Migration of TiO2 from PET/TiO2 Composite Films Used for Polymer-Laminated Steel Cans in Acidic Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Initial TiO2 Content in the Composite Film
2.3. Migration of TiO2 in 4% (v/v) Acetic Acid
2.4. Soaking Process of Polymer-Laminated Steel Cans
2.5. Stripping Process of the Polymer-Laminated Steel Cans
2.6. Analysis Method
3. Results and Discussion
3.1. Method Validation
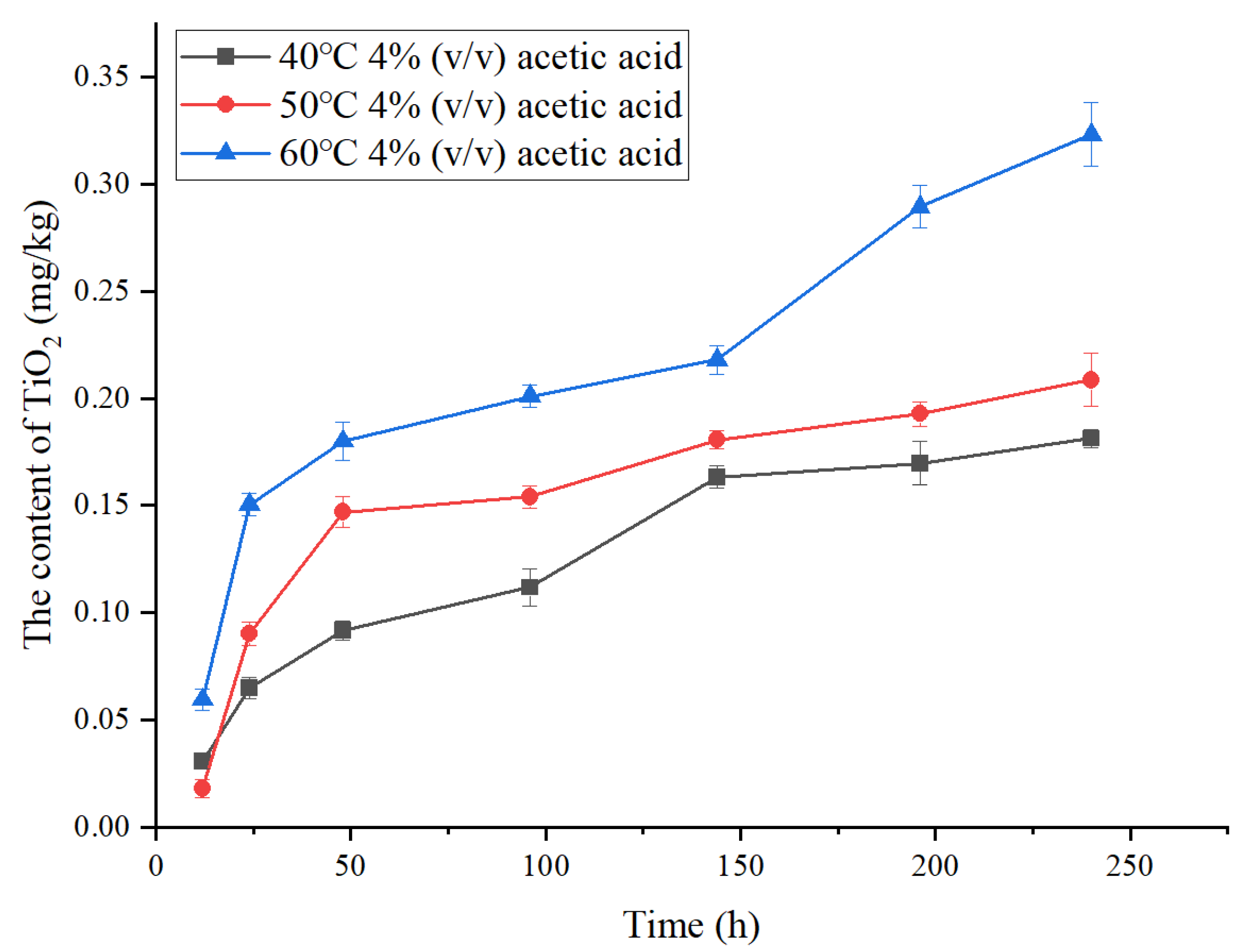
3.2. The Migration in 4% (v/v) Acetic Acid
3.3. Migration Model
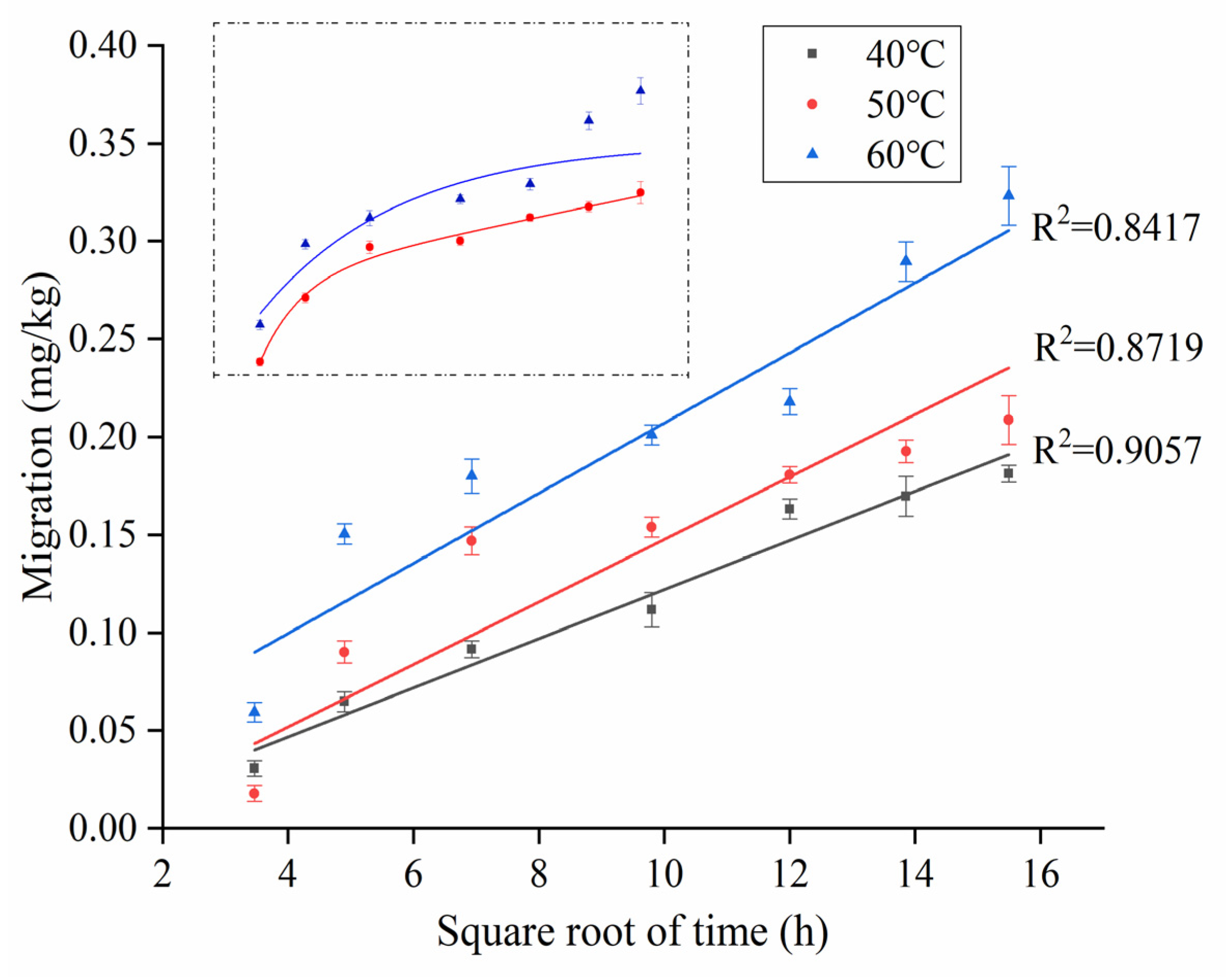
3.3.1. The Fick Law of Diffusion
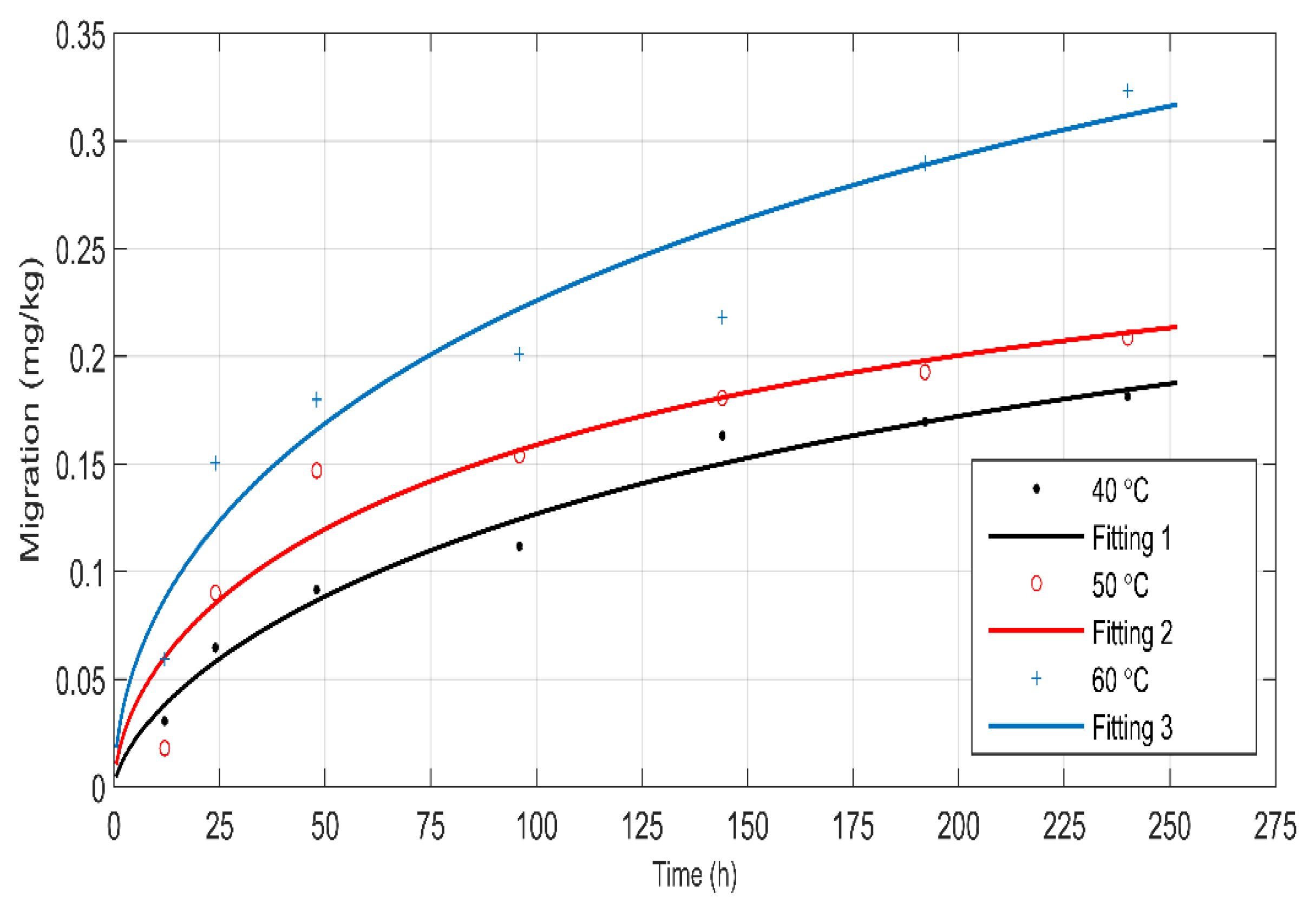
3.3.2. The Weibull Diffusion
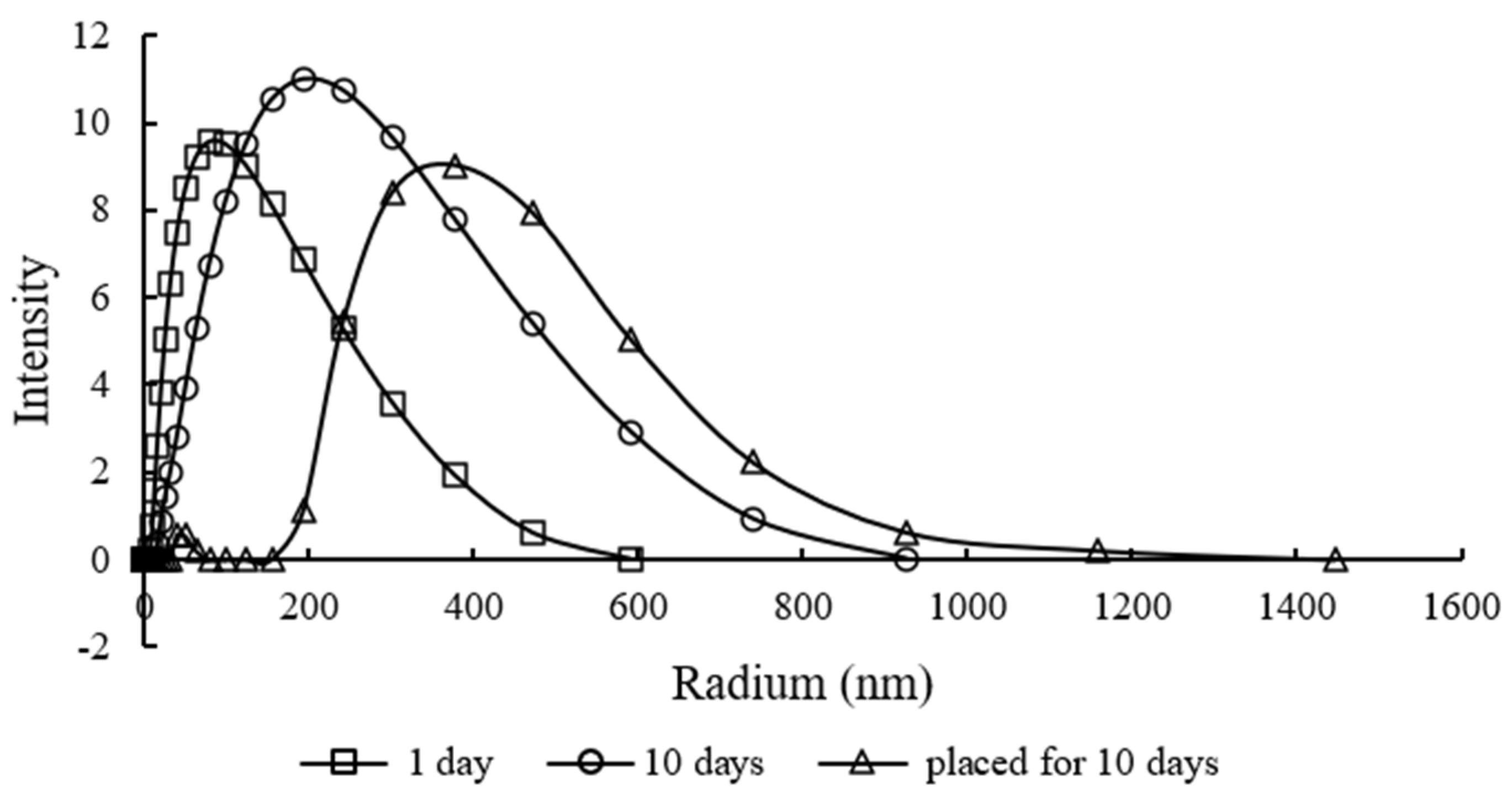
3.4. The Migration Mechanism of TiO2
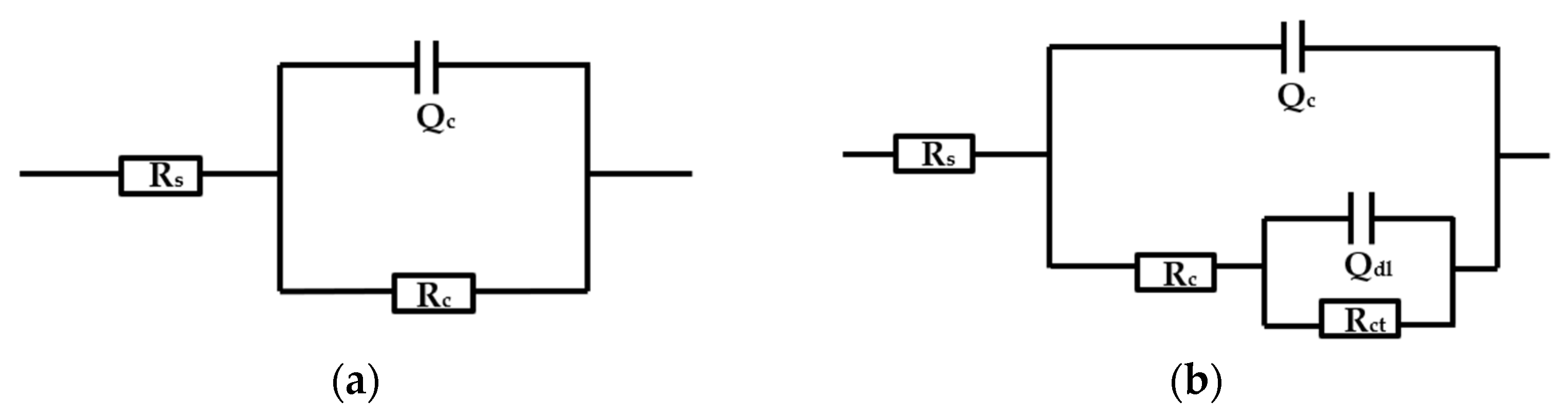
3.5. EIS Analysis
3.6. Properties Analysis of the Composite Film
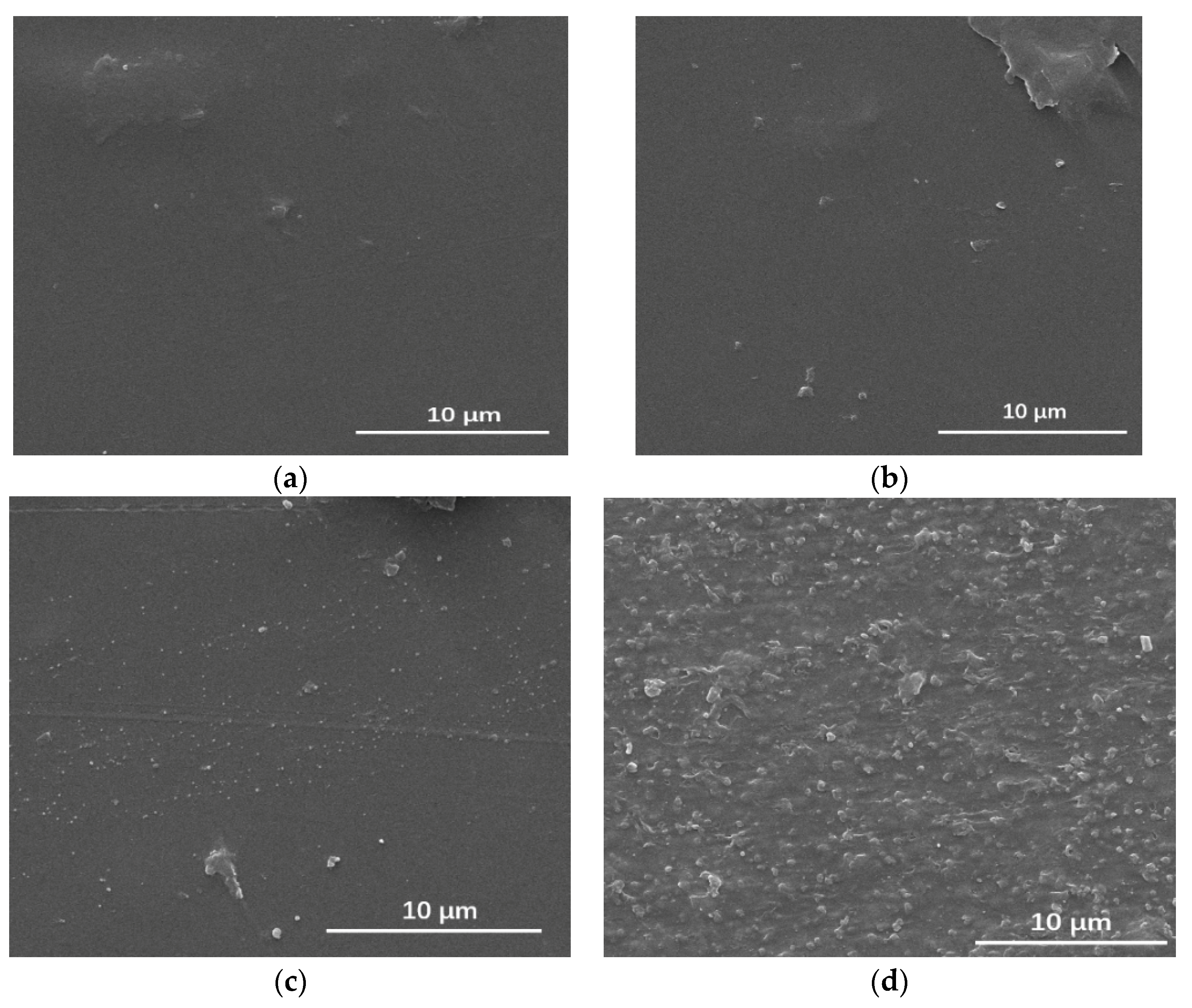
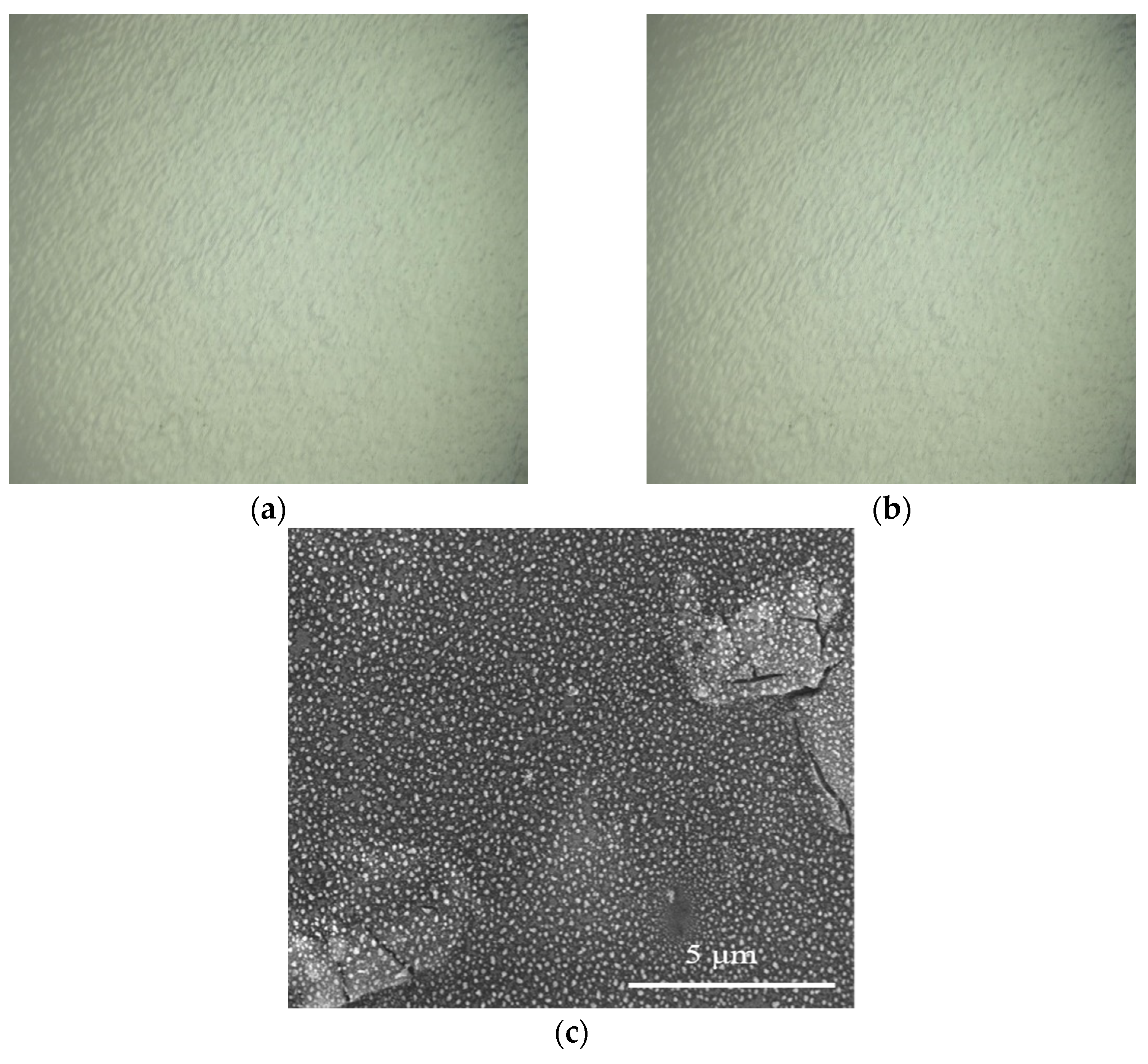
3.6.1. Surface Changes in the Composite Film
3.6.2. Cross-Sectional Structure of Polymer-Laminated Steel Cans
3.6.3. IR-ATR Results for the Composite Film
3.6.4. Thermal Properties of the PET/TiO2 Composite Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Kickelbick, G. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. [Google Scholar] [CrossRef]
- Li, D.D.; Ye, Q.Y.; Jiang, L.; Luo, Z.S. Effects of nano-TiO2-LDPE packaging on postharvest quality and antioxidant capacity of strawberry (Fragaria ananassa Duch.) stored at refrigeration temperature. J. Sci. Food Agric. 2017, 97, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Serrano, C.; Ferrer, M.; Lunsdorf, H.; Bielecki, P.; Cerrada, M.A.L.; Fernandez-Garcia, M.; Fernandez-Garcia, M. High-performance dual-action polymer-TiO2 nanocomposite films via melting processing. Nano Lett. 2007, 7, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, L.; Zhang, H.; Yuan, M.; Qin, Y. Evaluation of PLA nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese. J. Food Process. Preserv. 2018, 42, e13362. [Google Scholar] [CrossRef]
- Rim, K.-T.; Song, S.-W.; Kim, H.-Y. Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: A literature review. Saf. Health Work 2013, 4, 177–186. [Google Scholar] [CrossRef]
- Acar, M.S.; Bulut, Z.B.; Ates, A.; Nami, B.; Kocak, N.; Yildiz, B. Titanium dioxide nanoparticles induce cytotoxicity and reduce mitotic index in human amniotic fluid-derived cells. Human Exp. Toxicol. 2015, 34, 74–82. [Google Scholar] [CrossRef]
- Radziwill-Bienkowska, J.M.; Talbot, P.; Kamphuis, J.B.J.; Robert, V.; Cartier, C.; Fourquaux, I.; Lentzen, E.; Audinot, J.N.; Jamme, F.; Réfrégiers, M.; et al. Toxicity of Food-Grade TiO2 to Commensal Intestinal and Transient Food-Borne Bacteria: New Insights Using Nano-SIMS and Synchrotron UV Fluorescence Imaging. Front. Microbiol. 2018, 9, 794. [Google Scholar] [CrossRef]
- Bradley, E.L.; Castle, L.; Chaudhry, Q. Applications of nanomaterials in food packaging with a consideration of opportunities for developing countries. Trends Food Sci. Technol. 2011, 22, 604–610. [Google Scholar] [CrossRef]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Migration and exposure assessment of silver from a PVC nanocomposite. Food Chem. 2013, 139, 389–397. [Google Scholar] [CrossRef]
- Song, H.; Li, B.; Lin, Q.B.; Wu, H.J.; Chen, Y. Migration of silver from nanosilver-polyethylene composite packaging into food simulants. Food Addit. Contam. Part A-Chem. 2011, 28, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.Z.; Lin, Q.B.; Chen, C.F.; Wu, Y.M.; Wu, L.B.; Chen, X.Q.; Wang, Z.W. Effect of antioxidants and light stabilisers on silver migration from nanosilver-polyethylene composite packaging films into food simulants. Food Addit. Contam. Part A-Chem. 2015, 32, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Ntim, S.A.; Thomas, T.A.; Begley, T.H.; Noonan, G.O. Characterisation and potential migration of silver nanoparticles from commercially available polymeric food contact materials. Food Addit. Contam. Part A-Chem. 2015, 32, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, B. Critical Review of Public Health Regulations of Titanium Dioxide, a Human Food Additive. Integr. Environ. Assess. Manag. 2015, 11, 10–20. [Google Scholar] [CrossRef]
- Noonan, G.O.; Whelton, A.J.; Carlander, D.; Duncan, T.V. Measurement Methods to Evaluate Engineered Nanomaterial Release from Food Contact Materials. Compr. Rev. Food. Sci. Food Saf. 2014, 13, 679–692. [Google Scholar] [CrossRef]
- Chen, J.Y.; Dong, X.D.; Zhang, Q.; Ding, S.M. Migration of titanium dioxide from PET/TiO2 composite film for polymer-laminated steel. Food Addit. Contam. Part A-Chem. 2019, 36, 483–491. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food Text with EEA Relevance. 2011. p. 1–89. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R0010 (accessed on 1 January 2023).
- GB31604.1-2015; National Standards for Food Safety General Rules for Migration Testing of Food Contact Materials and Products. National Standards of China: Beijing, China, 2015; pp. 1–13.
- Yoshioka, T.; Motoki, T.; Okuwaki, A. Kinetics of hydrolysis of poly(ethylene terephthalate) powder in sulfuric acid by a modified shrinking-core model. Ind. Eng. Chem. Res. 2001, 40, 75–79. [Google Scholar] [CrossRef]
- Zhao, Y. Corrosion Behavior of Metal Materials in Acetic Acid. World Nonferrous Met. 2019, 532, 290–292. [Google Scholar]
- Lu, W.W.; Jiang, K.; Chu, Z.Z.; Yuan, M.L.; Tang, Z.Y.; Qin, Y.Y. Changes of thermal properties and microstructure of nano-ZnO/polylactic acid composite films during Zn migration. Packag. Technol. Sci. 2021, 34, 3–10. [Google Scholar] [CrossRef]
- Maiti, M.; Bhowmick, A.K. Effect of polymer-clay interaction on solvent transport behavior of fluoroelastomer-clay nanocomposites and prediction of aspect ratio of nanoclay. J. Appl. Polym. Sci. 2007, 105, 435–445. [Google Scholar] [CrossRef]
- Lin, Q.B.; Li, H.; Zhong, H.N.; Zhao, Q.; Xiao, D.H.; Wang, Z.W. Migration of Ti from nano-TiO2-polyethylene composite packaging into food simulants. Food Addit. Contam. Part A-Chem. 2014, 31, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Galic, K.; Cikovic, N. Permeability characterisation of solvent treated polymer materials. Polym. Test. 2001, 20, 599–606. [Google Scholar] [CrossRef]
- Khajavi, M.Z.; Mohammadi, R.; Ahmadi, S.; Farhoodi, M.; Yousefi, M. Strategies for controlling release of plastic compounds into foodstuffs based on application of nanoparticles and its potential health issues. Trends Food Sci. Technol. 2019, 90, 1–12. [Google Scholar] [CrossRef]
- Piringer, O.G. Prediction of Diffusion Coefficients in Plastic Materials. Rev. De Chim. 2008, 59, 1186–1189. [Google Scholar] [CrossRef]
- Franz, R.; Gmeiner, M.; Gruner, A.; Kemmer, D.; Welle, F. Diffusion behaviour of the acetaldehyde scavenger 2-aminobenzamide in polyethylene terephthalate for beverage bottles. Food Addit. Contam. Part A-Chem. 2016, 33, 364–372. [Google Scholar] [CrossRef]
- Bott, J.; Störmer, A.; Franz, R. A model study into the migration potential of nanoparticles from plastics nanocomposites for food contact. Food Packag. Shelf Life 2014, 2, 73–80. [Google Scholar] [CrossRef]
- Franz, R.; Bott, J.; Stormer, A. Considerations for and Guidance to Testing and Evaluating Migration/Release of Nanoparticles from Polymer Based Nanocomposites. Nanomaterials 2020, 10, 1113. [Google Scholar] [CrossRef]
- Golja, V.; Drazic, G.; Lorenzetti, M.; Vidmar, J.; Scancar, J.; Zalaznik, M.; Kalin, M.; Novak, S. Characterisation of food contact non-stick coatings containing TiO2 nanoparticles and study of their possible release into food. Food Addit. Contam. Part A-Chem. 2017, 34, 421–433. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Choi, J.; Park, H.J.; Ko, S. Zinc migration and its effect on the functionality of a low density polyethylene-ZnO nanocomposite film. Food Packag. Shelf Life 2019, 20, 100301. [Google Scholar] [CrossRef]
- Vadasz, P. Heat conduction in nanofluid suspensions. J. Heat Transf.-Trans. ASME 2006, 128, 465–477. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, K.S.; Jang, S.P.; Lee, B.H.; Kim, J.H.; Choi, S.; Choi, C.J. Effective viscosities and thermal conductivities of aqueous nanofluids containing of Al2O3 low volume concentrations nanoparticles. Int. J. Heat Mass Transf. 2008, 51, 2651–2656. [Google Scholar] [CrossRef]
- Chu, Z.; Deng, W.; Zheng, X.; Zhou, Y.; Zhang, C.; Xu, J.; Gao, L. Corrosion Mechanism of Plasma-Sprayed Fe-Based Amorphous Coatings with High Corrosion Resistance. J. Therm. Spray Technol. 2020, 29, 1111–1118. [Google Scholar] [CrossRef]
- Huang, X.Q.; Li, N.; Wang, H.Y.; Sun, H.X.; Sun, S.S.; Zheng, H. Electrodeposited cerium film as chromate replacement for tinplate. Thin Solid Film. 2008, 516, 1037–1043. [Google Scholar] [CrossRef]
- Huang, X.Q.; Li, N. Structural characterization and properties of lanthanum film as chromate replacement for tinplate. Appl. Surf. Sci. 2007, 254, 1463–1470. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, G.A.; Qiu, Y.B.; Guo, X.P. The crevice corrosion behaviour of stainless steel in sodium chloride solution. Corros. Sci. 2011, 53, 4065–4072. [Google Scholar] [CrossRef]
- Lei, X.W.; Fu, A.Q.; Feng, Y.R.; Zhang, J.X.; Yin, C.X. Influence of HAc Concentration and Temperature on the Electrochemcial Corrosion Behavior Of Super 13Cr Stainless Steel. Corros. Prot. 2017, 38, 676–682. [Google Scholar]
- Mohan, V.L.; Nagendra, S.M.S.; Maiya, M.P. Photocatalytic degradation of gaseous toluene using self-assembled air filter based on chitosan/activated carbon/TiO2. J. Environ. Chem. Eng. 2019, 7, 103455. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, X.Y.; Hong, M.H.; Chen, J.Y. Migration characteristics of membrane components in coated iron materials for can making. Shanghai Packing 2019, 297, 51–53. [Google Scholar]
- Deivanayaki, S.; Ponnuswamy, V.; Ashokan, S.; Jayamurugan, P.; Mariappan, R. Synthesis and characterization of TiO2-doped Polyaniline nanocomposites by chemical oxidation method. Mater. Sci. Semicond. Process. 2013, 16, 554–559. [Google Scholar] [CrossRef]
- Zhang, C.J.; Cai, X.; Wang, F.M. Preparation and evaluation of the self-cleaning poly (lactic acid) (PLA) film blended with Titanium dioxide (TiO2) nano particles. Ind. Text. 2016, 67, 121–126. [Google Scholar]
- Luo, Y.B.; Li, W.D.; Wang, X.L.; Xu, D.Y.; Wang, Y.Z. Preparation and properties of nanocomposites based on poly(lactic acid) and functionalized TiO2. Acta Mater. 2009, 57, 3182–3191. [Google Scholar] [CrossRef]
- Doganay, D.; Coskun, S.; Kaynak, C.; Unalan, H.E. Electrical, mechanical and thermal properties of aligned silver nanowire/polylactide nanocomposite films. Compos. Part B-Eng. 2016, 99, 288–296. [Google Scholar] [CrossRef]
- Boiteux, G.; Boullanger, C.; Cassagnau, P.; Fulchiron, R.; Seytre, G. Influence of morphology on PTC in conducting polypropylene-silver composites. Macromol. Symp. 2006, 233, 246–253. [Google Scholar] [CrossRef]
- Li, J.P.; Li, Y.; Jiang, X.G.; Dai, J.M. Study on the Non isothermal Crystallization Performance of PET with Low TiO2 Content. China Synth. Fiber Ind. 2017, 40, 45–48. [Google Scholar]
- Wang, S.X.; Dai, J.M.; Si, H.; Wang, Y.H.; Wang, K. Study on the Effect of Stretch Setting Temperature on the Hygrothermal Degradation Performance of Polyester Film. Synth. Technol. Appl. 2018, 33, 6–11. [Google Scholar]
- Mao, X.G.; Wang, S.X.; Dai, J.M.; Li, J.P.; Si, H. The Effect of Titanium Dioxide on the Hygrothermal Aging Properties of Polyester Film. Synth. Technol. Appl. 2020, 35, 1–5. [Google Scholar]

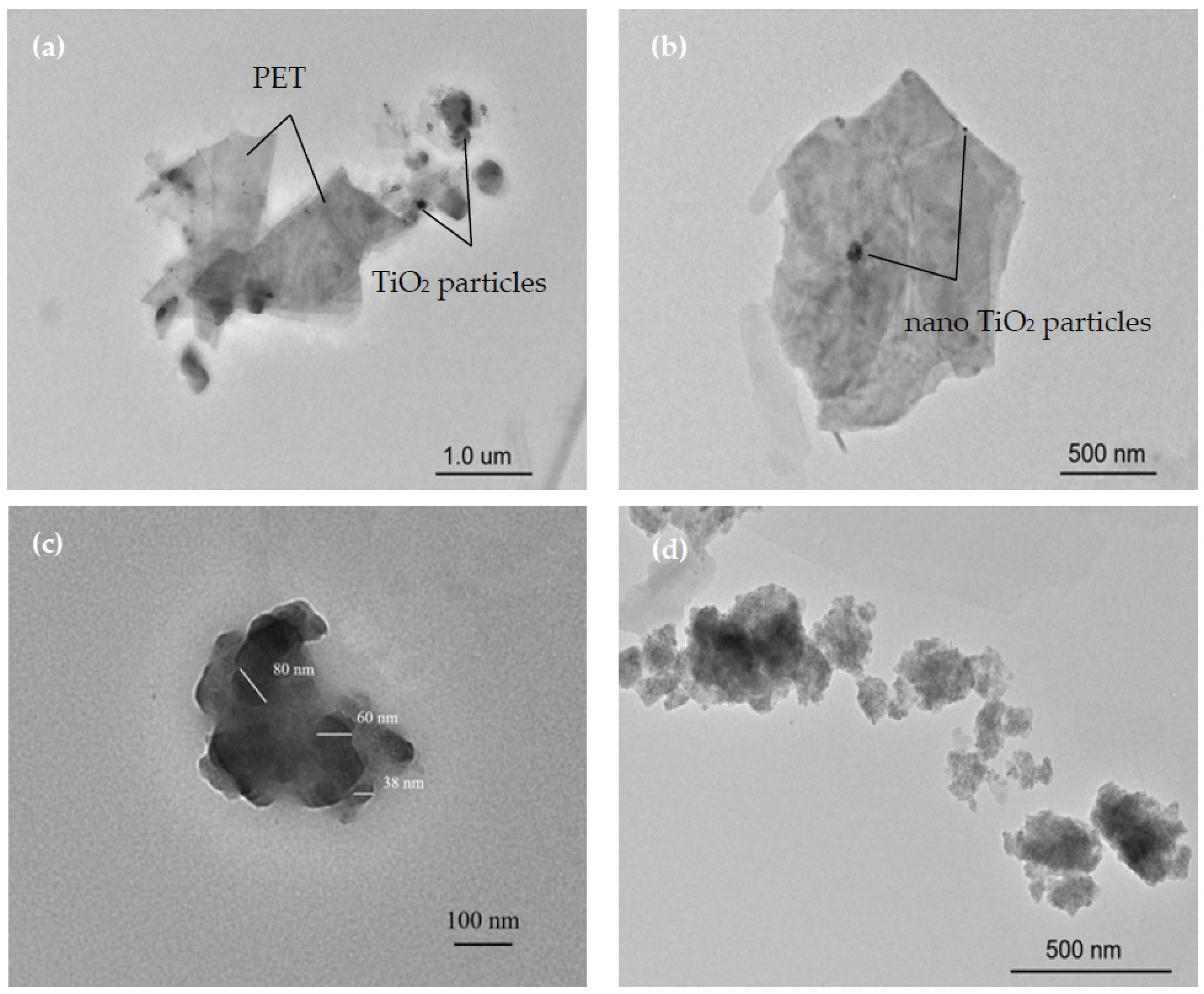


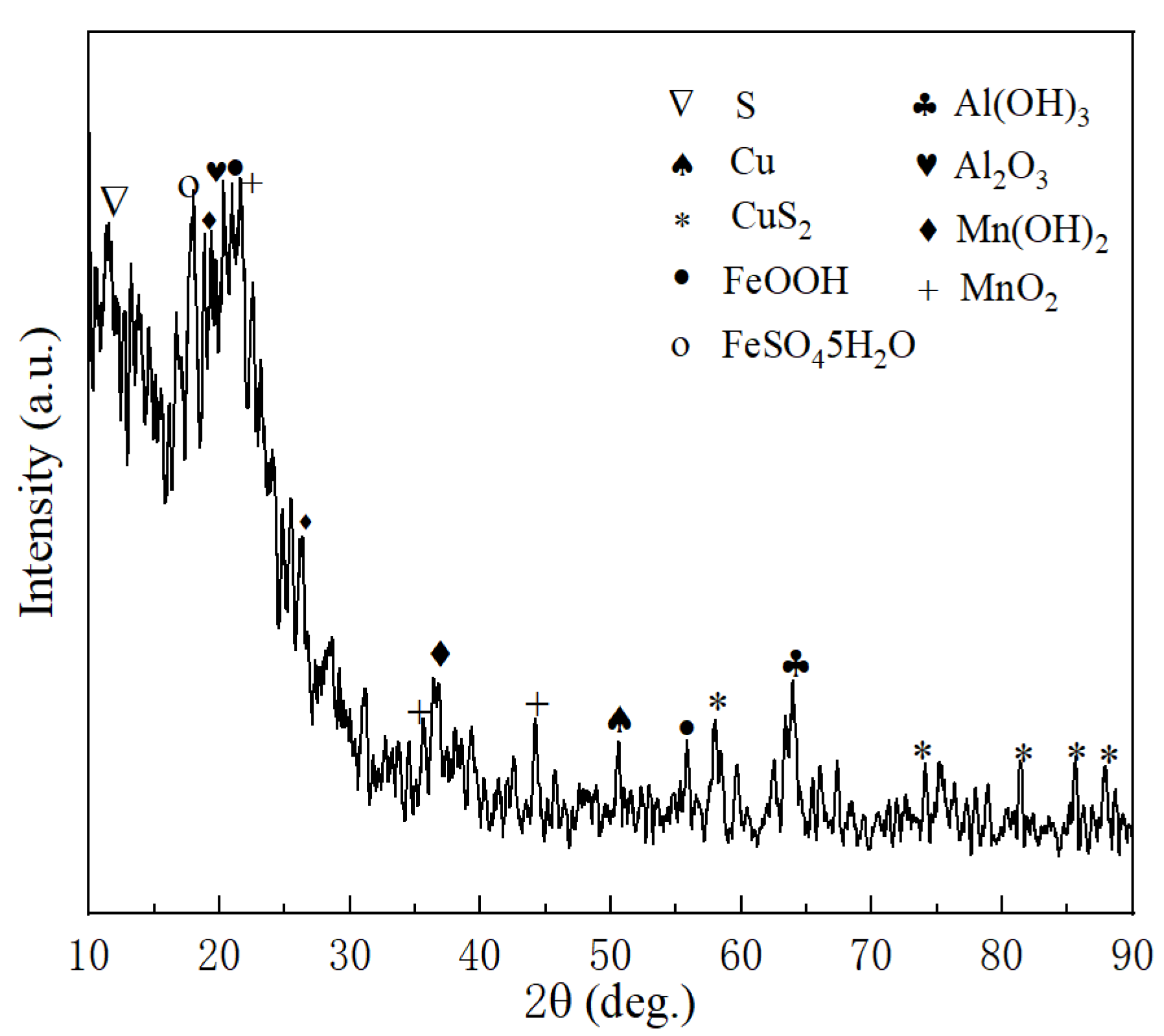
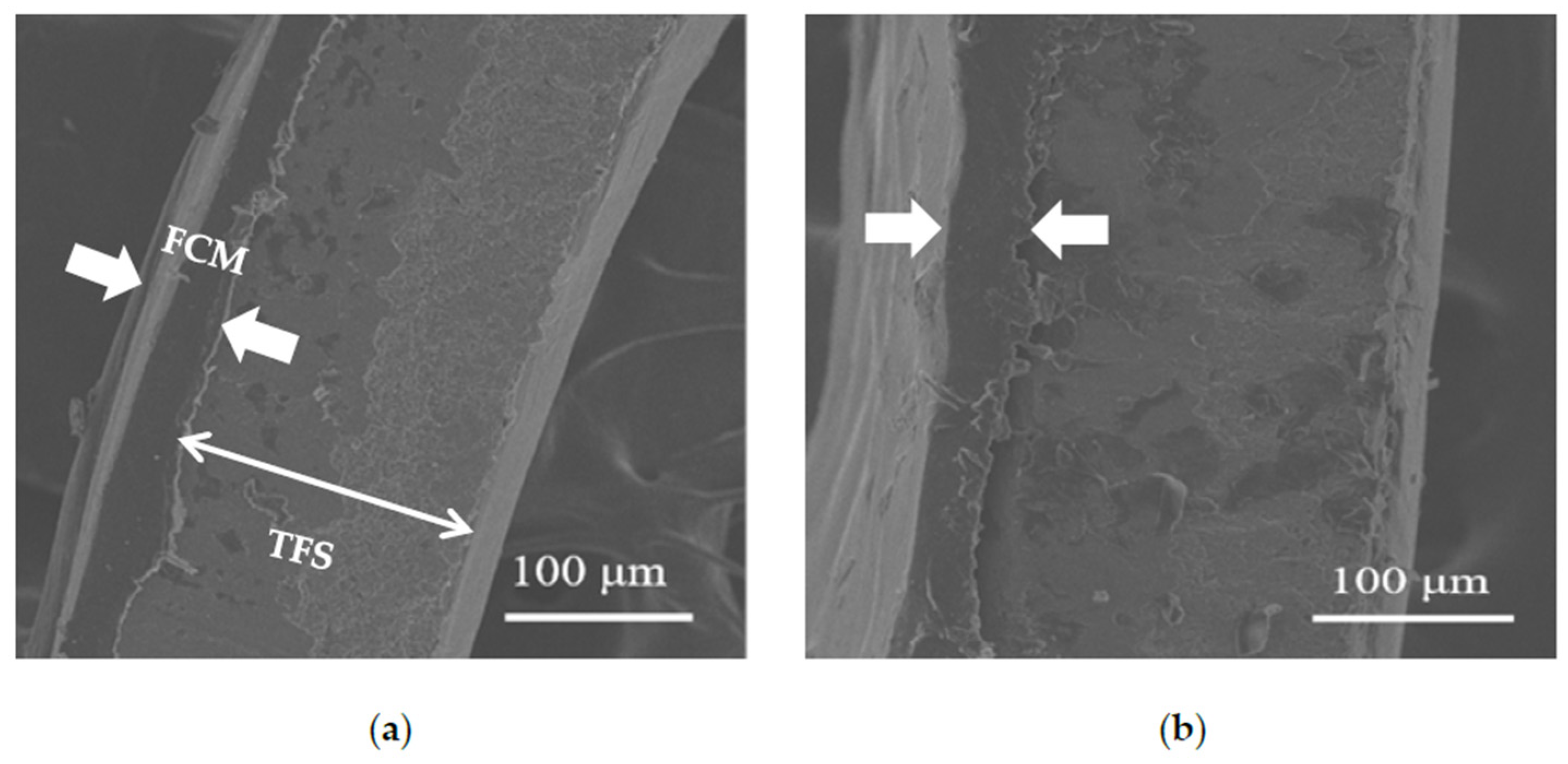
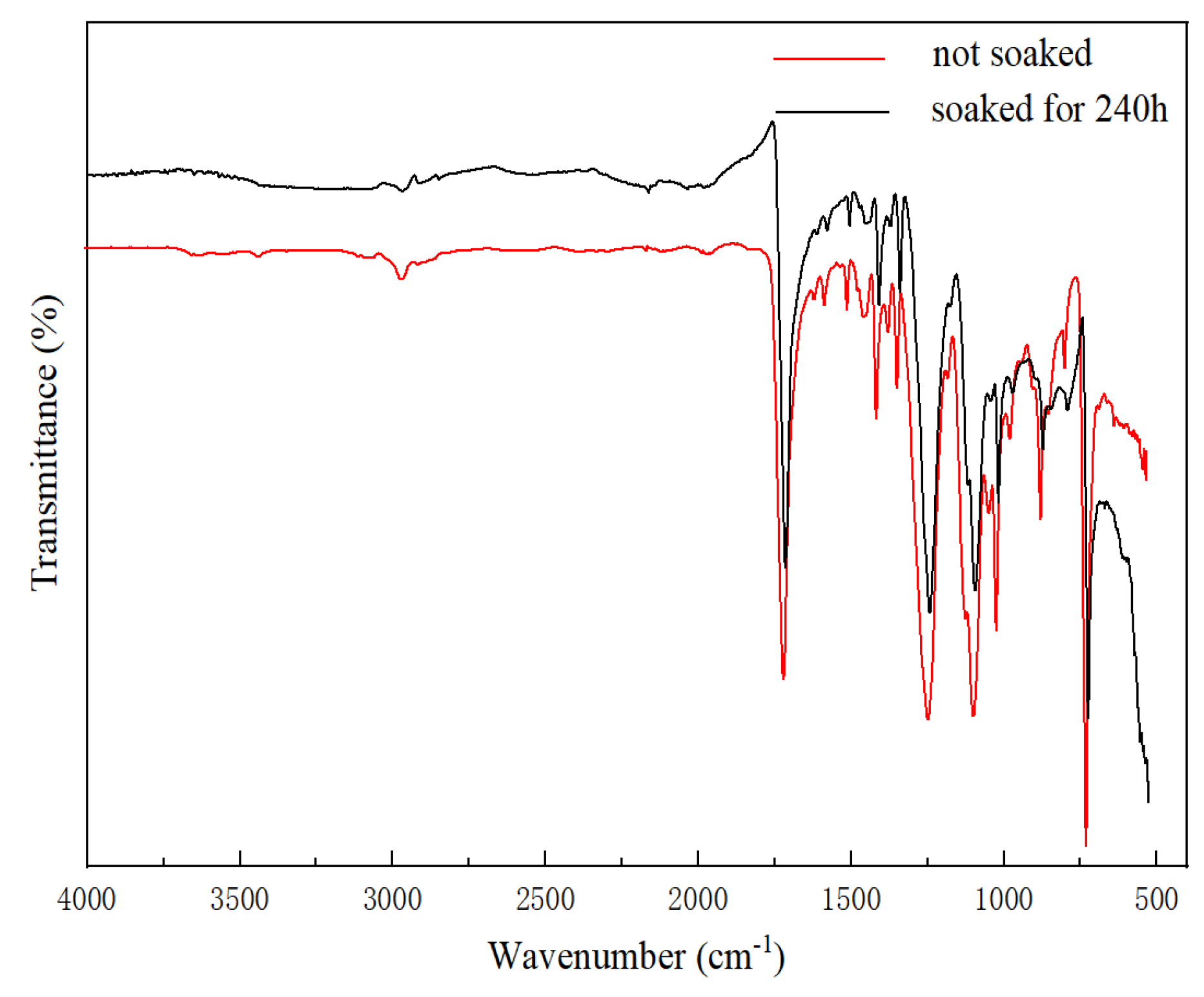
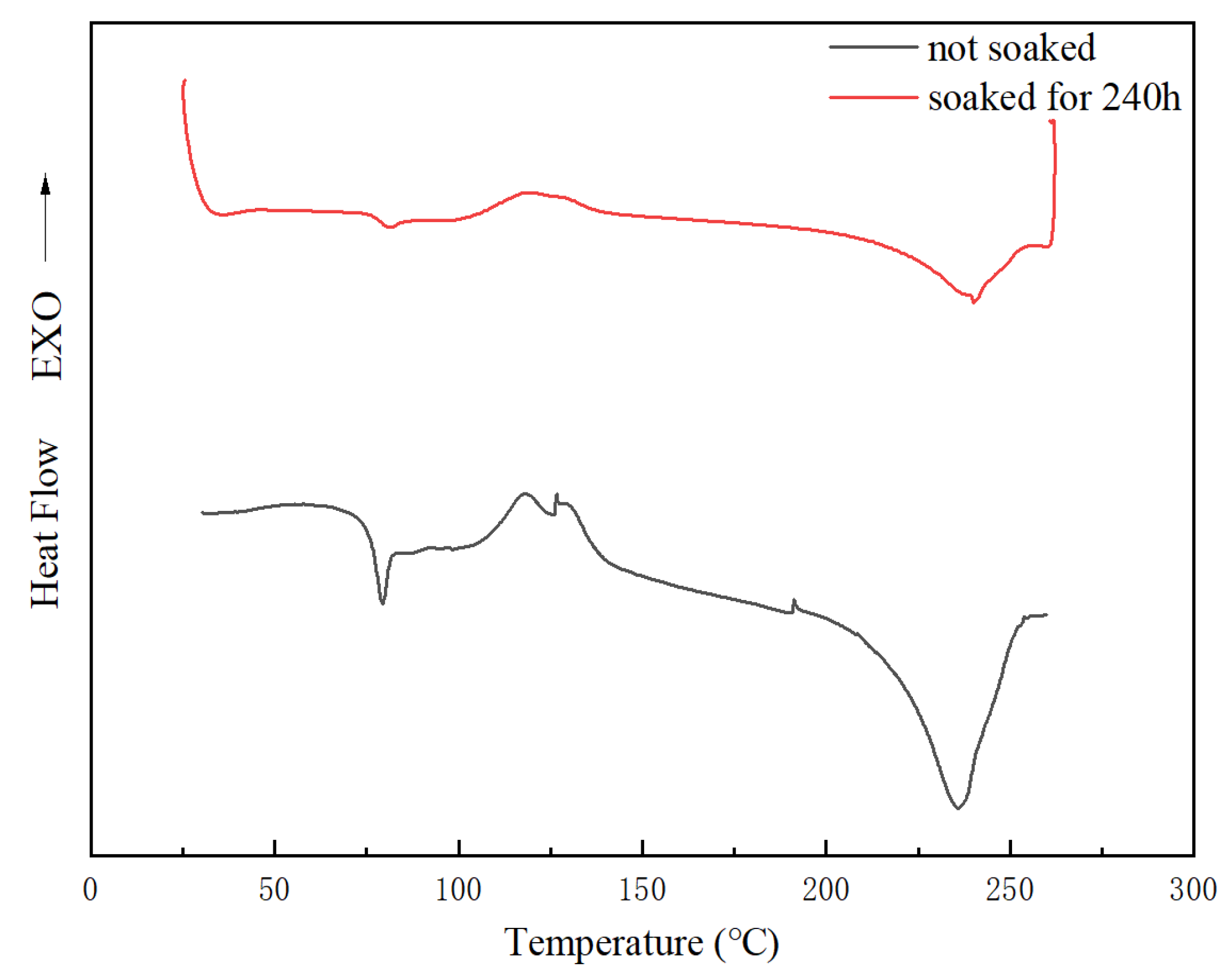
| Spiked Concentration of TiO2 | 20 μg/L | 100 μg/L | 500 μg/L |
|---|---|---|---|
| Recovery | 103 | 98 | 105 |
| RSD (%) | 1.7 | 3.5 | 2.2 |
| Temperature (°C) | τ | β | C∞ (mg/kg) | Experimental Value (mg/kg) | R2 |
|---|---|---|---|---|---|
| 40 | 204.3 | 0.67 | 0.27 | 0.18 | 0.9571 |
| 50 | 136.1 | 0.59 | 0.28 | 0.21 | 0.9069 |
| 60 | 323.2 | 0.53 | 0.54 | 0.32 | 0.9303 |
| Temperature (°C) | Parallel | Ti Ions (mg/kg) | Ti-Containing Particles (mg/kg) | Total Ti (mg/kg) |
|---|---|---|---|---|
| 60 | 1 | 0.11 | 0.21 | 0.32 |
| 2 | 0.07 | 0.20 | 0.27 | |
| 3 | 0.07 | 0.18 | 0.25 |
| Temperature (°C) | Zeta Potential (Absolute Value (mV)) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 Day | 10 Days | Placed for 10 Days | |||||||
| 60 | 5.8 | 5.5 | 9.0 | 12.5 | 10.1 | 9.5 | 5.3 | 8.0 | 7.5 |
| Time (h) | 12 | 24 | 48 | 96 | 144 | 192 | 240 |
|---|---|---|---|---|---|---|---|
| Rc (Ω·cm2) | 5.074 × 109 | 3.225 × 109 | 6.17 × 108 | 2.472 × 108 | 2.5078 × 106 | 8.1361 × 105 | 1.1056 × 104 |
| Qc (μF·cm2) | 7.832 × 10−10 | 7.598 × 10−10 | 7.642 × 10−10 | 7.696 × 10−10 | 1.022 × 10−9 | 9.29 × 10−10 | 1.586 × 10−9 |
| Rct (Ω·cm2) | - | - | - | 1.2682 × 107 | 1.3957 × 107 | 2.9846 × 105 | 4096 |
| Qdl (μF·cm2) | - | - | - | 3.0855 × 10−7 | 1.4903 × 10−8 | 3.0588 × 10−7 | 7.7898 × 10−5 |
| Composite Film | Tg (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| Prior to migration | 76.4 | 236.2 | 26.27 | 18.76 |
| After migration | 74.9 | 240.1 | 9.062 | 6.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Chen, J.; Gu, J.; Hong, X.; Hong, M.; Wu, Z.; Hu, Y. Migration of TiO2 from PET/TiO2 Composite Films Used for Polymer-Laminated Steel Cans in Acidic Solution. Coatings 2023, 13, 887. https://doi.org/10.3390/coatings13050887
Cui J, Chen J, Gu J, Hong X, Hong M, Wu Z, Hu Y. Migration of TiO2 from PET/TiO2 Composite Films Used for Polymer-Laminated Steel Cans in Acidic Solution. Coatings. 2023; 13(5):887. https://doi.org/10.3390/coatings13050887
Chicago/Turabian StyleCui, Jingying, Jinyang Chen, Jie Gu, Xiaomin Hong, Menghan Hong, Zheying Wu, and Yu Hu. 2023. "Migration of TiO2 from PET/TiO2 Composite Films Used for Polymer-Laminated Steel Cans in Acidic Solution" Coatings 13, no. 5: 887. https://doi.org/10.3390/coatings13050887
APA StyleCui, J., Chen, J., Gu, J., Hong, X., Hong, M., Wu, Z., & Hu, Y. (2023). Migration of TiO2 from PET/TiO2 Composite Films Used for Polymer-Laminated Steel Cans in Acidic Solution. Coatings, 13(5), 887. https://doi.org/10.3390/coatings13050887






