Polypyrrole Nanosheets Prepared by Rapid In Situ Polymerization for NIR-II Photoacoustic-Guided Photothermal Tumor Therapy
Abstract
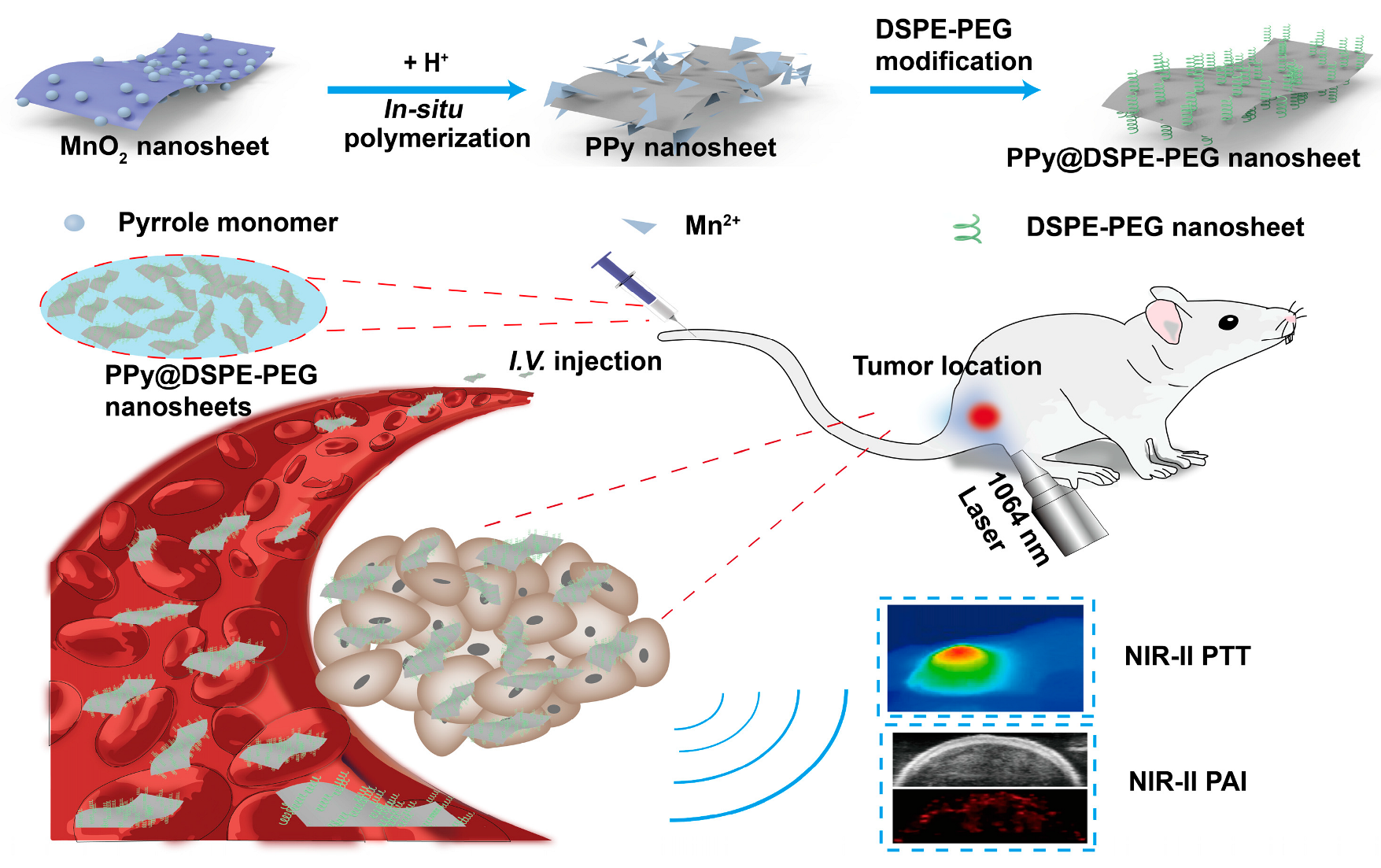
:1. Introduction
2. Materials and Methods
2.1. Materials
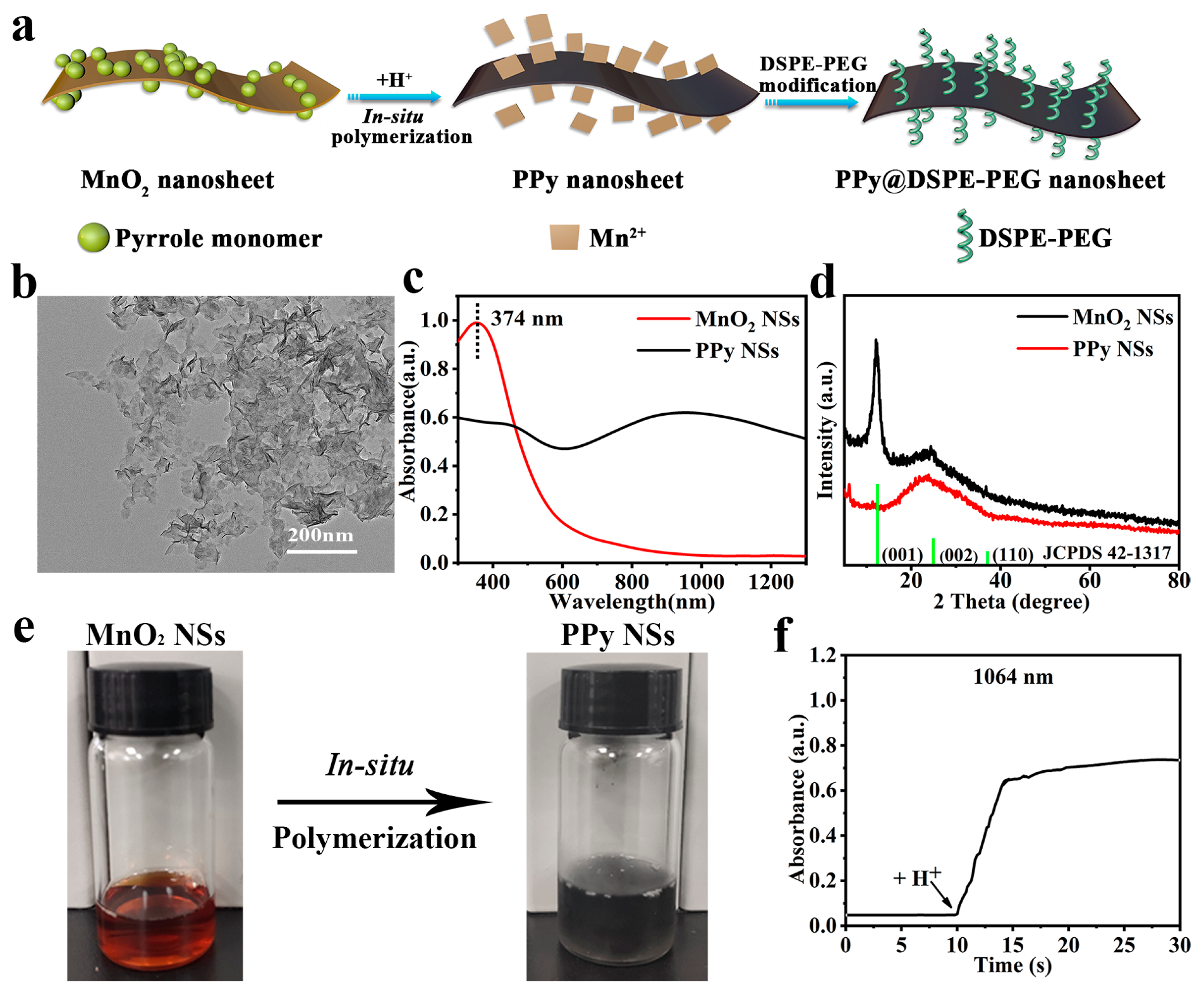
2.2. Synthesis of MnO2 NSs
2.3. Synthesis of PPy NSs and PPy@DSPE-PEG NSs
2.4. Characterization
2.5. Photothermal Effect
2.6. Cytotoxicity Assay of PPy@DSPE-PEG NSs
2.7. In Vitro Photothermal Therapy
2.8. Tumor Model
2.9. In Vivo Photoacoustic Imaging
2.10. In Vivo Photothermal Therapy
3. Results and Discussion
3.1. Synthesis and Characterization of PPy NSs
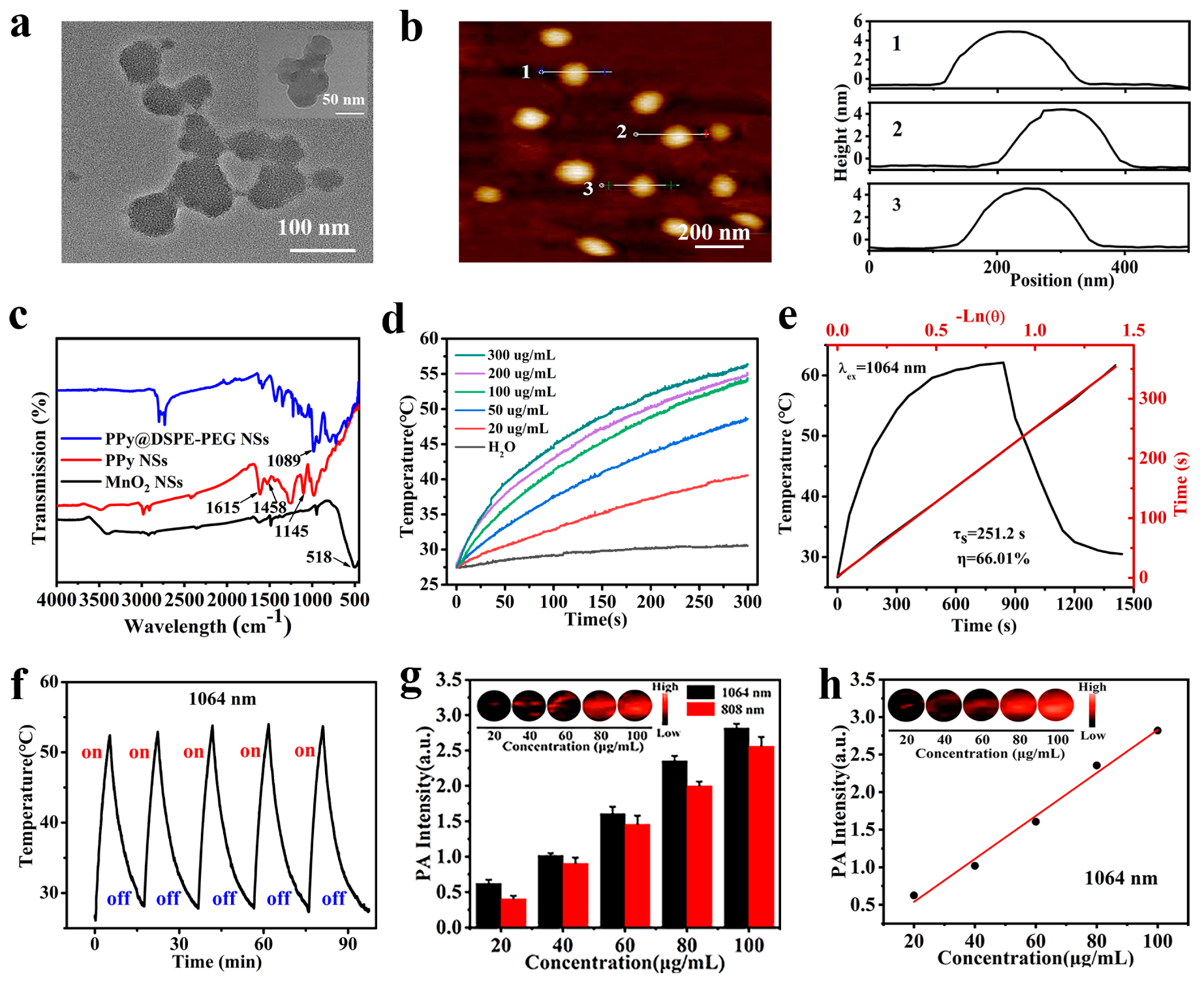
3.2. Photothermal Properties of PPy@DSPE-PEG NSs
3.3. Evaluation of In Vitro PA Imaging
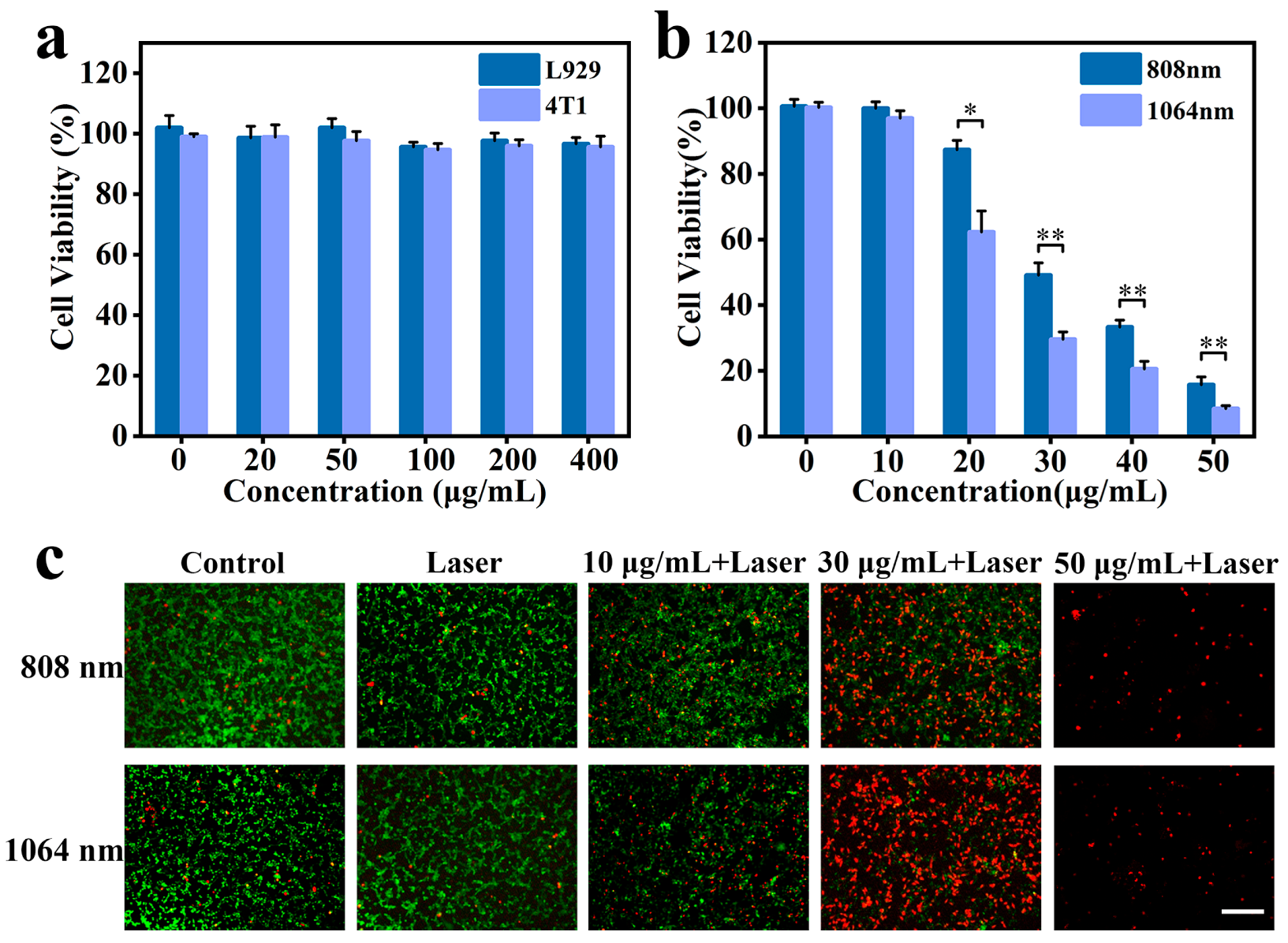
3.4. Cytotoxicity and In Vitro PTT Assay
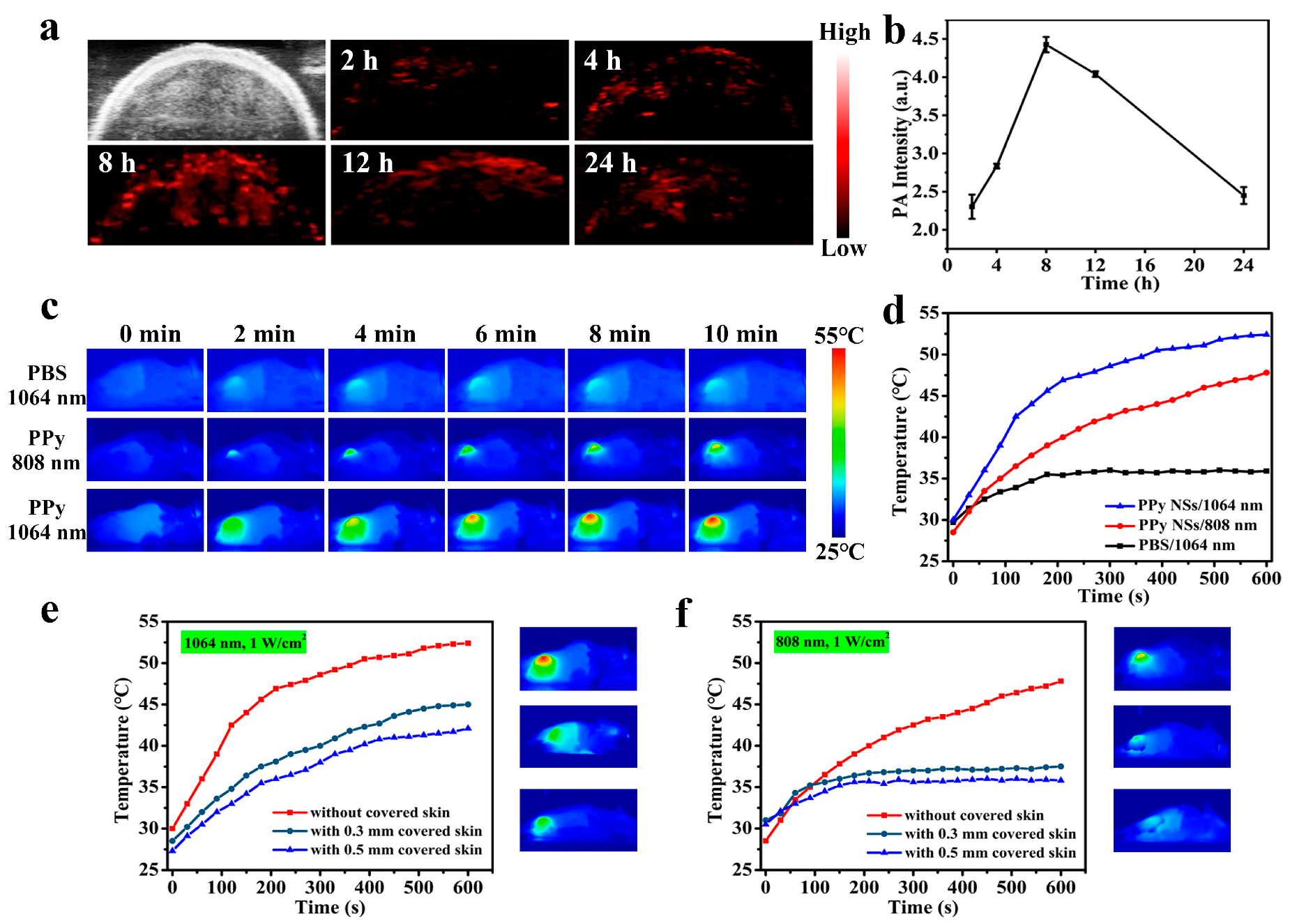
3.5. Evaluation of In Vivo PA Imaging
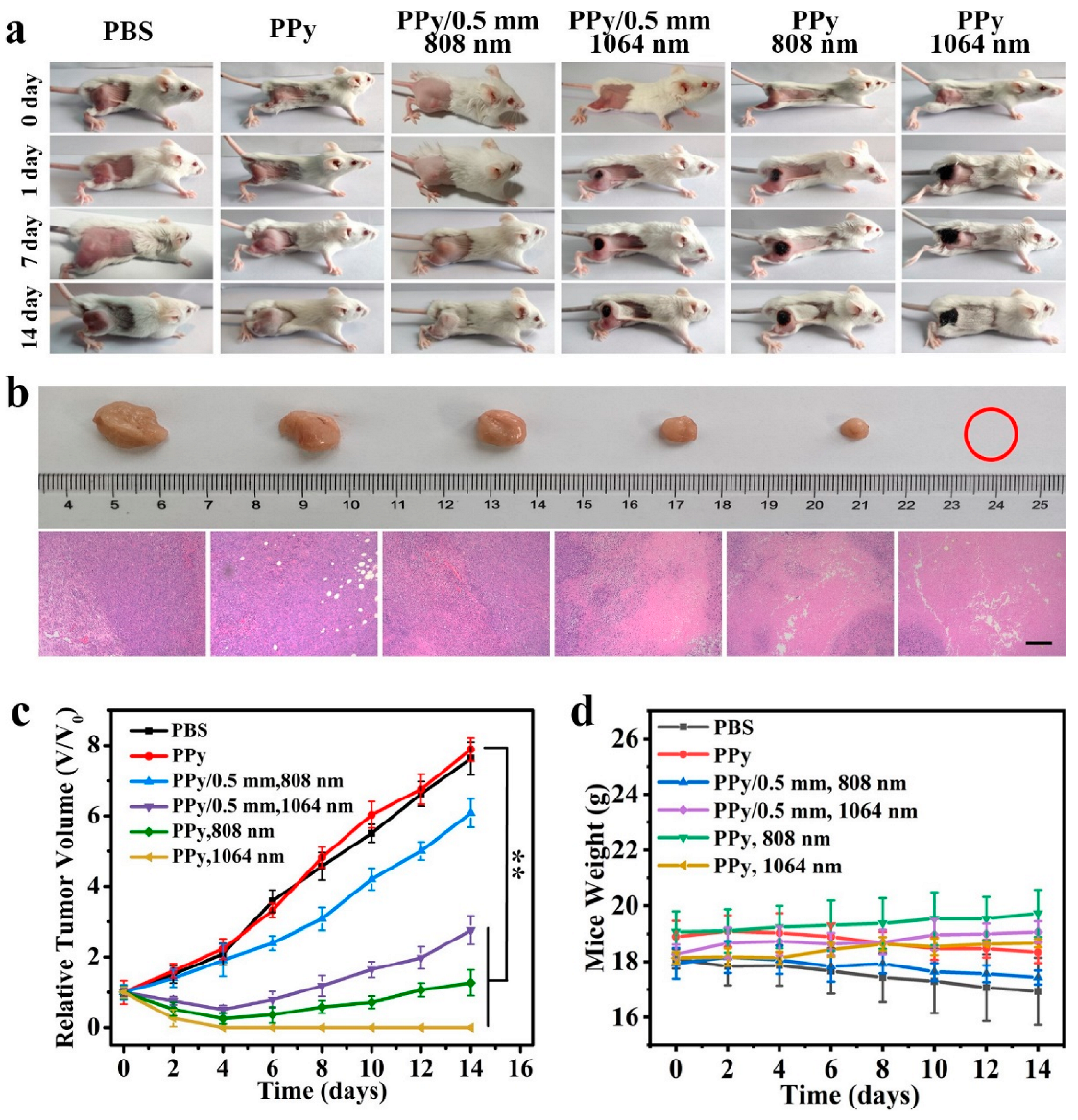
3.6. In Vivo PTT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.V.P.; Dubey, S.K.; Tiwari, S.; Puri, A.; Hejmady, S.; Gorain, B.; Kesharwani, P. Recent advances in nanoparticles mediated photothermal therapy induced tumor regression. Int. J. Pharmacol. 2021, 606, 120848. [Google Scholar] [CrossRef] [PubMed]
- Saneja, A.; Kumar, R.; Arora, D.; Kumar, S.; Panda, A.K.; Jaglan, S. Recent advances in near-infrared light-responsive nanocarriers for cancer therapy. Drug Discov. Today 2018, 23, 1115–1125. [Google Scholar] [CrossRef]
- Dash, B.S.; Jose, G.; Lu, Y.J.; Chen, J.P. Functionalized reduced graphene oxide as a versatile tool for cancer therapy. Int. J. Mol. Sci. 2021, 22, 2989. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in cancer therapeutics: Conventional thermal therapy to nanotechnology-based photothermal therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Rajani, C.; Tambe, V.; Kalyane, D.; Anup, N.; Deb, P.K.; Kalia, K.; Tekade, R.K. Nanomaterials assisted chemo-photothermal therapy for combating cancer drug resistance. J. Drug Deliv. Sci. Technol. 2022, 70, 103164. [Google Scholar] [CrossRef]
- Rizwan, M.; Rasheed, T.; Raza, A.; Bilal, M.; Yahya, R.; Yar, M.; Iqbal, H.M.N. Photodynamic-based therapeutic modalities to fight against cancer—A review from synergistic viewpoint. J. Drug Deliv. Sci. Technol. 2019, 51, 70–82. [Google Scholar] [CrossRef]
- Roy, S.; Roy, J.; Guo, B. Nanomaterials as multimodal photothermal agents (PTAs) against ‘Superbugs’. J. Mater. Chem. B 2023, 11, 2287–2306. [Google Scholar] [CrossRef]
- Plan Sangnier, A.; Van de Walle, A.; Aufaure, R.; Fradet, M.; Motte, L.; Guenin, E.; Lalatonne, Y.; Wilhelm, C. Endosomal confinement of gold nanospheres, nanorods, and nanoraspberries governs their photothermal identity and is beneficial for cancer cell therapy. Adv. Biosyst. 2020, 4, 1900284. [Google Scholar] [CrossRef]
- Shaw, S.K.; Kailashiya, J.; Gangwar, A.; Alla, S.K.; Gupta, S.K.; Prajapat, C.L.; Meena, S.S.; Dash, D.; Maiti, P.; Prasad, N.K. γ-Fe2O3 nanoflowers as efficient magnetic hyperthermia and photothermal agent. Appl. Surf. Sci. 2021, 560, 150025. [Google Scholar] [CrossRef]
- An, D.; Fu, J.Y.; Zhang, B.; Xie, N.; Nie, G.H.; Agren, A.; Qiu, M.; Zhang, H. NIR-II responsive inorganic 2D nanomaterials for cancer photothermal therapy: Recent advances and future challenges. Adv. Funct. Mater. 2021, 31, 2101625. [Google Scholar] [CrossRef]
- Macchi, S.; Jalihal, A.; Hooshmand, N.; Zubair, M.; Jenkins, S.; Alwan, N.; El-Sayed, M.; Ali, N.; Griffin, R.J.; Siraj, N. Enhanced photothermal heating and combination therapy of NIR dye via conversion to self-assembled ionic nanomaterials. J. Mater. Chem. B 2022, 10, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.D.; Phan, L.M.T.; Cho, S.; Park, J. Enhancement approaches for photothermal conversion of donor–acceptor conjugated polymer for photothermal therapy: A review. Sci. Technol. Adv. Mater. 2022, 23, 707–734. [Google Scholar] [CrossRef]
- Kawasaki, R.; Kondo, K.; Miura, R.; Yamana, K.; Isozaki, H.; Shimada, R.; Kawamura, S.; Hirano, H.; Nishimura, T.; Tarutani, N.; et al. Theranostic agent combining fullerene nanocrystals and gold nanoparticles for photoacoustic imaging and photothermal therapy. Int. J. Mol. Sci. 2022, 23, 4686. [Google Scholar] [CrossRef] [PubMed]
- Suarasan, S.; Campu, A.; Vulpoi, A.; Banciu, M.; Astilean, S. Assessing the efficiency of triangular gold nanoparticles as NIR photothermal agents in vitro and melanoma tumor model. Int. J. Mol. Sci. 2022, 23, 13724. [Google Scholar] [CrossRef] [PubMed]
- Khurana, D.; Dudi, R.; Shukla, S.K.; Singh, D.; Mondhe, D.M.; Soni, S. Gold nanoblackbodies mediated plasmonic photothermal cancer therapy for melanoma. Nanomedicine 2022, 17, 1323–1338. [Google Scholar] [CrossRef]
- Manivasagan, P.; Joe, A.; Han, H.W.; Thambi, T.; Selvaraj, M.; Chidambaram, K.; Kim, J.; Jang, E.S. Recent advances in multifunctional nanomaterials for photothermal-enhanced Fenton-based chemodynamic tumor therapy. Mater. Today Bio 2022, 13, 100197. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, H.W.; Mo, S.J.; Chung, B.G. Dual-stimuli responsive mesoporous copper (II) sulfide nanocomposite for chemo-photothermal synergistic therapy. Microporous Mesoporous Mater. 2020, 302, 110228. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Molybdenum disulfide (MoS2)-based nanostructures for tissue engineering applications: Prospects and challenges. J. Mater. Chem. B 2022, 10, 2761–2780. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, S.; Martin, E.M.; Kuppusamy, S.; Vetrivel, C. Chitosan coated molybdenum sulphide nanosheet incorporated with tantalum oxide nanomaterials for improving cancer photothermal therapy. Arab. J. Chem. 2020, 13, 4741–4750. [Google Scholar] [CrossRef]
- Karthikeyan, L.; Yasothamani, V.; Haldorai, Y.; Selvan Christyraj, J.R.S.; Vivek, R. TLR-7/8 agonist-loaded polypyrrole-based theranostic nanovaccine for second near-infrared photothermal immunotherapy of cancer. ACS Appl. Nano Mater. 2023, 6, 6279–6291. [Google Scholar] [CrossRef]
- Zeng, W.W.; Wu, X.X.; Chen, T.; Sun, S.J.; Shi, Z.F.; Liu, J.; Ji, X.Y.; Zeng, X.W.; Guan, J.; Mei, L.; et al. Renal-clearable ultrasmall polypyrrole nanoparticles with size-regulated property for second near-infrared light-mediated photothermal therapy. Adv. Funct. Mater. 2021, 31, 2008362. [Google Scholar] [CrossRef]
- Kim, T.E.; Jang, H.J.; Park, S.W.; Wei, J.; Cho, S.; Park, W.I.; Lee, B.R.; Yang, C.D.; Jung, Y.K. Folic acid functionalized carbon dot/polypyrrole nanoparticles for specific bioimaging and photothermal therapy. ACS Appl. Bio Mater. 2021, 4, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, S.; Samuvel Muthiah, K.; Sakthivel, R.; Liao, M.Y.; Kasai, H.; Chung, R.J. Polydopamine-coated Cu-BTC nanowires for effective magnetic resonance imaging and photothermal therapy. Pharmaceutics 2023, 15, 822. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Alifu, N.; Du, Z.; Chen, S.; Heng, Y.; Wang, J.; Zhu, L.; Ma, C.; Zhang, X. Indocyanine green-based theranostic nanoplatform for NIR fluorescence image-guided chemo/photothermal therapy of cervical cancer. Int. J. Nanomed. 2021, 16, 4847–4861. [Google Scholar] [CrossRef]
- Maziukiewicz, D.; Grzeskowiak, B.F.; Coy, E.; Jurga, S.; Mrowczynski, R. NDs@PDA@ICG conjugates for photothermal therapy of glioblastoma multiforme. Biomimetics 2019, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Egnuni, T.; Ingram, N.; Mirza, I.; Coletta, P.L.; McLaughlan, J.R. Evaluation of the targeting and therapeutic efficiency of anti-EGFR functionalised nanoparticles in head and neck cancer cells for use in NIR-II optical window. Pharmaceutics 2021, 13, 1651. [Google Scholar] [CrossRef]
- Salazar Sandoval, S.; Cortés-Adasme, E.; Gallardo-Toledo, E.; Araya, I.; Celis, F.; Yutronic, N.; Jara, P.; Kogan, M.J. β-cyclodextrin-based nanosponges inclusion compounds associated with gold nanorods for potential NIR-II drug delivery. Pharmaceutics 2022, 14, 2206. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, L.; Wang, C.; Peng, R.; Liu, Z. Conjugated polymers for photothermal therapy of cancer. Polym. Chem. 2014, 5, 1573–1580. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.T.; Liu, H.Y. Two-dimensional nanomaterials for photothermal therapy. Angew. Chem.-Int. Edit. 2020, 59, 5890–5900. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Ratautaite, V.; Mikoliunaite, L.; Kaseta, V.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Some biocompatibility aspects of conducting polymer polypyrrole evaluated with bone marrow-derived stem cells. Colloids Surf. 2014, 442, 152–156. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Kaseta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Synthesis of polythiophene nanoparticles by surfactant-free chemical oxidative polymerization method: Characterization, in vitro biomineralization, and cytotoxicity evaluation. J. Hazard. Mater. 2013, 250, 167–174. [Google Scholar] [CrossRef]
- Kesa, P.; Paurova, M.; Babic, M.; Heizer, T.; Matous, P.; Turnovcova, K.; Marekova, D.; Sefc, L.; Herynek, V. Photoacoustic properties of polypyrrole nanoparticles. Nanomaterials 2021, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.V.; Bui, N.Q.; Moorthy, M.S.; Lee, K.D.; Oh, J. Synthesis and in vitro performance of polypyrrole-coated iron-platinum nanoparticles for photothermal therapy and photoacoustic imaging. Nanoscale Res. Lett. 2017, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Sheng, X.; Wang, Y.; Xu, H. Ultrathin polypyrrole nanosheets via space-confined synthesis for efficient photothermal therapy in the second near-infrared window. Nano Lett. 2018, 18, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Lv, G.; Wang, F.; Zhou, X.; Hu, J.; Wang, Q. Facile synthesis of hydrophilic polypyrrole nanoparticles for photothermal cancer therapy. J. Mater. Sci. 2014, 49, 3484–3490. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhu, Z.X.; Dai, T.Y.; Lu, Y. Facile fabrication of functional polypyrrole nanotubes via a reactive self-degraded template. Macromol. Rapid Commun. 2005, 26, 1736–1740. [Google Scholar] [CrossRef]
- Guan, H.D.; Ding, T.; Zhou, W.; Wang, Z.Q.; Zhang, J.X.; Cai, K.Y. Hexagonal polypyrrole nanosheets from interface driven heterogeneous hybridization and selfassembly for photothermal cancer treatment. Chem. Commun. 2019, 55, 4359–4362. [Google Scholar] [CrossRef]
- Jeon, S.S.; An, H.H.; Yoon, C.S.; Im, S.S. Synthesis of ultra-thin polypyrrole nanosheets for chemical sensor application. Polymer 2011, 53, 652–657. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer–polypyrrole–for the improvement of electric charge transfer through fungal cell wall. Colloids Surf. B 2019, 175, 671–679. [Google Scholar] [CrossRef]
- Wu, S.S.; Chen, C.H.; Yang, H.T.; Wei, W.; Wei, M.; Zhang, Y.J.; Liu, S.Q. A sensitive fluorescence “Turn-off-on” biosensor for poly(ADP-ribose) polymerase-1 detection based on cationic conjugated polymer-MnO2 nanosheets. Sens. Actuator B-Chem. 2018, 273, 1047–1053. [Google Scholar] [CrossRef]
- Bi, H.T.; Dai, Y.L.; Yang, P.P.; Xu, J.T.; Yang, D.; Gai, S.L.; He, F.H.; An, G.H.; Zhong, C.N.; Lin, J. Glutathione and H2O2 consumption promoted photodynamic and chemotherapy based on biodegradable MnO2-Pt@Au-25 nanosheets. Chem. Eng. J. 2019, 356, 543–553. [Google Scholar] [CrossRef]
- Zhang, L.; Lian, J.; Wu, L.; Duan, Z.; Jiang, J.; Zhao, L. Synthesis of a thin-layer MnO2 nanosheet-coated Fe3O4 nanocomposite as a magnetically separable photocatalyst. Langmuir 2014, 30, 7006–7013. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.D.; Han, Y.M.; Zhou, K.Q.; Shi, C.L. In situ polymerization of aniline on the surface of manganese oxide nanosheets for reducing fire hazards of epoxy. Mater. Chem. Phys. 2020, 243, 122600. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.H.; Mu, J.; Dai, L.F.; Zhang, Z.; Sun, Y.N.; Shi, W.; Ge, D.T. Synthesis of polypyrrole nanowires with positive effect on MC3T3-E1 cell functions through electrical stimulation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 71, 43–50. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Xu, J.; Jin, H.; Yi, Y.; Shen, Y.; Zhang, X.; Liu, X.; Sun, Y.; Shi, W.; He, Y.; et al. Polypyrrole Nanosheets Prepared by Rapid In Situ Polymerization for NIR-II Photoacoustic-Guided Photothermal Tumor Therapy. Coatings 2023, 13, 1037. https://doi.org/10.3390/coatings13061037
Xie Y, Xu J, Jin H, Yi Y, Shen Y, Zhang X, Liu X, Sun Y, Shi W, He Y, et al. Polypyrrole Nanosheets Prepared by Rapid In Situ Polymerization for NIR-II Photoacoustic-Guided Photothermal Tumor Therapy. Coatings. 2023; 13(6):1037. https://doi.org/10.3390/coatings13061037
Chicago/Turabian StyleXie, Yixin, Ji Xu, Hui Jin, Yunfeng Yi, Yuqing Shen, Xiuming Zhang, Xinxin Liu, Yanan Sun, Wei Shi, Yuan He, and et al. 2023. "Polypyrrole Nanosheets Prepared by Rapid In Situ Polymerization for NIR-II Photoacoustic-Guided Photothermal Tumor Therapy" Coatings 13, no. 6: 1037. https://doi.org/10.3390/coatings13061037





