Convolvulus microphyllus Extract as a Green, Effective, and Affordable Corrosion Inhibitor: Theoretical Calculations and Experimental Studies
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
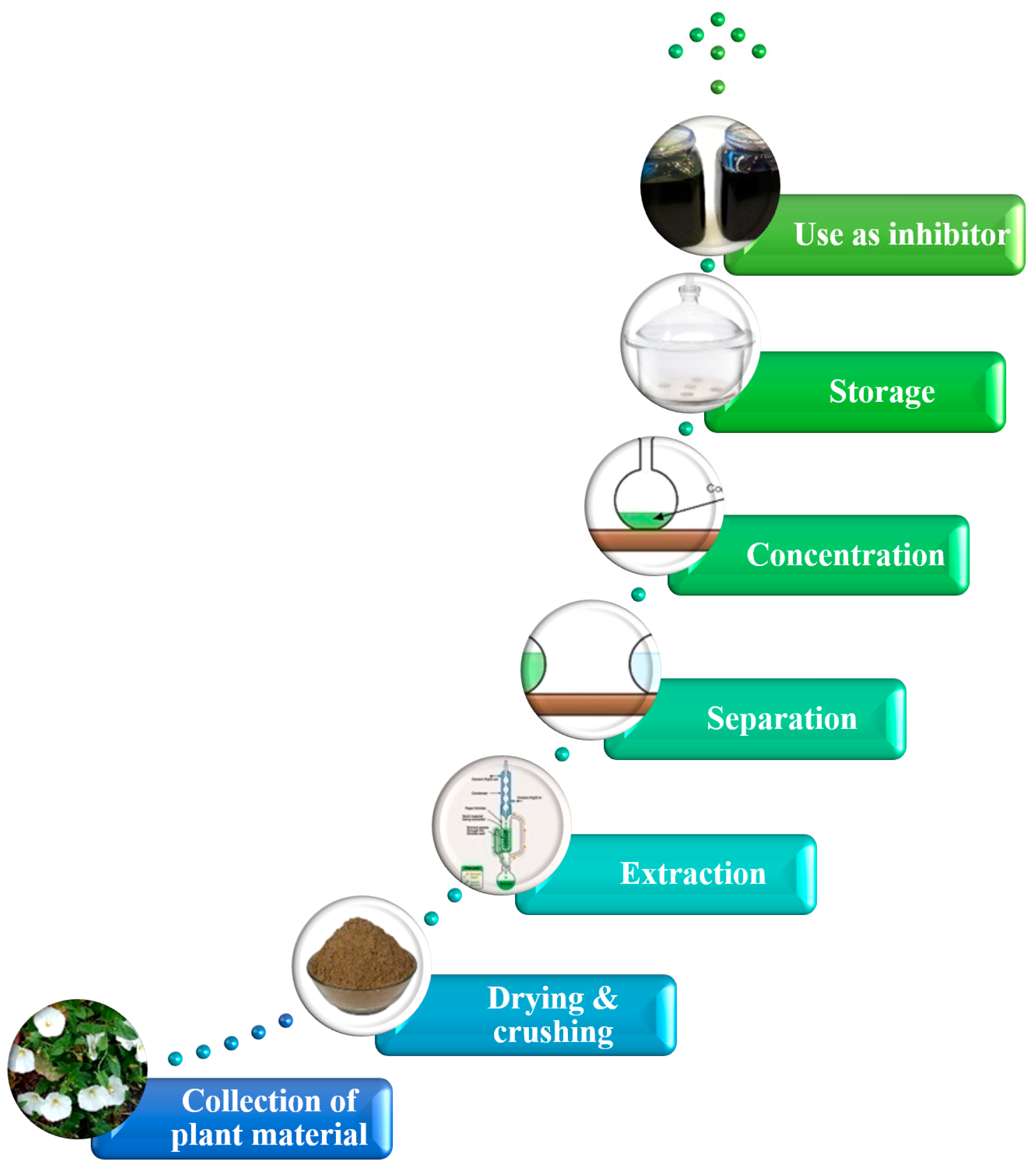
2.2. Preparation of Plant Extract
3. Methods
3.1. Extract Characterization
3.1.1. FT-IR Analysis
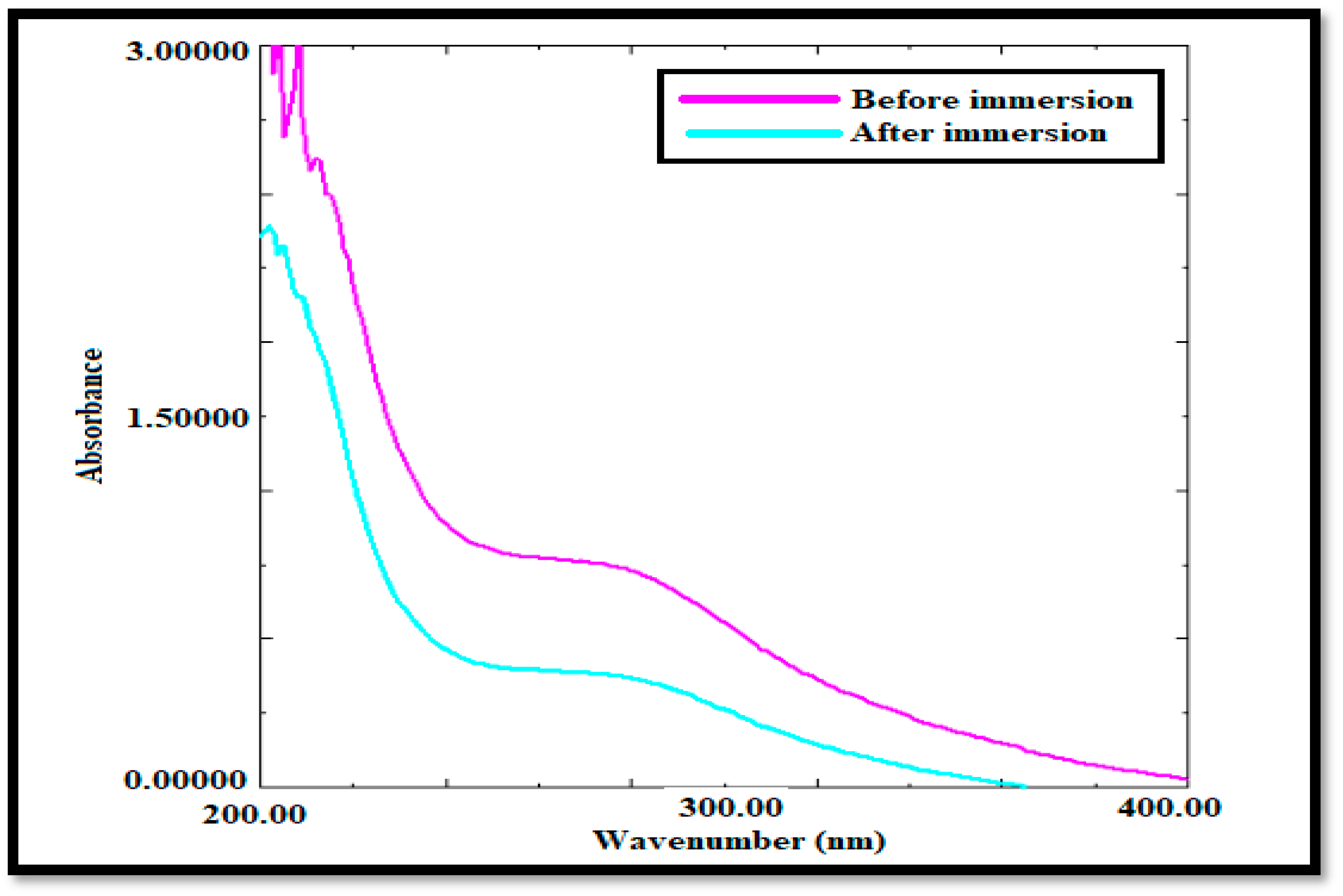
3.1.2. UV-Visible Analysis
3.2. Weight Loss Study
3.3. Electrochemical Study
3.4. Surface Study
3.5. Computational Studies
3.5.1. DFT
3.5.2. MD Simulations
3.6. Statistical Analysis
4. Results and Discussions
4.1. Characterization of C. microphyllus Extract
4.1.1. FT-IR Study
4.1.2. UV-Visible Study
4.2. Weight-Loss Study
4.3. Electrochemical Studies
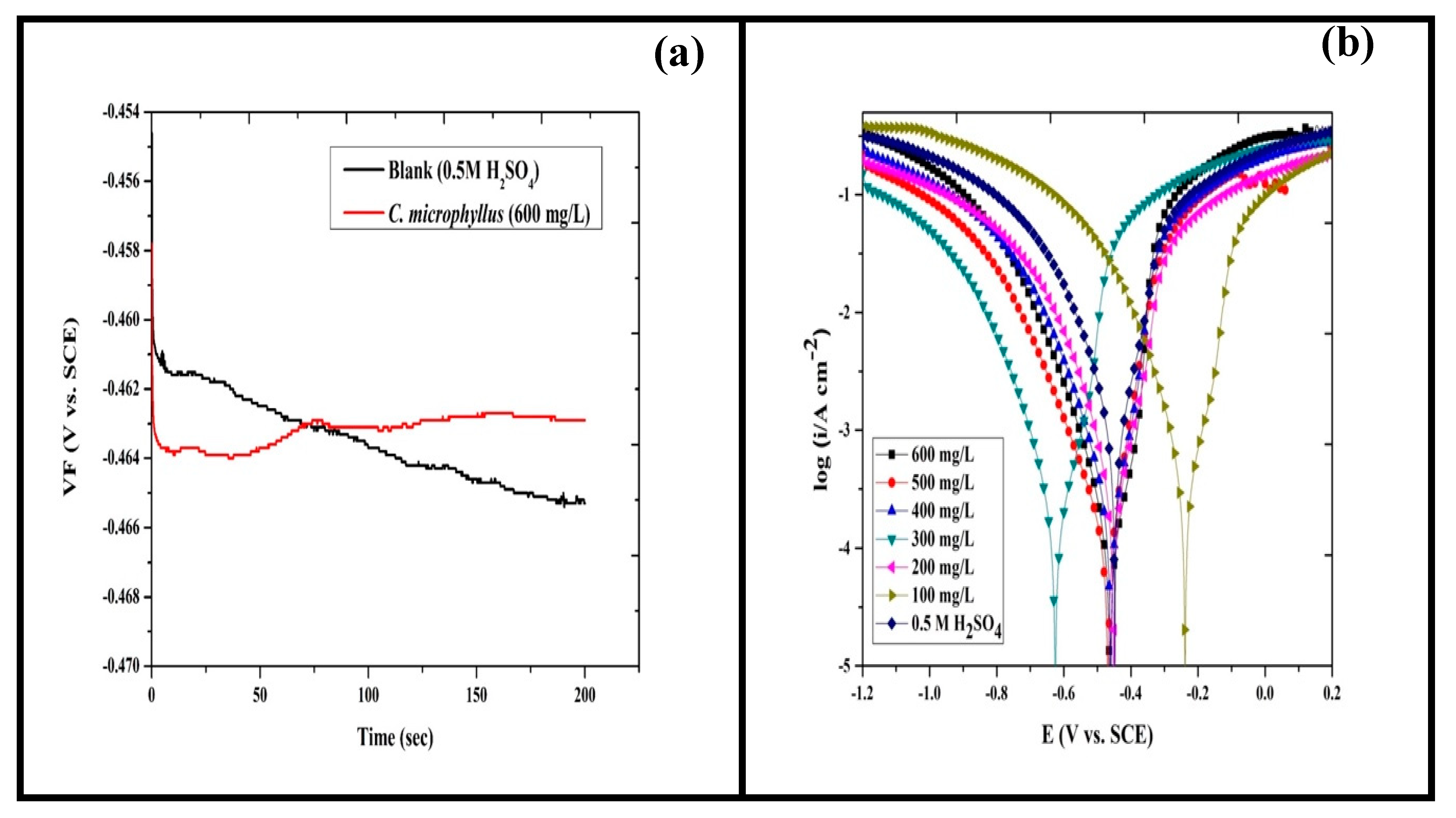
4.3.1. OCP
4.3.2. PDP
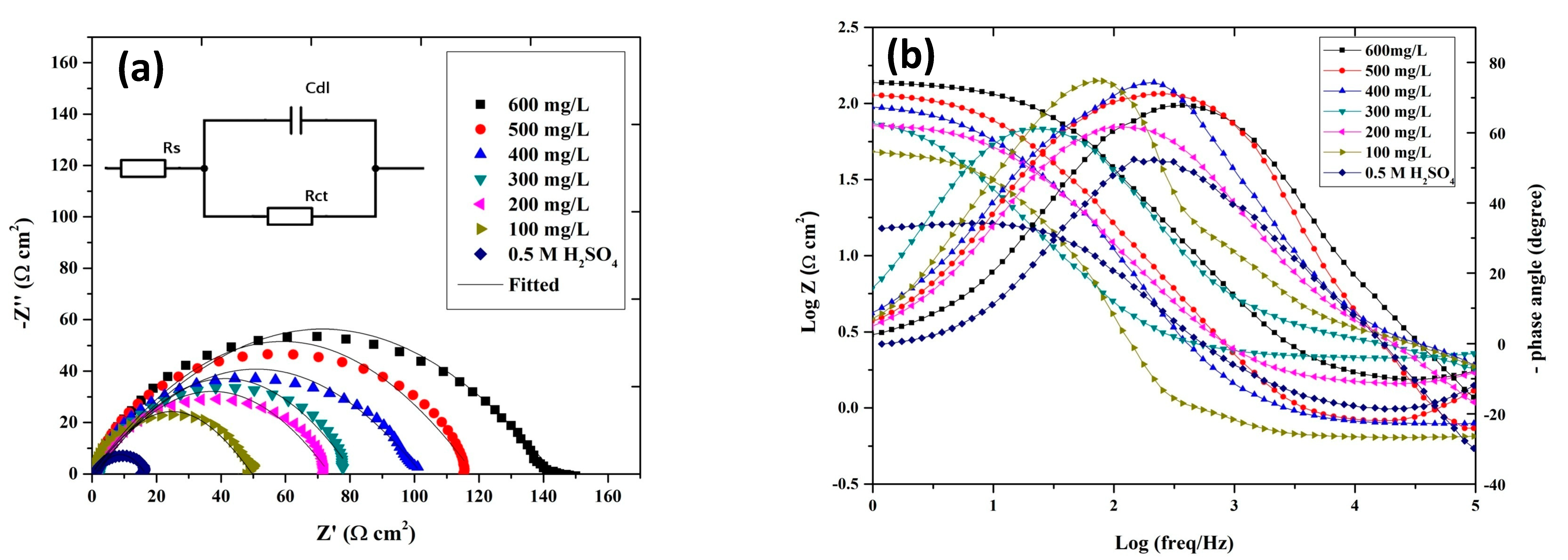
4.3.3. EIS
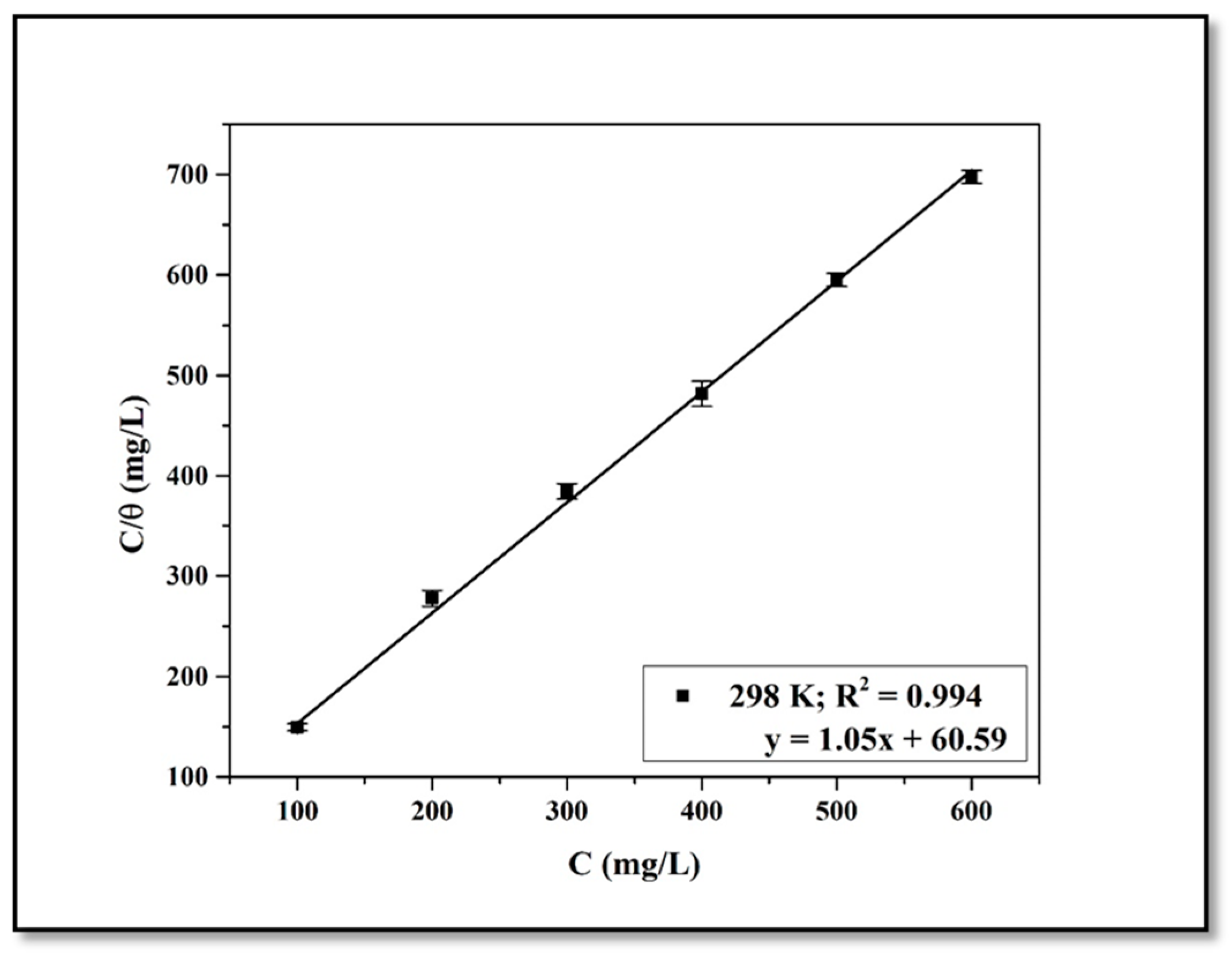
4.4. Adsorption Isotherm
4.5. Surface Studies
SEM and AFM Analysis
4.6. Computational Studies
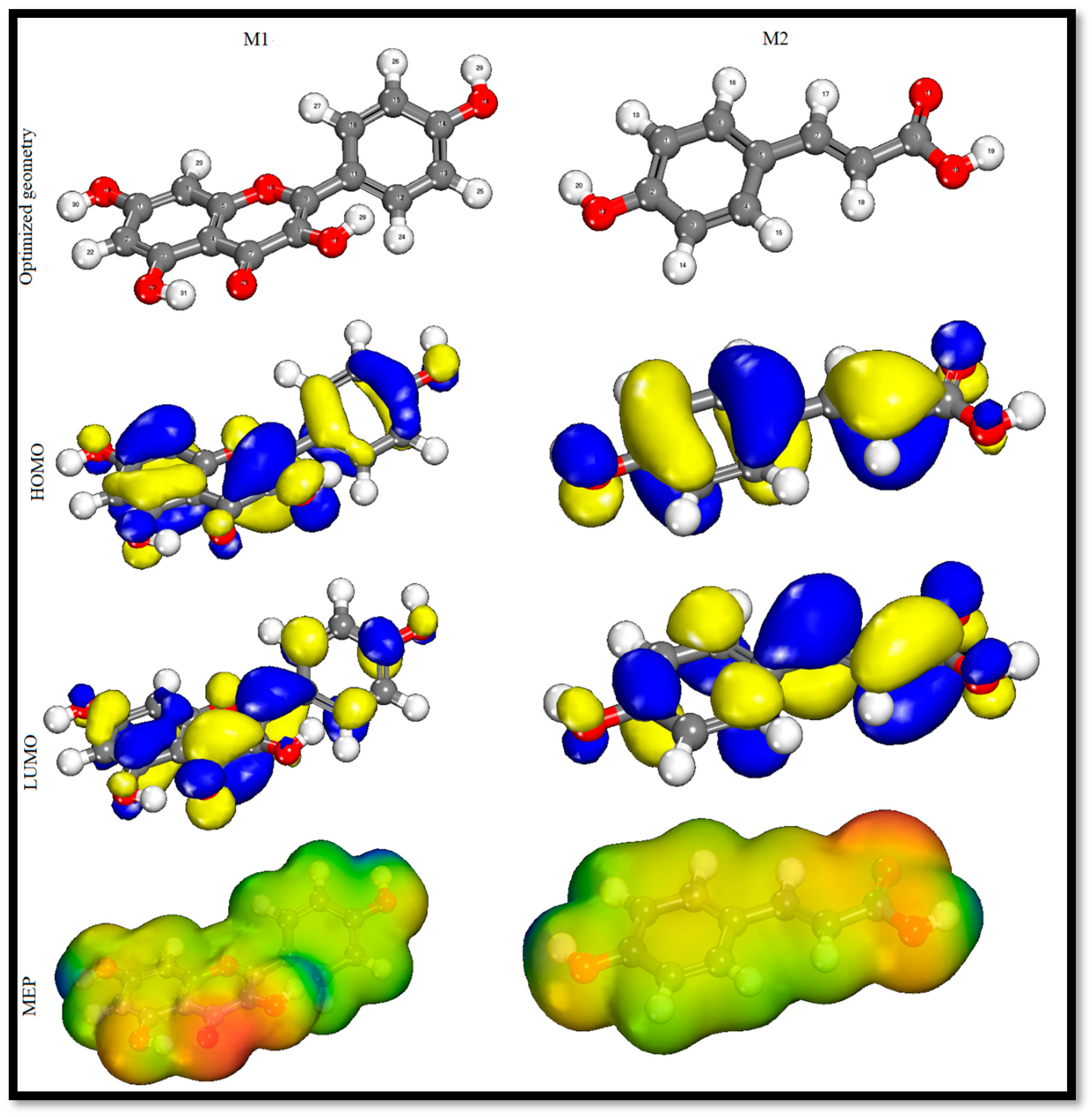
4.6.1. DFT
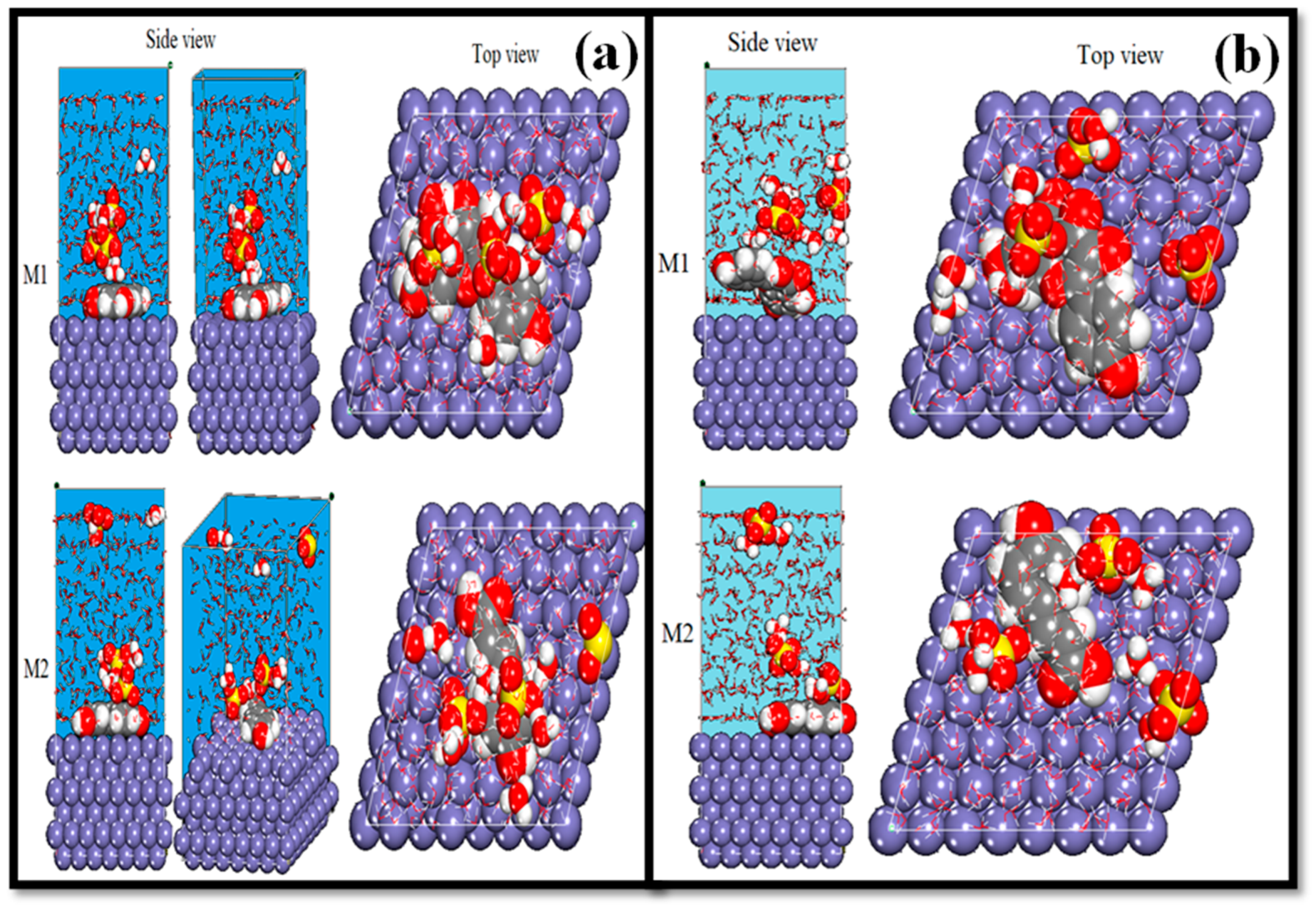
4.6.2. MDS
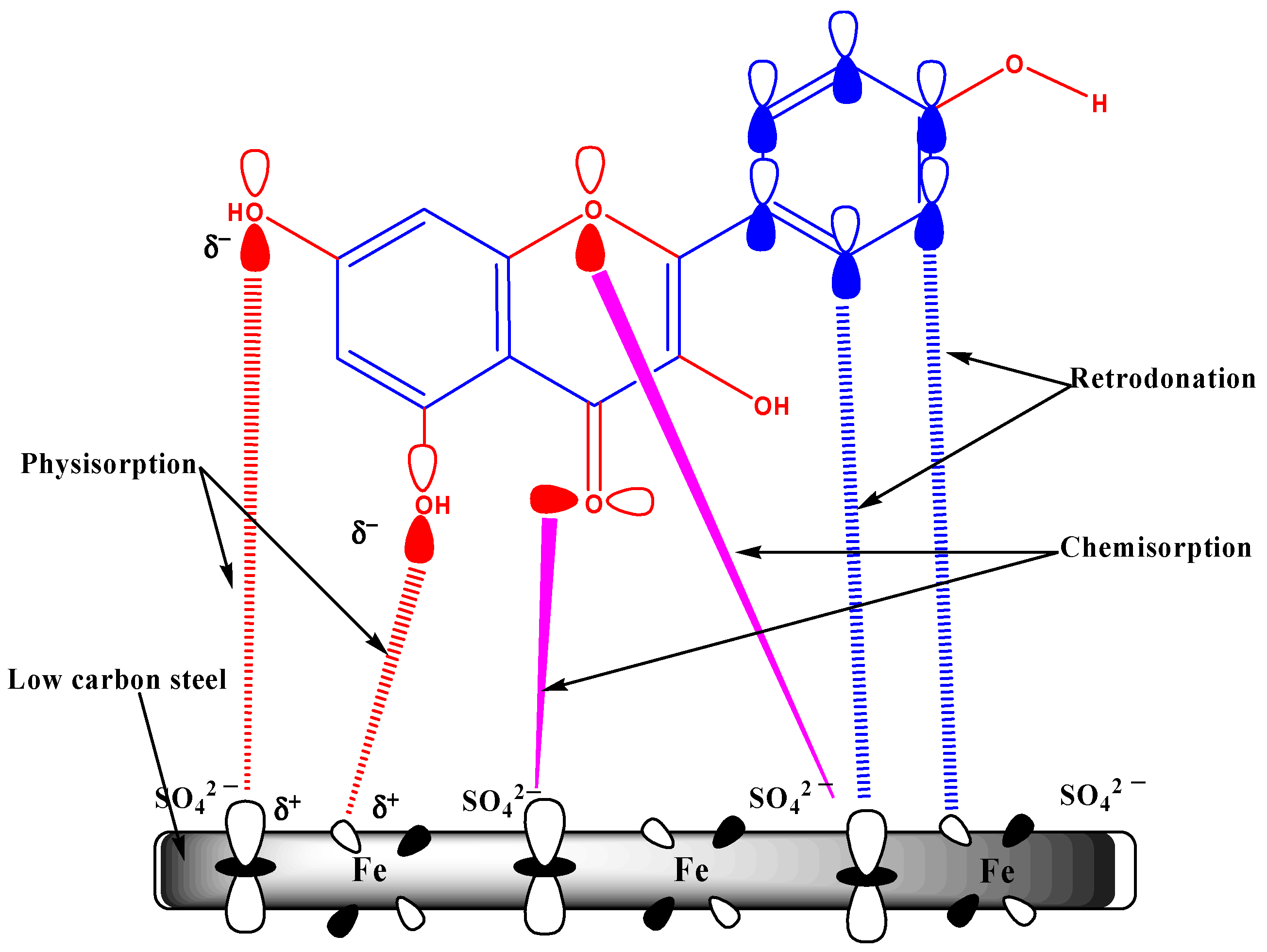
4.7. Inhibition Mechanism
5. Conclusions
- ❖
- Electrochemical analysis revealed that the inhibitory effect of C. microphyllus-derived film (extract) at a concentration of 600 mg/L provides approximately 92% protection against corrosion for low carbon steel at 298 K.
- ❖
- Potentiodynamic polarization estimates indicated that this natural product is a mixed-type inhibitor and also functions as an adsorptive inhibitor. Therefore, the efficiency of the inhibition may be improved by increasing the inhibitor concentration.
- ❖
- When the concentration of the inhibitor was increased, electrochemical impedance spectroscopy showed a decrease in the electrical double layer’s CPE and an increase in the charge transfer resistance.
- ❖
- These findings further demonstrate that the C. microphyllus-derived film (extract) extract only affects the metal/solution interface by adsorption.
- ❖
- The FT-IR technique demonstrated the presence of heteroatoms and unsaturated mixtures that were used as inhibitors.
- ❖
- The coordination connections between the inhibitor molecules and Fe2+ were confirmed by the UV-visible spectroscopic technique.
- ❖
- The SEM the AFM approaches explored the adsorption of the C. microphyllus-derived film (extract) inhibitor on a metal substrate.
- ❖
- The experimental results indicate that these inhibitor compounds are strongly physisorbed and chemisorbed onto the LCS surface.
- ❖
- Studies in DFT, MC, and MDS demonstrated that inhibitors frequently cured and safeguarded metals due to their distinct ability to attract and withdraw electrons from metals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy | M1 | Kaempferol |
| ASTM | American society for testing and materials | M2 | p-hydroxycinnamic acid |
| DFT | Density functional theory | MSD | Molecular dynamic simulation |
| EIS | Electrochemical impedance spectroscopy | OCP | Open circuit potential |
| FTIR | Fourier transform infrared spectroscopy | PDP | Potentiodynamic polarization |
| IE | Inhibition efficiency | SEM | Scanning electron microscopy |
| LCS | Low carbon steel | SCE | Saturated calomel electrode |
References
- Verma, D.K.; Kazi, M.; Alqahtani, M.S.; Syed, R.; Berdimurodov, E.; Kaya, S.; Salim, R.; Asatkar, A.; Haldhar, R. N–hydroxybenzothioamide derivatives as green and efficient corrosion inhibitors for mild steel: Experimental, DFT and MC simulation approach. J. Mol. Struct. 2021, 1241, 130648. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 2019, 279, 603–624. [Google Scholar] [CrossRef]
- Majd, M.T.; Asaldoust, S.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Green method of carbon steel effective corrosion mitigation in 1 M HCl medium protected by Primula vulgaris flower aqueous extract via experimental, atomic-level MC/MD simulation and electronic-level DFT theoretical elucidation. J. Mol. Liq. 2019, 284, 658–674. [Google Scholar] [CrossRef]
- Sanaei, Z.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Application of green molecules from Chicory aqueous extract for steel corrosion mitigation against chloride ions attack; the experimental examinations and electronic/atomic level computational studies. J. Mol. Liq. 2019, 290, 111176. [Google Scholar] [CrossRef]
- Tabatabaei majd, M.; Bahlakeh, G.; Dehghani, A.; Ramezanzadeh, B.; Ramezanzadeh, M. A green complex film based on the extract of Persian Echium amoenum and zinc nitrate for mild steel protection in saline solution; Electrochemical and surface explorations besides dynamic simulation. J. Mol. Liq. 2019, 291, 111281. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. Green Eucalyptus leaf extract: A potent source of bio-active corrosion inhibitors for mild steel. Bioelectrochemistry 2019, 130, 107339. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Aloysia citrodora leaves extract corrosion retardation effect on mild-steel in acidic solution: Molecular/atomic scales and electrochemical explorations. J. Mol. Liq. 2020, 310, 113221. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Green synthesis of reduced graphene oxide nanosheets decorated with zinc-centered metal-organic film for epoxy-ester composite coating reinforcement: DFT-D modeling and experimental explorations. J. Taiwan Inst. Chem. Eng. 2020, 114, 311–330. [Google Scholar] [CrossRef]
- Majd, M.T.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Steel corrosion lowering in front of the saline solution by a nitrogen-rich source of green inhibitors: Detailed surface, electrochemical and computational studies. Constr. Build. Mater. 2020, 254, 119266. [Google Scholar] [CrossRef]
- Asfia, M.P.; Rezaei, M.; Bahlakeh, G. Corrosion prevention of AISI 304 stainless steel in hydrochloric acid medium using garlic extract as a green corrosion inhibitor: Electrochemical and theoretical studies. J. Mol. Liq. 2020, 315, 113679. [Google Scholar] [CrossRef]
- Keramatinia, M.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Synthesis of a multi-functional zinc-centered nitrogen-rich graphene-like thin film from natural sources on the steel surface for achieving superior anti-corrosion properties. Corros. Sci. 2021, 178, 109077. [Google Scholar] [CrossRef]
- Mofidabadi, A.H.J.; Bahlakeh, G.; Ramezanzadeh, B. Anti-corrosion performance of the mild steel substrate treated by a novel nanostructure europium oxide-based conversion coating: Electrochemical and surface studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125689. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Bahlakeh, G.; Ramezanzadeh, B. Detailed theoretical DFT computation/molecular simulation and electrochemical explorations of Thymus vulgaris leave extract for effective mild-steel corrosion retardation in HCl solution. J. Mol. Liq. 2021, 335, 115897. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A.; Singh, P. Valeriana wallichii root extract as a green & sustainable corrosion inhibitor for mild steel in acidic environments: Experimental and theoretical study. Mater. Chem. Front. 2018, 2, 1225–1237. [Google Scholar] [CrossRef]
- Shahini, M.H.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Superior inhibition action of the Mish Gush (MG) leaves extract toward mild steel corrosion in HCl solution: Theoretical and electrochemical studies. J. Mol. Liq. 2021, 332, 115876. [Google Scholar] [CrossRef]
- Salmasifar, A.; Edraki, M.; Alibakhshi, E.; Ramezanzadeh, B.; Bahlakeh, G. Theoretical design coupled with experimental study of the effectiveness of the inhibitive molecules based on Cynara scolymus L extract toward chloride-induced corrosion of steel. J. Mol. Liq. 2021, 332, 115742. [Google Scholar] [CrossRef]
- Mostafatabar, A.H.; Bahlakeh, G.; Ramezanzadeh, B.; Dehghani, A.; Ramezanzadeh, M. A comprehensive electronic-scale DFT modeling, atomic-level MC/MD simulation, and electrochemical/surface exploration of active nature-inspired phytochemicals based on Heracleum persicum seeds phytoextract for effective retardation of the acidic-induced c. J. Mol. Liq. 2021, 331, 115764. [Google Scholar] [CrossRef]
- Tehrani, M.E.H.N.; Ghahremani, P.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Theoretical and experimental assessment of a green corrosion inhibitor extracted from Malva sylvestris. J. Environ. Chem. Eng. 2021, 9, 105256. [Google Scholar] [CrossRef]
- Salmasifar, A.; Edraki, M.; Alibakhshi, E.; Ramezanzadeh, B.; Bahlakeh, G. Combined electrochemical/surface investigations and computer modeling of the aquatic Artichoke extract molecules corrosion inhibition properties on the mild steel surface immersed in the acidic medium. J. Mol. Liq. 2021, 327, 114856. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Synthesis of graphene oxide nanosheets decorated by nanoporous zeolite-imidazole (ZIF-67) based metal-organic framework with controlled-release corrosion inhibitor performance: Experimental and detailed DFT-D theoretical explorations. J. Hazard. Mater. 2021, 404, 124068. [Google Scholar] [CrossRef]
- Wagner, H.; Schwarting, G. Struktur der microphyllinsäure aus dem harz von Convolvulus microphyllus. Phytochemistry 1977, 16, 715–717. [Google Scholar] [CrossRef]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study, 1st ed.; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Mofidabadi, A.H.J. Construction of a high-potency anti-corrosive metal-organic film based on europium (III)-benzimidazole: Theoretical and electrochemical investigations. Constr. Build. Mater. 2021, 269, 121271. [Google Scholar] [CrossRef]
- Keshmiri, N.; Najmi, P.; Ramezanzadeh, B.; Ramezanzadeh, M.; Bahlakeh, G. Nano-scale P, Zn-codoped reduced-graphene oxide incorporated epoxy composite; synthesis, electronic-level DFT-D modeling, and anti-corrosion properties. Prog. Org. Coat. 2021, 159, 106416. [Google Scholar] [CrossRef]
- Kumar, S.; Vashisht, H.; Olasunkanmi, L.O.; Bahadur, I.; Verma, H.; Goyal, M.; Singh, G.; Ebenso, E.E. Polyurethane Based Triblock Copolymers as Corrosion Inhibitors for Mild Steel in 0.5 M H2SO4. Ind. Eng. Chem. Res. 2017, 56, 441–456. [Google Scholar] [CrossRef]
- Belghiti, M.; Echihi, S.; Mahsoune, A.; Karzazi, Y.; Aboulmouhajir, A.; Dafali, A.; Bahadur, I. Piperine derivatives as green corrosion inhibitors on iron surface; DFT, Monte Carlo dynamics study and complexation modes. J. Mol. Liq. 2018, 261, 62–75. [Google Scholar] [CrossRef]
- Verma, D.; Khan, F.; Bahadur, I.; Salman, M.; Quraishi, M.; Verma, C.; Ebenso, E.E. Inhibition performance of Glycine max, Cuscuta reflexa and Spirogyra extracts for mild steel dissolution in acidic medium: Density functional theory and experimental studies. Results Phys. 2018, 10, 665–674. [Google Scholar] [CrossRef]
- Goyal, M.; Vashisht, H.; Kumar, S.; Bahadur, I. Anti-corrosion performance of eco-friendly inhibitor (2-aminobenzyl) triphenylphosphonium bromide ionic liquid on mild steel in 0.5M sulfuric acid. J. Mol. Liq. 2018, 261, 162–173. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Verma, C.; Bahadur, I.; Ebenso, E.E.; Lgaz, H.; Chung, I.-M. Interfacial adsorption behavior of quaternary phosphonium based ionic liquids on metal-electrolyte interface: Electrochemical, surface characterization and computational approaches. J. Mol. Liq. 2020, 298, 111995. [Google Scholar] [CrossRef]
- Goyal, M.; Vashisht, H.; Kumar, A.; Kumar, S.; Bahadur, I.; Benhiba, F.; Zarrouk, A. Isopentyltriphenylphosphonium bromideionic liquid as a newly effective corrosion inhibitor on metal-electrolyte interface in acidic medium: Experimental, surface morphological (SEM-EDX & AFM) and computational analysis. J. Mol. Liq. 2020, 316, 113838. [Google Scholar] [CrossRef]
- Sadik, K.; El Hamdani, N.; Hachim, M.E.; Byadi, S.; Bahadur, I.; Aboulmouhajir, A. Towards a theoretical understanding of alkaloid-extract Cytisine derivatives of Retama monosperma (L.) Boiss. Seeds, as eco-friendly inhibitor for carbon steel corrosion in acidic 1M HCl solution. J. Theor. Comput. Chem. 2020, 19, 2050013. [Google Scholar] [CrossRef]
- Goyal, M.; Vashist, H.; Kumar, S.; Bahadur, I.; Benhiba, F.; Zarrouk, A. Acid corrosion inhibition of ferrous and non-ferrous metal by nature friendly Ethoxycarbonylmethyltriphenylphosphonium Bromide (ECMTPB): Experimental and MD simulation evaluation. J. Mol. Liq. 2020, 315, 113705. [Google Scholar] [CrossRef]
- Kumar, B.; Vashisht, H.; Goyal, M.; Kumar, A.; Benhiba, F.; Prasad, A.K.; Kumar, S.; Bahadur, I.; Zarrouk, A. Study of adsorption mechanism of chalcone derivatives on mild steel-sulfuric acid interface. J. Mol. Liq. 2020, 318, 113890. [Google Scholar] [CrossRef]
- Daoudi, W.; El Aatiaoui, A.; Dagdag, O.; Zaidi, K.; Haldhar, R.; Kim, S.C.; Oussaid, A.; Aouinti, A.; Berisha, A.; Benhiba, F.; et al. Anti-corrosion coating formation by a biopolymeric extract of Artemisia herba-alba plant: Experimental and theoretical investigations. Coatings 2023, 13, 611. [Google Scholar] [CrossRef]
- Kashani, F.R.; Rezaei, M. Electrochemical studies and molecular simulations on the use of molybdic acid for stabilization of AISI 304 stainless steel passive film in sulfuric acid medium. J. Mol. Liq. 2021, 344, 117733. [Google Scholar] [CrossRef]
- Verma, D.K.; Al Fantazi, A.; Verma, C.; Khan, F.; Asatkar, A.; Hussain, C.M.; Ebenso, E.E. Experimental and computational studies on hydroxamic acids as environmental friendly chelating corrosion inhibitors for mild steel in aqueous acidic medium. J. Mol. Liq. 2020, 314, 113651. [Google Scholar] [CrossRef]
- Kashani, F.R.; Rezaei, M. Improving the localized corrosion resistance of 304 stainless steel in HCl solution by adsorption of molybdate ions: Interaction mechanisms at the interface using molecular dynamics simulation and electrochemical noise analysis. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129085. [Google Scholar] [CrossRef]
- Verma, D.K.; Aslam, R.; Aslam, J.; Quraishi, M.A.; Ebenso, E.E.; Verma, C. Computational Modeling: Theoretical Predictive Tools for Designing of Potential Organic Corrosion Inhibitors. J. Mol. Struct. 2021, 1236, 130294. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Jmiai, A.; Tara, A.; El Issami, S.; Hilali, M.; Jbara, O.; Bazzi, L. A new trend in corrosion protection of copper in acidic medium by using Jujube shell extract as an effective green and environmentally safe corrosion inhibitor: Experimental, quantum chemistry approach and Monte Carlo simulation study. J. Mol. Liq. 2021, 322, 114509. [Google Scholar] [CrossRef]
- Naseri, E.; Hajisafari, M.; Kosari, A.; Talari, M.; Hosseinpour, S.; Davoodi, A. Inhibitive effect of Clopidogrel as a green corrosion inhibitor for mild steel; statistical modeling and quantum Monte Carlo simulation studies. J. Mol. Liq. 2018, 269, 193–202. [Google Scholar] [CrossRef]
- Berrissoul, A.; Ouarhach, A.; Benhiba, F.; Romane, A.; Zarrouk, A.; Guenbour, A.; Dikici, B.; Dafali, A. Evaluation of Lavandula mairei extract as green inhibitor for mild steel corrosion in 1 M HCl solution. Experimental and theoretical approach. J. Mol. Liq. 2020, 313, 113493. [Google Scholar] [CrossRef]
- Zuo, X.; Li, W.; Luo, W.; Zhang, X.; Qiang, Y.; Zhang, J.; Li, H.; Tan, B. Research of Lilium brownii leaves extract as a commendable and green inhibitor for X70 steel corrosion in hydrochloric acid. J. Mol. Liq. 2021, 321, 114914. [Google Scholar] [CrossRef]
- Abdallah, M.; Altass, H.M.; Al-Gorair, A.S.; Al-Fahemi, J.H.; Jahdaly, B.A.A.L.; Soliman, K.A. Natural nutmeg oil as a green corrosion inhibitor for carbon steel in 1.0 M HCl solution: Chemical, electrochemical, and computational methods. J. Mol. Liq. 2021, 323, 115036. [Google Scholar] [CrossRef]
- Khadom, A.A.; Kadhim, M.M.; Anaee, R.A.; Mahood, H.B.; Mahdi, M.S.; Salman, A.W. Theoritical evaluation of Citrus Aurantium Leaf Extract as green inhibitor for chemical and biological corrosion of mild steel in acidic solution: Statistical, molecular dynamics, Docking, and quantum mechanics study. J. Mol. Liq. 2021, 343, 116978. [Google Scholar] [CrossRef]
- Dagdag, O.; Safi, Z.; Hamed, O.; Jodeh, S.; Wazzan, N.; Haldhar, R.; Safi, S.K.; Berisha, A.; El Gouri, M. Comparative study of some epoxy polymers based on bisphenolic and aromatic diamines: Synthesis, viscosity, thermal properties computational and statistical approaches. J. Polym. Res. 2021, 28, 165. [Google Scholar] [CrossRef]
- Dagdag, O.; El Bachiri, A.; Hamed, O.; Haldhar, R.; Verma, C.; Ebenso, E.; El Gouri, M. Dendrimeric Epoxy Resins Based on Hexachlorocyclotriphosphazene as a Reactive Flame Retardant Polymeric Materials: A Review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3240–3261. [Google Scholar] [CrossRef]
- El-Aouni, N.; Hsissou, R.; Safi, Z.; Abbout, S.; Benhiba, F.; El Azzaoui, J.; Haldhar, R.; Wazzan, N.; Guo, L.; Erramli, H.; et al. Performance of two new epoxy resins as potential corrosion inhibitors for carbon steel in 1MHCl medium: Combining experimental and computational approaches. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127066. [Google Scholar] [CrossRef]
- Dagdag, O.; Guo, L.; Safi, Z.; Verma, C.; Ebenso, E.E.; Wazzan, N.; Masroor, S.; Haldhar, R.; Jodeh, S.; El Gouri, M. Epoxy resin and TiO2 composite as anticorrosive material for carbon steel in 3% NaCl medium: Experimental and computational studies. J. Mol. Liq. 2020, 317, 114249. [Google Scholar] [CrossRef]
- Erramli, H.; Dagdag, O.; Safi, Z.; Wazzan, N.; Guo, L.; Abbout, S.; Ebenso, E.; Verma, C.; Haldhar, R.; El Gouri, M. Trifunctional epoxy resin as anticorrosive material for carbon steel in 1 M HCl: Experimental and computational studies. Surf. Interfaces 2020, 21, 100707. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Investigation of Corrosion Inhibition Effect and Adsorption Activities of Achyranthes aspera Extract for Mild Steel in 0.5 M H2SO4. J. Fail. Anal. Prev. 2018, 18, 957–968. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bahadur, I.; Dagdag, O.; Kaya, S.; Verma, D.K.; Kim, S.-C. Investigation of plant waste as a renewable biomass source to develop efficient, economical and eco-friendly corrosion inhibitor. J. Mol. Liq. 2021, 335, 116184. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Use of Syzygium aromaticum extract as green corrosion inhibitor for mild steel in 0.5M H2SO4. Surf. Rev. Lett. 2018, 26, 1850200. [Google Scholar] [CrossRef]
- Zavidovskiy, I.A.; Streletskiy, O.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Savchenko, N.F.; Tatarintsev, A.A.; Pavlikov, A.V. Highly selective polyene-polyyne resistive gas sensors: Response tuning by low-energy ion irradiation. J. Compos. Sci. 2023, 7, 156. [Google Scholar] [CrossRef]
- Tucureanu, V.; Matei, A.; Avram, A.M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R.; Singh, G.; Kumar, A. Use of Sida cordifolia extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Environ. Chem. Eng. 2018, 6, 694–700. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Investigation of corrosion inhibition effect and adsorption activities of Cuscuta reflexa extract for mild steel in 0.5 M H2SO4. Bioelectrochemistry 2018, 124, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Prasad, D.; Haldhar, R.; Singh, G.; Kumar, A. Use of Saraca ashoka extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Mol. Liq. 2018, 258, 89–97. [Google Scholar] [CrossRef]
- Haldhar, R.; Kim, S.-C.; Prasad, D.; Bedair, M.; Bahadur, I.; Kaya, S.; Dagdag, O.; Guo, L. Papaver somniferum as an efficient corrosion inhibitor for iron alloy in acidic condition: DFT, MC simulation, LCMS and electrochemical studies. J. Mol. Struct. 2021, 1242, 130822. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Myristica fragrans extract as an eco-friendly corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. J. Environ. Chem. Eng. 2018, 6, 2290–2301. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A.; Kumar, R. Experimental and theoretical studies of Ficus religiosa as green corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. Sustain. Chem. Pharm. 2018, 9, 95–105. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bhardwaj, N. Extraction and experimental studies of Citrus aurantifolia as an economical and green corrosion inhibitor for mild steel in acidic media. J. Adhes. Sci. Technol. 2019, 33, 1169–1183. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Armoracia rusticana as sustainable and eco-friendly corrosion inhibitor for mild steel in 0.5M sulphuric acid: Experimental and theoretical investigations. J. Environ. Chem. Eng. 2018, 6, 5230–5238. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bhardwaj, N. Surface Adsorption and Corrosion Resistance Performance of Acacia concinna Pod Extract: An Efficient Inhibitor for Mild Steel in Acidic Environment. Arab. J. Sci. Eng. 2020, 45, 131–141. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A.; Kaur, A. Corrosion resistance of mild steel in 0.5 M H2SO4 solution by plant extract of Alkana tinctoria: Experimental and theoretical studies. Eur. Phys. J. Plus 2018, 133, 356. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bahadur, I.; Dagdag, O.; Berisha, A. Evaluation of Gloriosa superba seeds extract as corrosion inhibition for low carbon steel in sulfuric acidic medium: A combined experimental and computational studies. J. Mol. Liq. 2021, 323, 114958. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Mandal, N.; Benhiba, F.; Bahadur, I.; Dagdag, O. Anticorrosive properties of a green and sustainable inhibitor from leaves extract of Cannabis sativa plant: Experimental and theoretical approach. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126211. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bhardwaj, N. Experimental and Theoretical Evaluation of Acacia catechu Extract as a Natural, Economical and Effective Corrosion Inhibitor for Mild Steel in an Acidic Environment. J. Bio- Tribo-Corros. 2020, 6, 76. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Kamboj, D.; Kaya, S.; Dagdag, O.; Guo, L. Corrosion inhibition, surface adsorption and computational studies of Momordica charantia extract: A sustainable and green approach. SN Appl. Sci. 2021, 3, 25. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D. Corrosion Resistance and Surface Protective Performance of Waste Material of Eucalyptus globulus for Low Carbon Steel. J. Bio- Tribo-Corros. 2020, 6, 48. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Withania somnifera extract as green inhibitor for mild steel in 8 % H2SO4. Asian J. Chem. 2016, 28, 2471–2474. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saharan, H. Performance of Pfaffia paniculata extract towards corrosion mitigation of low-carbon steel in an acidic environment. Int. J. Ind. Chem. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Nguyen, L.T.; Kaya, S.; Bahadur, I.; Dagdag, O.; Kim, S.-C. Corrosion inhibition, surface adsorption and computational studies of Swertia chirata extract: A sustainable and green approach. Mater. Chem. Phys. 2021, 267, 124613. [Google Scholar] [CrossRef]
- Benhiba, F.; Hsissou, R.; Benzekri, Z.; Echihi, S.; El-Blilak, J.; Boukhris, S.; Bellaouchou, A.; Guenbour, A.; Oudda, H.; Warad, I.; et al. DFT/electronic scale, MD simulation and evaluation of 6-methyl-2-(p-tolyl)-1,4-dihydroquinoxaline as a potential corrosion inhibition. J. Mol. Liq. 2021, 335, 116539. [Google Scholar] [CrossRef]
- Dagdag, O.; Safi, Z.; Wazzan, N.; Erramli, H.; Guo, L.; Mkadmh, A.M.; Verma, C.; Ebenso, E.; El Gana, L.; El Harfi, A. Highly functionalized epoxy macromolecule as an anti-corrosive material for carbon steel: Computational (DFT, MDS), surface (SEM-EDS) and electrochemical (OCP, PDP, EIS) studies. J. Mol. Liq. 2020, 302, 112535. [Google Scholar] [CrossRef]
- Salman, M.; Ansari, K.R.; Srivastava, V.; Chauhan, D.S.; Haque, J.; Quraishi, M.A. Chromeno naphthyridines based heterocyclic compounds as novel acidizing corrosion inhibitors: Experimental, surface and computational study. J. Mol. Liq. 2021, 322, 114825. [Google Scholar] [CrossRef]
- Kumar, H.; Yadav, V. Highly efficient and eco-friendly acid corrosion inhibitor for mild steel: Experimental and theoretical study. J. Mol. Liq. 2021, 335, 116220. [Google Scholar] [CrossRef]
- Kumar, H.; Yadav, V.; Anu; Saha, S.K.; Kang, N. Adsorption and inhibition mechanism of efficient and environment friendly corrosion inhibitor for mild steel: Experimental and theoretical study. J. Mol. Liq. 2021, 338, 116634. [Google Scholar] [CrossRef]
- Kumar, H.; Dhanda, T. Cyclohexylamine an effective corrosion inhibitor for mild steel in 0.1 N H2SO4: Experimental and theoretical (molecular dynamics simulation and FMO) study. J. Mol. Liq. 2021, 327, 114847. [Google Scholar] [CrossRef]
- Mechbal, N.; Belghiti, M.; Benzbiria, N.; Lai, C.-H.; Kaddouri, Y.; Karzazi, Y.; Touzani, R.; Zertoubi, M. Correlation between corrosion inhibition efficiency in sulfuric acid medium and the molecular structures of two newly eco-friendly pyrazole derivatives on iron oxide surface. J. Mol. Liq. 2021, 331, 115656. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, B.; Xu, B.; Yang, B. Ambient-stable polyethyleneimine functionalized Ti3C2Tx nanohybrid corrosion inhibitor for copper in alkaline electrolyte. Mater. Lett. 2023, 337, 133979. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, Y.; Zhu, S.; Nie, Z.; Ma, H.; Liu, Q.; Li, J. First-Principles Study on the Adsorption Characteristics of Corrosive Species on Passive Film TiO2 in a NaCl Solution Containing H2S and CO2. Metals 2022, 12, 1160. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, B.; Zhao, J.; Yang, B.; Zheng, X. Benzothiazole derivatives-based supramolecular assemblies as efficient corrosion inhibitors for copper in artificial seawater: Formation, interfacial release and protective mechanisms. Corros. Dcience 2023, 212, 110957. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Prasad, D.; Haldhar, R. Study of the Aegle marmelos as a Green Corrosion Inhibitor for Mild Steel in Acidic Medium: Experimental and Theoretical Approach. J. Bio- Tribo-Corros. 2018, 4, 61. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Use of Asparagus racemosus extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Mater. Sci. 2018, 53, 8523–8535. [Google Scholar] [CrossRef]
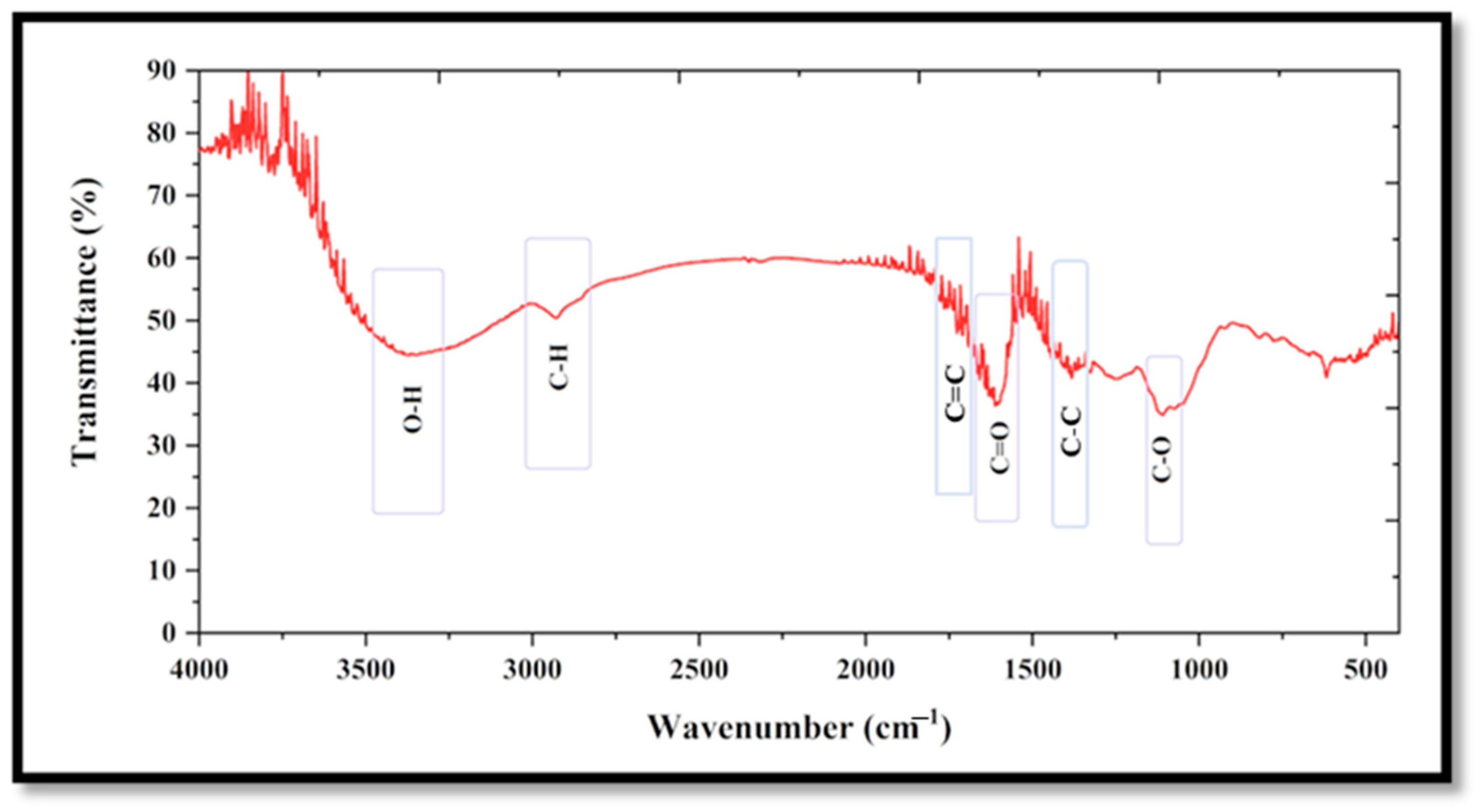


| FT-IR (Wavenumber; cm−1) | |
|---|---|
| 2931.89 | C–H stretching |
| 1118.89 | C–O stretching |
| 1610.61 | C=O stretching |
| 3389.04 | O–H stretching |
| 1670.63 | C=C stretching |
| 1305.25 | C–C stretching |
| Temperature (K) | Inhibitor Concentration C (mg/L) | Corrosion Rate (mmy−1) | Inhibition Efficiency IE (%) |
|---|---|---|---|
| Blank | 11.33 | - | |
| 100 | 3.67 | 67.55 | |
| 200 | 3.08 | 72.74 | |
| 298 | 300 | 2.42 | 78.59 |
| 400 | 1.89 | 83.27 | |
| 500 | 1.72 | 84.78 | |
| 600 | 1.47 | 86.95 | |
| Blank | 15.29 | - | |
| 100 | 5.59 | 67.61 | |
| 200 | 4.70 | 72.81 | |
| 308 | 300 | 4.07 | 78.64 |
| 400 | 3.51 | 83.31 | |
| 500 | 2.40 | 84.81 | |
| 600 | 2.39 | 87.02 | |
| Blank | 19.14 | - | |
| 100 | 7.57 | 63.44 | |
| 200 | 6.33 | 69.26 | |
| 318 | 300 | 5.75 | 73.38 |
| 400 | 4.91 | 77.04 | |
| 500 | 4.07 | 78.73 | |
| 600 | 3.88 | 79.73 |
| C (mg/L) | (mV vs. SCE) | (μA cm−2) | (mV/dec) | (mV/dec) | IE (%) |
|---|---|---|---|---|---|
| Blank | 465 | 890.90 | 141.66 | 164.25 | - |
| 100 | 239 | 251.70 | 53.00 | 106.62 | 71.74 |
| 200 | 452 | 226.20 | 62.22 | 107.00 | 74.61 |
| 300 | 625 | 144.40 | 68.28 | 106.70 | 83.79 |
| 400 | 460 | 141.80 | 45.21 | 108.45 | 84.08 |
| 500 | 469 | 103.00 | 58.96 | 122.80 | 88.44 |
| 600 | 463 | 67.06 | 34.79 | 107.40 | 92.47 |
| C (mg/L) | (Ω cm−2) | (μF cm−2) | IE (%) |
|---|---|---|---|
| Blank | 15.71 | 269.00 | - |
| 100 | 49.98 | 247.43 | 68.56 |
| 200 | 72.58 | 232.19 | 78.35 |
| 300 | 76.40 | 220.21 | 79.43 |
| 400 | 104.60 | 211.34 | 84.98 |
| 500 | 117.09 | 205.05 | 86.58 |
| 600 | 155.13 | 155.15 | 89.87 |
| Molecule | EHOMO (eV) | ELUMO (eV) | ΔE (eV) | ΔN (eV) | ΔEBack-Donation (eV) | η (eV) | σ (eV−1) | χ (eV) | π (eV) |
|---|---|---|---|---|---|---|---|---|---|
| Kaempferol (M1) | −5.36 | −2.41 | 2.94 | 0.22 | −0.36 | 1.47 | 0.67 | 3.88 | −3.88 |
| p-hydroxycinnamic acid (M2) | −5.70 | −2.51 | 3.19 | 0.27 | −0.39 | 1.59 | 0.62 | 4.11 | −4.11 |
| Systems | (kJ/mol) | (kJ/mol) |
|---|---|---|
| Fe(110)/M1 | −620.00 | 620.00 |
| Fe(110)/M2 | −545.00 | 545.00 |
| Sr. No. | Inhibitor | Optimum Concentration | Corrosive Media | IE (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Citrullus lanatus | 800 ppm | 1 M HCl | 91 | [2] |
| 2 | Eucalyptus | 800 ppm | 1 M HCl | 88 | [6] |
| 3 | Garlic | 10 cm3/L | 0.5 M H2SO4 | 88 | [10] |
| 4 | Cuscuta reflexa | 2 g/L | 1 M HCl | 81 | [27] |
| 5 | Glycyrrhiza glabra | 800 ppm | 1 M HCl | 88 | [40] |
| 6 | Lavandula mairei | 0.4 g/L | 1 M HCl | 92 | [43] |
| 7 | Lilium brownii | 200 mg/L | 0.5 M H2SO4 | 85 | [44] |
| 8 | Achyranthes aspera | 500 ppm | 0.5 M H2SO4 | 90 | [52] |
| 9 | Aegle marmelos | 500 ppm | 0.5 M H2SO4 | 83 | [85] |
| 10 | Asparagus racemosus | 100 mg/L | 0.5 M H2SO4 | 93 | [86] |
| 11 | Myristica fragrans | 500 mg/L | 0.5 M H2SO4 | 87 | [61] |
| 12 | Ficus religiosa | 500 mg/L | 0.5 M H2SO4 | 92 | [62] |
| 13 | Alkana tinctoria | 500 mg/L | 0.5 M H2SO4 | 91 | [66] |
| 14 | Pfaffia paniculate | 600 mg/L | 0.5 M H2SO4 | 88 | [73] |
| 15 | Convolvulus microphyllus | 600 mg/L | 0.5 M H2SO4 | 92 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haldhar, R.; Vanaraj, R.; Dagdag, O.; Berisha, A.; Kim, S.-C. Convolvulus microphyllus Extract as a Green, Effective, and Affordable Corrosion Inhibitor: Theoretical Calculations and Experimental Studies. Coatings 2023, 13, 860. https://doi.org/10.3390/coatings13050860
Haldhar R, Vanaraj R, Dagdag O, Berisha A, Kim S-C. Convolvulus microphyllus Extract as a Green, Effective, and Affordable Corrosion Inhibitor: Theoretical Calculations and Experimental Studies. Coatings. 2023; 13(5):860. https://doi.org/10.3390/coatings13050860
Chicago/Turabian StyleHaldhar, Rajesh, Ramkumar Vanaraj, Omar Dagdag, Avni Berisha, and Seong-Cheol Kim. 2023. "Convolvulus microphyllus Extract as a Green, Effective, and Affordable Corrosion Inhibitor: Theoretical Calculations and Experimental Studies" Coatings 13, no. 5: 860. https://doi.org/10.3390/coatings13050860
APA StyleHaldhar, R., Vanaraj, R., Dagdag, O., Berisha, A., & Kim, S.-C. (2023). Convolvulus microphyllus Extract as a Green, Effective, and Affordable Corrosion Inhibitor: Theoretical Calculations and Experimental Studies. Coatings, 13(5), 860. https://doi.org/10.3390/coatings13050860











