Atomic Layer Deposition of Ultra-Thin Crystalline Electron Channels for Heterointerface Polarization at Two-Dimensional Metal-Semiconductor Heterojunctions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. PE-ALD of Ga2O3 and TiO2
3.2. Photoelectrical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, H.; Karbalaei Akbari, M.; Zhuiykov, S. 2D semiconductor nanomaterials and heterostructures: Controlled synthesis and functional applications. Nanoscale Res. Lett. 2021, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jiang, D.; Zhao, M.; Wang, N.; Duan, Y.; Wang, W.; Li, M.; Li, Q.; Shan, C.; Sun, J. Polarization induced two-dimensional electron gas in ZnO/ZnMgO heterointerface for high-performance enhanced UV photodetector. J. Alloys Compd. 2020, 820, 153416. [Google Scholar] [CrossRef]
- Li, D.; Huang, X.; Xiao, Z.; Zhang, L.; Hao, Y.; Song, J.; Shao, D.-F.; Tsymbal, E.Y.; Tsymbal, E.Y.; Hong, X. Polar coupling enabled nonlinear optical filtering at MoS2/ferroelectric heterointerfaces. Nat. Commun. 2020, 11, 1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, W.; Wang, X.; Xu, Y.; Zhang, R. Recent advances on spin-polarized two-dimensional electron gases at oxide interfaces. ACS Appl. Electron. Mater. 2021, 3, 128–144. [Google Scholar] [CrossRef]
- Ummethala, S.; Harter, T.; Koehnle, K. THz-to-optical conversion in wireless communications using an ultra-broadband plasmonic modulator. Nat. Photonics 2019, 13, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Karbalaei Akbari, M.; Hai, Z.; Wei, Z.; Detavernier, C.; Solano, E.; Verpoort, F.; Zhuiykov, S. ALD-Developed plasmonic two-dimensional Au–WO3–TiO2 heterojunction architectonics for design of photovoltaic devices. ACS Appl. Mater. Interfaces 2018, 10, 10304–10314. [Google Scholar] [CrossRef]
- Xu, H.; Karbalaei Akbari, M.; Verpoort, F.; Zhuiykov, S. Nano-engineering and functionalization of hybrid Au–MexOy–TiO2 (Me = W, Ga) hetero-interfaces for optoelectronic receptors and nociceptors. Nanoscale 2020, 12, 20177–20188. [Google Scholar] [CrossRef]
- Wu, P.; Appenzeller, J. Toward CMOS like devices from two-dimensional channel materials. APL Mater. 2019, 7, 100701. [Google Scholar] [CrossRef] [Green Version]
- Karbalaei Akbari, M.; Zhuiykov, S. Photonic and plasmonic devices based on two-dimensional semiconductors. In Ultrathin Two-Dimensional Semiconductors for Novel Electronic Applications; CRC Press Tylor and Francis: Boca Raton, FL, USA, 2020; Volume 1, pp. 145–169. [Google Scholar]
- Zaidi, S.J.A.; Basit, M.B.; Park, T.J. Advances in atomic layer deposition of metal sulfides: From a precursors perspective. Chem. Mater. 2022, 34, 7106–7138. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Karbalaei Akbari, M.; Hai, Z.; Xue, C.; Xu, H.L. Wafer-scale fabrication of conformal atomic-layered TiO2 by atomic layer deposition using tetrakis (dimethylamino) titanium and H2O precursors. Mat. Des. 2017, 120, 99–108. [Google Scholar] [CrossRef]
- Xu, H.; Karbalaei Akbari, M.; Hai, Z.; Wei, Z.; Hyde, L.; Verpoort, F.; Xue, C.; Zhuiykov, S. Ultra-thin MoO3 film goes wafer-scaled nano-architectonics by atomic layer deposition. Mat. Des. 2018, 149, 135–144. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Hu, J.; Verpoort, F.; Lu, H.; Zhuiykov, S. Nanoscale all-oxide-heterostructured bio-inspired optoresponsive nociceptor. Nano-Micro Lett. 2020, 12, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskiväli, L.; Heikkilä, P.; Kenttä, E.; Virtanen, T.; Rautkoski, H.; Pasanen, A.; Vähä-Nissi, M.; Putkonen, M. Comparison of the Growth and Thermal Properties of Nonwoven Polymers after Atomic Layer Deposition and Vapor Phase Infiltration. Coatings 2021, 11, 1028. [Google Scholar] [CrossRef]
- Hai, Z.; Karbalaei Akbari, M.; Xue, C.; Xu, H.; Hyde, L.; Zhuiykov, S. Wafer-scaled monolayer WO3 windows ultra-sensitive, extremely-fast and stable UV-A photodetection. Appl. Surf. Sci. 2017, 405, 169–177. [Google Scholar] [CrossRef]
- Ramachandran, R.; Dendooven, J.; Botterman, J.; Sree, S.P.; Poelman, D.; Martens, J.; Poelman, H.; Detavernier, C. Plasma enhanced atomic layer deposition of Ga2O3 thin films. J. Mater. Chem. A 2014, 2, 19232–19238. [Google Scholar] [CrossRef]
- Lopa, N.S.; Karbalaei Akbari, M.; Wu, D.; Verpoort, F.; Zhuiykov, S. Two-dimensional SnO2-ZnO nanohybrid electrode fabricated via atomic layer deposition for electrochemical supercapacitors. Energy Fuels 2023, 37, 3142–3151. [Google Scholar] [CrossRef]
- Altuntas, H.; Donmez, I.; Ozgit-Akgun, C.; Biyikli, N. Electrical characterization of β-Ga2O3 thin films grown by PEALD. J. Alloys Compd. 2014, 593, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Knoops, H.; Faraz, T.; Arts, K.; Kessels, W.M.M. Status and prospects of plasma-assisted atomic layer deposition. J. Vac. Sci. Technol. A 2019, 37, 030902. [Google Scholar] [CrossRef] [Green Version]
- Donmez, I.; Ozgit-Akgun, C.; Biyikli, N. Low Temperature deposition of Ga2O3 thin films using trimethylgallium and oxygen plasma. J. Vac. Sci. Technol. A 2013, 31, 01A110. [Google Scholar] [CrossRef]
- Comstock, D.J.; Elam, J.W. Atomic layer deposition of Ga2O3 films using trimethylgallium and ozone. Chem. Mater. 2012, 24, 4011–4018. [Google Scholar] [CrossRef]
- Wheeler, V.D.; Nepal, N.; Boris, D.R.; Qadri, S.B.; Nyakiti, L.O.; Lang, A.; Koehler, A.; Foster, G.; Walton, S.G.; Eddy, C.R.; et al. Phase control of crystalline Ga2O3 films by plasma-enhanced atomic layer deposition. J. Chem. Mater. 2020, 32, 1140–1152. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Zhuiykov, S. Atomic layer deposition of two-dimensional semiconductors. In Ultrathin Two-Dimensional Semiconductors for Novel Electronic Applications; CRC Press Tylor and Francis: Boca Raton, FL, USA, 2020; Volume 1, pp. 43–73. [Google Scholar]
- Playford, H.Y.; Hannon, A.C.; Barney, E.R.; Walton, R.I. Structures of Uncharacterized Polymorphs of Gallium Oxide from Total Neutron Diffraction. Chem. Eur. J. 2013, 19, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Cora, I.; Mezzadri, F.; Boschi, F.; Bosi, M.; Caplovicova, M.; Calestani, G.; Dodony, I.; Pecz, B.; Fornari, R. The real structure of ε-Ga2O3 and its relation to κ-phase. CrystEngComm 2017, 19, 1509–1516. [Google Scholar] [CrossRef] [Green Version]
- Chikoidze, E.; Fellous, A.; Perez-Tomas, A.; Sauthier, G.; Tchelidze, T.; Ton-That, C.; Thanh Huynh, T.; Phillips, M.; Russell, S.; Jennings, M.; et al. P-type β-gallium oxide: A new perspective for power and optoelectronic devices. Mat. Today Phys. 2017, 3, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Fujita, S.; Oda, M.; Kaneko, K.; Hitora, T. Evolution of corundum-structured III-oxide semiconductors: Growth, properties, and devices. Jpn. J. Appl. Phys. 2016, 55, 1202A3. [Google Scholar] [CrossRef] [Green Version]
- Zhuiykov, S.; Karbalaei Akbari, M.; Hai, Z.; Xue, C.; Xu, H.; Hyde, L. Data set for fabrication of conformal two-dimensional TiO2 by atomic layer deposition using tetrakis (dimethylamino) titanium (TDMAT) and H2O precursors. Data Br. 2017, 13, 401–407. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Verpoort, F.; Zhuiykov, S. Plasma-enhanced elemental enrichment of liquid metal interfaces: Towards realization of GaS nanodomains in two-dimensional Ga2O3. Appl. Mater Today 2022, 27, 101461. [Google Scholar] [CrossRef]
- Yao, Y.; Okur, S.; Lyle, L.A.M.; Tompa, G.S.; Salagaj, T.; Sbrockey, N.; Davis, R.F.; Porter, L.M. Growth and characterization of α-, β-, ε-phases of Ga2O3 using MOCVD and HVPE techniques. Mater. Res. Lett. 2018, 6, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Mahi, A.; Mahi, F.Z.; Abbes, A. Systematic analysis of semiconductor photoconductivity dynamics under different laser excitations: Two- and three-level models. J. Comput. Electron 2023, 22, 296–309. [Google Scholar] [CrossRef]
- Naik, G.V.; Boltasseva, A. A comparative study of semiconductor-based plasmonic metamaterials. Metamaterials 2011, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Karbalaei Akbari, M.; Wang, S.; Chen, S.; Kats, E.; Verpoort, F.; Hue, J.; Zhuiyko, S. Tunability of near infrared opto-synaptic properties of thin MoO3 films fabricated by atomic layer deposition. Appl. Surf. Sci. 2022, 539, 153399. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Ramachandran, R.K.; Detavernier, C.; Hu, J.; Kim, J.; Verpoort, F.; Zhuiykov, S. Heterostructured plasmonic memristors with tunable optosynaptic functionalities. J. Mater. Chem. C 2021, 9, 2539–2549. [Google Scholar] [CrossRef]
- Salonikidou, B.; Yasunori, T.; Le Borgne, B.; England, J.; Shizuo, T.; Sporea, R.A. Toward fully printed memristive elements: a-TiO2 electronic synapse from functionalized nanoparticle ink. ACS Appl. Electron. Mater. 2019, 1, 2692–2700. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Karbalaei Akbari, M. Metal/semiconductor hetero-interface engineering for photocurrent controlling in plasmonic photodetectors. In Proceedings of the SMSI 2021—Sensors and Instrumentation, Online, 3–6 May 2021; AMA Service Gmbh: Nuremberg, Germany, 2021; pp. 165–166. [Google Scholar]
- Auer, E.; Lugstein, A.; Loffler, S.; Hyun, Y.J.; Brezna, W.; Bertagnolli, E.; Pongratz, P. Ultrafast VLS growth of epitaxial β-Ga2O3 nanowires. Nanotechnology 2009, 20, 434017. [Google Scholar] [CrossRef] [PubMed]
- Phumying, S.; Labauyai, S.; Chareonboon, W.; Phokha, S.; Maensiri, S. Optical properties of β-Ga2O3 nanorods synthesized by a simple and cost-effective method using egg white solution. Jpn. J. Appl. Phys. 2015, 54, 06FJ13. [Google Scholar] [CrossRef]
- Cuscó, R.; Domènech-Amador, N.; Hatakeyama, T.; Yamaguchi, T.; Honda, T.; Artús, L. Lattice dynamics of a mistchemical vapor deposition-grown corundum-like Ga2O3 single crystal. J. Appl. Phys. 2015, 117, 185706. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Cheng, Y.; Li, Z.; Cheng, Y.N.; Feng, Q.; Chen, D.; Zhang, J.; Hao, Y. Influence of carrier gases on the quality of epitaxial corundum-structured α-Ga2O3 films grown by mist chemical vapor deposition method. Materials 2019, 12, 3670. [Google Scholar] [CrossRef] [Green Version]
- Kranert, C.; Sturm, C.; Schmidt-Grund, R. Raman tensor elements of β-Ga2O3. Sci. Rep. 2016, 6, 35964. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Varshney, M.; Saraswat, H.; Chaudhary, S.; Parkash, J.; Shin, H.-J.; Chae, K.-H.; Won, S.-O. Nano-structured phases of gallium oxide (GaOOH, α-Ga2O3, β-Ga2O3, γ-Ga2O3, δ-Ga2O3, and ε-Ga2O3): Fabrication, structural, and electronic structure investigations. Int. Nano Lett. 2020, 10, 71–79. [Google Scholar] [CrossRef]
- Achoura, A.; Islamb, M.; Solaymanic, S.; Vizireanud, S.; Saeede, K.; Dinescu, G. Influence of plasma functionalization treatment and gold nanoparticles on surface chemistry and wettability of reactive-sputtered TiO2 thin films. Appl. Surf. Sci. 2018, 458, 678–685. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Duan, P.; Sun, X.; Chu, B.; He, Q. Properties and photocatalytic activity of 𝛽-Ga2O3 nanorods under simulated solar irradiation. J. Nanomater. 2015, 16, 126. [Google Scholar]
- Shi, F.; Han, J.; Xing, Y.; Li, J.; Zhang, L.; He, T.; Li, T.; Deng, X.; Zhang, X.; Zhang, B. Annealing effects on properties of Ga2O3 films deposited by plasma-enhanced atomic layer deposition. Mater. Lett. 2019, 237, 105–108. [Google Scholar] [CrossRef]
- Passlack, M.; Schubert, E.F.; Hobson, W.S.; Hong, M.; Moriya, N.; Chu, S.N.G.; Konstadinidis, K.; Mannaerts, J.P.; Schnoes, M.L.; Zydzik, G.J. Ga2O3 films for electronic and optoelectronic applications. J. Appl. Phys. 1995, 77, 686–693. [Google Scholar] [CrossRef]
- Remple, C.; Huso, J.; McCluskey, M.D. Photoluminescence and Raman mapping of β-Ga2O3. AIP Advances 2021, 11, 105006. [Google Scholar] [CrossRef]
- Mi, W.; Luan, C.; Li, Z.; Zhao, C.; Feng, X.; Ma, J. Ultraviolet–green photoluminescence of ß-Ga2O3 films deposited on MgAl6O10 (100) substrate. Opt. Mater. 2013, 35, 2624–2628. [Google Scholar] [CrossRef]
- Hao, J.H.; Cocivera, M. Optical and luminescent properties of undoped and rare-earth-doped Ga2O3 thin films deposited by spray pyrolysis. J. Phys. D: Appl. Phys. 2002, 35, 433. [Google Scholar] [CrossRef]
- Lorenz, M.R.; Woods, J.F.; Gambino, R.J. Some electrical properties of the semiconductor β-Ga2O3. J. Phys. Chem. Solids 1967, 28, 403. [Google Scholar] [CrossRef]
- Chikoidze, E.; Sartel, C.; Mohamed, H.; Madaci, I.; Tchelidze, T.; Modreanu, M.; Vales-Castro, P.; Rubio, C.; Arnold, C.; Sallet, V.; et al. Enhancing the intrinsic p-type conductivity of the ultra-wide bandgap Ga2O3 semiconductor. J. Mater. Chem. C 2019, 7, 10231–10239. [Google Scholar] [CrossRef]

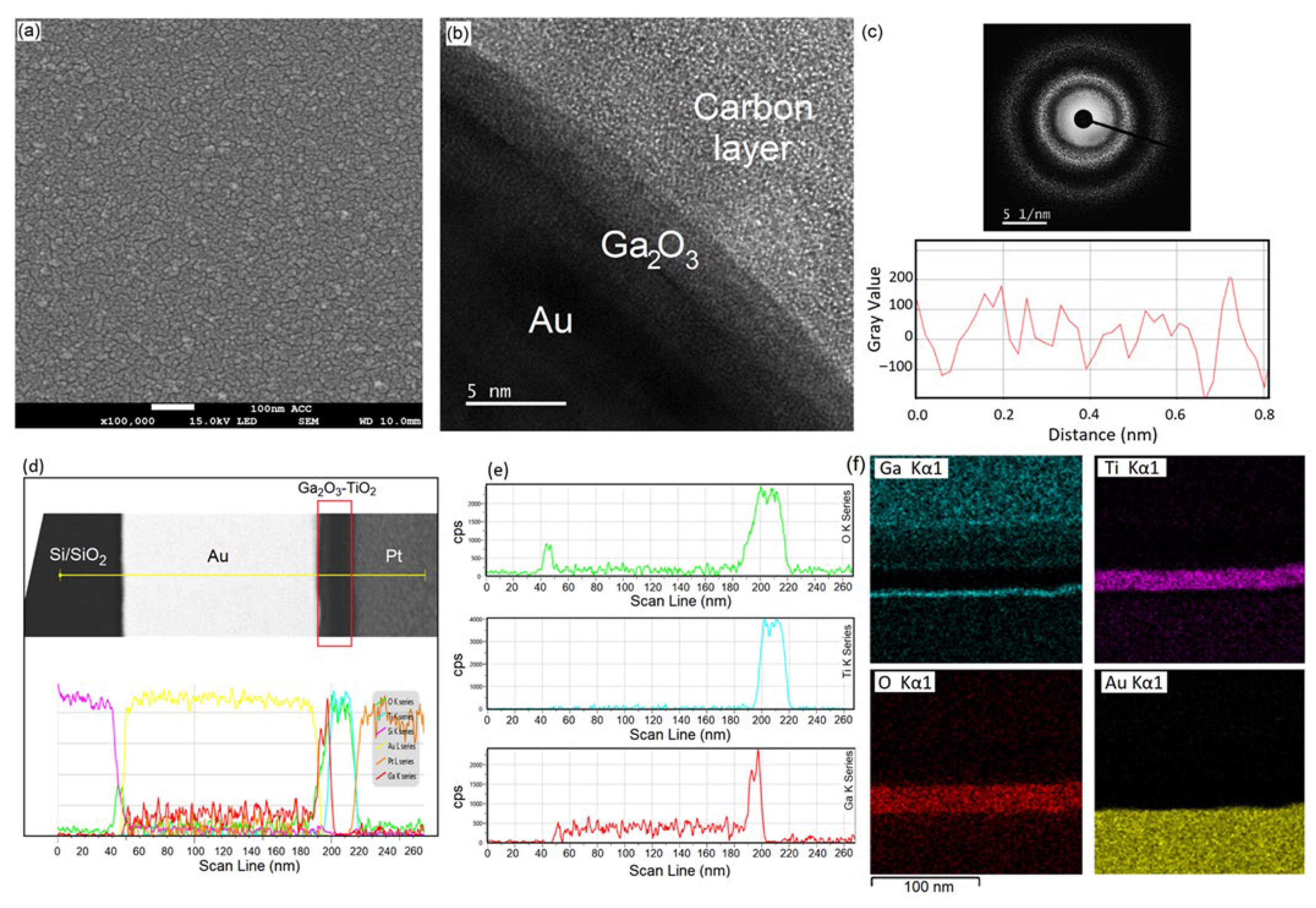
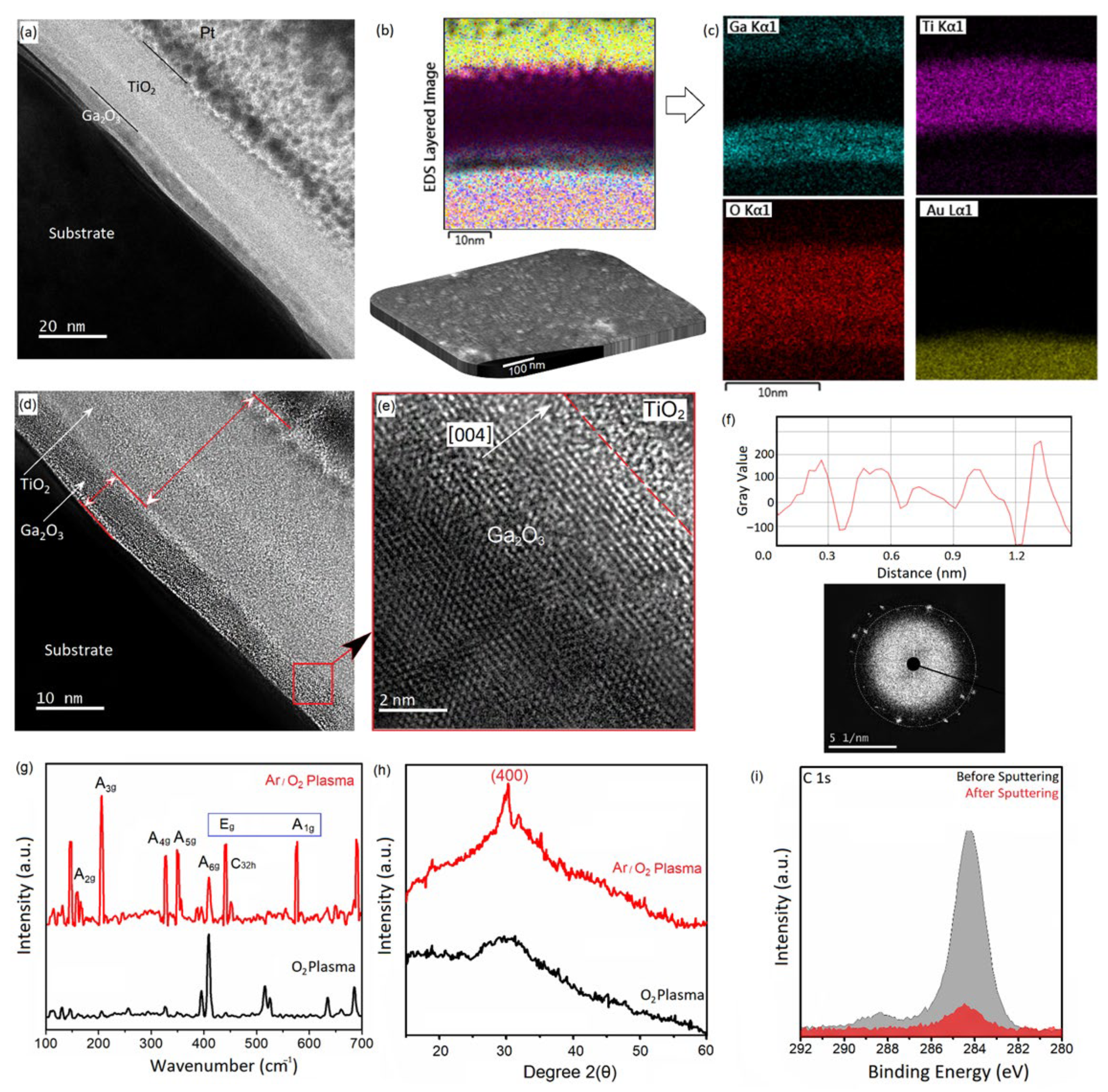
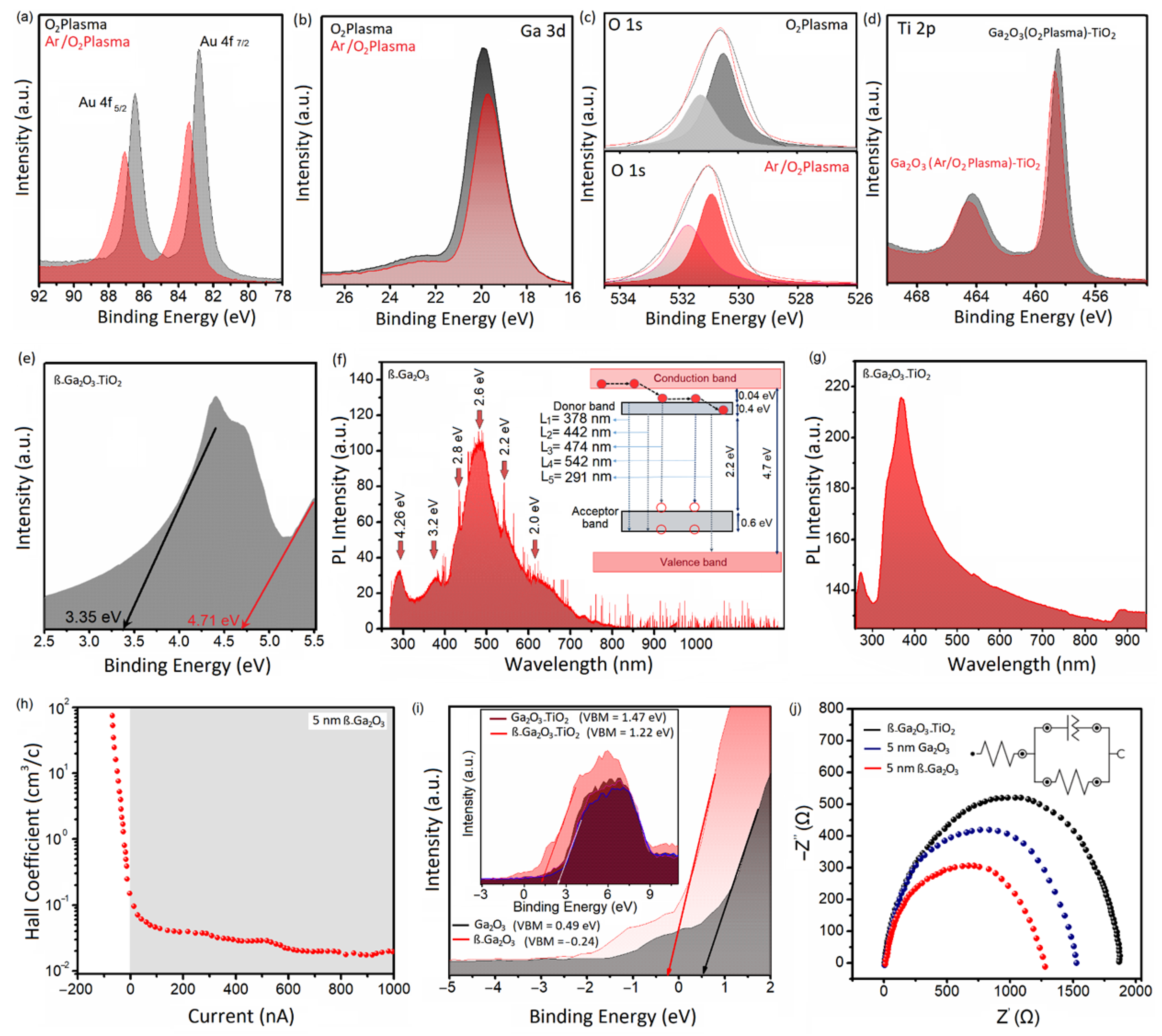
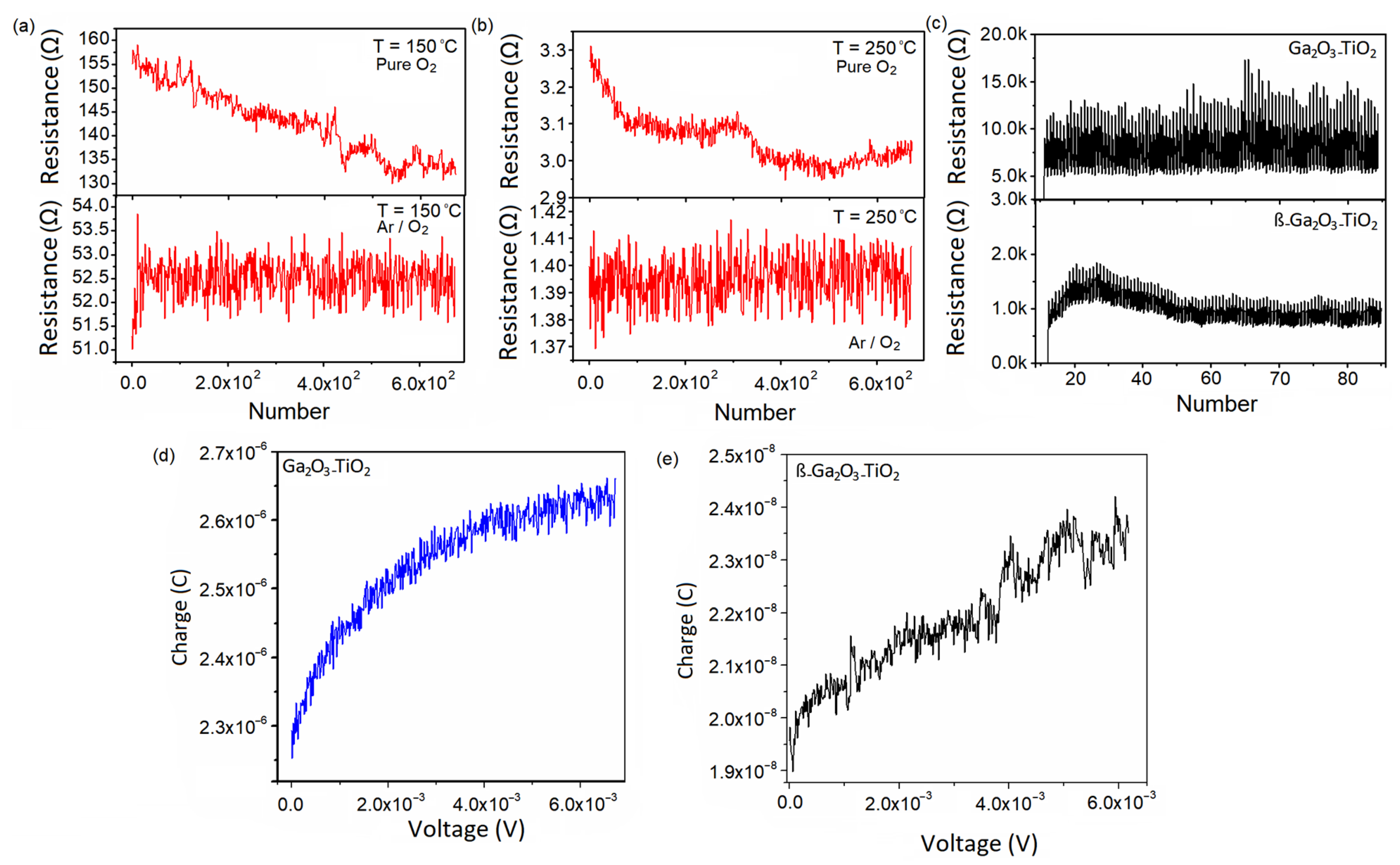
| Film | ALD Temperature | Plasma Atmosphere | Structure | Phases (Raman) | Ga/O Ratio (XPS) |
|---|---|---|---|---|---|
| Ga2O3 (5 nm) | 150 °C | Pure O2 | Amorphous | ß and ε | 0.68 |
| Ga2O3 (5 nm) | 150 °C | Ar/O2 (4:1 ratio) | Amorphous | ß and ε | 0.67 |
| Ga2O3 (5 nm) | 250 °C | Pure O2 | Semicrystalline | ß and ε | 0.69 |
| Ga2O3 (5 nm) | 250 °C | Ar/O2 (4:1 ratio) | Crystalline | Mostly ß | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbalaei Akbari, M.; Siraj Lopa, N.; Zhuiykov, S. Atomic Layer Deposition of Ultra-Thin Crystalline Electron Channels for Heterointerface Polarization at Two-Dimensional Metal-Semiconductor Heterojunctions. Coatings 2023, 13, 1041. https://doi.org/10.3390/coatings13061041
Karbalaei Akbari M, Siraj Lopa N, Zhuiykov S. Atomic Layer Deposition of Ultra-Thin Crystalline Electron Channels for Heterointerface Polarization at Two-Dimensional Metal-Semiconductor Heterojunctions. Coatings. 2023; 13(6):1041. https://doi.org/10.3390/coatings13061041
Chicago/Turabian StyleKarbalaei Akbari, Mohammad, Nasrin Siraj Lopa, and Serge Zhuiykov. 2023. "Atomic Layer Deposition of Ultra-Thin Crystalline Electron Channels for Heterointerface Polarization at Two-Dimensional Metal-Semiconductor Heterojunctions" Coatings 13, no. 6: 1041. https://doi.org/10.3390/coatings13061041





