Dispersion and Demineralization Inhibition Capacity of Novel Magnesium Oxide Nanoparticles Varnish on Enamel Surfaces against Streptococcus mutans (an In Vitro Study)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ethical Approval
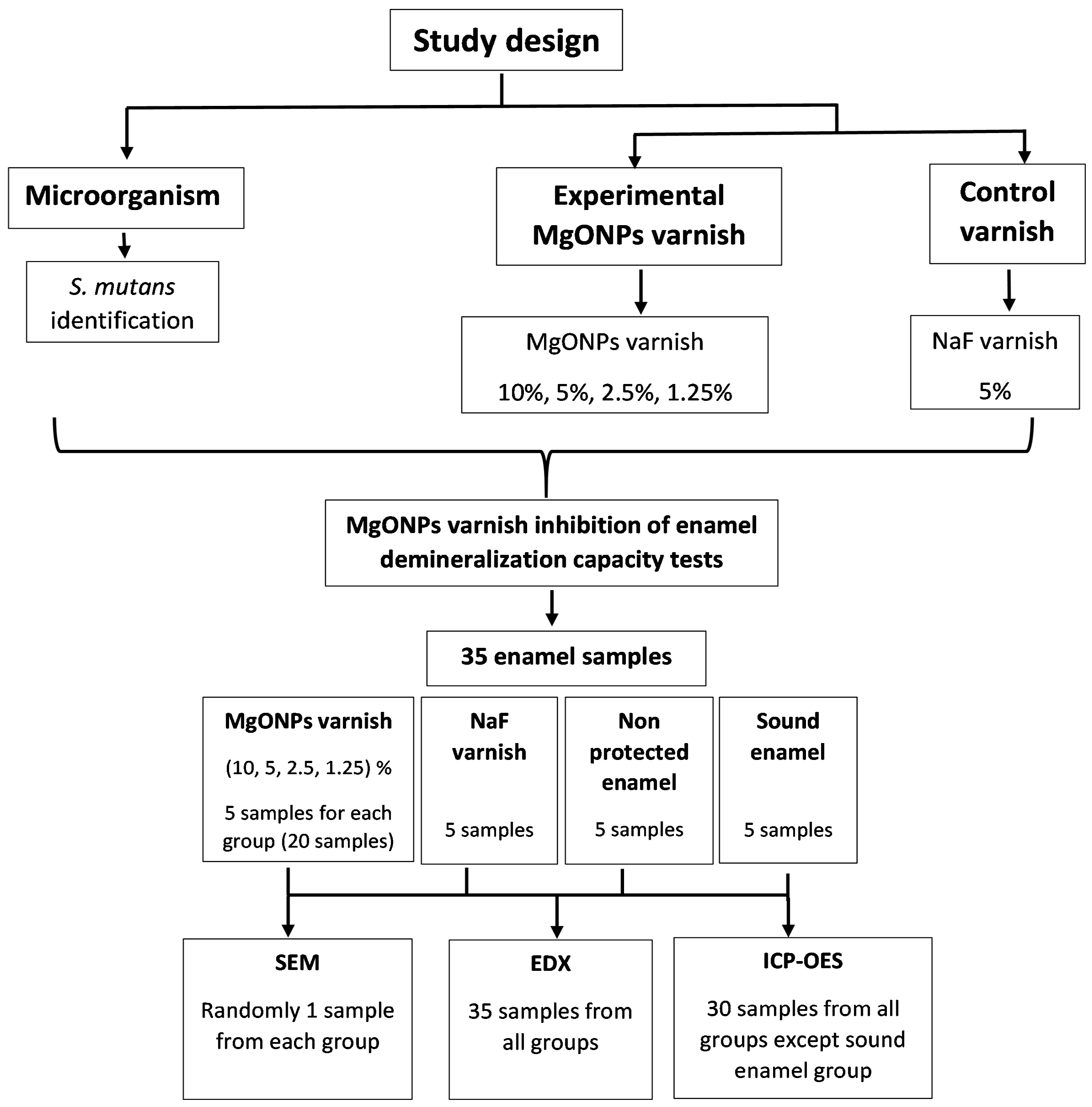
2.2.2. Study Groups
2.2.3. MgONPs Characterization
2.2.4. Experimental MgONPs Varnish Preparation
2.2.5. Microorganism
Streptococcus mutans Isolation and Identification
2.2.6. Tooth Sections Preparation
2.2.7. MgONPs Varnish Application
2.2.8. Dispersion of the MgONPs Varnish tests on the Enamel Surfaces
Scanning Electron Microscope (SEM)
Energy Dispersive X-ray Spectroscopy (EDX)
2.2.9. Enamel Demineralization Inhibition Capacity of the MgONPs Varnish Test
Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
2.3. Statistical Analysis
3. Results
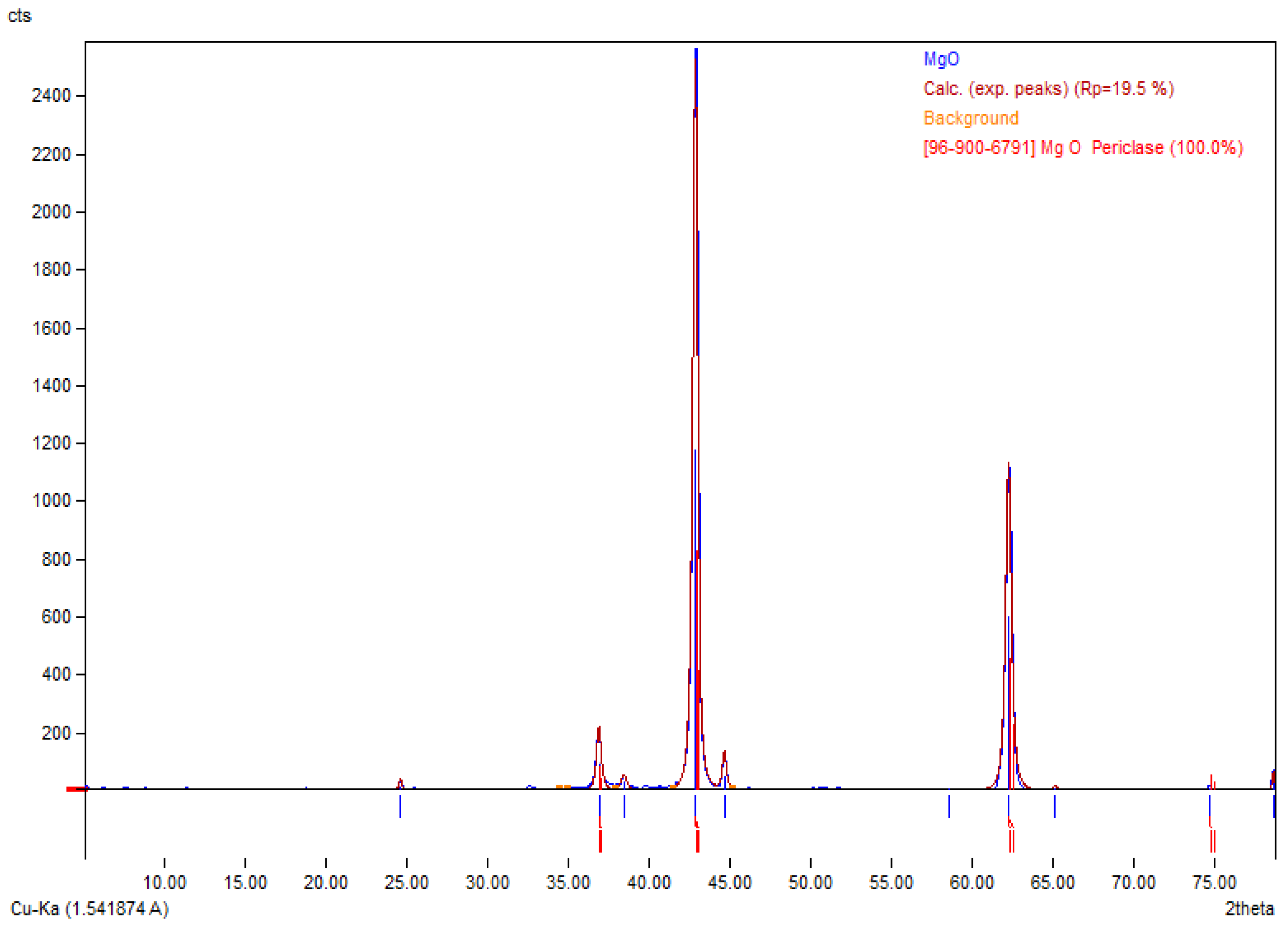
3.1. MgONPs Characterization
3.2. Microorganism
Streptococcus mutans Isolation and Identification
3.3. Dispersion of MgONPs Varnish on the Enamel Surface
3.3.1. Scanning Electron Microscope (SEM)
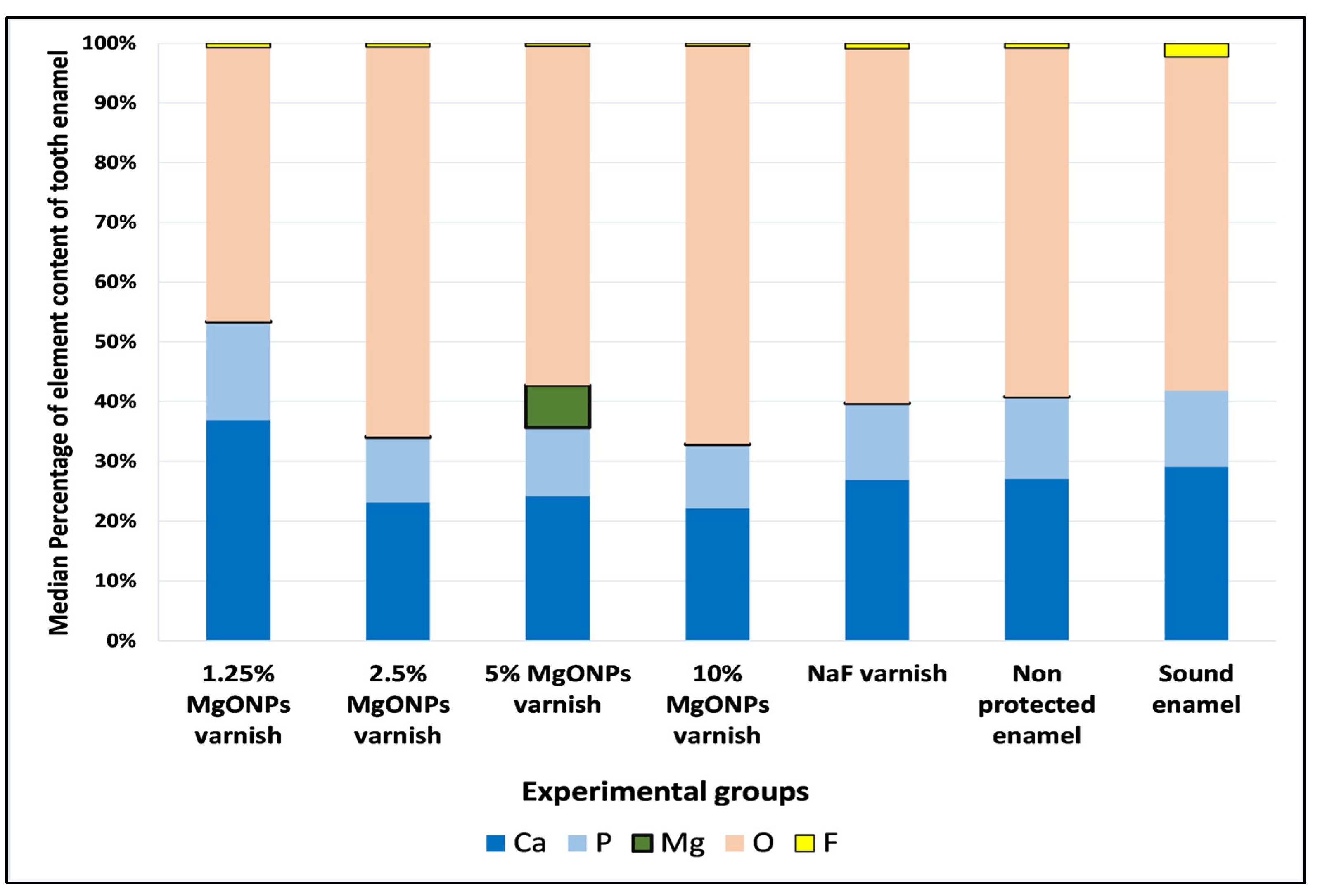
3.3.2. Energy Dispersive X-ray Spectrophotometry (EDX)
3.4. Enamel Demineralization Inhibition Capacity of the MgONPs Varnish
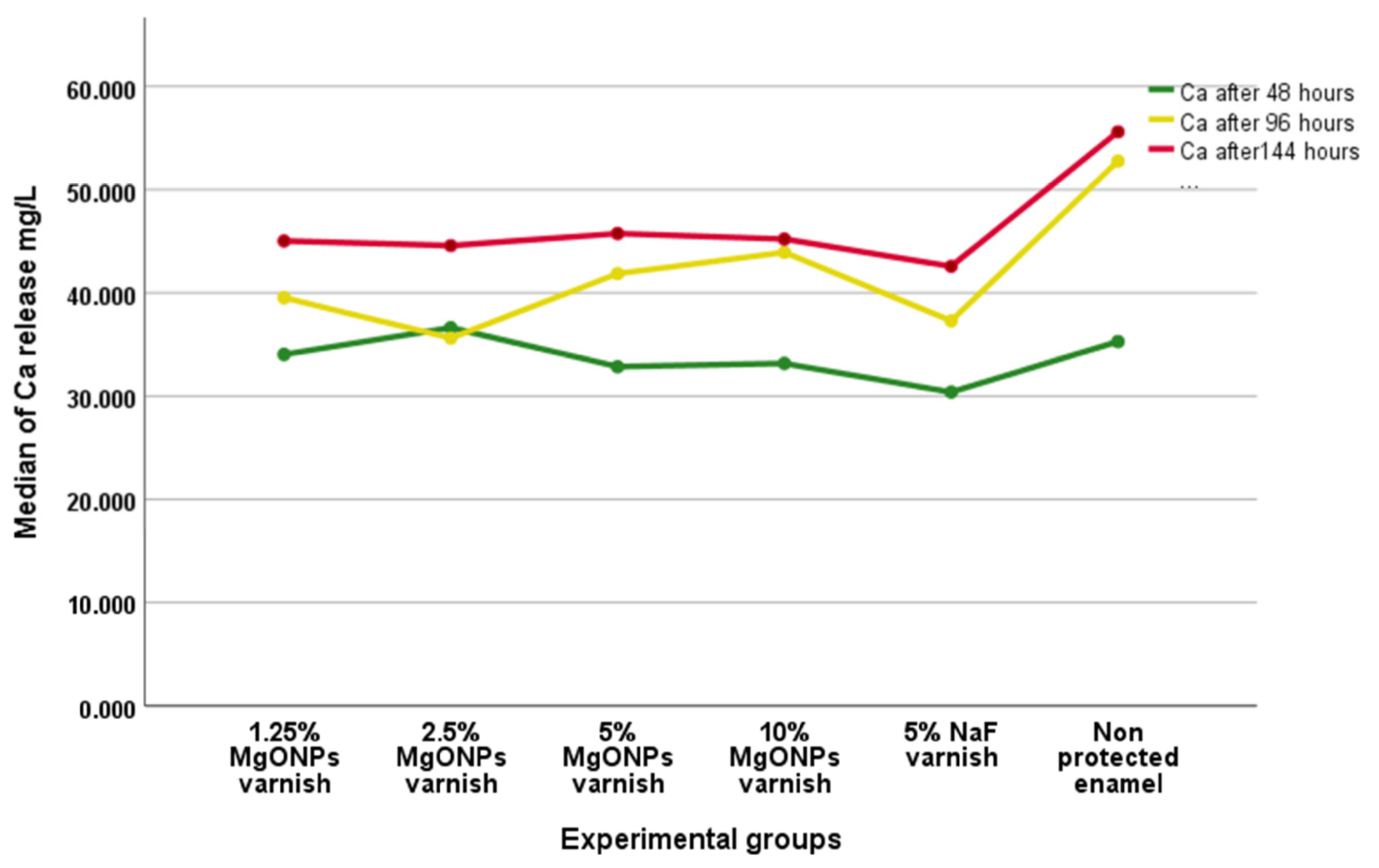
Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, I. Developmental Biology and Building a Tooth. Quintessence Int. 2003, 34, 613–620. [Google Scholar] [PubMed]
- Eimar, H.; Ghadimi, E.; Marelli, B.; Vali, H.; Nazhat, S.N.; Amin, W.M.; Torres, J.; Ciobanu, O.; Albuquerque Junior, R.F.; Tamimi, F. Regulation of Enamel Hardness by Its Crystallographic Dimensions. Acta Biomater. 2012, 8, 3400–3410. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, E.; Eimar, H.; Song, J.; Marelli, B.; Ciobanu, O.; Abdallah, M.-N.; Stähli, C.; Nazhat, S.N.; Vali, H.; Tamimi, F. Regulated Fracture in Tooth Enamel: A Nanotechnological Strategy from Nature. J. Biomech. 2014, 47, 2444–2451. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Bidlack, F.B. Tooth Enamel and Its Dynamic Protein Matrix. Int. J. Mol. Sci. 2020, 21, 4458. [Google Scholar] [CrossRef]
- Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; Arora, A.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- Wen, P.Y.F.; Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2022, 101, 392–399. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network Global Burden of Disease Study 2019. Available online: https://ghdx.healthdata.org/gbd-2019 (accessed on 20 May 2022).
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef]
- Clarke, J.K. On the Bacterial Factor in the Ætiology of Dental Caries. Br. J. Exp. Pathol. 1924, 5, 141–147. [Google Scholar]
- Loesche, W.J. Role of Streptococcus Mutans in Human Dental Decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Li, Z.-R.; Sun, J.; Du, Y.; Pan, A.; Zeng, L.; Maboudian, R.; Burne, R.A.; Qian, P.-Y.; Zhang, W. Mutanofactin Promotes Adhesion and Biofilm Formation of Cariogenic Streptococcus Mutans. Nat. Chem. Biol. 2021, 17, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Nakano, M. Role of Streptococcus Mutans Surface Proteins for Biofilm Formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Kudo, Y.; Baker, J.L.; LaBonte, S.; Jordan, P.A.; McKinnie, S.M.K.; Guo, J.; Huan, T.; Moore, B.S.; Edlund, A. Cariogenic Streptococcus Mutans Produces Tetramic Acid Strain-Specific Antibiotics That Impair Commensal Colonization. ACS Infect. Dis. 2020, 6, 563–571. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, S.M.; Dykes, G.A. Potential Mechanisms for the Effects of Tea Extracts on the Attachment, Biofilm Formation and Cell Size of Streptococcus Mutans. Biofouling 2013, 29, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular Mechanisms of Inhibiting Glucosyltransferases for Biofilm Formation in Streptococcus Mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef]
- Bawden, J.W. Fluoride Varnish: A Useful New Tool for Public Health Dentistry. J. Public Health Dent. 1998, 58, 266–269. [Google Scholar] [CrossRef]
- Kanduti, D.; Sterbenk, P.; Artnik, B. Fluoride: A Review of Use and Effects on Health. Mater. Socio-Med. 2016, 28, 133–137. [Google Scholar] [CrossRef]
- Corrie, S.R.; Thurecht, K.J. Nano-Bio Interactions: Guiding the Development of Nanoparticle Therapeutics, Diagnostics, and Imaging Agents. Pharm. Res. 2016, 33, 2311–2313. [Google Scholar] [CrossRef]
- Deyhle, H.; Bunk, O.; Buser, S.; Krastl, G.; Zitzmann, N.U.; Ilgenstein, B.; Beckmann, F.; Pfeiffer, F.; Weiger, R.; Müller, B. Bio-Inspired Dental Fillings. Biomim. Bioinspiration 2009, 7401, 74010E. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of Magnesium-Based Biomaterials and Their Applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-Based Biomaterials as Emerging Agents for Bone Repair and Regeneration: From Mechanism to Application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Bhushan, J.; Maini, C. Nanoparticles: Apromising Novel Adjunct for Dentistry. Indian J. Dent. Sci. 2019, 11, 167–173. [Google Scholar] [CrossRef]
- Yamamoto, O.; Ohira, T.; Alvarez, K.; Fukuda, M. Antibacterial Characteristics of CaCO3-MgO Composites. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 173, 208–212. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (NMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef] [PubMed]
- Arass, J.; Fadil, A. The Effect of Magnesium Oxide Nanoparticles on the Antibacterial and Antibiofilm Properties of Glass-Ionomer Cement. Heliyon 2019, 5, e02568. [Google Scholar] [CrossRef]
- Maguire, M.E.; Cowan, J.A. Magnesium Chemistry and Biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef]
- Medicom Safety Data Sheet Duraflour Varnish. Revision No. 1, Issued 23 November 2017. Available online: https://medicom.com/ (accessed on 20 May 2023).
- Shahmoradi, M.; Hunter, N.; Swain, M. Efficacy of Fluoride Varnishes with Added Calcium Phosphate in the Protection of the Structural and Mechanical Properties of Enamel. BioMed Res. Int. 2017, 2017, 7834905. [Google Scholar] [CrossRef]
- Haghgoo, R.; Saderi, H.; Eskandari, M.; Haghshenas, H.; Rezvani, M. Evaluation of the Antimicrobial Effect of Conventional and Nanosilver-Containing Varnishes on Oral Streptococci. J. Dent. 2014, 15, 57–62. [Google Scholar]
- Kis, V.K.; Sulyok, A.; Hegedűs, M.; Kovács, I.; Rózsa, N.; Kovács, Z. Magnesium Incorporation into Primary Dental Enamel and Its Effect on Mechanical Properties. Acta Biomater. 2021, 120, 104–115. [Google Scholar] [CrossRef]
- Pradhan, S.; Hedberg, J.; Blomberg, E.; Wold, S.; Odnevall Wallinder, I. Effect of Sonication on Particle Dispersion, Administered Dose and Metal Release of Non-Functionalized, Non-Inert Metal Nanoparticles. J. Nanopart. Res. 2016, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.; Seow, W.; Walsh, L.; Bird, P. Comparison of Five Selective Media for the Growth and Enumeration of Streptococcus Mutans. Aust. Dent. J. 2002, 47, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Villhauer, A.L.; Lynch, D.J.; Drake, D.R. Improved Method for Rapid and Accurate Isolation and Identification of Streptococcus Mutans and Streptococcus Sobrinus from Human Plaque Samples. J. Microbiol. Methods 2017, 139, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Oho, T.; Yamashita, Y.; Shimazaki, Y.; Kushiyama, M.; Koga, T. Simple and Rapid Detection of Streptococcus Mutans and Streptococcus Sobrinus in Human Saliva by Polymerase Chain Reaction. Oral Microbiol. Immunol. 2000, 15, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Van Loveren, C.; Buijs, J.F.; Buijs, M.J.; ten Cate, J.M. Protection of Bovine Enamel and Dentine by Chlorhexidine and Fluoride Varnishes in a Bacterial Demineralization Model. Caries Res. 1996, 30, 45–51. [Google Scholar] [CrossRef]
- Wassel, M.O.; Khattab, M.A. Antibacterial Activity against Streptococcus Mutans and Inhibition of Bacterial Induced Enamel Demineralization of Propolis, Miswak, and Chitosan Nanoparticles Based Dental Varnishes. J. Adv. Res. 2017, 8, 387–392. [Google Scholar] [CrossRef]
- Vallapragada, V.V.; Inti, G.; Ramulu, J.S. A Validated Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) Method to Estimate Free Calcium and Phosphorus in In Vitro Phosphate Binding Study of Eliphos Tablets. Am. J. Anal. Chem. 2011, 2, 718–725. [Google Scholar] [CrossRef]
- Hou, X.; Amais, R.S.; Jones, B.T.; Donati, G.L. Inductively Coupled Plasma Optical Emission Spectrometry. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 1–29. ISBN 978-0-470-02731-8. [Google Scholar]
- Roberts, W.E.; Mangum, J.E.; Schneider, P.M. Pathophysiology of Demineralization, Part II: Enamel White Spots, Cavitated Caries, and Bone Infection. Curr. Osteoporos. Rep. 2022, 20, 106–119. [Google Scholar] [CrossRef]
- Vidic, J.; Stankic, S.; Haque, F.; Ciric, D.; Le Goffic, R.; Vidy, A.; Jupille, J.; Delmas, B. Selective Antibacterial Effects of Mixed ZnMgO Nanoparticles. J. Nanoparticle Res. 2013, 15, 1595. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.-P.; Vega-Jiménez, A.L.; Vázquez-Olmos, A.R.; Ortega-Maldonado, M.; Ximenez-Fyvie, L.-A. Antibacterial Properties In Vitro of Magnesium Oxide Nanoparticles for Dental Applications. Nanomaterials 2023, 13, 502. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Pessan, J.P.; Honório, H.M.; ten Cate, J.M. Mechanisms of Action of Fluoride for Caries Control. Monogr Oral Sci. 2011, 22, 97–114. [Google Scholar] [PubMed]
- Gupta, A.; Sharda, S.; Nishant; Shafiq, N.; Kumar, A.; Goyal, A. Topical Fluoride-Antibacterial Agent Combined Therapy versus Topical Fluoride Monotherapy in Preventing Dental Caries: A Systematic Review and Meta-Analysis. Eur. Arch. Paediatr. Dent. 2020, 21, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mier, E.A. Fluoride: Its Metabolism, Toxicity, and Role in Dental Health. J. Evid.-Based Complement. Altern. Med. 2012, 17, 28–32. [Google Scholar] [CrossRef]
- Ullah, R.; Zafar, M.S.; Shahani, N. Potential Fluoride Toxicity from Oral Medicaments: A Review. Iran J. Basic Med. Sci. 2017, 20, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, L.; Amini, A.; Bashir Bahati, A.; Rahimi, G. In Vitro Antibacterial Biofilm Effect of Magnesium Oxide Nanoparticles on Streptococcus Mutans. Micro Nano Biomed. 2016, 1, 21–27. [Google Scholar] [CrossRef]
- Trayler, R.B.; Kohn, M.J. Tooth Enamel Maturation Reequilibrates Oxygen Isotope Compositions and Supports Simple Sampling Methods. Geochim. Cosmochim. Acta 2017, 198, 32–47. [Google Scholar] [CrossRef]
- Abdallah, M.-N.; Eimar, H.; Bassett, D.C.; Schnabel, M.; Ciobanu, O.; Nelea, V.; McKee, M.D.; Cerruti, M.; Tamimi, F. Diagenesis-Inspired Reaction of Magnesium Ions with Surface Enamel Mineral Modifies Properties of Human Teeth. Acta Biomater. 2016, 37, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Lale, S.; Solak, H.; Hınçal, E.; Vahdettin, L. In Vitro Comparison of Fluoride, Magnesium, and Calcium Phosphate Materials on Prevention of White Spot Lesions around Orthodontic Brackets. BioMed Res. Int. 2020, 2020, 1989817. [Google Scholar] [CrossRef]





| Varnish | Component | ||||
|---|---|---|---|---|---|
| Concentration | Fluoride (g) | Nanoparticle (g) | Hydrogenated Rosin (g) | Ethanol (mL) | |
| MgONPs varnish | 10% | - | 10 | 10 | 80 |
| 5% | - | 5 | 10 | 85 | |
| 2.5% | - | 2.5 | 10 | 87.5 | |
| 1.25% | - | 1.25 | 10 | 88.75 | |
| NaF varnish (Duraflour) [29] | 5% | 1–10 | - | 50–70 | 10–30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamalaw, S.J.; Kareem, F.A.; Noori, A.J. Dispersion and Demineralization Inhibition Capacity of Novel Magnesium Oxide Nanoparticles Varnish on Enamel Surfaces against Streptococcus mutans (an In Vitro Study). Coatings 2023, 13, 1018. https://doi.org/10.3390/coatings13061018
Hamalaw SJ, Kareem FA, Noori AJ. Dispersion and Demineralization Inhibition Capacity of Novel Magnesium Oxide Nanoparticles Varnish on Enamel Surfaces against Streptococcus mutans (an In Vitro Study). Coatings. 2023; 13(6):1018. https://doi.org/10.3390/coatings13061018
Chicago/Turabian StyleHamalaw, Sonya Jamal, Fadil Abdulla Kareem, and Arass Jalal Noori. 2023. "Dispersion and Demineralization Inhibition Capacity of Novel Magnesium Oxide Nanoparticles Varnish on Enamel Surfaces against Streptococcus mutans (an In Vitro Study)" Coatings 13, no. 6: 1018. https://doi.org/10.3390/coatings13061018







