Theoretical and Experimental Studies of 1-Dodecyl-3-phenylquinoxalin-2(1H)-one as a Sustainable Corrosion Inhibitor for Carbon Steel in Acidic Electrolyte
Abstract
1. Introduction
2. Experimental
2.1. Synthesized Inhibitor
2.2. CS and HCl Prepared
2.3. Experimental
2.4. SEM/EDX and UV-Visible Analysis
2.5. DFT and MD Simulation Procedure
3. Results and Discussion
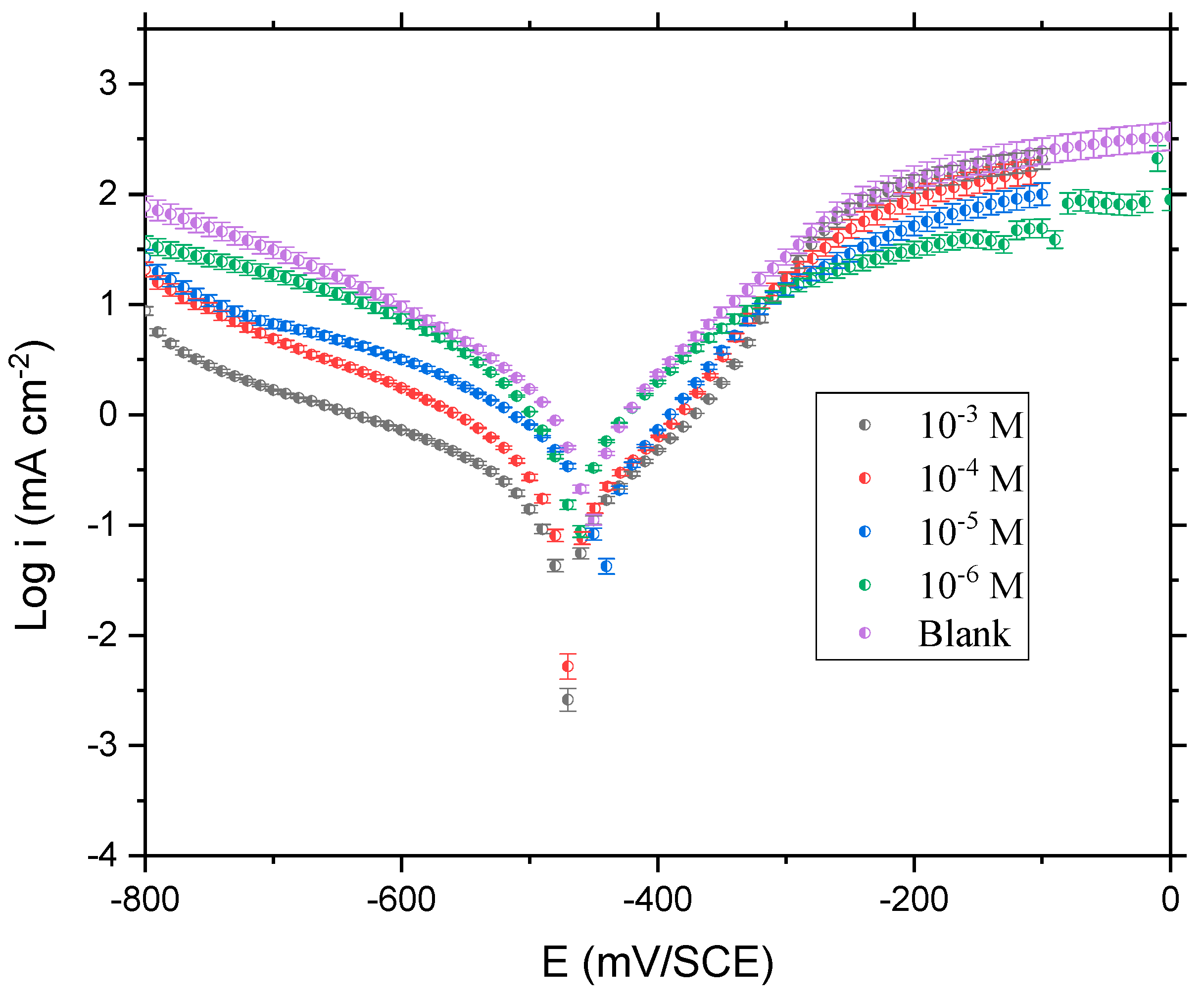
3.1. Potentiodynamic Polarization
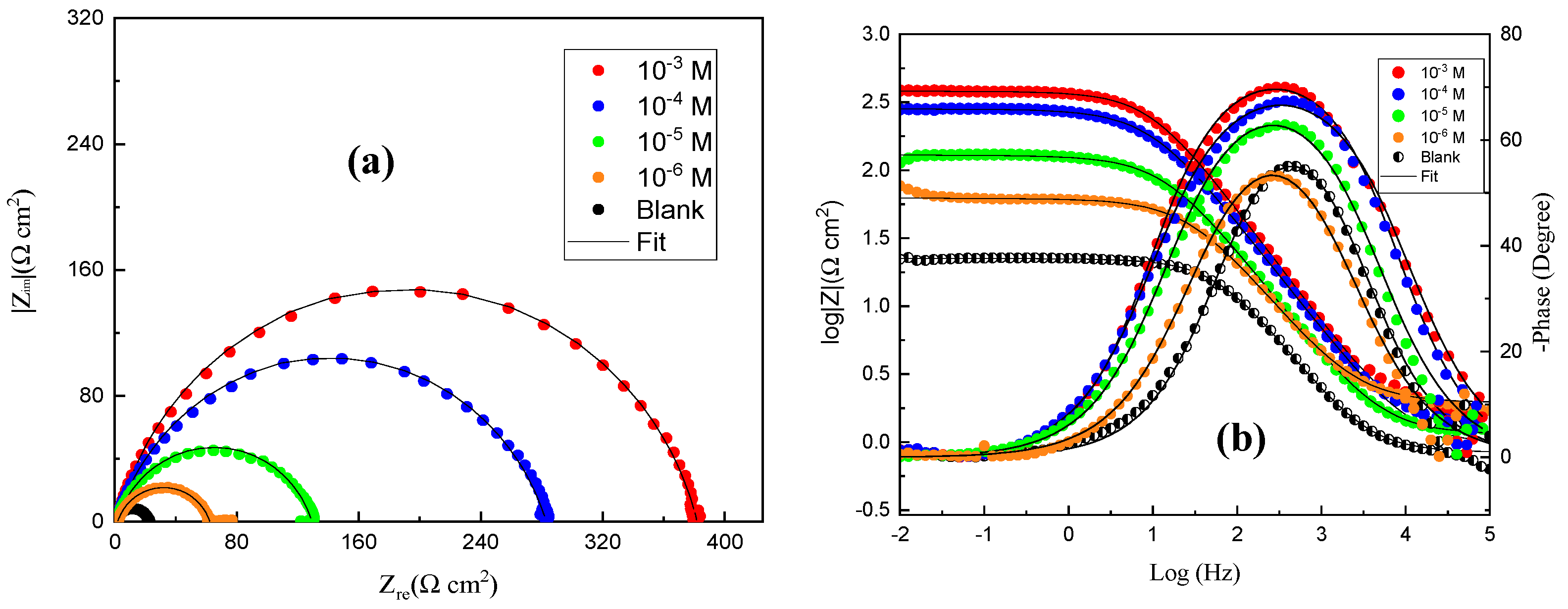
3.2. EIS Investigation
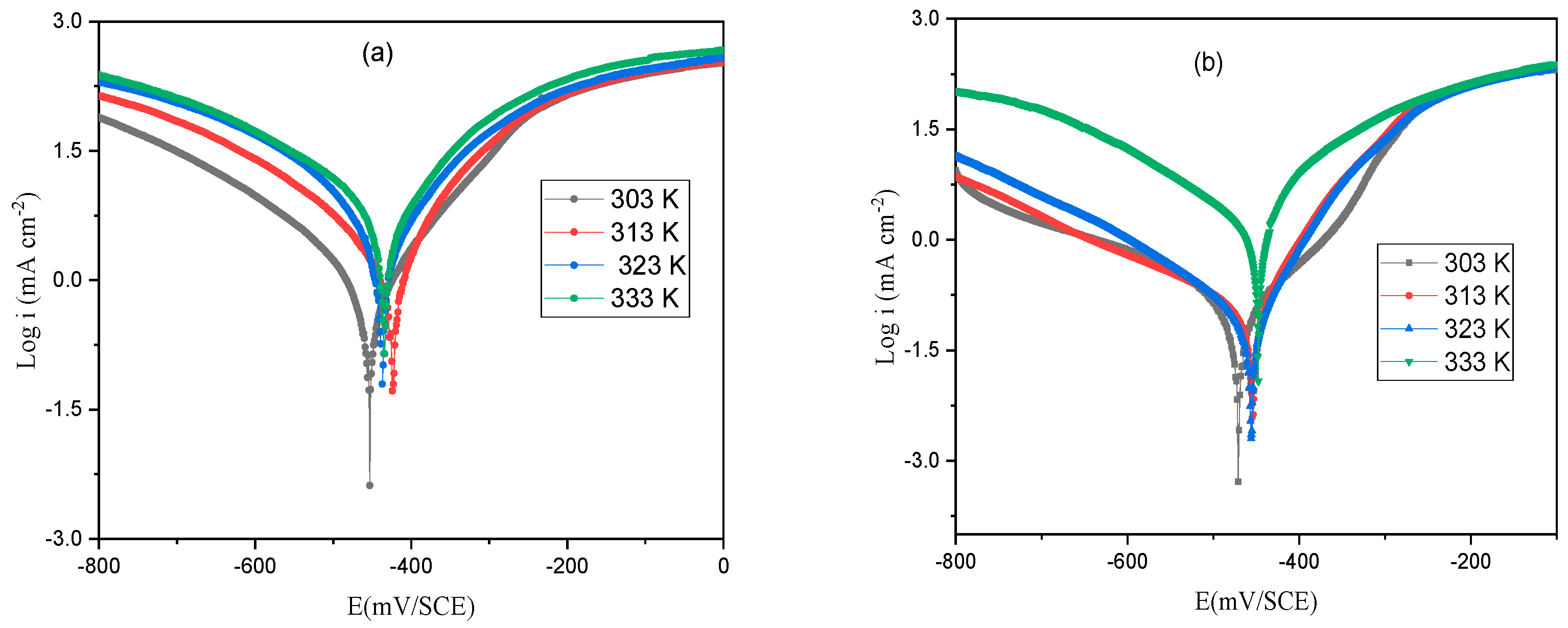
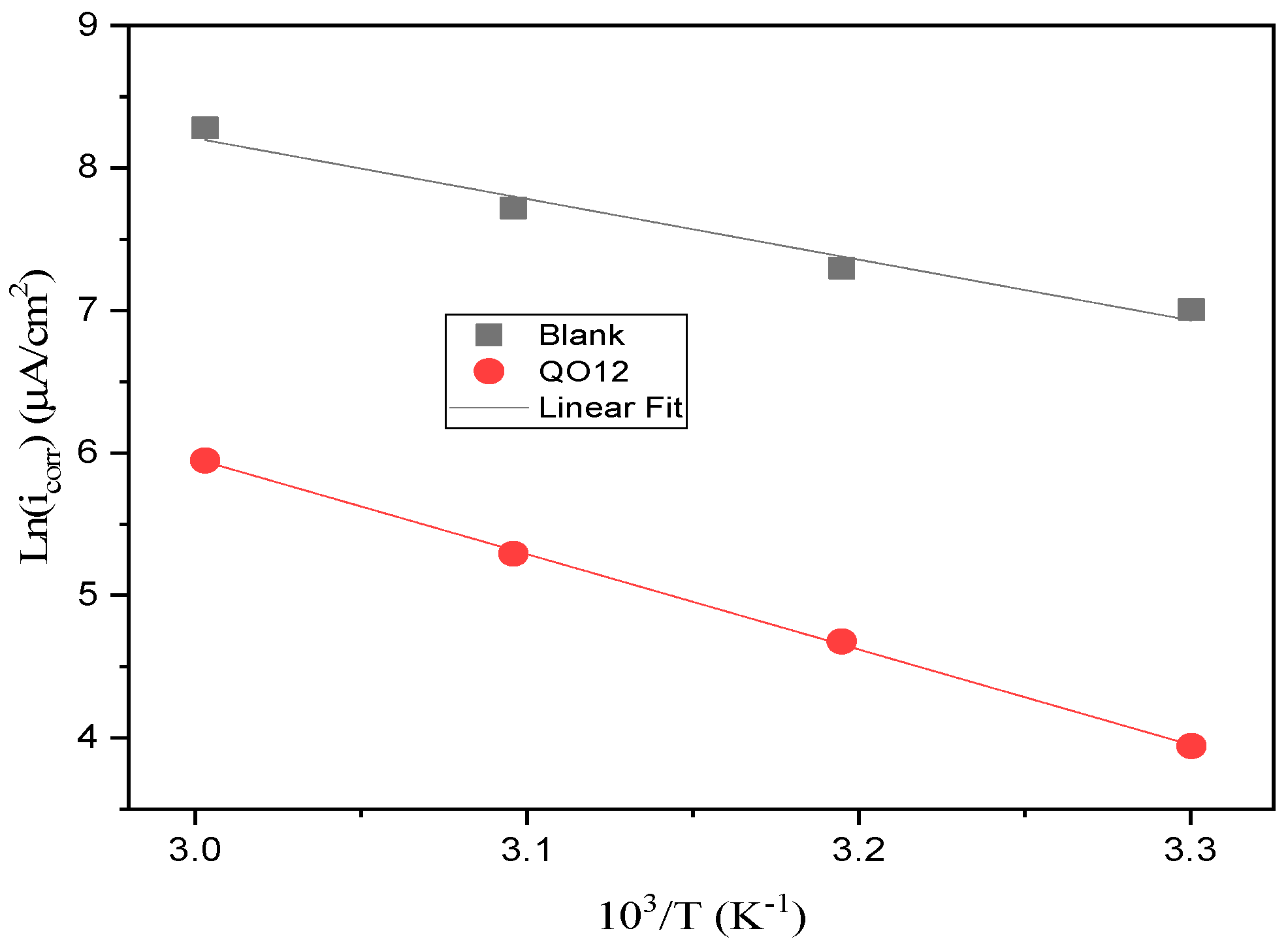
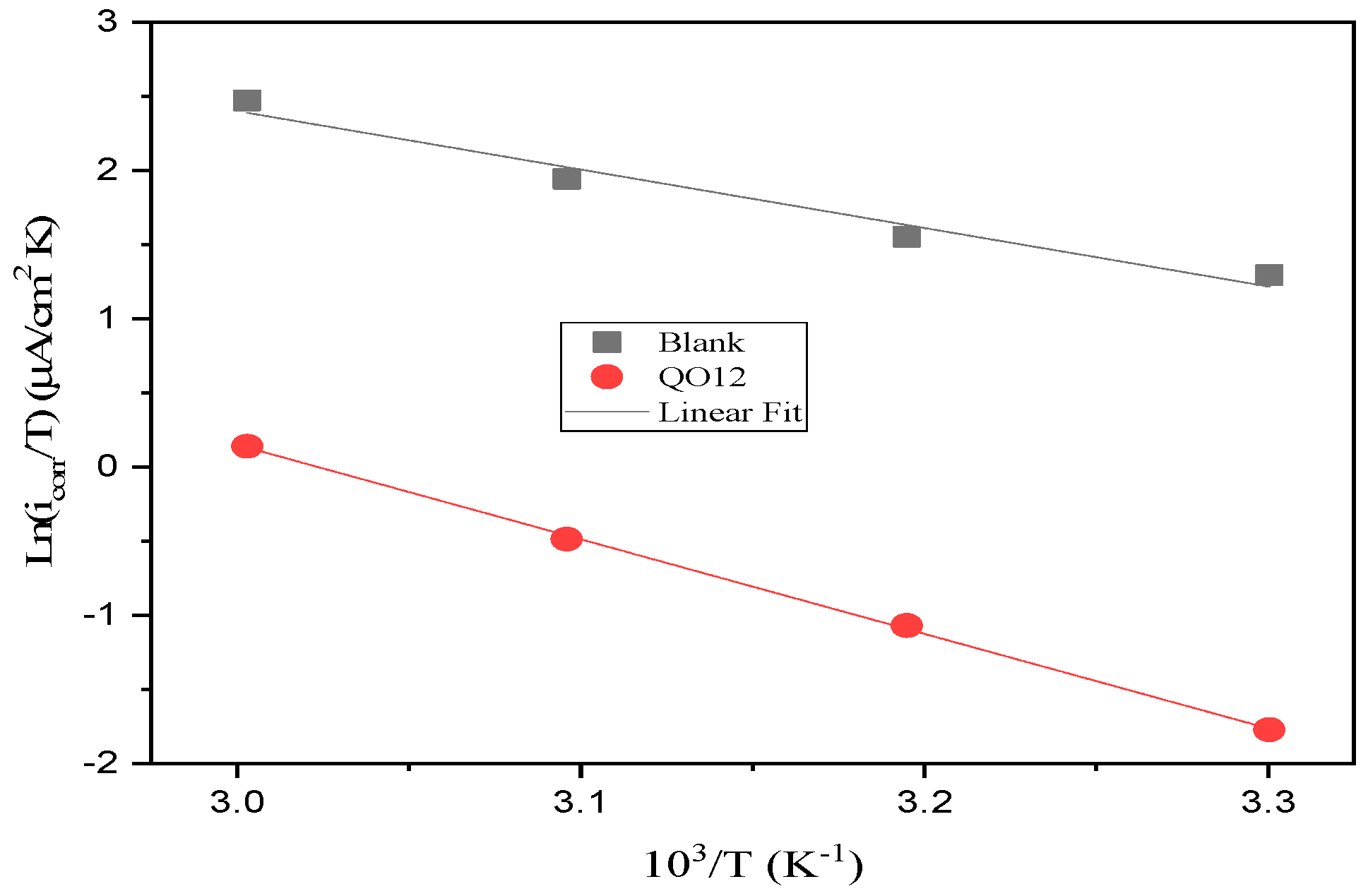
3.3. Temperature Impact and Thermodynamic Indices
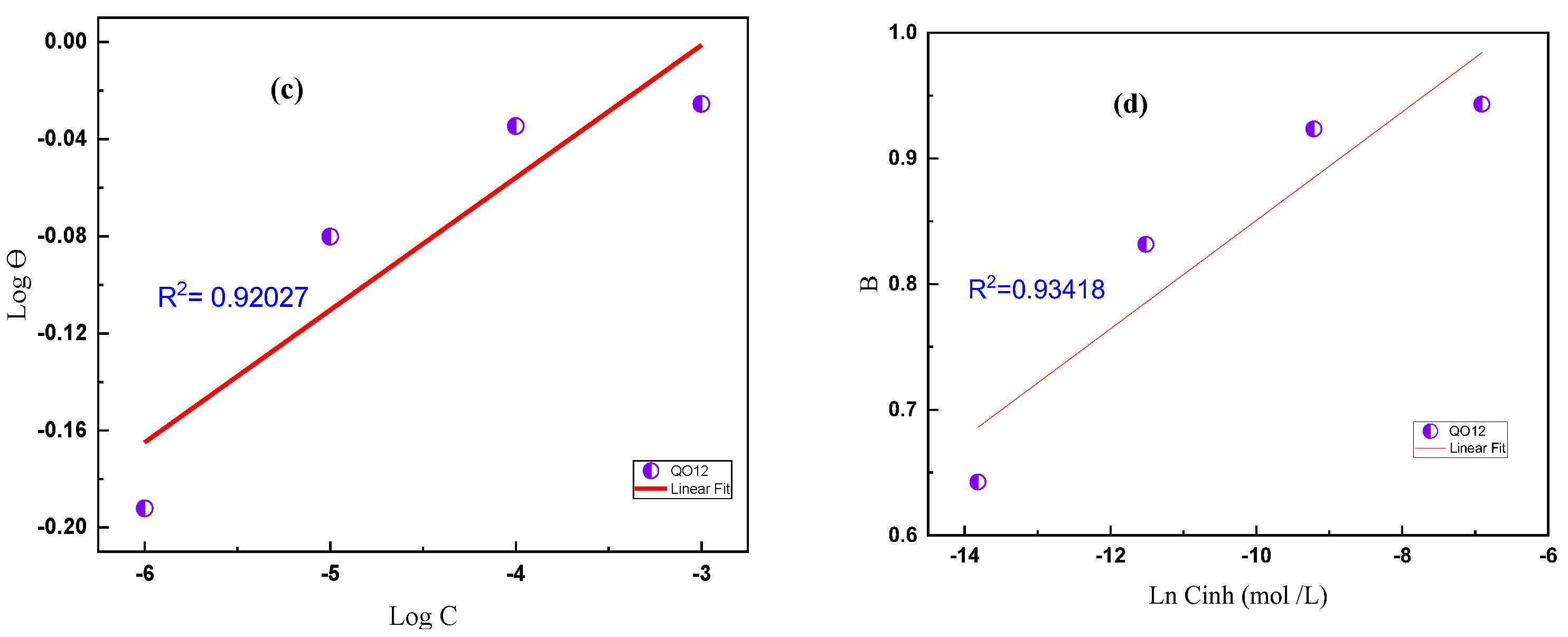
3.4. Isotherm of Adsorption
3.5. UV-Visible Spectroscopy
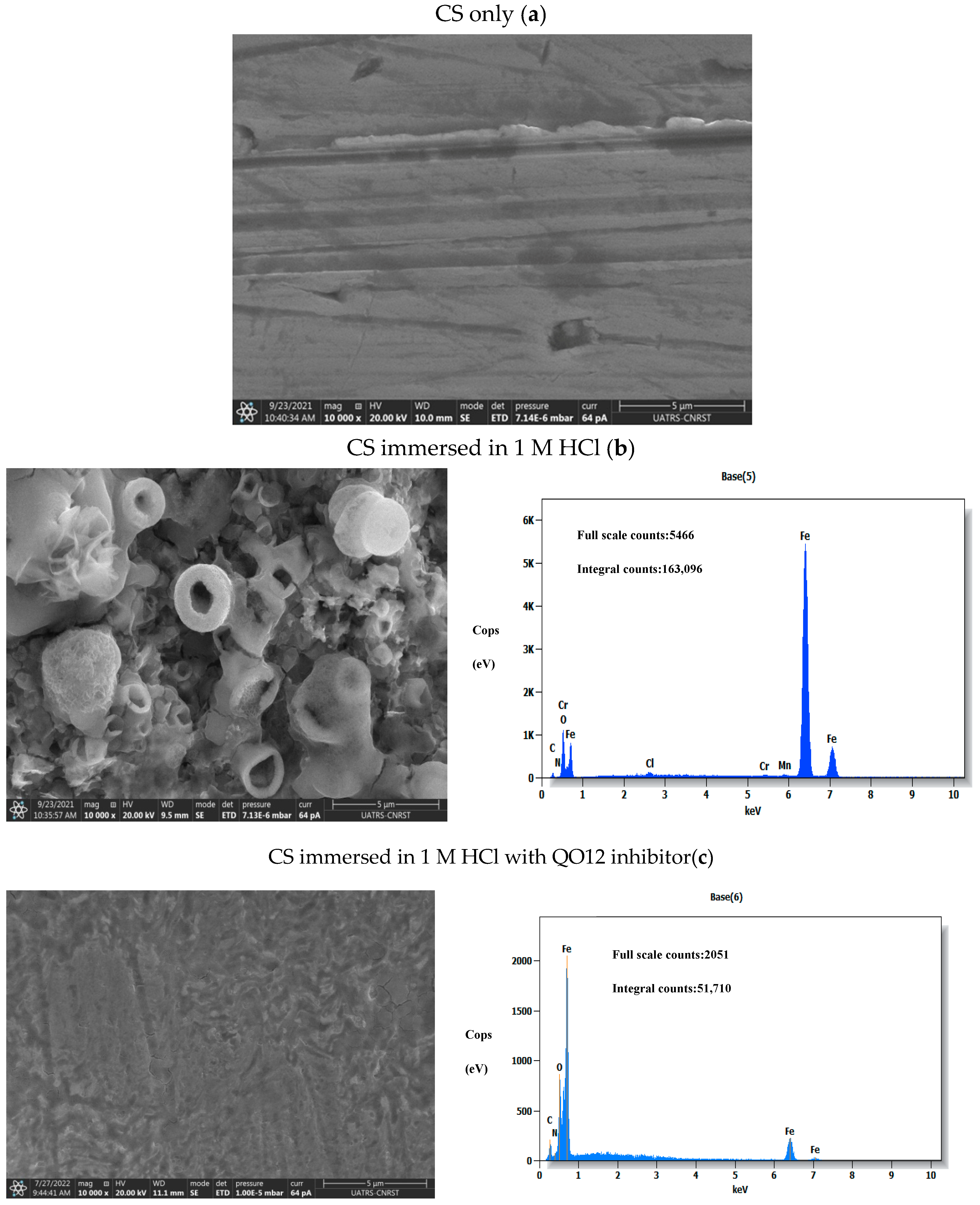
3.6. SEM-EDX Investigation
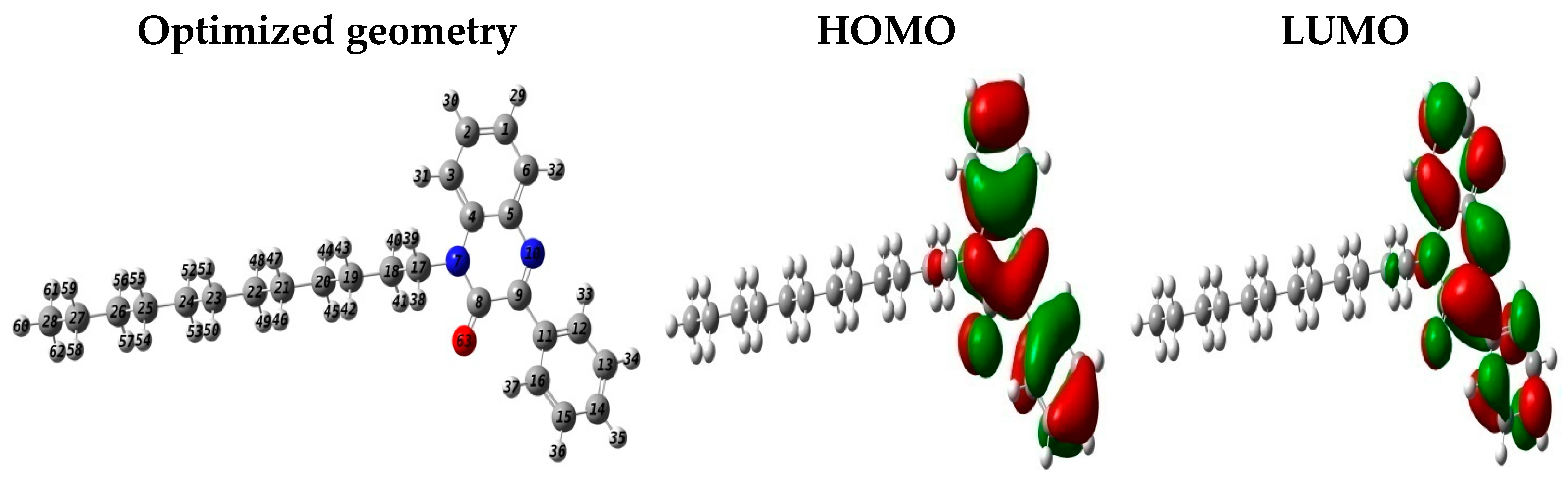
3.7. DFT Approaches
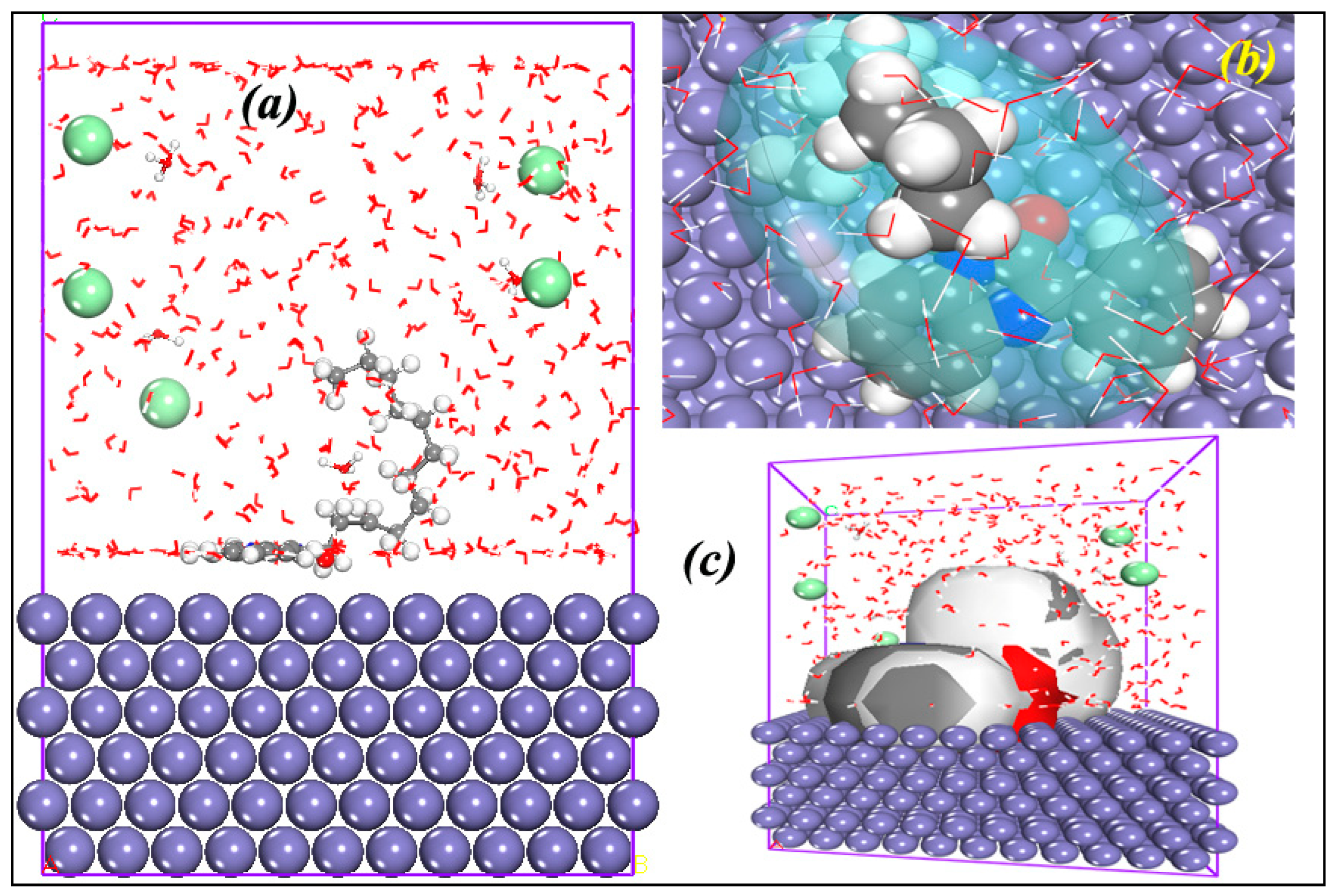
3.8. MD Simulation Investigations
3.8.1. QO12/Fe (110) System
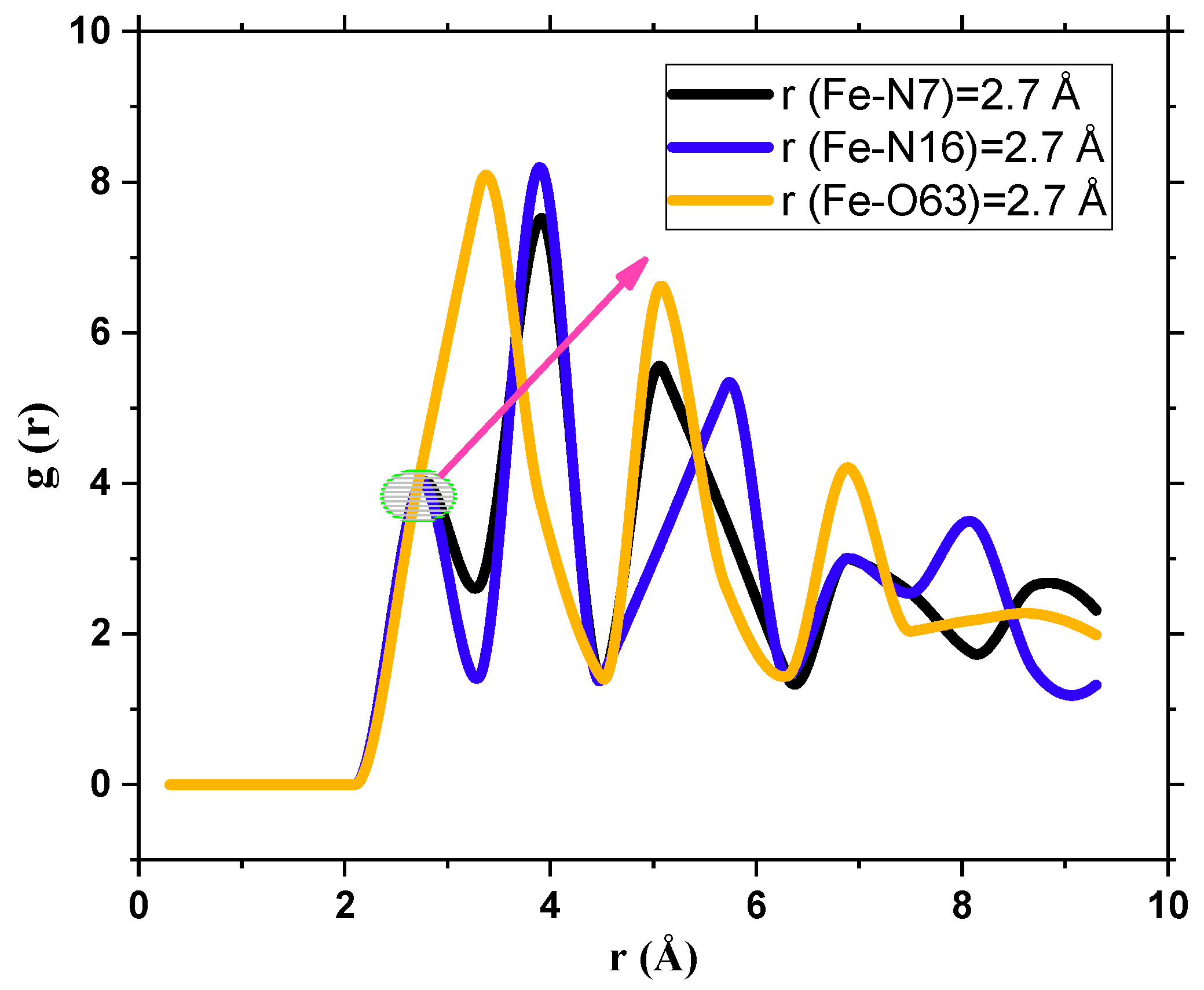
3.8.2. RDF
3.8.3. MSD Tool
4. Conclusions
- QO12 has excellent effectiveness inhibiting CS corrosion in an acidic electrolyte and its performance improves as the amount rises, reaching a max of 95.33%, at 10−3 M. This efficiency value is closer to that of Q1 and Q2 in Table 1, while it is lower than that of the Q3 inhibitor, due to the presence of the nitro (NO2) attracting group.
- The PDP profiles show that QO12 significantly inhibits anodic metal dissolution and cathodic hydrogen evolution processes, indicating that it is a mixed-type inhibitor with a cathodic tendency. EIS assessments show that the presence of the QO12 increases Rp values while decreasing the constant phase element of the double layer (Cdl), hence validating the inhibitor’s inhibitory impact on CS corrosion.
- The chemisorption mechanism of QO12 adsorption on the CS interface is consistent with the Langmuir adsorption isotherm.
- Surface and electrolyte analyses (SEM, EDX, and UV-visible) suggest QO12 adsorption on the CS interface.
- Theoretical approaches indicate a good adsorption of QO12 on the selected surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ech-chihbi, E.; Belghiti, M.E.; Salim, R.; Oudda, H.; Taleb, M.; Benchat, N.; Hammouti, B.; El-Hajjaji, F. Experimental and computational studies on the inhibition performance of the organic compound “2-phenylimidazo [1,2-a]pyrimidine-3-carbaldehyde” against the corrosion of carbon steel in 1.0 M HCl solution. Surf. Interfaces 2017, 9, 206–217. [Google Scholar] [CrossRef]
- Thoume, A.; Benhiba, F.; Elmakssoudi, A.; Benmessaoud Left, D.; Benzbiria, N.; Warad, I.; Dakir, M.; Azzi, M.; Zertoubi, M.; Zarrouk, A. Corrosion inhibition behavior of chalcone oxime derivatives on carbon steel in 0.5 M H2SO4. J. Appl. Electrochem. 2021, 51, 1755–1770. [Google Scholar] [CrossRef]
- Liao, B.; Ma, S.; Zhang, S.; Li, X.; Quan, R.; Wan, S.; Guo, X. Fructus cannabis protein extract powder as a green and high effective corrosion inhibitor for Q235 carbon steel in 1 M HCl solution. Int. J. Biol. Macromol. 2023, 239, 124358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Min, X.; Wan, S.; Liu, J.; Liao, B.; Guo, X. A novel green corrosion inhibitor extracted from waste feverfew root for carbon steel in H2SO4 solution. Results Eng. 2023, 17, 100971. [Google Scholar] [CrossRef]
- El Faydy, M.; Touir, R.; Touhami, M.E.; Zarrouk, A.; Jama, C.; Lakhrissi, B.; Olasunkanmi, L.; Ebenso, E.; Bentiss, F. Corrosion inhibition performance of newly synthesized 5-alkoxymethyl-8-hydroxyquinoline derivatives for carbon steel in 1 M HCl solution: Experimental, DFT and Monte Carlo simulation studies. Phys. Chem. Chem. Phys. 2018, 20, 20167–20187. [Google Scholar] [CrossRef]
- Boulechfar, C.; Ferkous, H.; Djellali, S.; Amin, M.A.; Boufas, S.; Djedouani, A.; Delimi, A.; Amor, Y.B.; Yadav, K.; Byong-Hun Jeon, K.; et al. DFT/molecular scale, MD simulation and assessment of the eco-friendly anti-corrosion performance of a novel Schiff base on XC38 carbon steel in acidic medium. J. Mol. Liq. 2021, 344, 117874. [Google Scholar] [CrossRef]
- Sebhaoui, J.; El Bakri, Y.; El Aoufir, Y.; Anouar, E.H.; Guenbour, A.; Nasser, A.A.; Essassi, E.M. Synthesis, NMR characterization, DFT and anti-corrosion on carbon steel in 1M HCl of two novel 1,5-benzodiazepines. J. Mol. Struct. 2019, 1182, 123–130. [Google Scholar] [CrossRef]
- El-Hashemy, M.A.; Sallam, A. The inhibitive action of Calendula officinalis flower heads extract for mild steel corrosion in 1 M HCl solution. J. Mater. Res. Technol. 2020, 9, 13509–13523. [Google Scholar] [CrossRef]
- Tsoeunyane, M.G.; Makhatha, M.E.; Arotiba, O.A. Corrosion Inhibition of Mild Steel by Poly(butylene succinate)-L-histidine Extended with 1,6-diisocynatohexane Polymer Composite in 1 M HCl. Int. J. Corros. 2019, 2019, 7406409. [Google Scholar] [CrossRef]
- Maranescu, B.; Lupa, L.; Tara-Lunga Mihali, M.; Plesu, N.; Maranescu, V.; Visa, A. The corrosion inhibitor behavior of iron in saline solution by the action of magnesium carboxyphosphonate. Pure Appl. Chem. 2018, 90, 1713–1722. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Ebenso, E.E. Quinoline and its derivatives as corrosion inhibitors: A review. Surf. Interfaces. 2020, 21, 100634. [Google Scholar] [CrossRef]
- Lupa, L.; Maranescu, B.; Visa, A. Equilibrium and kinetic studies of chromium ions adsorption on Co (II)-based phosphonate metal organic frameworks. Sep. Sci. Technol. 2018, 53, 1017–1026. [Google Scholar] [CrossRef]
- Xu, Z.G.; Wang, X.H.; Jiang, M.; Li, L.P. Investigation on formation of equiaxed zone in low carbon steel slabs. Metall. Res. Technol. 2016, 113, 106. [Google Scholar] [CrossRef]
- Hjouji, M.; Djedid, M.; Elmsellem, H.; Rodi, Y.K.; Ouzidan, Y.; Chahdi, F.O.; Sebbar, N.; Essassi, E.; Abdel-Rahman, I.; Hammouti, B. Corrosion Inhibition of mild-S in hydrochloric acid solution by pyrido [2, 3-b] pyrazine derivative: Electrochemical and theoretical evaluation. J. Mater. Environ. Sci. 2016, 7, 1425–1435. [Google Scholar]
- Ammal, P.R.; Prajila, M.; Joseph, A. Electroanalytical and Kinetic Studies on PBIMOT, a Benzimidazole Motif of 1, 3, 4-Oxadiazole as a Powerful Corrosion Inhibitor for Mild Steel in Nitric Acid. J. Bio-Tribo-Corros. 2017, 3, 47. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A. Experimental and computational investigation on the corrosion inhibition characteristics of mild steel by some novel synthesized imines in hydrochloric acid solutions. Corros. Sci. 2015, 92, 104–117. [Google Scholar] [CrossRef]
- Hsissou, R.; Benassaoui, H.; Benhiba, F.; Hajjaji, N.; Elharfi, A. Application of a new tri-functional epoxy prepolymer, triglycidyl ethylen? Ether of bisphenol A, in the coating of E24 steel in 3.5% NaCl. J. Chem. Technol. Metall. 2017, 52, 431–438. [Google Scholar]
- Ebenso, E.E.; Kabanda, M.M.; Arslan, T.; Saracoglu, M.; Kandemirli, F.; Murulana, L.C.; Singh, A.K.; Shukla, S.K.; Hammouti, B.; Khaled, K.F.; et al. Quantum chemical investigations on quinoline derivatives as effective corrosion inhibitors for mild steel in acidic medium. Int. J. Electrochem. Sci. 2012, 7, 5643–5676. [Google Scholar]
- Laabaissi, T.; Benhiba, F.; Missioui, M.; Rouifi, Z.; Rbaa, M.; Oudda, H.; Ramli, Y.; Guenbour, A.; Warad, I.; Zarrouk, A. Coupling of chemical, electrochemical and theoretical approach to study the corrosion inhibition of mild steel by new quinoxaline compounds in 1 M HCl. Heliyon 2020, 6, e03939. [Google Scholar] [CrossRef]
- Laabaissi, T.; Benhiba, F.; Rouifi, Z.; Missioui, M.; Ourrak, K.; Oudda, H.; Ramli, Y.; Warad, I.; Allali, M.; Zarrouk, A. New quinoxaline derivative as a green corrosion inhibitor for mild steel in mild acidic medium: Electrochemical and theoretical studies. Int. J. Corros. Scale Inhib. 2019, 8, 241–256. [Google Scholar]
- Benhiba, F.; ELAoufir, Y.; Belayachi, M.; Zarrok, H.; El Assyry, A.; Zarrouk, A.; Hammouti, B.; Ebenso, E.E.; Guenbour, A.; Al Deyab, S.S.; et al. Theoretical and experimental studies on the inhibition of 1,1′-(2-phenylquinoxaline 1,4-diyl)diethanone for the corrosion of carbon steel in 1.0 M HCl. J. Der Pharm. Lett. 2014, 6, 306–318. [Google Scholar]
- Zarrok, H.; Zarrouk, A.; Salghi, R.; Oudda, H.; Hammouti, B.; Assouag, M.; Taleb, M.; Ebn Touhami, M.; Bouachrine, M.; Boukhris, S. Gravimetric and quantum chemical studies of 1-[4-acetyl-2-(4-chlorophenyl) quinoxalin-1 (4H)-yl] acetone as corrosion inhibitor for carbon steel in hydrochloric acid solution. J. Chem. Pharm. Res. 2012, 4, 5056–5066. [Google Scholar]
- Zarrouk, A.; Zarrok, H.; Ramli, Y.; Bouachrine, M.; Hammouti, B.; Sahibed-Dine, A.; Bentiss, F. Inhibitive properties, adsorption and theoretical study of 3,7-dimethyl-1-(prop-2-yn-1-yl) quinoxalin-2 (1H)-one as efficient corrosion inhibitor for carbon steel in hydrochloric acid solution. J. Mol. Liq. 2016, 222, 239–252. [Google Scholar] [CrossRef]
- Benhiba, F.; Lotfi, N.; Ourrak, K.; Benzekri, Z.; Zarrok, H.; Guenbour, A.; Boukhris, S.; Souizi, A.; El Hezzat, M.; Warad, I.; et al. Corrosion inhibition study of 2-(2, 4-dichlorophenyl) -6-Nitro-1, 4-dihydroquinoxaline for carbon steel in hydrochloric acid solution. J. Mater. Environ. Sci. 2017, 9, 1086–1097. [Google Scholar]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Oubaaqa, M.; Ouakki, M.; Rbaa, M.; Ashraf, S.; Abousalem, M.; Benhiba, F.; Jarid, A.; Ebn Touhami, M.; Zarrouk, A. Insight into the corrosion inhibition of new amino-acids as efficient inhibitors for mild steel in HCl solution: Experimental studies and theoretical calculations. J. Mol. Liq. 2021, 334, 116520. [Google Scholar] [CrossRef]
- Abd allah, M.; Hegazy, M.A.; Ahmed, H.; Arej, S.; Al-Gorair, H.; Hawsawi, M.; Benhiba, F.; Warad, I.; Zarrouk, A. Appraisal of synthetic cationic Gemini surfactants as highly effi cient inhibitors for carbon steel in the acidization of oil and gas wells: An experimental and computational approach. RSC Adv. 2022, 12, 17050–17064. [Google Scholar] [CrossRef]
- Laadam, G.; Benhiba, F.; El Faydy, M.; Titi, A.; Al-Gorair, A.S.; Alshareef, M.; Hawsawi, H.; Touzani, R.; Warad, I.; Bellaouchou, A.; et al. Anti-corrosion performance of novel pyrazole derivative for carbon steel corrosion in 1 M HCl: Computational and experimental studies. Inorg. Chem. Commun. 2022, 145, 109963. [Google Scholar] [CrossRef]
- Benhiba, F.; Hsissou, R.; Benzikri, Z.; Echihi, S.; El-Blilak, J.; Boukhris, S.; Bellaouchou, A.; Guenbour, A.; Oudda, H.; Warad, I.; et al. DFT/electronic scale, MD simulation and evaluation of 6-methyl-2-(p-tolyl)-1,4-dihydroquinoxaline as a potential corrosion inhibition. J. Mol. Liq. 2021, 335, 116539. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision B. 01; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- El yaktini, A.; Lachiri, A.; El Faydy, M.; Benhiba, F.; Zarrok, H.; El Azzouzi, M.; Zertoubi, M.; Azzi, M.; Lakhrissi, B.; Zarrouk, A. Practical and Theoretical Study on the Inhibitory Inflences of New Azomethine Derivatives Containing 8-hydroxyquinoline Moiety for the Corrosion of Carbon Steel in 1 M HCl, Orient. J. Chem. 2018, 34, 3016–3029. [Google Scholar]
- El Faydy, M.; Benhiba, F.; Berisha, A.; Kerroum, Y.; Jama, C.; Lakhrissi, B.; Zarrouk, A. An experimental-coupled empirical investigation on the corrosion inhibitory action of 7-alkyl-8-hydroxyquinolines on C35E steel in HCl electrolyte. J. Mol. Liq. 2020, 317, 113973. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Zhang, W.; Nie, B.; Li, H.J.; Li, Q.; Li, C.; Wu, Y.C. Inhibition of mild-S corrosion in 1 M HCl by chondroitin sulfate and its synergistic effect with sodium alginate. Carbohydr. Polym. 2021, 260, 117842. [Google Scholar] [CrossRef]
- Abdallah, M. Rhodanine azosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in hydrochloric acid solution. Corros. Sci. 2002, 44, 717–728. [Google Scholar] [CrossRef]
- Daoudi, W.; El Aatiaoui, A.; Dagdag, O.; Zaidi, K.; Haldhar, R.; Kim, S.-C.; Oussaid, A.; Aouinti, A.; Berisha, A.; Benhiba, F.; et al. Anti-Corrosion Coating Formation by a Biopolymeric Extract of Artemisia herba-alba Plant: Experimental and Theoretical Investigations. Coatings 2023, 13, 611. [Google Scholar] [CrossRef]
- JO’M, B.; Srinivasan, S. Elucidation of the mechanism of electrolytic hydrogen evolution by the use of HT separation factors. Ind. Eng. Chem. Res. Electrochim. Acta. 1964, 9, 31–44. [Google Scholar]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Saranya, J.; Lavanya, K.; Kiranmai, M.H.; Subbiah, R.; Zarrouk, A.; Chitra, S. Quinoxaline derivatives as anticorrosion additives for metals. Corros. Rev. 2021, 39, 79–92. [Google Scholar] [CrossRef]
- Gholami, M.; Danaee, I.; Maddahy, M.H.; RashvandAvei, M. Correlated ab initio and electroanalytical study on inhibition behavior of 2-mercaptobenzothiazole and its thiole–thione tautomerism effect for the corrosion of steel (API 5L X52) in sulphuric acid solution. Ind. Eng. Chem. Res. 2013, 52, 14875–14889. [Google Scholar] [CrossRef]
- El Faydy, M.; Benhiba, F.; About, H.; Kerroum, Y.; Guenbour, A.; Lakhrissi, B.; Warad, I.; Verma, C.; Sherif, E.-S.M.; Ebenso, E.E.; et al. Experimental and computational investigations on the anti-corrosive and adsorption behavior of 7-N,N’-dialkyaminomethyl-8-Hydroxyquinolines on C40E steel surface in acidic medium. J. Coll. Inter. Sci. 2020, 576, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Zouitini, A.; Rodi, Y.K.; Chahdi, H.E.; Steli, H.; Ad, C.; Ouzidan, Y.; Essassi, E.; Chetouani, A.; Hammouti, B. Corrosion Inhibition Behavior of Indazole Derivative as a Green Corrosion Inhibitor for Mild-S in Hydrochloric Acid: Electrochemical, Weight Loss and DFT Simulations Studies. Moroc. J. Chem. 2018, 6, 391–403. [Google Scholar]
- Fu, J.; Zang, H.; Wang, Y.; Li, S.; Chen, T.; Liu, X. Experimental and theoretical study on the inhibition performances of quinoxaline and its derivatives for the corrosion of mild-S in hydrochloric acid. Ind. Eng. Chem. Res. 2012, 51, 6377–6386. [Google Scholar] [CrossRef]
- El Faydy, M.; Benhiba, F.; Kerroum, Y.; Guenbour, A.; Bentiss, F.; Warad, I.; Lakhrissi, B.; Zarrouk, A. Synthesis and anti-corrosion characteristics of new 8-quinolinol analogs with amide-substituted on C35E steel in acidic medium: Experimental and computational ways. J. Mol. Liq. 2021, 325, 115224. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Rodríguez-Gómez, F. Caffeine and nicotine in 3% NaCl solution with CO2 as corrosion inhibitors for low carbon steel. RSC Adv. 2016, 6, 70226–70236. [Google Scholar] [CrossRef]
- Ousslim, A.; Chetouani, A.; Hammouti, B.; Bekkouch, K.; Al-Deyab, S.; Aouniti, A.; Elidrissi, A. Thermodynamics, quantum and electrochemical studies of corrosion of iron by piperazine compounds in sulphuric acid. Int. J. Electrochem. Sci. 2013, 8, 5980–6004. [Google Scholar]
- Hsissou, R.; Dagdag, O.; Berradi, M.; El Bouchti, M.; Assouag, M.; Elharfi, A. Development rheological and anti-corrosion property of epoxy polymer and its composite. Heliyon 2019, 5, e02789. [Google Scholar] [CrossRef]
- Noor, E.A. The inhibition of mild-S corrosion in phosphoric acid solutions by some N-heterocyclic compounds in the salt form. Corros. Sci. 2005, 47, 33–55. [Google Scholar] [CrossRef]
- Mourya, P.; Singh, P.; Tewari, A.; Rastogi, R.; Singh, M. Relationship between structure and inhibition behaviour of quinolinium salts for mild-S corrosion: Experimental and theoretical approach. Corros. Sci. 2015, 95, 71–87. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Soliman, K.A.; Tantawy, A.H. Novel synthesized Schiff Base-based cationic gemini surfactants: Electrochemical investigation, theoretical modeling and applicability as biodegradable inhibitors for mild-S against acidic corrosion. J. Mol. Liq. 2017, 232, 478–498. [Google Scholar] [CrossRef]
- Abd El Rehim, S.; Sayyah, S.; El-Deeb, M.; Kamal, S.; Azooz, R. Adsorption and corrosion inhibitive properties of P (2-aminobenzothiazole) on mild-S in hydrochloric acid media. Ind. Eng. Chem. Res. 2016, 7, 39–52. [Google Scholar] [CrossRef]
- El-Aouni, N.; Hsissou, R.; Saf, Z.; Abbout, S.; Benhiba, F.; El Azzaoui, J.; Haldhar, R.; Wazzan, N.; Guo, L.; Erramli, H.; et al. Performance of two new epoxy resins as potential corrosion inhibitors for carbon steel in 1MHCl medium: Combining experimental and computational approaches. Colloids Surf. A. Physicochem. Eng. Asp. 2021, 626, 127066. [Google Scholar] [CrossRef]
- Benhiba, F.; Benzekri, Z.; Kerroum, Y.; Timoudan, N.; Hsissou, R.; Guenbour, A.; Belfaquir, M.; Boukhris, S.; Bellaouchou, A.; Oudda, H.; et al. Assessment of inhibitory behavior of ethyl 5-cyano-4-(furan-2-yl)-2-methyl-6-oxo-1,4,5,6-tetrahydropyridine-3-carboxylate as a corrosion inhibitor for carbon steel in molar HCl: Theoretical approaches and experimental investigation. J. Indian Chem. Soc. 2023, 100, 100916. [Google Scholar] [CrossRef]
- El Faydy, M.; Benhiba, F.; Timoudan, N.; Lakhrissi, B.; Warad, I.; Saoiabi, S.; Guenbour, A.; Bentiss, F.; Zarrouk, A. Experimental and theoretical examinations of two quinolin-8-ol-piperazine derivatives as organic corrosion inhibitors for C35E steel in hydrochloric acid. J. Mol. Liq. 2022, 354, 118900. [Google Scholar] [CrossRef]
- Lebrini, M.; Lagrenée, M.; Vezin, H.; Gengembre, L.; Bentiss, F. Electrochemical and quantum chemical studies of new thiadiazole derivatives adsorption on mild steel in normal hydrochloric acid medium. Corrosi. Sci. 2005, 47, 485–505. [Google Scholar] [CrossRef]
- Cherinka, K.; Andrews, B.; Sánchez-Gallego, B.H.; Brownstein, J.; Argudo-Fernández, J.; Blanton, M.; Bundy, M.; Jones, K.; Masters, A.; Law, D.R. A tool kit for streamlined access and visualization of the SDSS-IV MaNGA data set. Astron. J. 2019, 158, 74. [Google Scholar]
- Roy, D.; Keith, T.A.; Millam, J.M. Current Version: GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Erdoğan, Ş.; Safi, Z.S.; Kaya, S.; Işın, D.Ö.; Guo, L.; Kaya, C. A computational study on corrosion inhibition performances of novel quinoline derivatives against the corrosion of iron. J. Mol. Struct. 2017, 1134, 751–761. [Google Scholar] [CrossRef]
- Kumar, U.P.; Albrakaty, R.H.; Wazzan, N.; Obot, I.B.; Safi, Z.S.; Shanmugan, S.; Liang, T. Insight into the nature of the ionic interactions between some aldehydes and Ni-W alloy: A theoretical study. Mater. Today Commun. 2020, 22, 100693. [Google Scholar] [CrossRef]
- Wazzan, N.; Obot, I.B.; Fagieh, T.M. The role of some triazoles on the corrosion inhibition of C1020 steel and copper in a desalination descaling solution. Desalination. 2022, 527, 115551. [Google Scholar] [CrossRef]
- Walters, F.H. Design of corrosion inhibitors: Use of the hard and soft acid-base (HSAB) theory. J. Chem. Educ. 1991, 68, 29. [Google Scholar] [CrossRef]
- Attou, A.; Tourabi, M.; Benikdes, A.; Benali, O.; Ouici, H.B.; Benhiba, F.; Zarrouk, A.; Jama, C.; Bentiss, F. Experimental studies and computational exploration on the 2-amino-5-(2-methoxyphenyl)-1,3,4-thiadiazole as novel corrosion inhibitor for mild steel in acidic environment. Colloids Surf. A 2020, 604, 125320. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef]
- Berrissoul, A.; Loukili, E.; Mechbal, N.; Benhiba, F.; Guenbour, A.; Dikici, B.; Zarrouk, A.; Dafali, A. Anticorrosion effect of a green sustainable inhibitor on mild steel in hydrochloric acid. J. Colloid Interface Sci. 2020, 580, 740–752. [Google Scholar] [CrossRef]
- Hsissou, R.; Bekhtaa, A.; Elharfia, A.; Benzidia, B.; Hajjaji, N. Theoretical and electrochemical studies of the coating behavior of a new epoxy polymer: Hexaglycidyl ethylene of methylene dianiline (HGEMDA) on E24 steel in 3.5% NaCl. Port. Electrochim. Acta. 2018, 18, 101–117. [Google Scholar] [CrossRef]
- El yaktini, A.; Lachiri, A.; El Faydy, M.; Benhiba, F.; Zarrok, H.; El Azzouzi, M.; Zertoubi, M.; Azzi, M.; Lakhrissi, B.; Zarrouk, A. Inhibitor effect of new azomethine derivative containing an 8-hydroxyquinoline moiety on corrosion behavior of mild carbon steel in acidic media. Int. J. Corros. Scale Inhib. 2017, 7, 609–632. [Google Scholar]
- Rouifi, Z.; Rbaa, M.; Benhiba, F.; Laabaissi, T.; Oudda, H.; Lakhrissi, B.; Guenbour, A.; Warad, I.; Zarrouk, A. Preparation and anti-corrosion activity of novel 8-hydroxyquinoline derivative for carbon steel corrosion in HCl molar: Computational and experimental analyses. J. Mol. Liq. 2020, 307, 112923. [Google Scholar] [CrossRef]
- Goyal, M.; Vashisht, H.; Kumar, A.; Kumar, S.; Bahadur, I.; Benhiba, F.; Zarrouk, A. Isopentyl triphenylphosphonium bromideionic liquid as a newly effective corrosion inhibitor on metal-electrolyte interface in acidic medium: Experimental, surface morphological (SEM-EDX & AFM) and computational analysis. J. Mol. Liq. 2020, 316, 113838. [Google Scholar]
- Huong, D.Q.; Huong NT, L.; Nguyet TT, A.; Duong, T.; Tuan, D.; Thong, N.M.; Nam, P.C. Pivotal Role of Heteroatoms in Improving the Corrosion Inhibition Ability of Thiourea Derivatives. ACS Omega 2020, 5, 27655–27666. [Google Scholar] [CrossRef]
- Mehmeti, V.; Podvorica, F.I. Experimental and theoretical studies on corrosion inhibition of niobium and tantalum surfaces by Carboxylated graphene oxide. Materials 2018, 11, 893. [Google Scholar] [CrossRef]
- Charati, S.G.; Stern, S.A. Diffusion of Gases in Silicone Polymers: Molecular Dynamics Simulations. Macromolecules 1998, 31, 5529–5535. [Google Scholar] [CrossRef]
- Benhiba, F.; Sebbar, N.K.; Bourazmi, H.; Belghiti, M.E.; Hsissou, R.; Hökelek, T.; Bellaouchou, A.; Guenbour, A.; Warad, I.; Oudda, H.; et al. Corrosion inhibition performance of 4-(prop-2-ynyl)-[1,4]-benzothiazin-3-one against mild steel in 1M HCl solution: Experimental and theoretical studies. Int. J. Hydrogen Energy 2021, 46, 25800–25818. [Google Scholar] [CrossRef]
- Hu, S.Q.; Guo, A.L.; Yan, Y.G.; Jia, X.L.; Geng, Y.F.; Guo, W.Y. Computer simulation of diffusion of corrosive particle in corrosion inhibitor membrane. Comput. Theor. Chem. 2011, 964, 1766181. [Google Scholar] [CrossRef]
- Guo, A.; Duan, G.; He, K.; Sun, B.; Fan, C.; Hu, S. Synergistic effect between 2-oleyl-1-oleylamidoethyl imidazoline ammonium methylsulfate and halide ion by molecular dynamics simulation. Comput. Theor. Chem. 2013, 1015, 21–26. [Google Scholar] [CrossRef]






| Quinoxaline Derivatives | Anticorrosive Properties (%) | References |
|---|---|---|
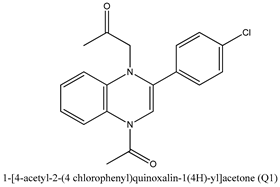 | 95.80 | [22] |
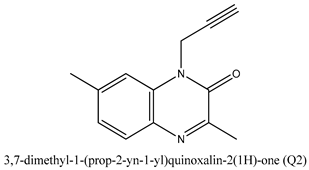 | 94.90 | [23] |
 | 88.00 | [24] |
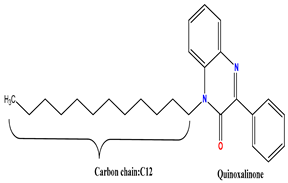 | 95.33 | Present work |
| Alloys | C | Si | S | Cu | Mn | Cr | Co | Ti | Ni | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage in mass | 0.370 | 0.230 | 0.016 | 0.160 | 0.680 | 0.077 | 0.009 | 0.011 | 0.059 | Rest |
| Inhibitor | C. (M) | −Ecorr (mV/SCE) | icorr (µA/cm2) | −βc (mV/dec) | βa (mV/dec) | IE (%) |
|---|---|---|---|---|---|---|
| HCl | 1 | 456.3 ± 6 | 1104.00 ± 4.9 | 112.8 ± 6 | 155.4 ± 5 | - |
| QO12 | 10−3 | 435.7 ± 5 | 51.55 ± 4.4 | 153.8 ± 7 | 51.8 ± 5 | 95.33 |
| 10−4 | 452.3 ± 5 | 72.89 ± 5.1 | 145.6 ± 6 | 54.8 ± 6 | 93.39 | |
| 105 | 465.1 ± 4 | 188.51 ± 4.5 | 73.4 ± 4 | 59.6 ± 5 | 82.92 | |
| 10−6 | 463.9 ± 7 | 483.92 ± 6.2 | 92.6 ± 4 | 107.2 ± 7 | 56.17 |
| Inh. | C (M) | Rs (Ω cm2) | Rp (Ω cm2) | Cdl (µF cm−2) | Q (µF sn−1 cm−2) | ndl | ȠEIS (%) | χ2 × 10−3 |
|---|---|---|---|---|---|---|---|---|
| HCl | 1 | 0.83 ± 1.01 | 21.57 ± 0.56 | 120.4 | 293.90 ± 2.35 | 0.845 ± 0.003 | - | 2.0 |
| QO12 | 10−3 | 1.26 ± 1.00 | 380.60 ± 0.52 | 43.9 | 83.31 ± 2.12 | 0.836 ± 0.002 | 94.3 | 1.1 |
| 10−4 | 0.95 ± 1.19 | 281.90 ± 0.50 | 57.9 | 127.06 ± 2.32 | 0.809 ± 0.001 | 92.3 | 1.3 | |
| 10−5 | 1.14 ± 1.09 | 128.20 ± 0.53 | 87.1 | 206.06 ± 2.28 | 0.808 ± 0.003 | 83.1 | 0.3 | |
| 10−6 | 1.94 ± 1.29 | 60.42 ± 0.58 | 89.3 | 263.34 ± 2.37 | 0.793 ± 0.004 | 64.2 | 0.3 |
| Inhibitor | Temp. (K) | −Ecorr (mV vs. SCE) | icorr (µA/cm2) | EPDP (%) |
|---|---|---|---|---|
| Blank | 303 | 456.3 ± 6 | 1104.1 ± 4.9 | - |
| 313 | 423.5 ± 9 | 1477.4 ± 7.8 | - | |
| 323 | 436.3 ± 7 | 2254.0 ± 10.2 | - | |
| 333 | 433.3 ± 5 | 3944.9 ± 12.2 | - | |
| QO12 | 303 | 435.7 ± 5.0 | 51.5 ± 4.4 | 95.3 |
| 313 | 455.7 ± 5.4 | 107.4 ± 5.6 | 92.7 | |
| 323 | 454.0 ± 5.3 | 198.8 ± 5.9 | 91.1 | |
| 333 | 447.7 ± 5.6 | 382.8 ± 5.1 | 90.2 |
| Elements | E*a (kJ/mol) | ΔH*a (kJ/mol) | ΔS*a (J/mol K) |
|---|---|---|---|
| Blank | 35.41 | 32.77 | −81.11 |
| QO12 | 55.63 | 52.99 | −37.30 |
| Langmuir Isotherm | R2 | Slope | Kads103 (M) | ΔG°ads (kJ/mol) |
|---|---|---|---|---|
| QO12 | 1 | 1.059 | 709.486 | −44.05 |
| EHO | ELU | ΔEgap | ꭓ | η | ΔN110 | TE |
|---|---|---|---|---|---|---|
| −5.847 | −1.994 | 3.853 | 3.920 | 1.926 | 0.233 | −32,546.928 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benhiba, F.; Missioui, M.; Lamghafri, S.; Hsissou, R.; Bellaouchou, A.; Oudda, H.; Lamhamdi, A.; Warad, I.; Ramli, Y.; Zarrouk, A. Theoretical and Experimental Studies of 1-Dodecyl-3-phenylquinoxalin-2(1H)-one as a Sustainable Corrosion Inhibitor for Carbon Steel in Acidic Electrolyte. Coatings 2023, 13, 1109. https://doi.org/10.3390/coatings13061109
Benhiba F, Missioui M, Lamghafri S, Hsissou R, Bellaouchou A, Oudda H, Lamhamdi A, Warad I, Ramli Y, Zarrouk A. Theoretical and Experimental Studies of 1-Dodecyl-3-phenylquinoxalin-2(1H)-one as a Sustainable Corrosion Inhibitor for Carbon Steel in Acidic Electrolyte. Coatings. 2023; 13(6):1109. https://doi.org/10.3390/coatings13061109
Chicago/Turabian StyleBenhiba, Fouad, Mohcine Missioui, Selma Lamghafri, Rachid Hsissou, Abdelkbir Bellaouchou, Hassan Oudda, Abdellatif Lamhamdi, Ismail Warad, Youssef Ramli, and Abdelkader Zarrouk. 2023. "Theoretical and Experimental Studies of 1-Dodecyl-3-phenylquinoxalin-2(1H)-one as a Sustainable Corrosion Inhibitor for Carbon Steel in Acidic Electrolyte" Coatings 13, no. 6: 1109. https://doi.org/10.3390/coatings13061109
APA StyleBenhiba, F., Missioui, M., Lamghafri, S., Hsissou, R., Bellaouchou, A., Oudda, H., Lamhamdi, A., Warad, I., Ramli, Y., & Zarrouk, A. (2023). Theoretical and Experimental Studies of 1-Dodecyl-3-phenylquinoxalin-2(1H)-one as a Sustainable Corrosion Inhibitor for Carbon Steel in Acidic Electrolyte. Coatings, 13(6), 1109. https://doi.org/10.3390/coatings13061109








