Adsorption and Surface Diffusion of Atomic Ru on TiN and SiO2: A First-Principles Study
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, M.H.; Shue, W.S. The Overview of Current Interconnect Technology Challenges and Future Opportunities. In Proceedings of the 2020 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 12–18 December 2020; pp. 32.1.1–32.1.4. [Google Scholar]
- Baklanov, M.R.; Adelmann, C.; Zhao, L.; Gendt, S.D. Advanced Interconnects: Materials, Processing, and Reliability. ECS J. Solid State Sci. Technol. 2014, 4, Y1. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent Advances in Barrier Layer of Cu Interconnects. Materials 2020, 13, 5049. [Google Scholar] [CrossRef]
- Adelmann, C.; Sankaran, K.; Dutta, S.; Gupta, A.; Kundu, S.; Jamieson, G.; Moors, K.; Pinna, N.; Ciofi, I.; Van Eishocht, S.; et al. Alternative Metals: From Ab Initio Screening to Calibrated Narrow Line Models. In Proceedings of the 2018 IEEE International Interconnect Technology Conference (IITC), Santa Clara, CA, USA, 4–7 June 2018; pp. 154–156. [Google Scholar]
- Gall, D. The Search for the Most Conductive Metal for Narrow Interconnect Lines. J. Appl. Phys. 2020, 127, 050901. [Google Scholar] [CrossRef]
- Dutta, S.; Moors, K.; Vandemaele, M.; Adelmann, C. Finite Size Effects in Highly Scaled Ruthenium Interconnects. IEEE Electron Device Lett. 2018, 39, 268–271. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Merkx, M.J.M.; Kessels, W.M.M. From the Bottom-Up: Toward Area-Selective Atomic Layer Deposition with High Selectivity. Chem. Mater. 2019, 31, 2–12. [Google Scholar] [CrossRef]
- Parsons, G.N.; Clark, R.D. Area-Selective Deposition: Fundamentals, Applications, and Future Outlook. Chem. Mater. 2020, 32, 4920–4953. [Google Scholar] [CrossRef]
- Kim, S.K.; Han, J.H.; Kim, G.H.; Hwang, C.S. Investigation on the Growth Initiation of Ru Thin Films by Atomic Layer Deposition. Chem. Mater. 2010, 22, 2850–2856. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, S.W.; Kim, S.K.; Han, S.; Lee, W.; Hwang, C.S.; Dussarat, C.; Gatineau, J. Study on Initial Growth Behavior of RuO2 Film Grown by Pulsed Chemical Vapor Deposition: Effects of Substrate and Reactant Feeding Time. Chem. Mater. 2012, 24, 1407–1414. [Google Scholar] [CrossRef]
- Jung, H.J.; Han, J.H.; Jung, E.A.; Park, B.K.; Hwang, J.-H.; Son, S.U.; Kim, C.G.; Chung, T.-M.; An, K.-S. Atomic Layer Deposition of Ruthenium and Ruthenium Oxide Thin Films from a Zero-Valent (1,5-Hexadiene)(1-Isopropyl-4-Methylbenzene)Ruthenium Complex and O2. Chem. Mater. 2014, 26, 7083–7090. [Google Scholar] [CrossRef]
- Popovici, M.; Groven, B.; Marcoen, K.; Phung, Q.M.; Dutta, S.; Swerts, J.; Meersschaut, J.; van den Berg, J.A.; Franquet, A.; Moussa, A.; et al. Atomic Layer Deposition of Ruthenium Thin Films from (Ethylbenzyl) (1-Ethyl-1,4-Cyclohexadienyl) Ru: Process Characteristics, Surface Chemistry, and Film Properties. Chem. Mater. 2017, 29, 4654–4666. [Google Scholar] [CrossRef]
- Hwang, J.M.; Han, S.-M.; Yang, H.; Yeo, S.; Lee, S.-H.; Park, C.W.; Kim, G.H.; Park, B.K.; Byun, Y.; Eom, T.; et al. Atomic Layer Deposition of a Ruthenium Thin Film Using a Precursor with Enhanced Reactivity. J. Mater. Chem. C 2021, 9, 3820–3825. [Google Scholar] [CrossRef]
- Claessens, N.; Khan, Z.Z.; Haghighi, N.R.; Delabie, A.; Vantomme, A.; Vandervorst, W.; Meersschaut, J. Quantification of Area-Selective Deposition on Nanometer-Scale Patterns Using Rutherford Backscattering Spectrometry. Sci. Rep. 2022, 12, 17770. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.-S.; Lee, D.-J.; Kim, K.-S.; Kim, S.-H.; Yoon, T.-S.; Kim, K.-B. Nucleation Kinetics of Ru on Silicon Oxide and Silicon Nitride Surfaces Deposited by Atomic Layer Deposition. J. Appl. Phys. 2008, 103, 113509. [Google Scholar] [CrossRef]
- Soethoudt, J.; Grillo, F.; Marques, E.A.; van Ommen, J.R.; Tomczak, Y.; Nyns, L.; Van Elshocht, S.; Delabie, A. Diffusion-Mediated Growth and Size-Dependent Nanoparticle Reactivity during Ruthenium Atomic Layer Deposition on Dielectric Substrates. Adv. Mater. Interfaces 2018, 5, 1800870. [Google Scholar] [CrossRef]
- Soethoudt, J.; Hody, H.; Spampinato, V.; Franquet, A.; Briggs, B.; Chan, B.T.; Delabie, A. Defect Mitigation in Area-Selective Atomic Layer Deposition of Ruthenium on Titanium Nitride/Dielectric Nanopatterns. Adv. Mater. Interfaces 2019, 6, 1900896. [Google Scholar] [CrossRef]
- Grillo, F.; Soethoudt, J.; Marques, E.A.; de Martín, L.; Van Dongen, K.; van Ommen, J.R.; Delabie, A. Area-Selective Deposition of Ruthenium by Area-Dependent Surface Diffusion. Chem. Mater. 2020, 32, 9560–9572. [Google Scholar] [CrossRef]
- Kang, S.Y.; Hwang, C.S.; Kim, H.J. Improvements in Growth Behavior of CVD Ru Films on Film Substrates for Memory Capacitor Integration. J. Electrochem. Soc. 2004, 152, C15. [Google Scholar] [CrossRef]
- Coloma Ribera, R.; van de Kruijs, R.W.E.; Sturm, J.M.; Yakshin, A.E.; Bijkerk, F. In Vacuo Growth Studies of Ru Thin Films on Si, SiN, and SiO2 by High-Sensitivity Low Energy Ion Scattering. J. Appl. Phys. 2016, 120, 065303. [Google Scholar] [CrossRef]
- Kim, H.-M.; Lee, J.-H.; Lee, S.-H.; Harada, R.; Shigetomi, T.; Lee, S.; Tsugawa, T.; Shong, B.; Park, J.-S. Area-Selective Atomic Layer Deposition of Ruthenium Using a Novel Ru Precursor and H2O as a Reactant. Chem. Mater. 2021, 33, 4353–4361. [Google Scholar] [CrossRef]
- Antczak, G.; Ehrlich, G. Surface Diffusion: Metals, Metal Atoms, and Clusters; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-89983-3. [Google Scholar]
- Seebauer, E.G.; Allen, C.E. Estimating Surface Diffusion Coefficients. Prog. Surf. Sci. 1995, 49, 265–330. [Google Scholar] [CrossRef]
- Kaxiras, E. Review of Atomistic Simulations of Surface Diffusion and Growth on Semiconductors. Comput. Mater. Sci. 1996, 6, 158–172. [Google Scholar] [CrossRef]
- Ratsch, C.; Scheffler, M. Density-Functional Theory Calculations of Hopping Rates of Surface Diffusion. Phys. Rev. B 1998, 58, 13163–13166. [Google Scholar] [CrossRef]
- Tholander, C.; Alling, B.; Tasnádi, F.; Greene, J.E.; Hultman, L. Effect of Al Substitution on Ti, Al, and N Adatom Dynamics on TiN(001), (011), and (111) Surfaces. Surf. Sci. 2014, 630, 28–40. [Google Scholar] [CrossRef]
- Guo, F.; Wang, J.; Du, Y.; Wang, J.; Shang, S.-L.; Li, S.; Chen, L. First-Principles Study of Adsorption and Diffusion of Oxygen on Surfaces of TiN, ZrN and HfN. Appl. Surf. Sci. 2018, 452, 457–462. [Google Scholar] [CrossRef]
- Vakula, N.I.; Kuramshina, G.M.; Gorb, L.G.; Hill, F.; Leszczynski, J. Adsorption and Diffusion of a Silver Atom and Its Cation on α-SiO2 (001): Comparison of a Pure Surface with a Surface Containing an Al Defect. Chem. Phys. Lett. 2013, 567, 27–33. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Edström, D.; Hultman, L.; Petrov, I.; Greene, J.E.; Chirita, V. Ab Initio and Classical Molecular Dynamics Simulations of N2 Desorption from TiN(001) Surfaces. Surf. Sci. 2014, 624, 25–31. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Edström, D.; Hultman, L.; Petrov, I.; Greene, J.E.; Chirita, V. Ti Adatom Diffusion on TiN(001): Ab Initio and Classical Molecular Dynamics Simulations. Surf. Sci. 2014, 627, 34–41. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Tasnádi, F.; Hultman, L.; Petrov, I.; Greene, J.E.; Chirita, V. N and Ti Adatom Dynamics on Stoichiometric Polar TiN(111) Surfaces. Surf. Sci. 2016, 649, 72–79. [Google Scholar] [CrossRef]
- Kwon, J.A.; Kim, M.-S.; Shin, D.Y.; Kim, J.Y.; Lim, D.-H. First-Principles Understanding of Durable Titanium Nitride (TiN) Electrocatalyst Supports. J. Ind. Eng. Chem. 2017, 49, 69–75. [Google Scholar] [CrossRef]
- Tosoni, S.; Pacchioni, G. Influence of Surface Hydroxylation on the Ru Atom Diffusion on the ZrO2(101) Surface: A DFT Study. Surf. Sci. 2017, 664, 87–94. [Google Scholar] [CrossRef]
- Cottom, J.; Bochkarev, A.; Olsson, E.; Patel, K.; Munde, M.; Spitaler, J.; Popov, M.N.; Bosman, M.; Shluger, A.L. Modeling of Diffusion and Incorporation of Interstitial Oxygen Ions at the TiN/SiO2 Interface. ACS Appl. Mater. Interfaces 2019, 11, 36232–36243. [Google Scholar] [CrossRef]
- Kondati Natarajan, S.; Nies, C.-L.; Nolan, M. Ru Passivated and Ru Doped ε-TaN Surfaces as a Combined Barrier and Liner Material for Copper Interconnects: A First Principles Study. J. Mater. Chem. C 2019, 7, 7959–7973. [Google Scholar] [CrossRef]
- Das, T.; Tosoni, S.; Pacchioni, G. Role of Support in Tuning the Properties of Single Atom Catalysts: Cu, Ag, Au, Ni, Pd, and Pt Adsorption on SiO2/Ru, SiO2/Pt, and SiO2/Si Ultrathin Films. J. Chem. Phys. 2021, 154, 134706. [Google Scholar] [CrossRef] [PubMed]
- Nies, C.-L.; Kondati Natarajan, S.; Nolan, M. Control of the Cu Morphology on Ru-Passivated and Ru-Doped TaN Surfaces—Promoting Growth of 2D Conducting Copper for CMOS Interconnects. Chem. Sci. 2022, 13, 713–725. [Google Scholar] [CrossRef]
- Hafner, J. Ab-Initio Simulations of Materials Using VASP: Density-Functional Theory and Beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Sharada, M.S.; Karlsson, R.K.B.; Maimaiti, Y.; Voss, J.; Bligaard, T. Adsorption on Transition Metal Surfaces: Transferability and Accuracy of DFT Using the ADS41 Dataset. Phys. Rev. B 2019, 100, 035439. [Google Scholar] [CrossRef]
- Rehak, F.R.; Piccini, G.; Alessio, M.; Sauer, J. Including Dispersion in Density Functional Theory for Adsorption on Flat Oxide Surfaces, in Metal–Organic Frameworks and in Acidic Zeolites. Phys. Chem. Chem. Phys. 2020, 22, 7577–7585. [Google Scholar] [CrossRef]
- Goumans, T.P.M.; Wander, A.; Brown, W.A.; Catlow, C.R.A. Structure and Stability of the (001) α-Quartz Surface. Phys. Chem. Chem. Phys. 2007, 9, 2146–2152. [Google Scholar] [CrossRef]
- Marlo, M.; Milman, V. Density-Functional Study of Bulk and Surface Properties of Titanium Nitride Using Different Exchange-Correlation Functionals. Phys. Rev. B 2000, 62, 2899–2907. [Google Scholar] [CrossRef]
- Jung, J.H.; Oh, H.; Shong, B. Fluorination of TiN, TiO2, and SiO2 Surfaces by HF toward Selective Atomic Layer Etching (ALE). Coatings 2023, 13, 387. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A Climbing Image Nudged Elastic Band Method for Finding Saddle Points and Minimum Energy Paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Gomer, R. Diffusion of Adsorbates on Metal Surfaces. Rep. Prog. Phys. 1990, 53, 917. [Google Scholar] [CrossRef]
- Hosseini, M.; Schaekers, M.; van der Veen, M.H.; Teugels, L.; Batuk, D.; Martinez, G.T.; Yu, H.; Jourdan, N.; Schleicher, F.; Debruyn, H.; et al. Ru as an Alternative Material for Advanced Contacts. In Proceedings of the 2020 IEEE International Interconnect Technology Conference (IITC), San Jose, CA, USA, 5–8 October 2020; pp. 157–159. [Google Scholar]
- Kotsugi, Y.; Han, S.-M.; Kim, Y.-H.; Cheon, T.; Nandi, D.K.; Ramesh, R.; Yu, N.-K.; Son, K.; Tsugawa, T.; Ohtake, S.; et al. Atomic Layer Deposition of Ru for Replacing Cu-Interconnects. Chem. Mater. 2021, 33, 5639–5651. [Google Scholar] [CrossRef]
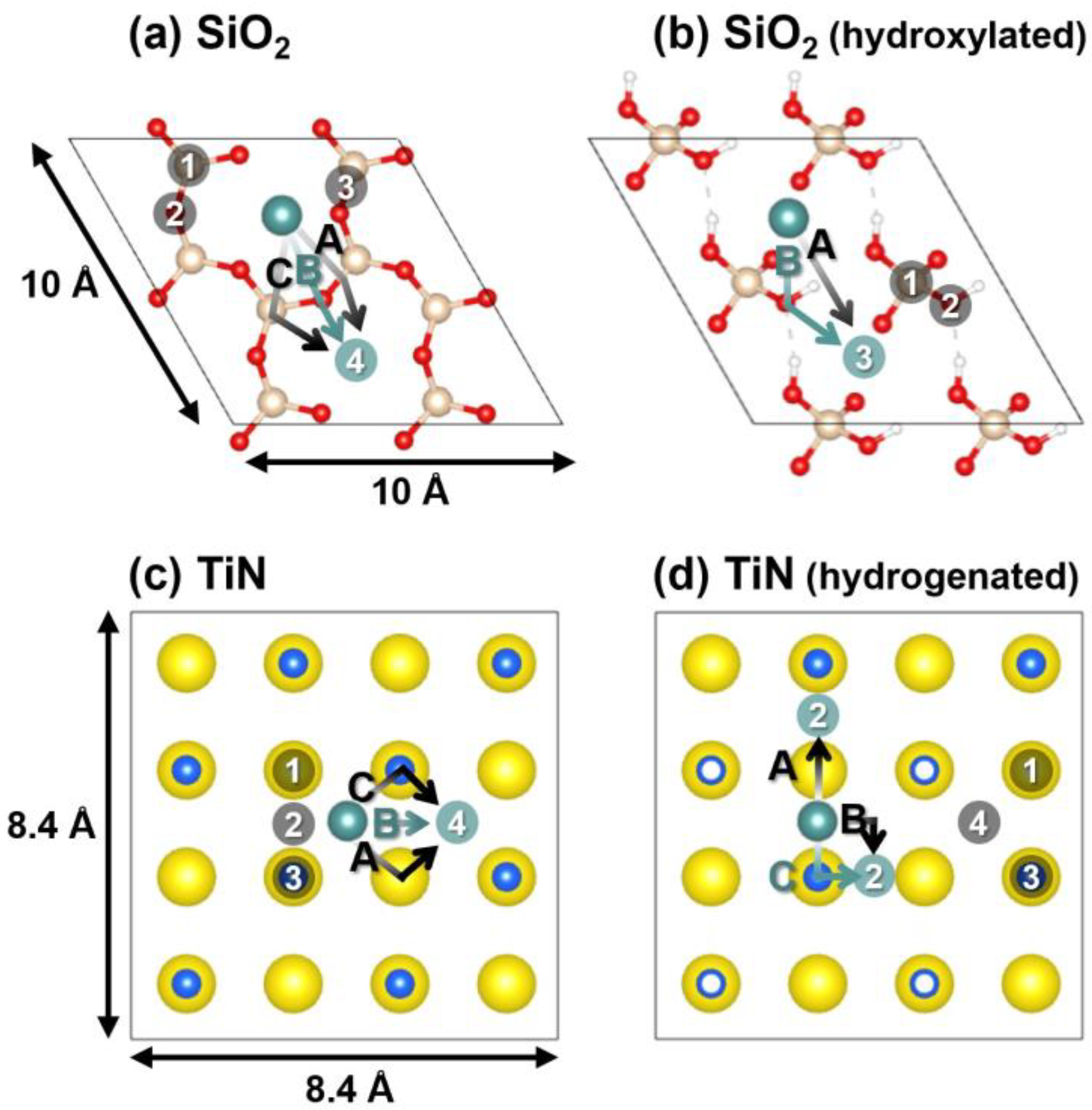
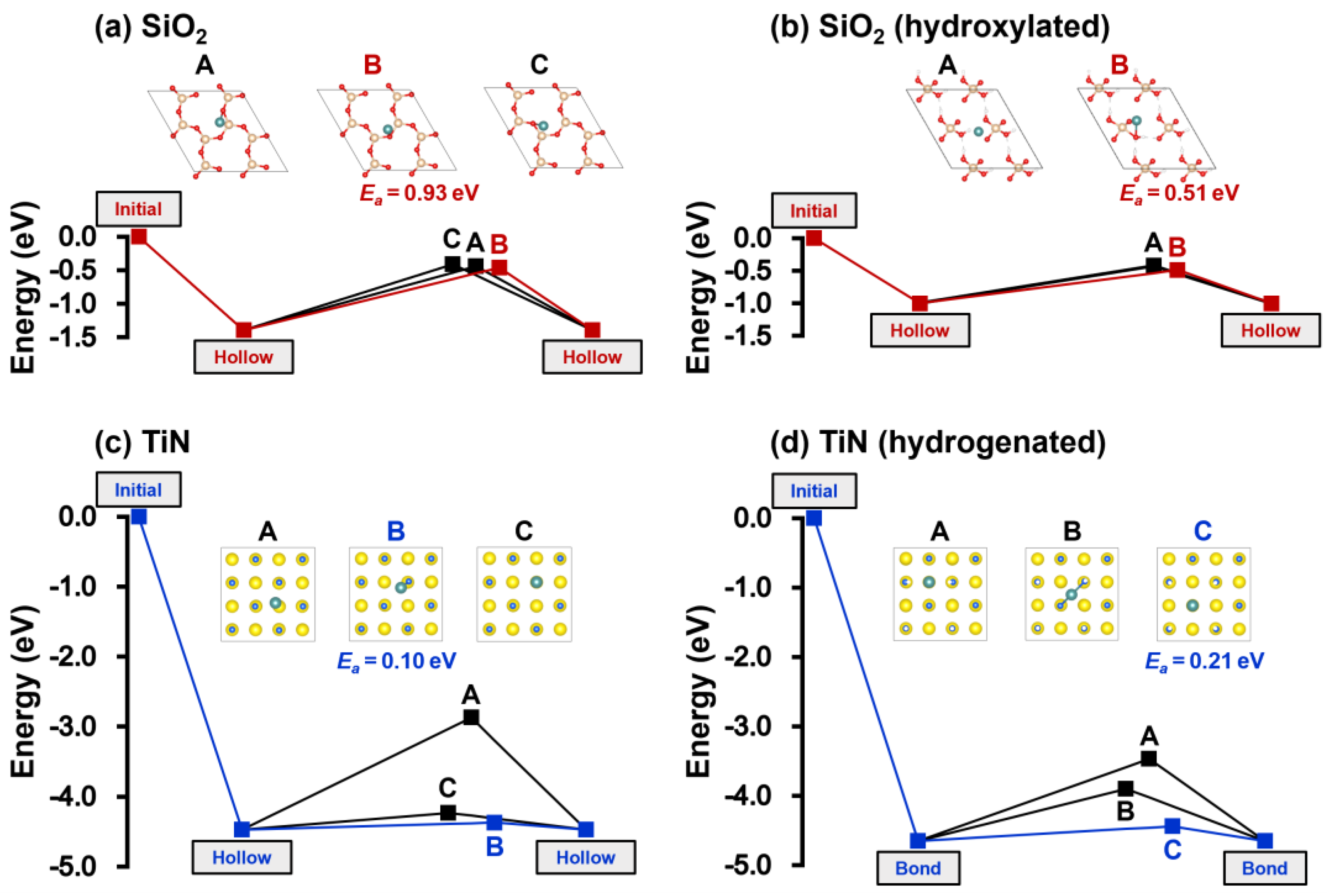
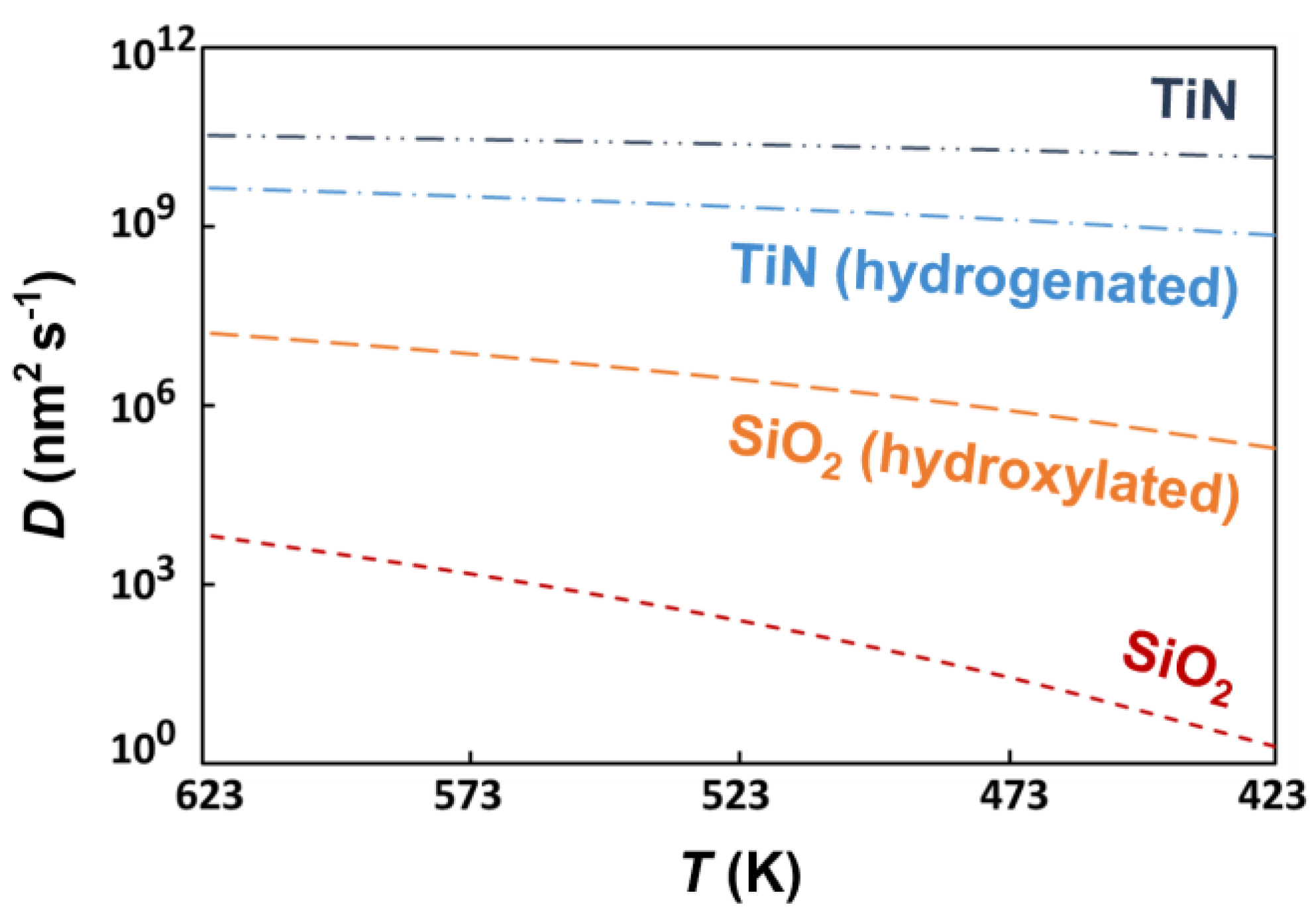
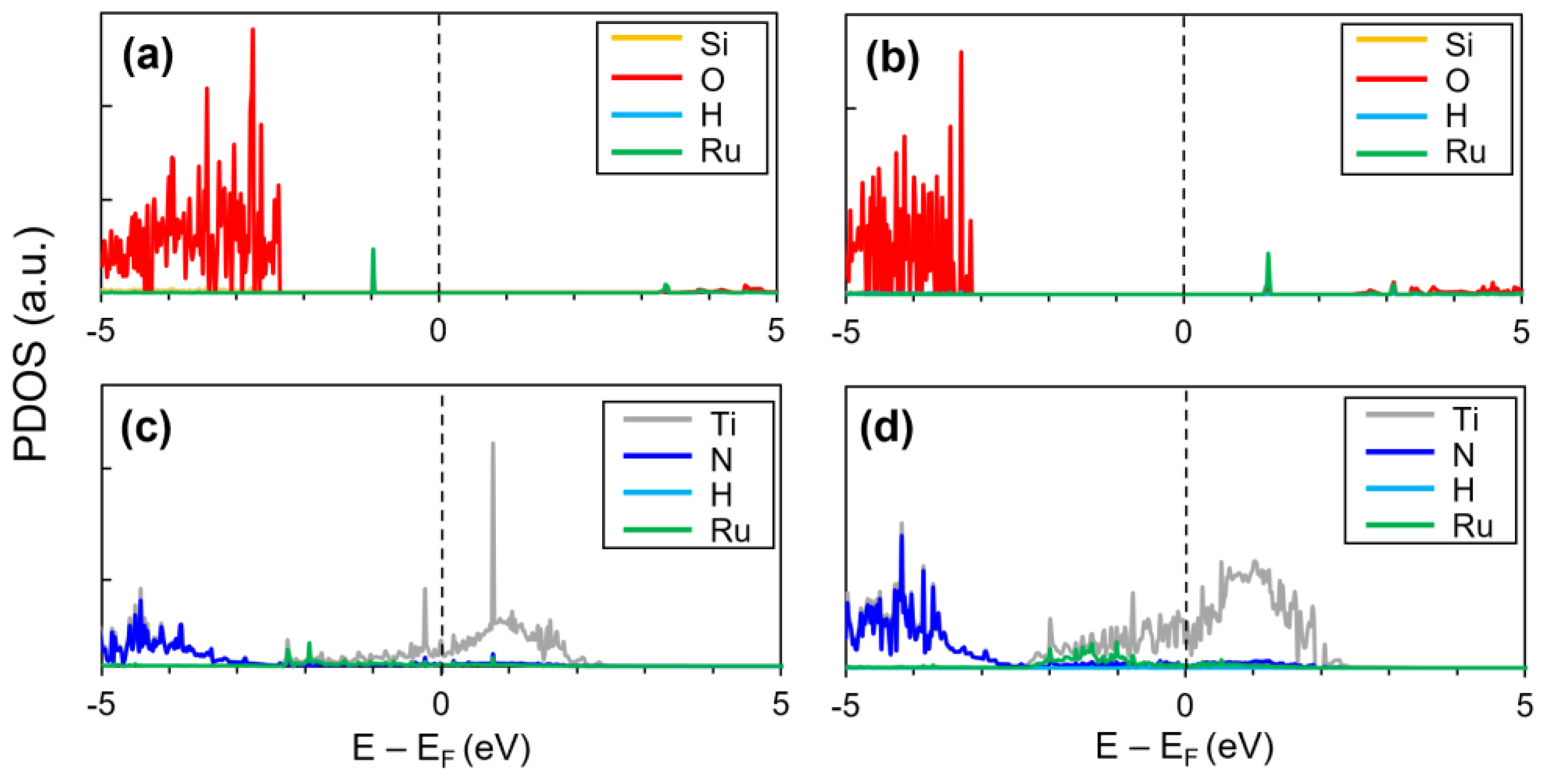
| Substrate | Adsorption Site | Adsorption Energy (eV) |
|---|---|---|
| (a) SiO2 | ① Si atom | −0.61 |
| ② O atom | −0.62 | |
| ③ Si-O bond | −0.61 | |
| ④ Hollow | −1.39 | |
| (b) SiO2 (hydroxylated) | ① Si atom | −0.53 |
| ② O atom | −0.43 | |
| ③ Hollow | −1.00 | |
| (c) TiN | ① Ti atom | −2.98 |
| ② Ti–N bond | −4.24 | |
| ③ N atom | −4.39 | |
| ④ Hollow | −4.47 | |
| (d) TiN (hydrogenated) | ① Ti atom | −3.47 |
| ② Ti–N bond | −4.65 | |
| ③ N atom | −4.45 | |
| ④ Hollow | −3.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, C.; Jung, J.H.; Kim, J.J.; Lee, D.-C.; Shong, B. Adsorption and Surface Diffusion of Atomic Ru on TiN and SiO2: A First-Principles Study. Coatings 2023, 13, 1020. https://doi.org/10.3390/coatings13061020
Ahn C, Jung JH, Kim JJ, Lee D-C, Shong B. Adsorption and Surface Diffusion of Atomic Ru on TiN and SiO2: A First-Principles Study. Coatings. 2023; 13(6):1020. https://doi.org/10.3390/coatings13061020
Chicago/Turabian StyleAhn, Changhyun, Ju Hyeon Jung, Jae Jung Kim, Dong-Chan Lee, and Bonggeun Shong. 2023. "Adsorption and Surface Diffusion of Atomic Ru on TiN and SiO2: A First-Principles Study" Coatings 13, no. 6: 1020. https://doi.org/10.3390/coatings13061020






