Inhibition of Surface Corrosion Behavior of Zinc-Iron Alloy by Silicate Passivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Passivation Treatment
2.2. Microstructure Characterization
2.3. Evaluation of Corrosion Performance
3. Results and Discussion
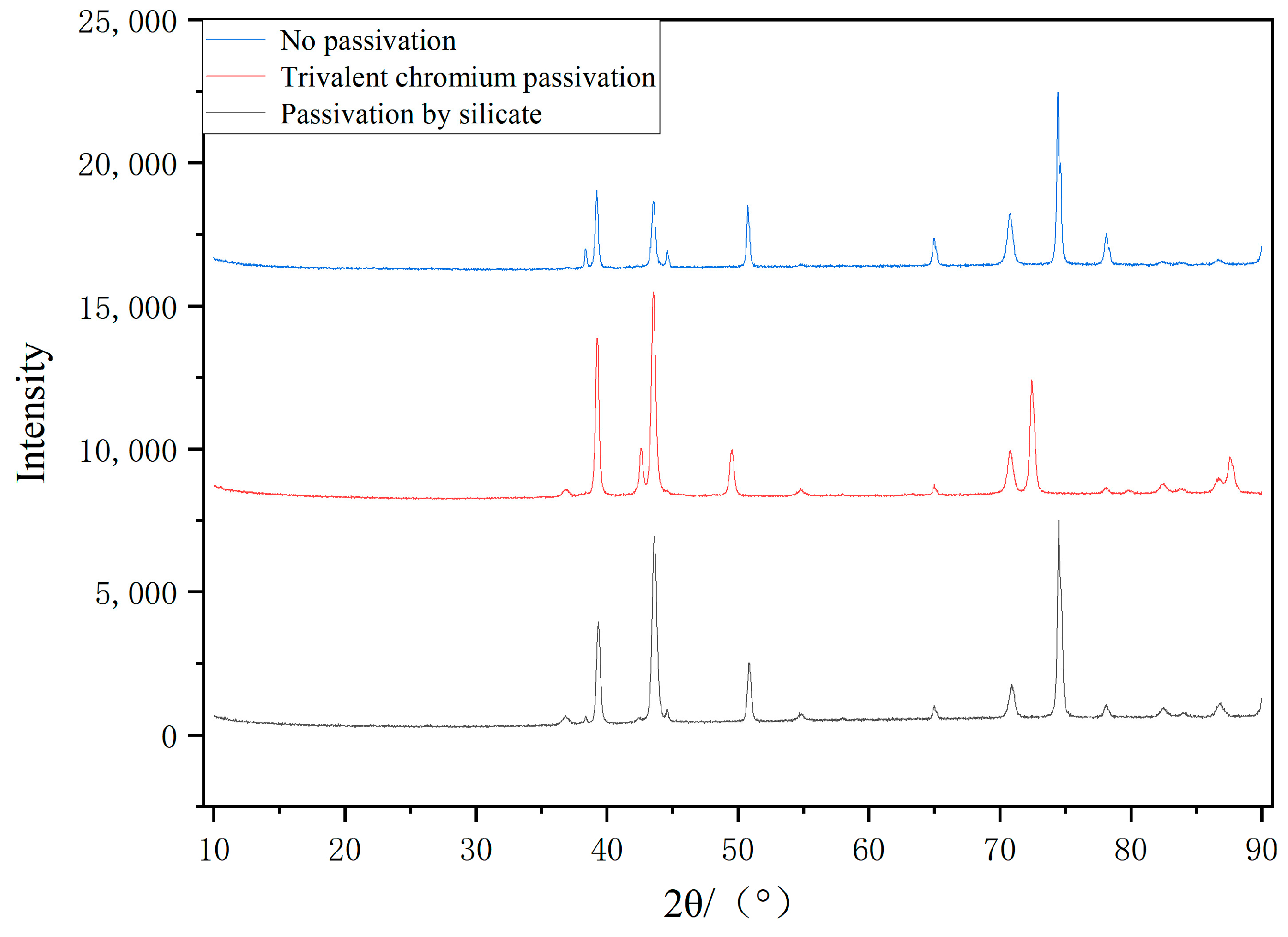
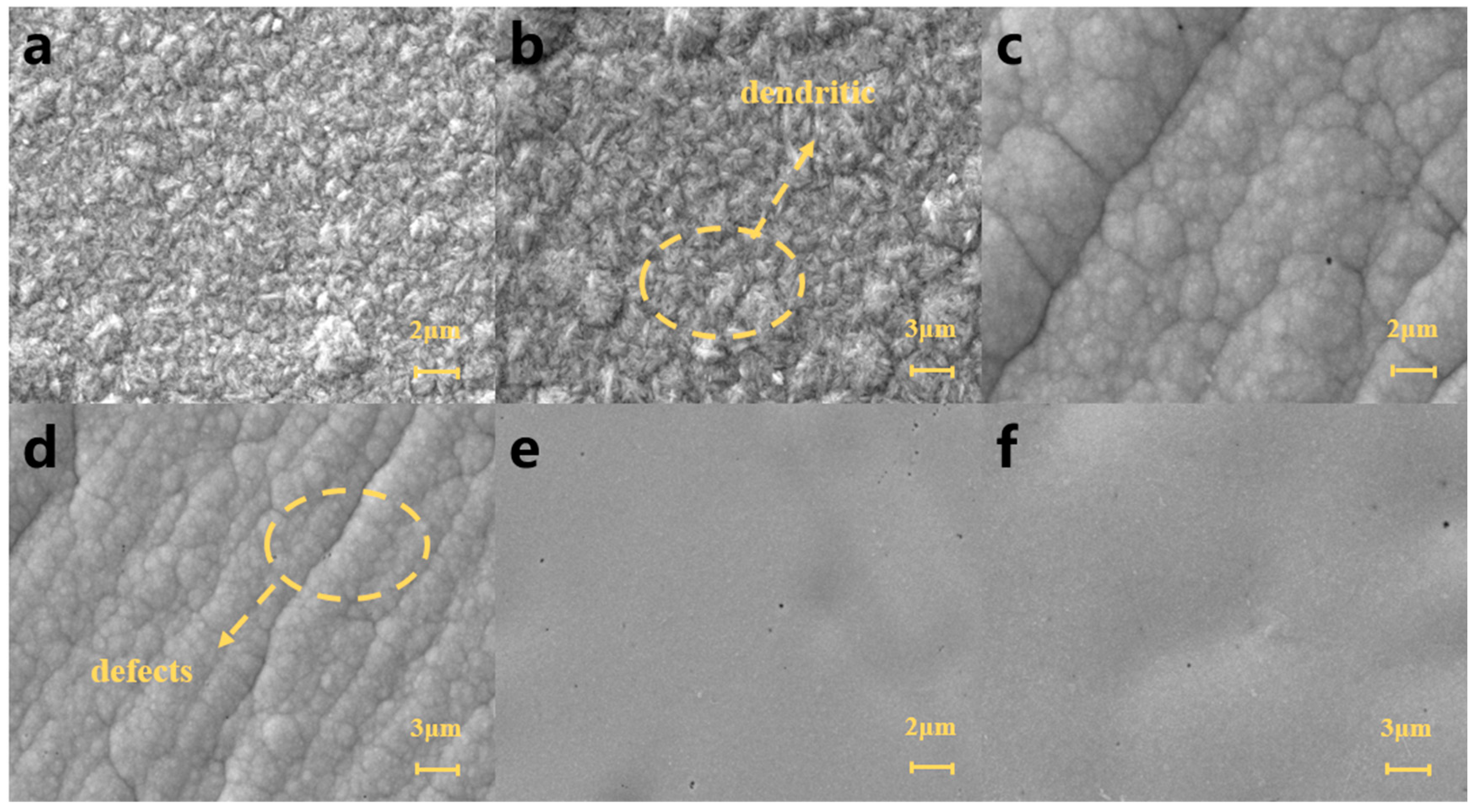
3.1. Surface Morphology and Composition Analysis
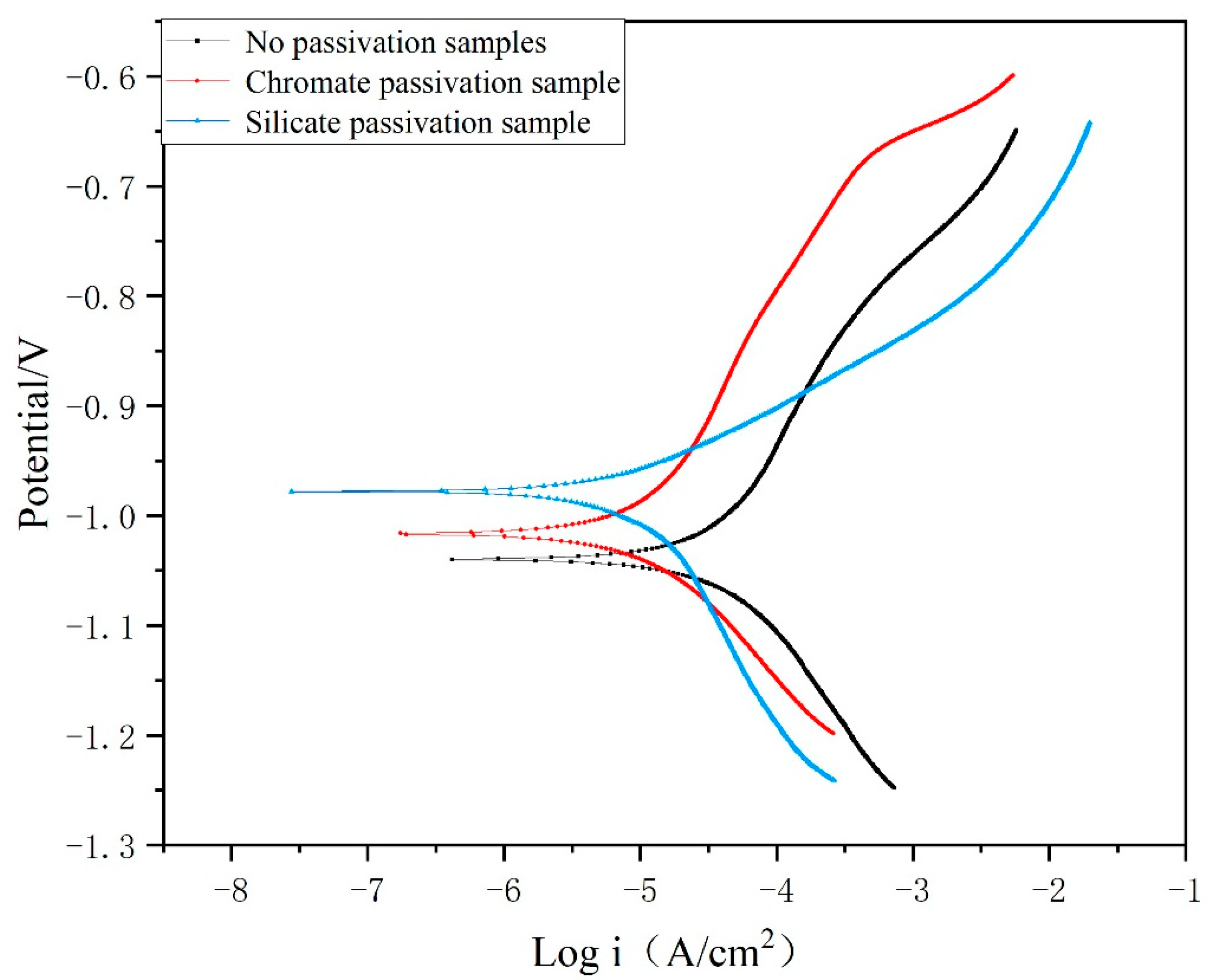
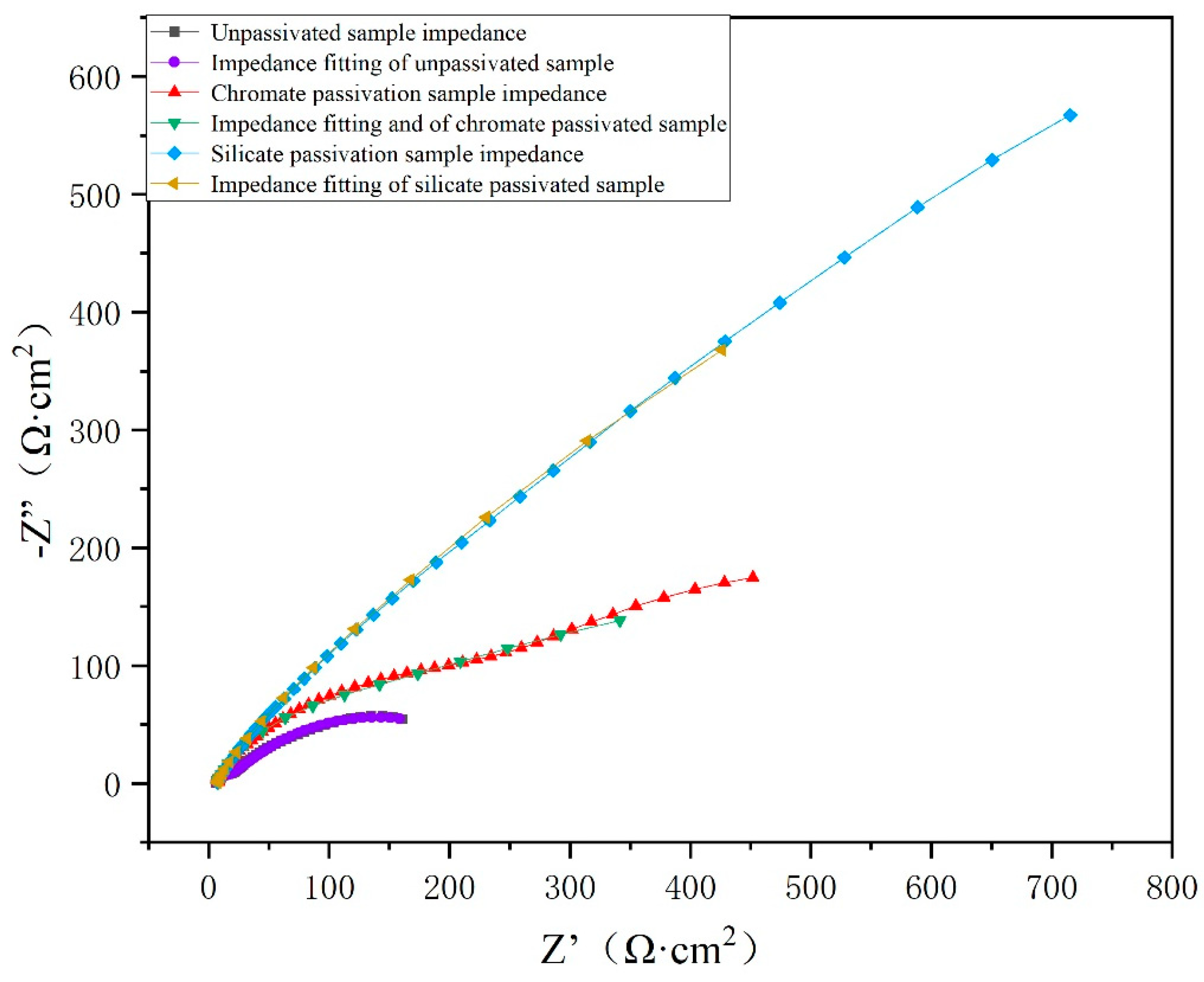
3.2. Corrosion Resistance of Passivation Film
3.2.1. Polarization Curve
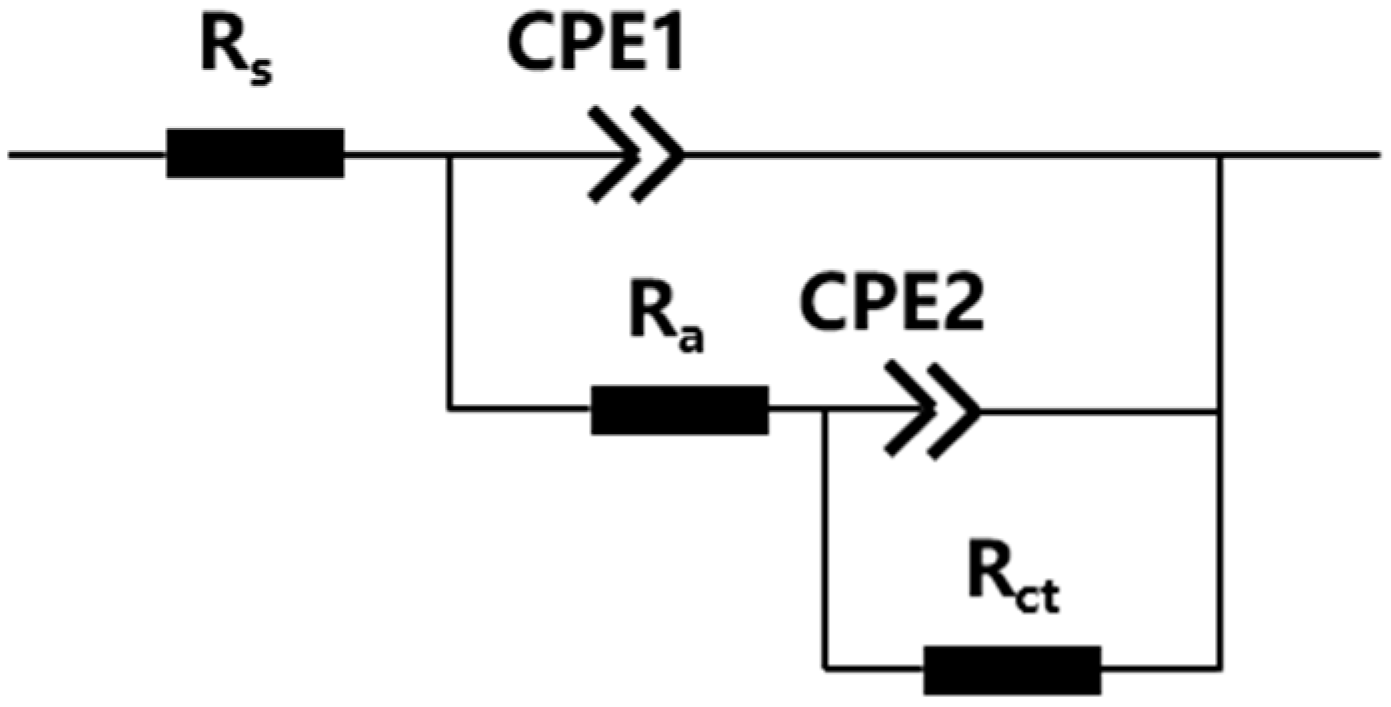
3.2.2. Impedance Study
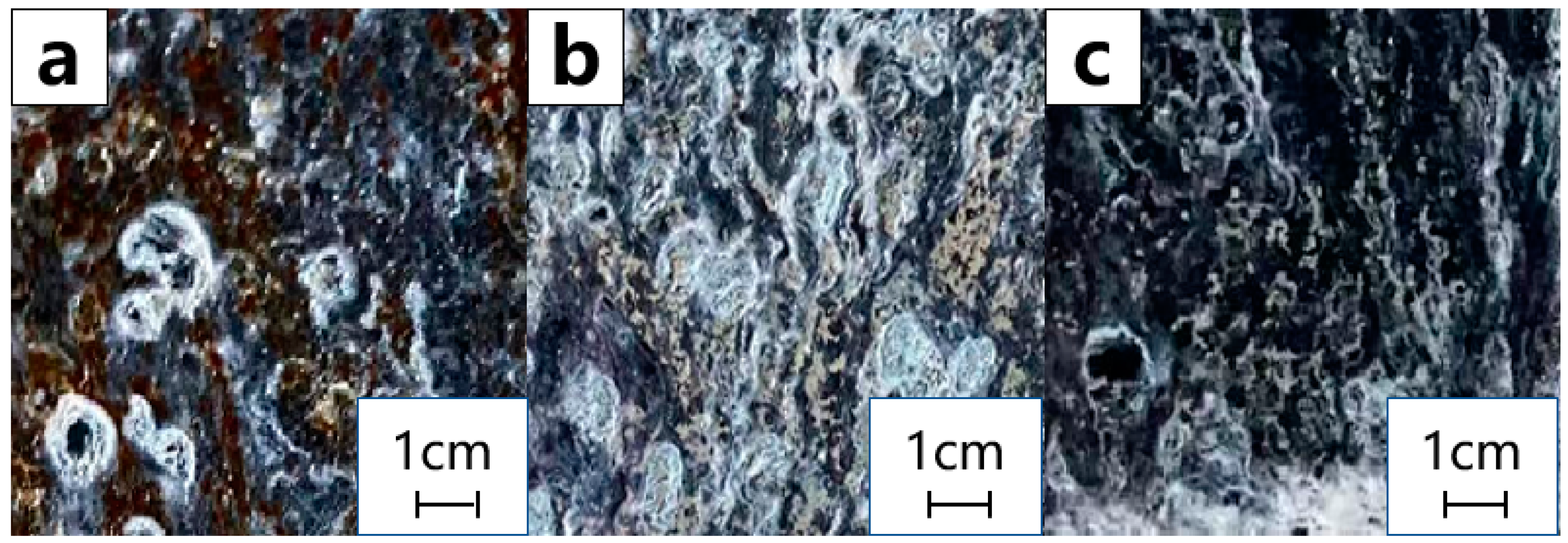
3.2.3. Salt Spray Test to Analyse Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, G.; Lu, J.; Zhang, S.; Che, C.; Wu, H. A comparative study of molybdate/silane composite films on galvanized steel with different treatment processes. Surf. Coat. Technol. 2010, 205, 545–550. [Google Scholar] [CrossRef]
- Long, H.; Chen, L.; Dong, B.; Sun, Y.; Yan, Y.; Chen, C. The electronic properties and surface chemistry of passive film on reinforcement: Effect of composition of simulated concrete pore solution. Constr. Build. Mater. 2022, 360, 129567. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, E.; Wu, L.; Tang, A.; Atrens, A.; Pan, F. Active corrosion protection of phosphate loaded PEO/LDHs composite coatings: SIET study. J. Magnes. Alloys 2022, 10, 1351–1357. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Kan, X.; Wang, Z.; Zhu, R.; Yang, W.; Wu, Q.; Shezad, M. Phosphate coatings evolution study and effects of ultrasonic on soft magnetic properties of FeSiAl by aqueous phosphoric acid solution passivation. J. Alloys Compd. 2019, 783, 434–440. [Google Scholar] [CrossRef]
- Sheu, H.H.; Lee, H.B.; Jian, S.Y.; Hsu, C.Y.; Lee, C.Y. Investigation on the corrosion resistance of trivalent chromium conversion passivate on electroplated Zn–Ni alloy. Surf. Coat. Technol. 2016, 305, 241–248. [Google Scholar] [CrossRef]
- Yuan, M.; Lu, J.; Kong, G.; Che, C. Self healing ability of silicate conversion coatings on hot dip galvanized steels. Surf. Coat. Technol. 2011, 205, 4507–4513. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Kumar, A. Recent innovations in nano container-based self-healing coatings in construction industry. Curr. Nanosci. 2022, 18, 203–216. [Google Scholar] [CrossRef]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: Implications on human health and remediation strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Y.; Lin, C. High-temperature oxidation resistance of hot stamping steel with chromium coating electroplated in trivalent chromium bath. Mater. Today Commun. 2022, 33, 104663. [Google Scholar] [CrossRef]
- Winn, D.; Dalton, W. Chromium-free corrosion solutions: Silica-based electrolytic method offers viable alternative to both hex- and trivalent-chromate passivates. Met. Finish. 2008, 106, 70–74. [Google Scholar] [CrossRef]
- Yang, T.; Chen, W.; Li, X.; Song, J.; Dong, L.; Fu, Y. Environment-friendly and chromium-free passivation of copper and its alloys. Mater. Today Commun. 2021, 29, 102826. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Zhou, W.; Ma, R.; Du, A.; Fan, Y.; Zhao, X.; Cao, X. Improved anti-corrosion behaviour of an inorganic passive film on hot-dip galvanised steel by modified graphene oxide incorporation. Corros. Sci. 2020, 174, 108846. [Google Scholar] [CrossRef]
- Safaruddin, A.; Bermundo, J.; Jallorina, M.; Yamamoto, A.; Uraoka, Y. Spray pyrolyzed fluorinated inorganic-organic passivation for solution-processed a-InZnO thin-film transistors. Mater. Sci. Semicond. Process. 2022, 146, 106669. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Li, J.; Cheng, J.; Li, Y.; Ko, M.; Zhao, Y.; Zhang, X. Inorganic material passivation of defects toward efficient perovskite solar cells. Sci. Bull. 2020, 65, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Banczek, E.P.; Terada, M.; Rodrigues, P.R.P.; Costa, I. Study of an alternative phosphate sealer for replacement of hexavalent chromium. Surf. Coat. Technol. 2010, 205, 2503–2510. [Google Scholar] [CrossRef]
- Guo, S.; Xu, D.; Jiang, G.; Guo, Y.; Jing, Z. Sulfate corrosion and phosphate passivation of Ni-based alloy in supercritical water. J. Supercrit. Fluids 2022, 184, 105564. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, R.; Du, A.; Zhang, X.; Fan, Y.; Zhao, X.; Zhang, S.; Cao, X. Investigation on structure and corrosion resistance of complex inorganic passive film based on graphene oxide. Corros. Sci. 2019, 150, 64–75. [Google Scholar] [CrossRef]
- Lei, S.; Wang, S.; Zhong, H.; Ma, X.; Cao, Z. Performance and corrosion resistance mechanism of SA-Al composite hydrophobic coating on electrolytic manganese surface. Surf. Coat. Technol. 2021, 419, 127290. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Roostaei, M.; Mahmoudi, M.; Fattahpour, V.; Zeng, H.; Luo, J. Characterization and corrosion behavior of electroless Ni-Mo-P/Ni-P composite coating in CO2/H2S/Cl− brine: Effects of Mo addition and heat treatment. Surf. Coat. Technol. 2020, 403, 126416. [Google Scholar] [CrossRef]
- Firouzi-Nerbin, H.; Nasirpouri, F.; Moslehifard, E. Pulse electrodeposition and corrosion properties of nanocrystalline nickel-chromium alloy coatings on copper substrate. J. Alloys Compd. 2020, 822, 153712. [Google Scholar] [CrossRef]
- Foster, K.; Claypool, J.; Fahrenholtz, W.G.; O’Keefe, M.; Nahlawi, T.; Almodovar, F. Characterization of cobalt containing and cobalt-free trivalent chromium passivations on γ-ZnNi coated steel substrates. Thin Solid Films 2021, 735, 138894. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Malathy, M.; Rajavel, R. Poly(ophenylenediaminecoaniline)/ZnO coated on passivated low nickel stainless steel. J. Sci. Adv. Mater. Devices 2017, 2, 86–92. [Google Scholar] [CrossRef]
- Silva, J.; Torresi, S.; Torresi, R. Polyaniline/poly (methylmethacrylate) blends for corrosion protection: The effect of passivating dopants on different metals. Prog. Org. Coat. 2007, 58, 33–39. [Google Scholar] [CrossRef]
- Qin, B.; Ma, L.; Wang, S.; Hou, C.; Zhou, S. Construction of a sandwich-like Gr/Ni composite coating on AZ91D magnesium alloy to achieve excellent corrosion and wear resistances in the seawater. Diam. Relat. Mater. 2022, 130, 109400. [Google Scholar] [CrossRef]
- Xu, R.; He, T.; Yang, R.; Da, Y.; Chen, C. Application zinc silicate-potassium silicate coating for anticorrosion of steel bar in autoclaved aerated concrete. Constr. Build. Mater. 2020, 237, 117521. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Wei, B.; Liu, Q. Electrochemical performance of microarc oxidation films formed on AZ91D magnesium alloy in silicate and phosphate electrolytes. Surf. Coat. Technol. 2006, 200, 3727–3733. [Google Scholar] [CrossRef]
- Oliveira, L.; Correa, O.; Santos, D.; Páez, A.; Oliveira, M.; Antunes, R. Effect of silicate-based films on the corrosion behavior of the API 5L X80 pipeline steel. Corros. Sci. 2018, 139, 21–34. [Google Scholar] [CrossRef]
- Yuan, M.; Lu, J.; Kong, G. Effect of SiO2:Na2O molar ratio of sodium silicate on the corrosion resistance of silicate conversion coatings. Surf. Coat. Technol. 2010, 204, 1229–1235. [Google Scholar] [CrossRef]
- Wu, T.; Blawert, C.; Serdechnova, M.; Karlova, P.; Dovzhenko, G.; Wieland, D.C.F.; Stojadinovic, S.; Vasilic, R.; Wang, L.; Wang, C.; et al. Role of phosphate, silicate and aluminate in the electrolytes on PEO coating formation and properties of coated Ti6Al4V alloy. Appl. Surf. Sci. 2022, 595, 153523. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, X.; Zheng, L.; Ye, P.; Li, M.; Shen, L.; Li, J.; Zhang, D.; Gu, Z.; Yu, Y. Enhanced interfacial and electrical characteristics of 4H-SiC MOS capacitor with lanthanum silicate passivation interlayer. Appl. Surf. Sci. 2017, 410, 326–331. [Google Scholar] [CrossRef]
- Tristijanto, H.; Ilman, M.; Iswanto, P. Corrosion Inhibition of Welded of X—52 Steel Pipelines by Sodium Molybdate in 3.5% NaCl Solution. Egypt. J. Pet. 2020, 29, 155–162. [Google Scholar] [CrossRef]
- Osipenko, M.; Kharytonau, D.; Kasach, A.; Ryl, J.; Adamiec, J.; Kurilo, I. Inhibitive effect of sodium molybdate on corrosion of AZ31 magnesium alloy in chloride solutions. Electrochim. Acta 2022, 414, 140175. [Google Scholar] [CrossRef]
- Alves, A.; Wenger, F.; Ponthiaux, P.; Celis, J.; Pinto, A.; Rocha, L.; Fernandes, J. Corrosion mechanisms in titanium oxide-based films produced by anodic treatment. Electrochim. Acta 2017, 234, 16–27. [Google Scholar] [CrossRef]
- Micallef, C.; Chiu, C.; Zhuk, Y.; Aria, A. Rapid surface finishing of chemical vapour deposited tungsten carbide hard coatings by electropolishing. Surf. Coat. Technol. 2021, 428, 127900. [Google Scholar] [CrossRef]
- Tsai, C.; Liu, J.; Chen, P.; Lin, C. A roll coating tungstate passivation treatment for hot-dip galvanized sheet steel. Surf. Coat. Technol. 2011, 205, 5124–5129. [Google Scholar] [CrossRef]
- Qin, W.; Xi, X.; Zhang, L.; Wang, M.; Nie, Z. Effect of cathodic current density on the microstructure and performance of W-ZrO2 composites coating prepared in Na2WO4-WO3-ZrO2 molten salt. Surf. Coat. Technol. 2022, 440, 128497. [Google Scholar] [CrossRef]
- Jiang, L.; Wolpers, M.; Volovitch, P.; Ogle, K. An atomic emission spectroelectrochemical study of passive film formation and dissolution on galvanized steel treated with silicate conversion coatings. Surf. Coat. Technol. 2012, 206, 3151–3157. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Kumar, A. Recent Trends in the Characterization and Application Progress of Nano-Modified Coatings in Corrosion Mitigation of Metals and Alloys. Appl. Sci. 2023, 13, 730. [Google Scholar] [CrossRef]
- Dalbin, S.; Maurin, G.; Nogueira, R.; Persello, J.; Pommier, N. Silica-based coating for corrosion protection of electrogalvanized steel. Surf. Coat. Technol. 2005, 194, 363–371. [Google Scholar] [CrossRef]
- Hamlaoui, Y.; Tifouti, L.; Pedraza, F. Corrosion behaviour of molybdate–phosphate–silicate coatings on galvanized steel. Corros. Sci. 2009, 51, 2455–2462. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, S.; Guan, Q.; Li, W.; Du, J. A high anticorrosive chromium-free conversion coating prepared with an alkaline conversion bath on electroless Ni–P coating. Appl. Surf. Sci. 2015, 349, 108–115. [Google Scholar] [CrossRef]
- Sandberg, J.; Odnevall Wallinder, I.; Leygraf, C.; Le Bozec, N. Corrosion-Induced Zinc Runoff from Construction Materials in a Marine Environment. J. Electrochem. Soc. 2007, 154, 120–131. [Google Scholar] [CrossRef]
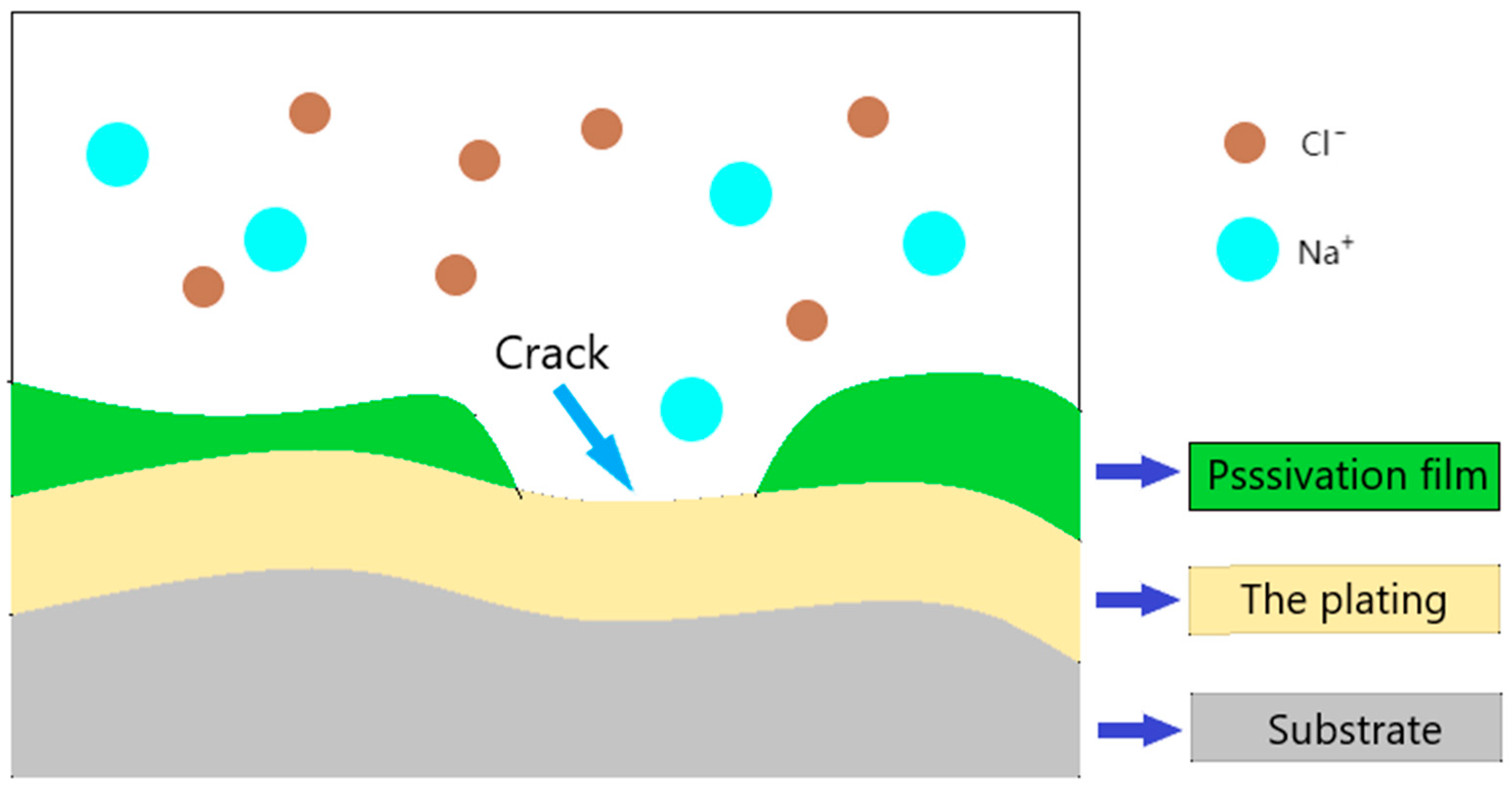
- Hou, D.; Zhang, K.; Hong, F.; Wu, S.; Wang, Z.; Li, M.; Wang, M. The corrosion deterioration of reinforced passivation Film: The impact of defects. Appl. Surf. Sci. 2022, 582, 152408. [Google Scholar] [CrossRef]
| Component | Content |
|---|---|
| ZnO | 6 g/L |
| NaOH | 160 g/L |
| SEC softener | 10 mL/L |
| SEC Complexing agent | 60 g/L |
| FeCl2 | 3 g/L |
| Ethylenediamine tetraacetic acid disodium | 0.8 g/L |
| Vanillin aldehyde | 0.04 g/L |
| Component | Content | Working Parameters |
|---|---|---|
| Sodium silicate | 25 g/L | The pH was 1 to 2, the temperature was 25 °C and the time was 30 s. |
| Cobalt nitrate | 2.5 g/L | |
| Sodium citrate | 15 g/L | |
| Tannins | 2–4 g/L | |
| Organic promoter | Right amount |
| The Surface Type | Ecorr (mV vs. SCE) | Icorr (10−2 μA cm−2) | Rp(Ω cm−2) |
|---|---|---|---|
| No passivation | −1040 | 5.597 | 739 |
| Trivalent chromium passivation | −1017 | 1.422 | 2549 |
| Passivation of silicate | −978 | 0.853 | 2679 |
| The Surface Type | Rs/Ω·cm2 | CPE1-T(10−4/Ω−1·cm−2·sn) | Rct/Ω·cm2 | Ra/Ω·cm2 | CPE2-T(10−4/Ω−1·cm−2·sn) | Rp/Ω·cm2 |
|---|---|---|---|---|---|---|
| No passivation | 6.20 | 341.31 | 249.10 | 10.48 | 11.88 | 265.78 |
| Trivalent chromium passivation | 7.20 | 27.31 | 958.80 | 109.10 | 1.52 | 1075.10 |
| Passivation of silicate | 7.04 | 1.00 | 2795.00 | 208.80 | 2.94 | 3010.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, F.; Cao, P.; Li, Y.; Wang, Y.; Shi, L.; Wu, D. Inhibition of Surface Corrosion Behavior of Zinc-Iron Alloy by Silicate Passivation. Coatings 2023, 13, 1057. https://doi.org/10.3390/coatings13061057
Cao F, Cao P, Li Y, Wang Y, Shi L, Wu D. Inhibition of Surface Corrosion Behavior of Zinc-Iron Alloy by Silicate Passivation. Coatings. 2023; 13(6):1057. https://doi.org/10.3390/coatings13061057
Chicago/Turabian StyleCao, Fan, Peng Cao, Yangyang Li, Yi Wang, Lei Shi, and Di Wu. 2023. "Inhibition of Surface Corrosion Behavior of Zinc-Iron Alloy by Silicate Passivation" Coatings 13, no. 6: 1057. https://doi.org/10.3390/coatings13061057




