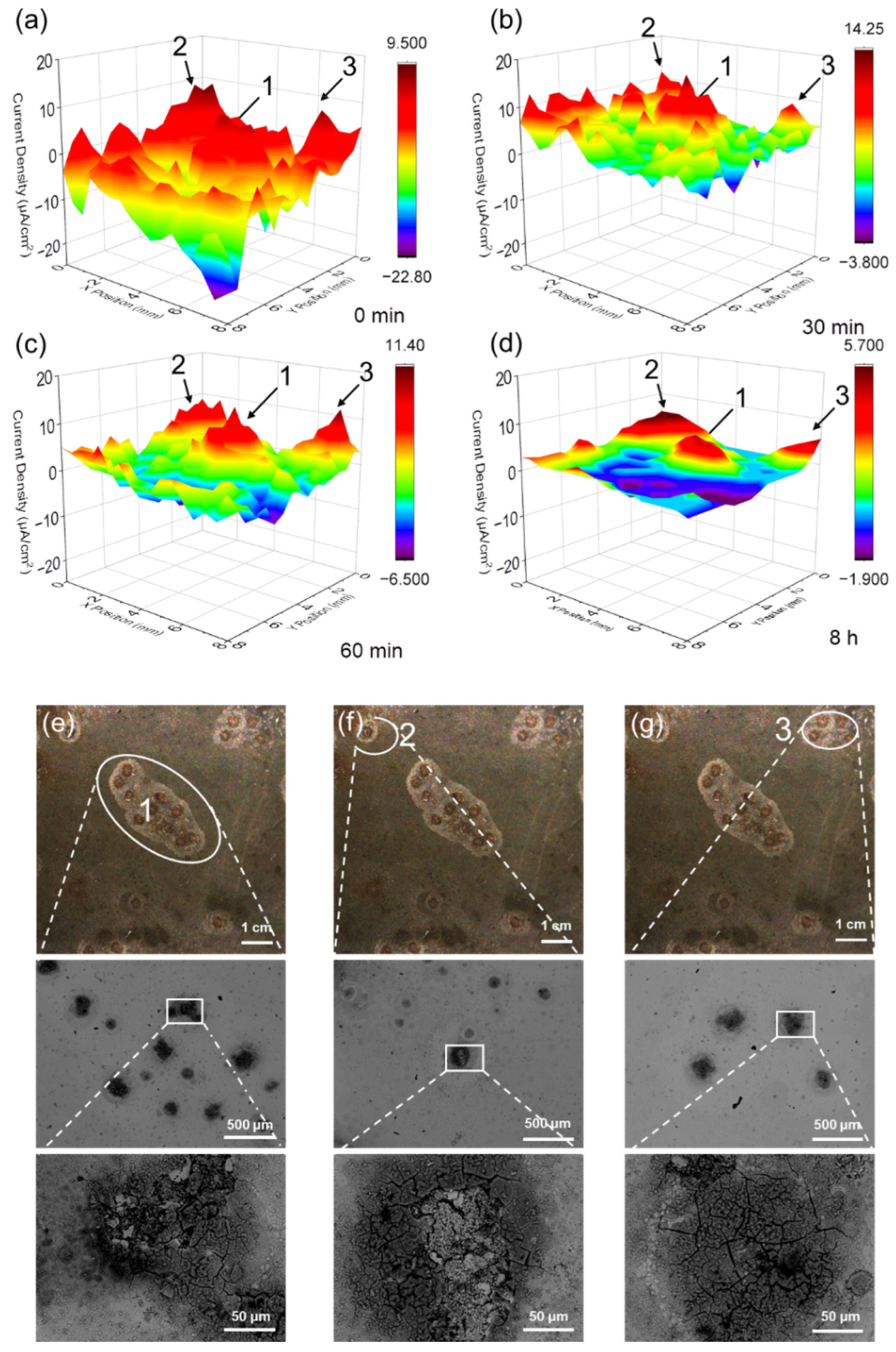
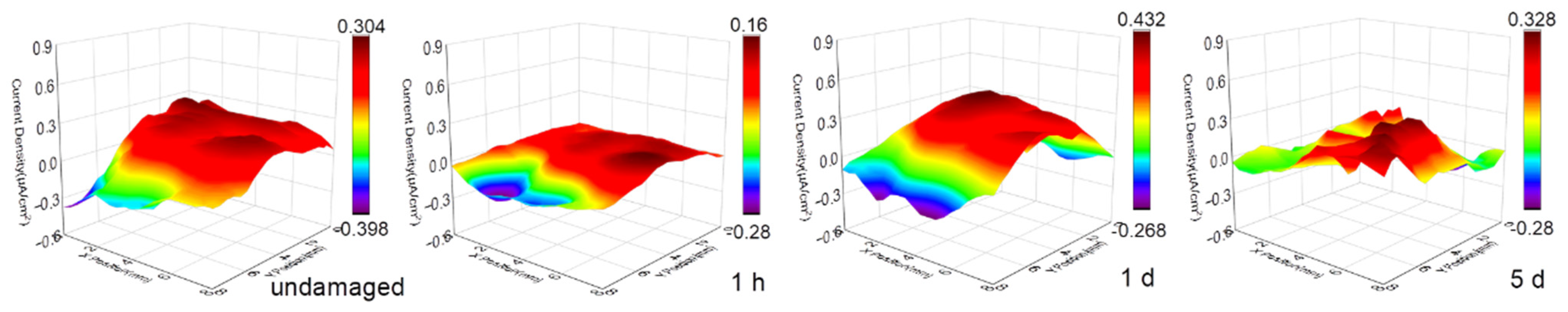
3.1. SVET Test Result
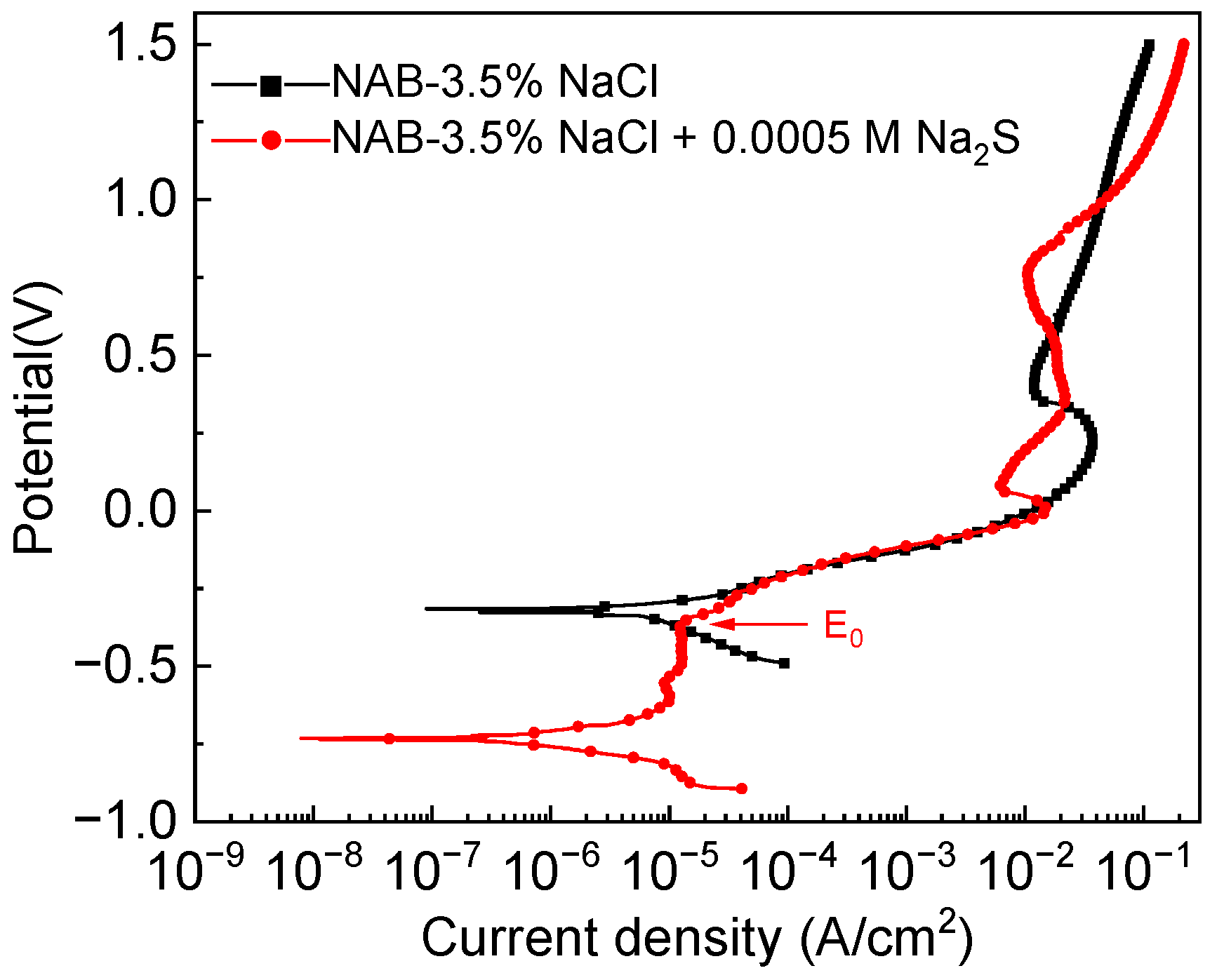
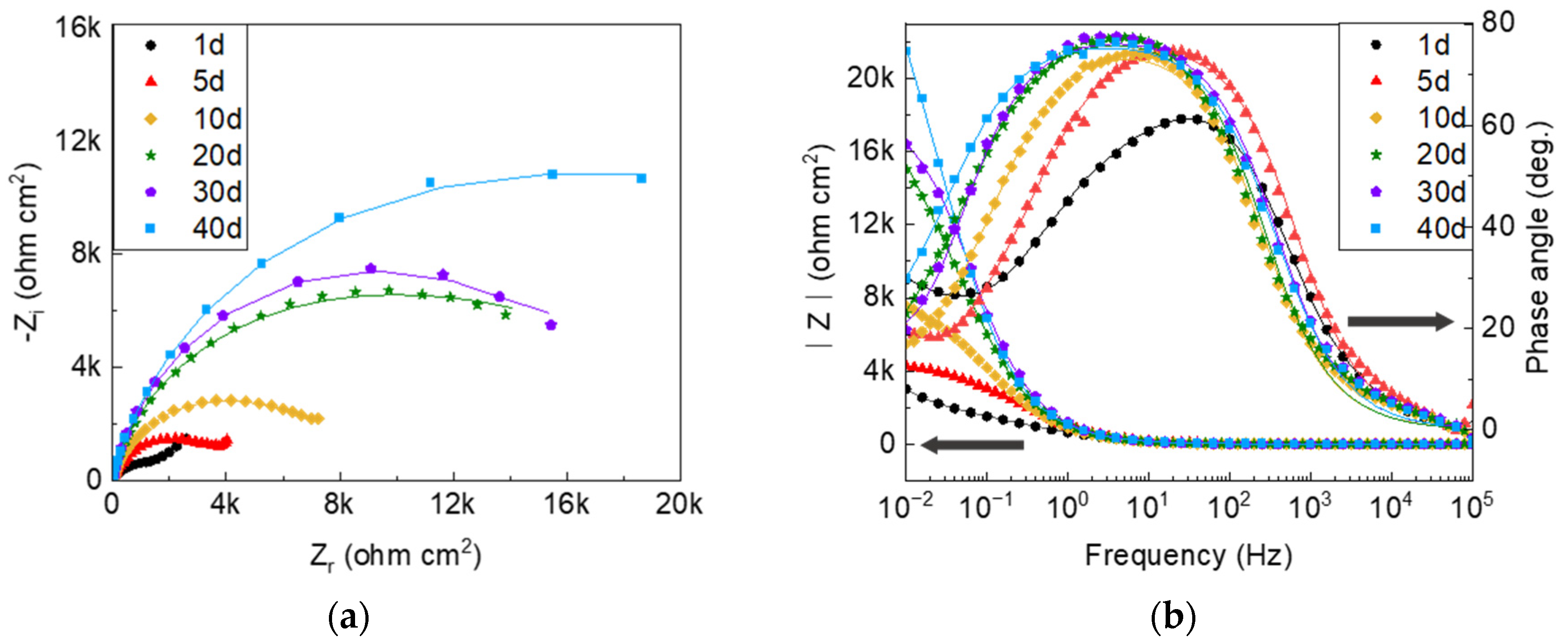
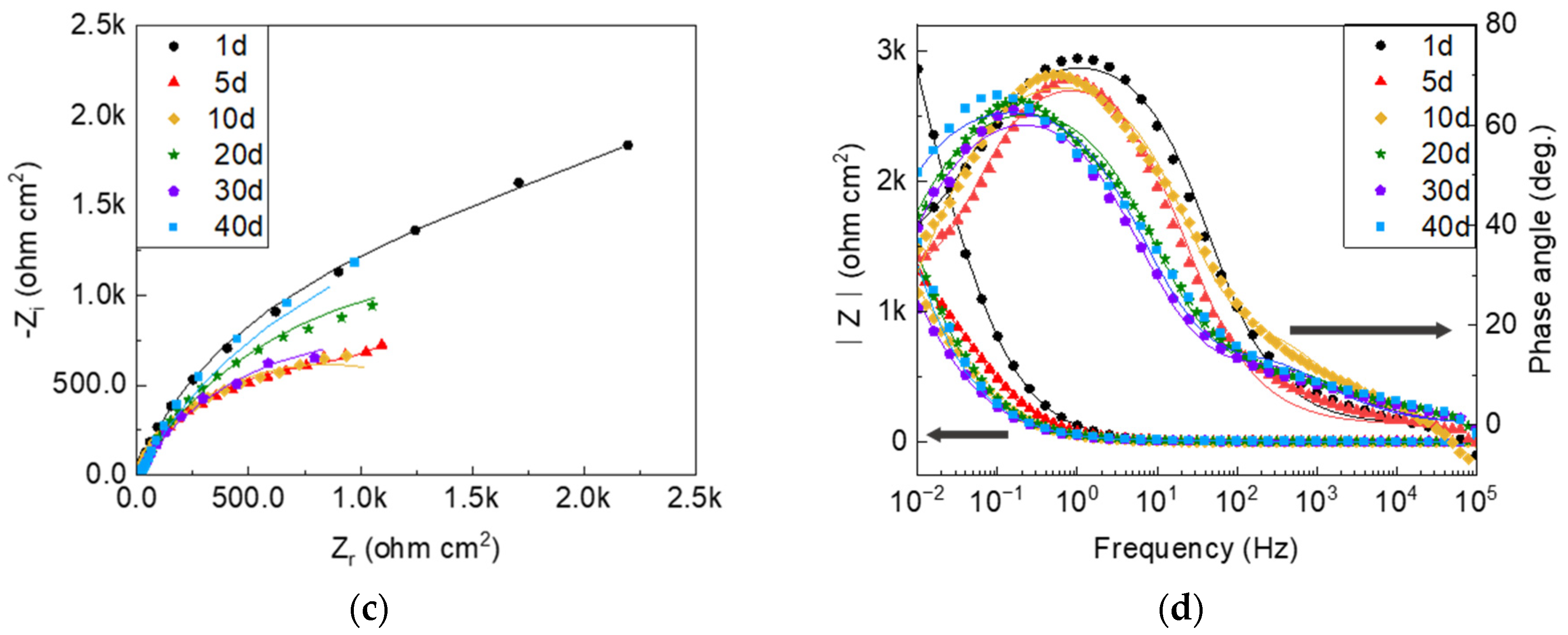
3.2. Electrochemical Properties
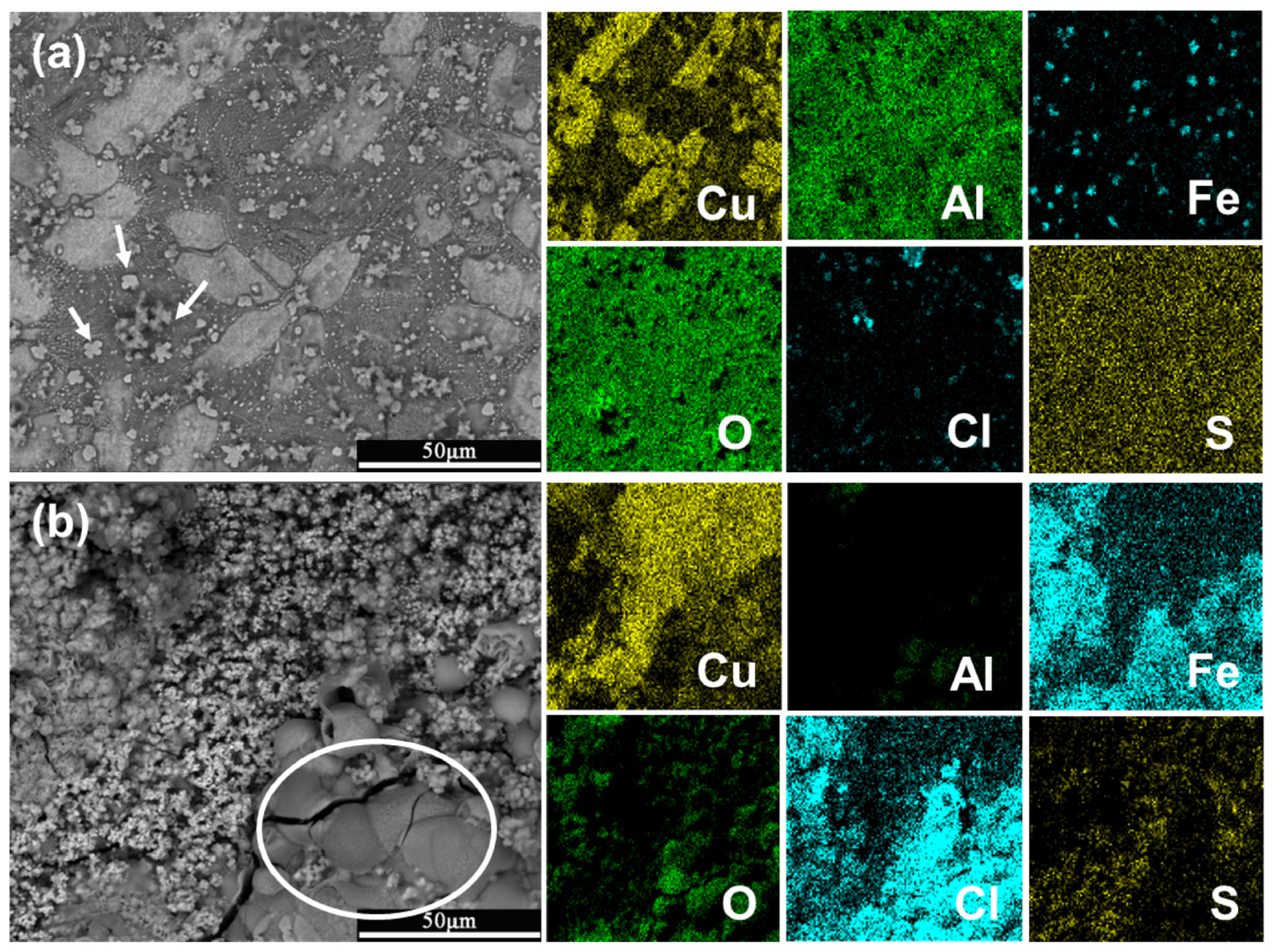
3.3. Surface Corrosion Morphology
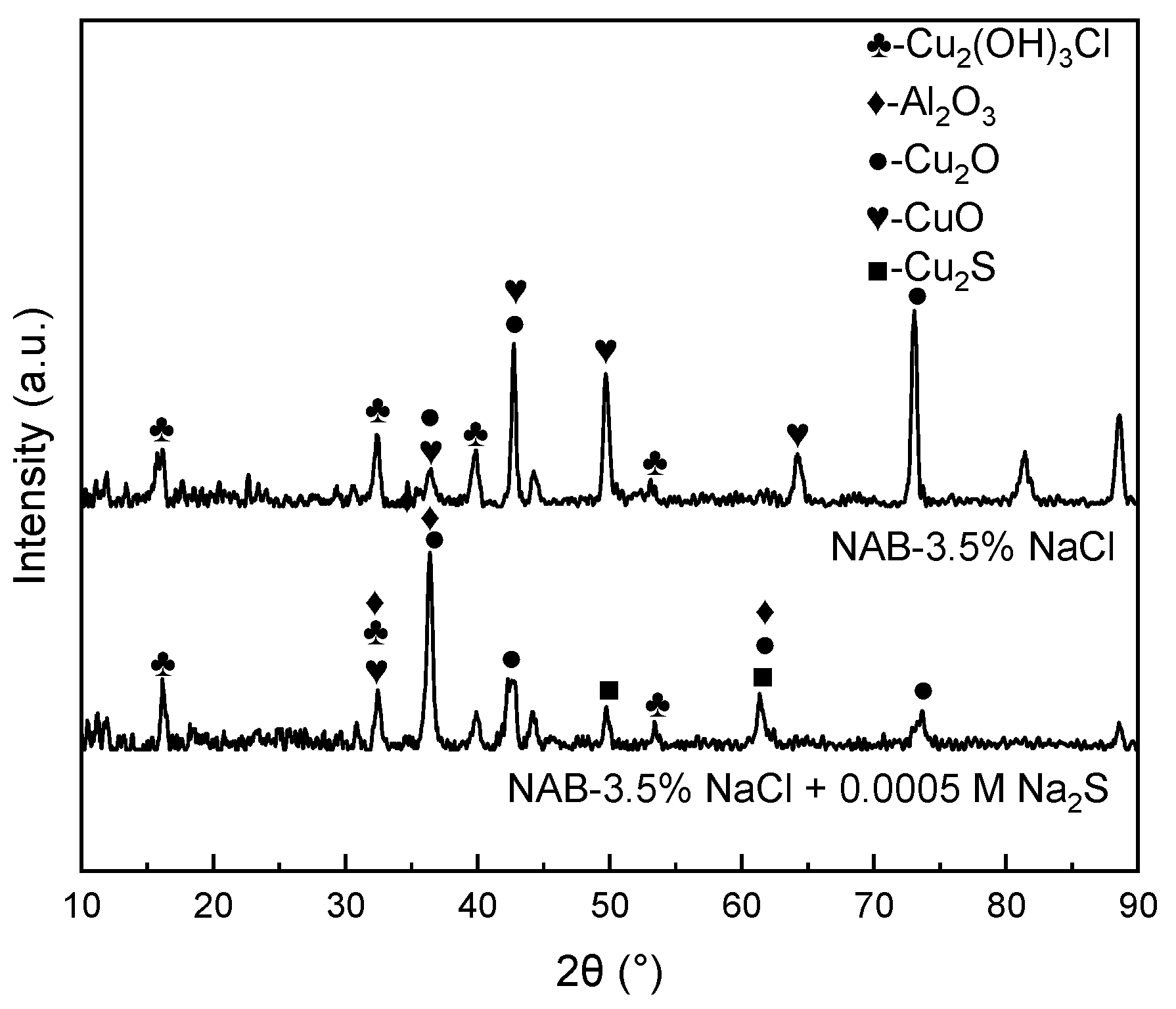
3.4. Corrosion Product Analysis
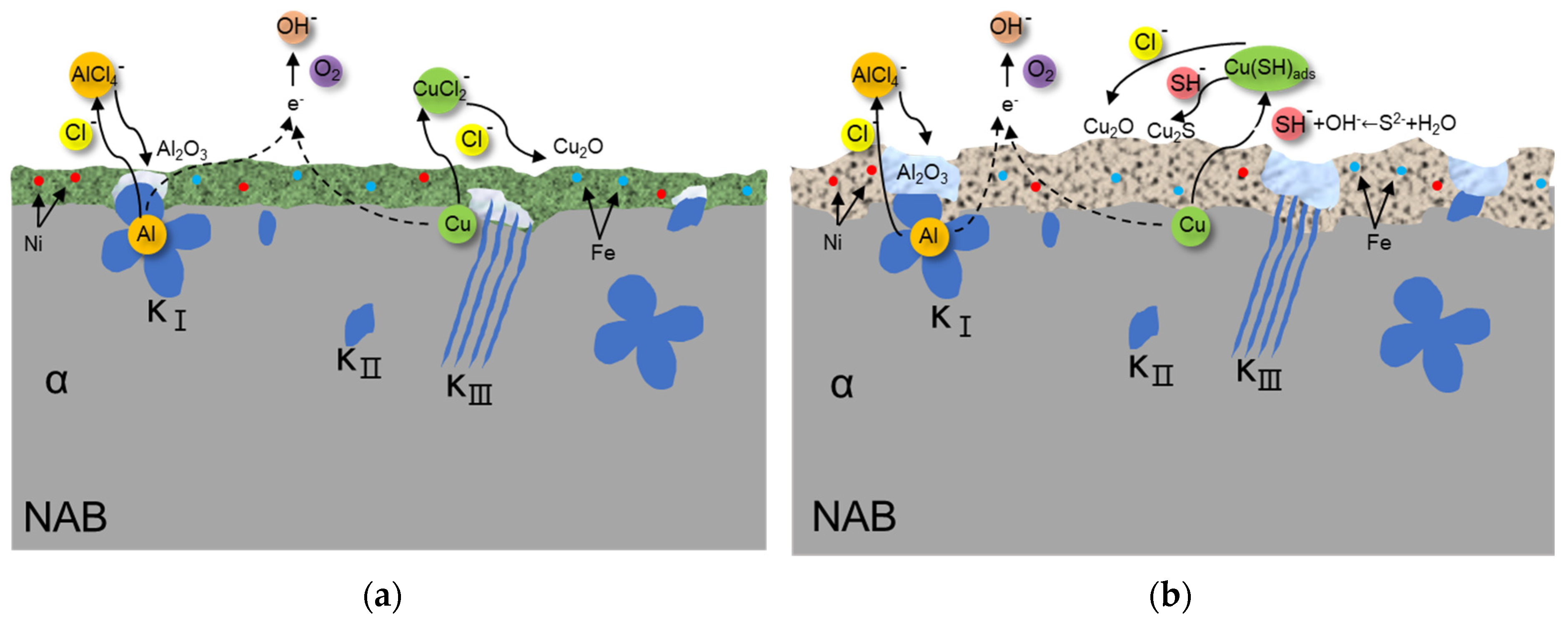
3.5. Discussion
4. Conclusions
- The SVET measurement was carried out to monitor the in situ corrosion evolution in real time. The SVET results provide visual evidence of the corrosion activation and self-passivation of NAB under sulfide-free conditions. The addition of sulfide does not significantly increase the corrosion strength of the single point, but initiates multiple corrosion points simultaneously and maintains long-term high corrosion current density. By this technique, we estimated that the corrosion mass loss after a period of immersion in the sulfide-polluted solution is more than four times that in the clean environment.
- Global electrochemical techniques were employed to complete the results of the SVET. In the presence of sulfide, the reduced potential in the PDP shows that the corrosion is more severe due to the modification of the anodic reaction process. It is inferred from the EIS results that the variable nature of the corrosion product films is the main factor that causes the discrepancy in the protection performance.
- The further morphology and composition analysis of the corrosion product films reveals that in the sulfide-free solution, a dense layer consisting mainly of Cu2O and Al2O3 is formed on the surface of NAB, while in the sulfide-polluted environment, the film containing Cu2O and Cu2S is loose and vulnerable to aggressive ions.
- The findings in this article may provide guidance for the copper alloy composition design, estimation of pitting corrosion degree, and available protective measures such as heat treatment, friction-stir processing, or coating protection.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, C.H.; Cheng, F.T.; Man, H.C. Effect of laser surface melting on the corrosion and cavitation erosion behaviors of a manganese–nickel–aluminium bronze. Mater. Sci. Eng. A 2004, 373, 195–203. [Google Scholar] [CrossRef]
- Fonlupt, S.; Bayle, B.; Delafosse, D.; Heuze, J.-L. Role of second phases in the stress corrosion cracking of a nickel–aluminium bronze in saline water. Corros. Sci. 2005, 47, 2792–2806. [Google Scholar] [CrossRef]
- Tang, C.H.; Cheng, F.T.; Man, H.C. Laser surface alloying of a marine propeller bronze using aluminium powder. Surf. Coat. Technol. 2006, 200, 2602–2609. [Google Scholar] [CrossRef]
- Alhajji, J.N.; Reda, M.R. On the Effects of Common Pollutants on the Corrosion of Copper-Nickel-Alloys in Sulfide Polluted Seawater. J. Electrochem. Soc. 1995, 142, 2944–2953. [Google Scholar] [CrossRef]
- Culpan, E.A.; Rose, G. Microstructual characterization of cast nickel aluminium bronze. J. Mater. Sci. 1978, 13, 1647–1657. [Google Scholar] [CrossRef]
- Chen, R.-P.; Liang, Z.-Q.; Zhang, W.-W.; Zhang, D.-T.; Luo, Z.-Q.; Li, Y.-Y. Effect of heat treatment on microstructure and properties of hot-extruded nickel-aluminum bronze. Trans. Nonferrous Met. Soc. China 2007, 17, 1254–1258. [Google Scholar] [CrossRef]
- Anantapong, J.; Uthaisangsuk, V.; Suranuntchai, S.; Manonukul, A. Effect of hot working on microstructure evolution of as-cast Nickel Aluminum Bronze alloy. Mater. Des. 2014, 60, 233–243. [Google Scholar] [CrossRef]
- Al-Hashem, A.; Riad, W. The role of microstructure of nickel–aluminium–bronze alloy on its cavitation corrosion behavior in natural seawater. Mater. Charact. 2002, 48, 37–41. [Google Scholar] [CrossRef]
- Lv, Y.; Guo, J.; Zhang, G.; Cao, L.; Sun, X.; Qin, Z.; Xia, D.-H. Insights into the selective phase corrosion of as cast NiAl bronze alloy: Effect of electrical properties of each phase’s protective film. J. Alloys Compd. 2022, 891, 162008. [Google Scholar] [CrossRef]
- Schussler, A.; Exner, H.E. The corrosion of nickel-aluminium bronzes in seawater-I. protecive layer formation and the passivation mechanism. Corros. Sci. 1993, 34, 1793–1802. [Google Scholar] [CrossRef]
- Wharton, J.A.; Barik, R.C.; Kear, G.; Wood, R.J.K.; Stokes, K.R.; Walsh, F.C. The corrosion of nickel–aluminium bronze in seawater. Corros. Sci. 2005, 47, 3336–3367. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Abousalem, A.S.; Kumar, A. Experimental, DFT and MC simulation analysis of Vicia Sativa weed aerial extract as sustainable and eco-benign corrosion inhibitor for mild steel in acidic environment. Sustain. Chem. Pharm. 2022, 29, 100785. [Google Scholar] [CrossRef]
- Hazra, M.; Balan, K.P. Failure of a nickel aluminium bronze (NAB) canned motor pump impeller working under polluted sea water—Influence of material selection, section thickness dependent microstructure and temper annealing heat treatment. Eng. Fail. Anal. 2016, 70, 141–156. [Google Scholar] [CrossRef]
- Hosseini, R.K.; Yareiee, S. Failure analysis of a nickel aluminium bronze butterfly valve in a seawater line. Eng. Fail. Anal. 2021, 129, 105686. [Google Scholar] [CrossRef]
- Schüssler, A.; Exner, H.E. The corrosion of nickel-aluminium bronzes in seawater—II. The corrosion mechanism in the presence of sulphide pollution. Corros. Sci. 1993, 34, 1803–1815. [Google Scholar] [CrossRef]
- Rahmouni, K.; Keddam, M.; Srhiri, A.; Takenouti, H. Corrosion of copper in 3% NaCl solution polluted by sulphide ions. Corros. Sci. 2005, 47, 3249–3266. [Google Scholar] [CrossRef]
- Bates, J.F.; Popplewell, J.M. Corrosion of Condenser Tube Alloys in Sulfide Contaminated Brine. Corrosion 1975, 31, 269–275. [Google Scholar] [CrossRef]
- Eiselstein, L.E.; Syrett, B.C.; Wing, S.S.; Caligiuri, R.D. The accelerated corrosion of Cu-Ni alloys in sulphide-polluted seawater: Mechanism no. 2. Corros. Sci. 1983, 23, 223–239. [Google Scholar] [CrossRef]
- Syrett, B.C. The mechanism of accelerated corrosion of copper-nickel alloys in sulphide-polluted seawater. Corros. Sci. 1981, 21, 187–209. [Google Scholar] [CrossRef]
- Yuan, S.J.; Pehkonen, S.O. Surface characterization and corrosion behavior of 70/30 Cu–Ni alloy in pristine and sulfide-containing simulated seawater. Corros. Sci. 2007, 49, 1276–1304. [Google Scholar] [CrossRef]
- Awad, N.K.; Ashour, E.A.; Allam, N.K. Unravelling the composition of the surface layers formed on Cu, Cu-Ni, Cu-Zn and Cu-Ni-Zn in clean and polluted environments. Appl. Surf. Sci. 2015, 346, 158–164. [Google Scholar] [CrossRef]
- Al-Hajji, J.N.; Reda, M.R. the corrosion of copper-nickel alloys in sulfide polluted seawater: The effect of sulfide concentration. Corros. Sci. 1993, 34, 163–177. [Google Scholar] [CrossRef]
- Song, Q.-N.; Xu, N.; Bao, Y.-F.; Jiang, Y.-F.; Gu, W.; Zheng, Y.-G.; Qiao, Y.-X. Corrosion and Cavitation Erosion Behaviors of Two Marine Propeller Materials in Clean and Sulfide-Polluted 3.5% NaCl Solutions. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 712–720. [Google Scholar] [CrossRef]
- Song, Q.-N.; Xu, N.; Tong, Y.; Huang, C.-M.; Sun, S.-Y.; Xu, C.-B.; Bao, Y.-F.; Jiang, Y.-F.; Qiao, Y.-X.; Zhu, Z.-Y.; et al. Corrosion and Cavitation Erosion Behaviours of Cast Nickel Aluminium Bronze in 3.5% NaCl Solution with Different Sulphide Concentrations. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 1470–1482. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, H.S. The measurement of the galvanic corrosion of soldered copper using the scanning vibrating electrode technique. Corros. Sci. 1988, 28, 547–558. [Google Scholar] [CrossRef]
- Nakhaie, D.; Davoodi, A.; Imani, A. The role of constituent phases on corrosion initiation of NiAl bronze in acidic media studied by SEM-EDS, AFM and SKPFM. Corros. Sci. 2014, 80, 104–110. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, J.; Zhao, W.; Wu, W.; Dong, J.; Zhou, K.; Xue, Q. Excellent corrosion resistance of graphene coating on copper due to the low defect overlapping structure. Surf. Topogr. Metrol. Prop. 2019, 7, 015014. [Google Scholar] [CrossRef]
- de Sousa Araujo, J.V.; Donatus, U.; Queiroz, F.M.; Terada, M.; Milagre, M.X.; de Alencar, M.C.; Costa, I. On the severe localized corrosion susceptibility of the AA2198-T851 alloy. Corros. Sci. 2018, 133, 132–140. [Google Scholar] [CrossRef]
- Hasan, F.; Jahanafrooz, A.; Lorimer, G.W.; Ridley, N. The Morphology, Crystallography, and Chemistry of Phases in As-Cast Nickel-Aluminum Bronze. Metall. Trans. A 1982, 13, 1337–1345. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, Y.H.; Wang, J.T.; Zhang, Z.P.; Zhu, J.; Quan, L.L.; He, D.C. Effect of Cast Defects on the Corrosion Behavior and Mechanism of UNS C95810 Alloy in Artificial Seawater. Materials 2020, 13, 1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Zhao, R.; Qin, Z.B.; Wu, Z.; Wang, L.Q.; Liu, L.; Lu, W.J. Evolution of the Corrosion Product Film on Nickel-Aluminum Bronze and Its Corrosion Behavior in 3.5 wt % NaCl Solution. Materials 2019, 12, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, D.C.; Dong, C.F.; Ni, X.Q.; Xu, A.N.; He, C.; Xiao, K.; Li, X.G. Long-term polarisation and immersion for copper corrosion in high-level nuclear waste environment. Mater. Corros. 2017, 68, 1070–1079. [Google Scholar] [CrossRef]
- Chen, J.; Qin, Z.; Shoesmith, D.W. Kinetics of Corrosion Film Growth on Copper in Neutral Chloride Solutions Containing Small Concentrations of Sulfide. J. Electrochem. Soc. 2010, 157, 338–345. [Google Scholar] [CrossRef]
- Awad, N.K.; Ashour, E.A.; Fouda, A.S.; Allam, N.K. Effect of alloying elements on the electrochemical behavior of Cu–Ni–Zn ternary system in sulfide-polluted saltwater. Appl. Surf. Sci. 2014, 307, 621–630. [Google Scholar] [CrossRef]
- Qin, Z.; Xia, D.-H.; Zhang, Y.; Wu, Z.; Liu, L.; Lv, Y.; Liu, Y.; Hu, W. Microstructure modification and improving corrosion resistance of laser surface quenched nickel–aluminum bronze alloy. Corros. Sci. 2020, 174, 108744. [Google Scholar] [CrossRef]
- Qin, Z.B.; Zhang, Q.; Luo, Q.; Wu, Z.; Shen, B.; Liu, L.; Hu, W.B. Microstructure design to improve the corrosion and cavitation corrosion resistance of a nickel-aluminum bronze. Corros. Sci. 2018, 139, 255–266. [Google Scholar] [CrossRef]
- North, R.F.; Pryor, M.J. the influence of corrosion product structure on the corrosion rate of Cu-Ni alloys. Corros. Sci. 1970, 10, 297–311. [Google Scholar] [CrossRef]
- Ma, A.L.; Jiang, S.L.; Zheng, Y.G.; Ke, W. Corrosion product film formed on the 90/10 copper–nickel tube in natural seawater: Composition/structure and formation mechanism. Corros. Sci. 2015, 91, 245–261. [Google Scholar] [CrossRef]





| Element | Al | Mn | Fe | Ni | Cu |
|---|---|---|---|---|---|
| wt.% | 9.2 | 1.5 | 4.6 | 4.3 | 80.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wei, Y. Corrosion Evolution of Nickel Aluminum Bronze in Clean and Sulfide-Polluted Solutions. Coatings 2023, 13, 846. https://doi.org/10.3390/coatings13050846
Yang L, Wei Y. Corrosion Evolution of Nickel Aluminum Bronze in Clean and Sulfide-Polluted Solutions. Coatings. 2023; 13(5):846. https://doi.org/10.3390/coatings13050846
Chicago/Turabian StyleYang, Liu, and Yinghua Wei. 2023. "Corrosion Evolution of Nickel Aluminum Bronze in Clean and Sulfide-Polluted Solutions" Coatings 13, no. 5: 846. https://doi.org/10.3390/coatings13050846


.jpg)


