Generation of Hybrid Lead Halide CH3NH3PbI3-xClx Perovskite Crystals via Convective Self-Assembly
Abstract
:1. Introduction
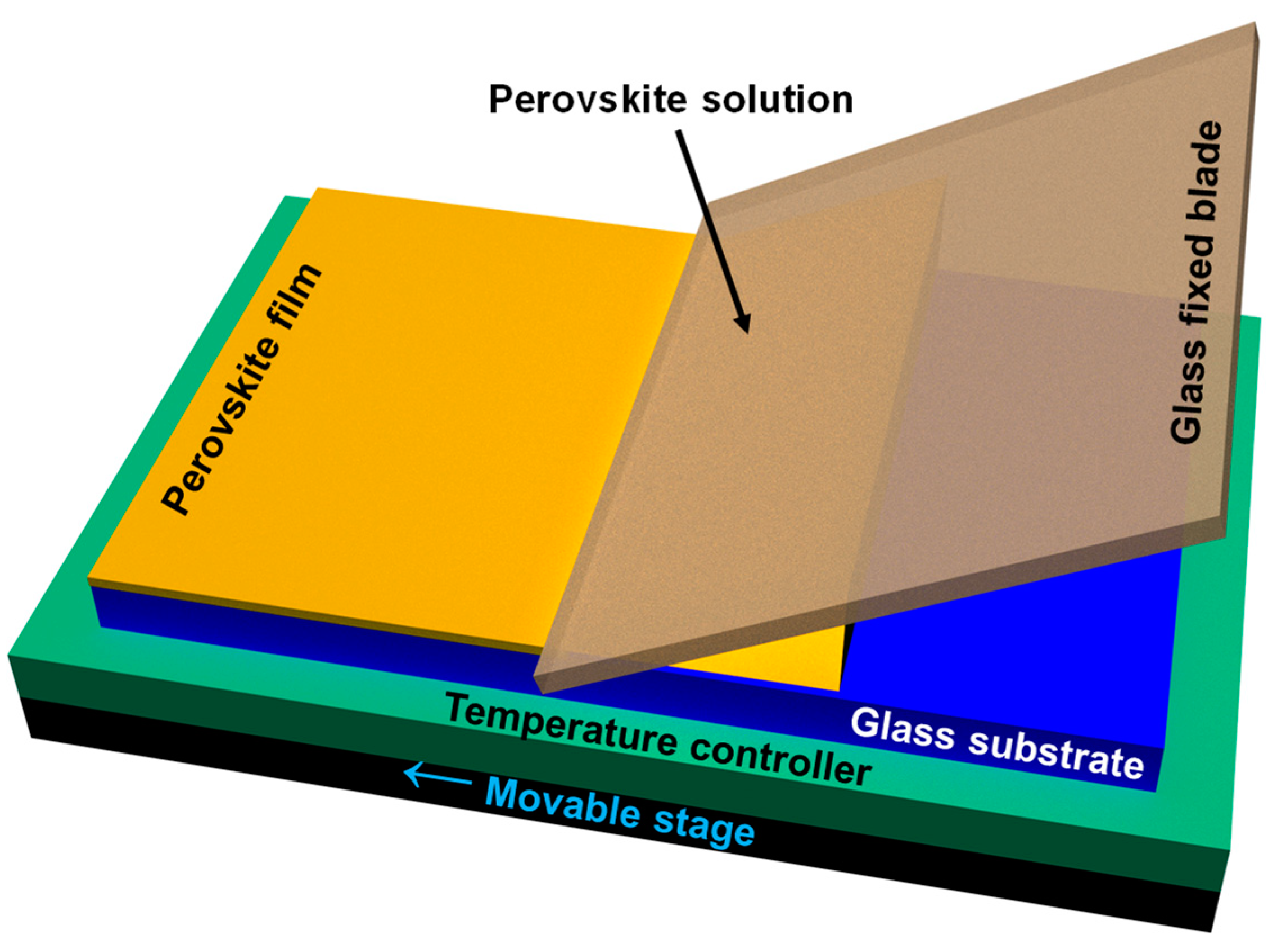
2. Materials and Methods
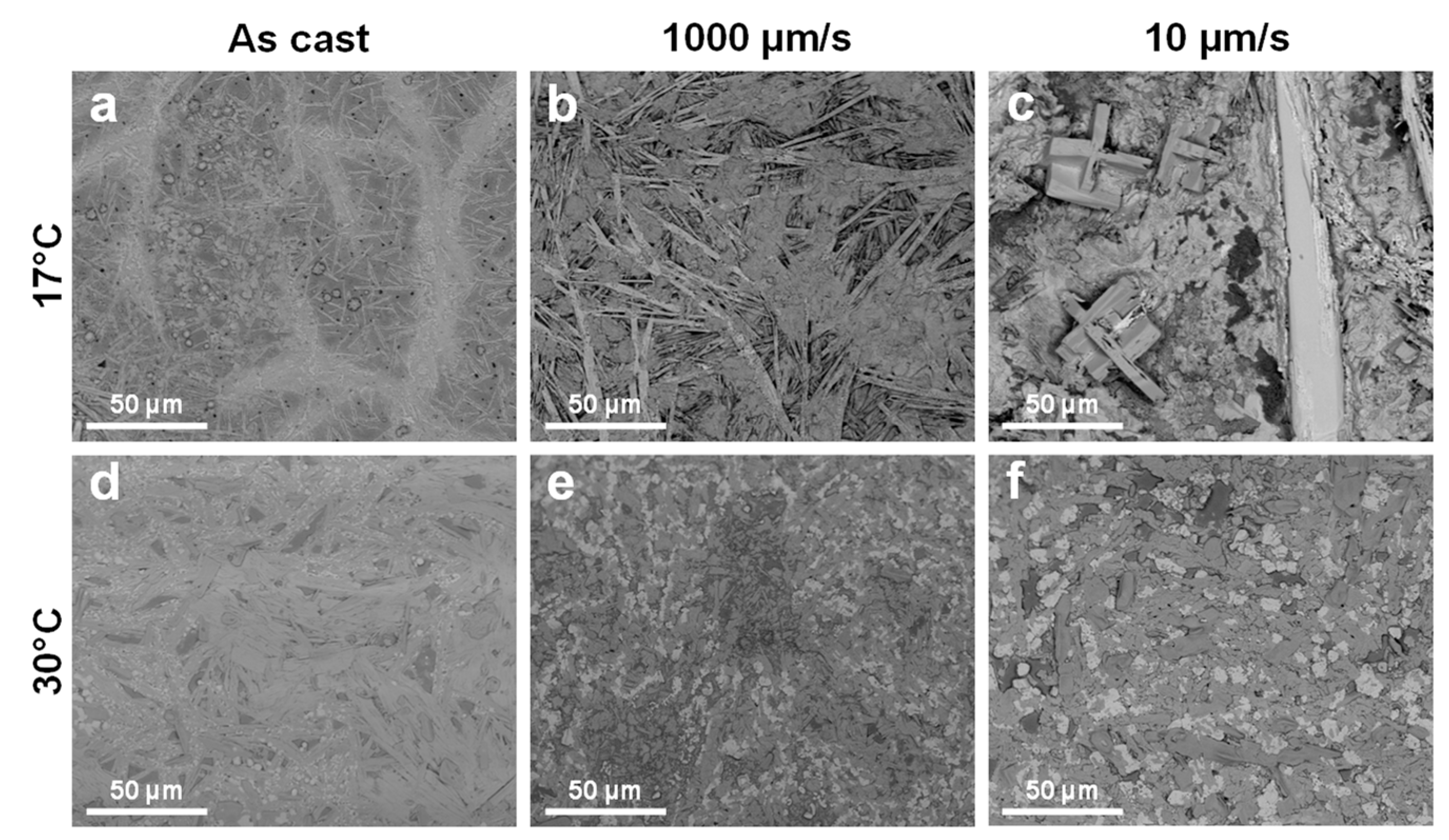
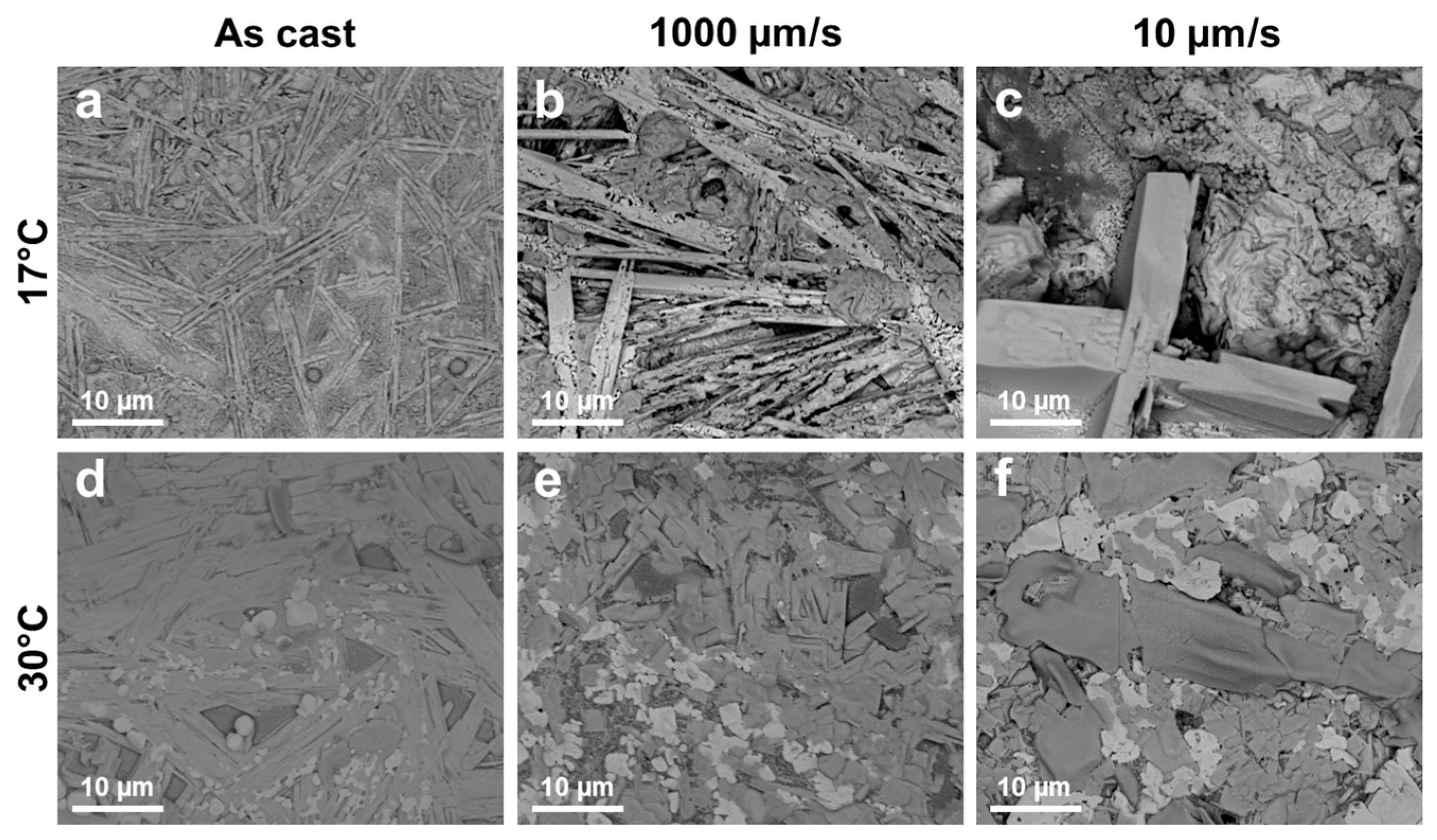
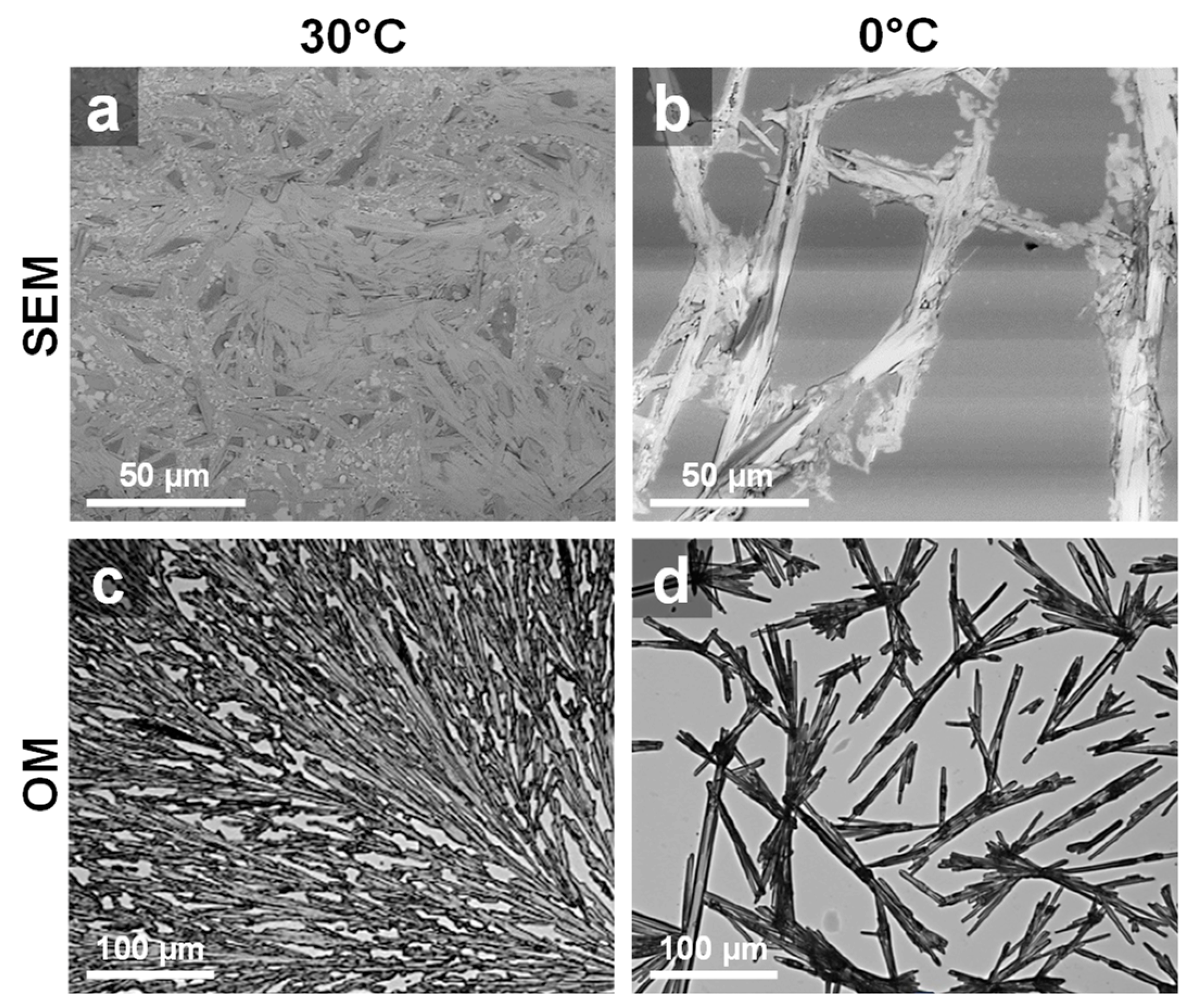
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Z.; Zhang, S. The Importance of Governance Efficiency in Environmental Policy Research in Energy Shortage Period. J. Sustain. Bus. Econ. 2022, 5, 25. [Google Scholar] [CrossRef]
- Tao, J.; Waqas, M.; Ali, M.; Umair, M.; Gan, W.; Haider, H. Pakistan’s Electrical Energy Crises, a Way Forward towards 50% of Sustain Clean and Green Electricity Generation. Energy Strategy Rev. 2022, 40, 100813. [Google Scholar] [CrossRef]
- Glazer, A.M. Simple Ways of Determining Perovskite Structures. Acta Crystallogr. Sect. A 1975, 31, 756–762. [Google Scholar] [CrossRef] [Green Version]
- Maafa, I.M. All-Inorganic Perovskite Solar Cells: Recent Advancements and Challenges. Nanomaterials 2022, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, W. Perovskite Tandem Solar Cells: From Fundamentals to Commercial Deployment. Chem. Rev. 2020, 120, 9835–9950. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Qaid, S.M.H.; Hezam, M.; Bedja, I.; Ghaithan, H.M.; Aldwayyan, A.S. Effect of Deposition Method on the Structural and Optical Properties of CH3NH3PbI3 Perovskite Thin Films. Opt. Mater. 2020, 103, 109836. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.-A.; Hu, Y. Recent Developments in Fabrication and Performance of Metal Halide Perovskite Field-Effect Transistors. J. Mater. Chem. C 2020, 8, 16691–16715. [Google Scholar] [CrossRef]
- Abiram, G.; Thanihaichelvan, M.; Ravirajan, P.; Velauthapillai, D. Review on Perovskite Semiconductor Field–Effect Transistors and Their Applications. Nanomaterials 2022, 12, 2396. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Chen, P.; Qiu, X.; Wei, H.; Xia, J.; Chen, H.; Zeng, Z.; Liao, L.; Hu, Y. Tuning the Electrical Performance of 2D Perovskite Field-Effect Transistors by Forming Organic Semiconductor/Perovskite van Der Waals Heterojunctions. Adv. Electron. Mater. 2022, 8, 2200148. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Yang, Y.; Xue, Q.; Yip, H.-L.; Cao, Y. Modulation of Recombination Zone Position for Quasi-Two-Dimensional Blue Perovskite Light-Emitting Diodes with Efficiency Exceeding 5%. Nat. Commun. 2019, 10, 1027. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wei, Z. The Strategies for Preparing Blue Perovskite Light-Emitting Diodes. J. Semicond. 2020, 41, 051203. [Google Scholar] [CrossRef]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef]
- Chouhan, L.; Ghimire, S.; Subrahmanyam, C.; Miyasaka, T.; Biju, V. Synthesis, Optoelectronic Properties and Applications of Halide Perovskites. Chem. Soc. Rev. 2020, 49, 2869–2885. [Google Scholar] [CrossRef]
- Savill, K.J.; Ulatowski, A.M.; Herz, L.M. Optoelectronic Properties of Tin–Lead Halide Perovskites. ACS Energy Lett. 2021, 6, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Ran, C.; Gao, W.; Li, M.; Xia, Y.; Huang, W. Metal Halide Perovskite for Next-Generation Optoelectronics: Progresses and Prospects. eLight 2023, 3, 3. [Google Scholar] [CrossRef]
- Cho, Y.; Jung, H.R.; Jo, W. Halide Perovskite Single Crystals: Growth, Characterization, and Stability for Optoelectronic Applications. Nanoscale 2022, 14, 9248–9277. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J. Present Status and Future Prospects of Perovskite Photovoltaics. Nat. Mater. 2018, 17, 372–376. [Google Scholar] [CrossRef]
- Guo, Z.; Jena, A.K.; Kim, G.M.; Miyasaka, T. The High Open-Circuit Voltage of Perovskite Solar Cells: A Review. Energy Environ. Sci. 2022, 15, 3171–3222. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Zhumagali, S.; Merino, L.V.T.; De Bastiani, M.; McCulloch, I.; De Wolf, S. Molecular Engineering of Contact Interfaces for High-Performance Perovskite Solar Cells. Nat. Rev. Mater. 2022, 8, 89–108. [Google Scholar] [CrossRef]
- Badrooj, M.; Jamali-Sheini, F.; Torabi, N. Zn-Doped Pb/Sn Hybrid Perovskite Solar Cells: Towards High Photovoltaic Performance. Sol. Energy 2022, 236, 63–74. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Huang, N.-Y.; Xu, Q. Metal Halide Perovskite Materials in Photocatalysis: Design Strategies and Applications. Coord. Chem. Rev. 2023, 481, 215031. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, X.; Yao, H.; Fan, Q.; Yu, B.; Li, J. Review on Efficiency Improvement Effort of Perovskite Solar Cell. Sol. Energy 2022, 233, 421–434. [Google Scholar] [CrossRef]
- Aftab, A.; Ahmad, M.I. A Review of Stability and Progress in Tin Halide Perovskite Solar Cell. Sol. Energy 2021, 216, 26–47. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite Solar Cells with Atomically Coherent Interlayers on SnO2 Electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Chen, B.; Yu, Z.J.; Manzoor, S.; Wang, S.; Weigand, W.; Yu, Z.; Yang, G.; Ni, Z.; Dai, X.; Holman, Z.C.; et al. Blade-Coated Perovskites on Textured Silicon for 26%-Efficient Monolithic Perovskite/Silicon Tandem Solar Cells. Joule 2020, 4, 850–864. [Google Scholar] [CrossRef]
- Agresti, A.; Pazniak, A.; Pescetelli, S.; Di Vito, A.; Rossi, D.; Pecchia, A.; Auf der Maur, M.; Liedl, A.; Larciprete, R.; Kuznetsov, D.V.; et al. Titanium-Carbide MXenes for Work Function and Interface Engineering in Perovskite Solar Cells. Nat. Mater. 2019, 18, 1228–1234. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wang, S.; Li, X.; Zhang, F. Seeding Agents in Metal Halide Perovskite Solar Cells: From Material to Mechanism. ChemSusChem 2023, 16, e202202109. [Google Scholar] [CrossRef]
- Xiong, Z.; Chen, X.; Zhang, B.; Odunmbaku, G.O.; Ou, Z.; Guo, B.; Yang, K.; Kan, Z.; Lu, S.; Chen, S.; et al. Simultaneous Interfacial Modification and Crystallization Control by Biguanide Hydrochloride for Stable Perovskite Solar Cells with PCE of 24.4%. Adv. Mater. 2022, 34, 2106118. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.; Agarwal, S.; Dhaka, M.S. Stability and Efficiency Issues, Solutions and Advancements in Perovskite Solar Cells: A Review. Sol. Energy 2022, 244, 516–535. [Google Scholar] [CrossRef]
- Lee, D.-K.; Park, N.-G. Materials and Methods for High-Efficiency Perovskite Solar Modules. Sol. RRL 2022, 6, 2100455. [Google Scholar] [CrossRef]
- Liao, J.; Wang, M.; Kong, L.; Chen, J.; Wang, X.; Yan, H.; Huang, J.; Tu, C. Dual-Mode Optical Temperature Sensing Behavior of Double-Perovskite CaGdMgSbO6:Mn4+/Sm3+ Phosphors. J. Lumin. 2020, 226, 117492. [Google Scholar] [CrossRef]
- Li, L.; Ye, S.; Qu, J.; Zhou, F.; Song, J.; Shen, G. Recent Advances in Perovskite Photodetectors for Image Sensing. Small 2021, 17, 2005606. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lu, H.; Han, X.; Wang, C.; Xu, Z.; Han, S.; Pan, C. Recent Progress on Wavelength-Selective Perovskite Photodetectors for Image Sensing. Small Methods 2023, 7, 2201499. [Google Scholar] [CrossRef]
- Sun, T.; Chen, T.; Chen, J.; Lou, Q.; Liang, Z.; Li, G.; Lin, X.; Yang, G.; Zhou, H. High-Performance p–i–n Perovskite Photodetectors and Image Sensors with Long-Term Operational Stability Enabled by a Corrosion-Resistant Titanium Nitride Back Electrode. Nanoscale 2023, 15, 7803–7811. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, S.; Xu, L.; Yue, W.; Zhang, C.; Kan, H.; Li, Y.; Shen, G. Nanostructured Perovskites for Nonvolatile Memory Devices. Chem. Soc. Rev. 2022, 51, 3341–3379. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, F.; Ruan, L.; Tong, J.; Yan, L.; Sun, C.; Zhang, X. A Facile Fabrication of Lead-Free Cs2NaBiI6 Double Perovskite Films for Memory Device Application. J. Alloys Compd. 2022, 909, 164613. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Chu, H.; Yao, X. High Performance Resistive Memory Device Based on Highly Stable Layered CsPb2Br5 Perovskite Polymer Nanocomposite. J. Alloys Compd. 2022, 921, 166014. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.; Wei, Q.; Peng, S.; Shang, Y.; Jiang, X.; Yu, D.; Wang, K.; Pu, R.; Zhao, C.; et al. In-Situ Growth of Low-Dimensional Perovskite-Based Insular Nanocrystals for Highly Efficient Light Emitting Diodes. Light Sci. Appl. 2023, 12, 62. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Gangishetty, M.K.; Abdi-Jalebi, M.; Chin, S.-H.; Bin Mohd Yusoff, A.R.; Congreve, D.N.; Tress, W.; Deschler, F.; Vasilopoulou, M.; Bolink, H.J. Perovskite Light-Emitting Diodes. Nat. Electron. 2022, 5, 203–216. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, B.; Yang, T.; Lai, R.; Lan, D.; Friend, R.H.; Di, D. Toward Stable and Efficient Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2022, 32, 2109495. [Google Scholar] [CrossRef]
- Mei, X.; Jia, D.; Chen, J.; Zheng, S.; Zhang, X. Approaching High-Performance Light-Emitting Devices upon Perovskite Quantum Dots: Advances and Prospects. Nano Today 2022, 43, 101449. [Google Scholar] [CrossRef]
- Morgenstern, T.; Lampe, C.; Naujoks, T.; Jurow, M.; Liu, Y.; Urban, A.S.; Brütting, W. Elucidating the Performance Limits of Perovskite Nanocrystal Light Emitting Diodes. J. Lumin. 2020, 220, 116939. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, J.; Li, J.; Li, Q. Halide Perovskite Materials for Energy Storage Applications. Adv. Funct. Mater. 2020, 30, 2003653. [Google Scholar] [CrossRef]
- Tambwe, K.; Ross, N.; Baker, P.; Bui, T.-T.; Goubard, F. Humidity Sensing Applications of Lead-Free Halide Perovskite Nanomaterials. Materials 2022, 15, 4146. [Google Scholar] [CrossRef]
- George, K.J.; Halali, V.V.; Sanjayan, C.G.; Suvina, V.S.; Sakar, M.; Balakrishna, R.G. Perovskite Nanomaterials as Optical and Electrochemical Sensors. Inorg. Chem. Front. 2020, 7, 2702–2725. [Google Scholar] [CrossRef]
- Blancon, J.C.; Even, J.; Stoumpos, C.C.; Kanatzidis, M.G.; Mohite, A.D. Semiconductor Physics of Organic–Inorganic 2D Halide Perovskites. Nat. Nanotechnol. 2020, 15, 969–985. [Google Scholar] [CrossRef]
- Ricciardulli, A.G.; Yang, S.; Smet, J.H.; Saliba, M. Emerging Perovskite Monolayers. Nat. Mater. 2021, 20, 1325–1336. [Google Scholar] [CrossRef]
- Kim, E.-B.; Akhtar, M.S.; Shin, H.-S.; Ameen, S.; Nazeeruddin, M.K. A Review on Two-Dimensional (2D) and 2D-3D Multidimensional Perovskite Solar Cells: Perovskites Structures, Stability, and Photovoltaic Performances. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100405. [Google Scholar] [CrossRef]
- Rothmann, M.U.; Kim, J.S.; Borchert, J.; Lohmann, K.B.; O’Leary, C.M.; Sheader, A.A.; Clark, L.; Snaith, H.J.; Johnston, M.B.; Nellist, P.D.; et al. Atomic-Scale Microstructure of Metal Halide Perovskite. Science 2020, 370, eabb5940. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Park, N.-G. A Realistic Methodology for 30% Efficient Perovskite Solar Cells. Chem 2020, 6, 1254–1264. [Google Scholar] [CrossRef]
- Xie, Y.; Xue, Q.; Yip, H. Metal-Halide Perovskite Crystallization Kinetics: A Review of Experimental and Theoretical Studies. Adv. Energy Mater. 2021, 11, 2100784. [Google Scholar] [CrossRef]
- Petrovai, I.; Todor-Boer, O.; David, L.; Botiz, I. Growth of Hybrid Perovskite Crystals from CH3NH3PbI3-XClx Solutions Subjected to Constant Solvent Evaporation Rates. Materials 2023, 16, 2625. [Google Scholar] [CrossRef]
- Brooks, K.G.; Nazeeruddin, M.K. Laser Processing Methods for Perovskite Solar Cells and Modules. Adv. Energy Mater. 2021, 11, 2101149. [Google Scholar] [CrossRef]
- Bai, D.; Bian, H.; Jin, Z.; Wang, H.; Meng, L.; Wang, Q.; (Frank) Liu, S. Temperature-Assisted Crystallization for Inorganic CsPbI2Br Perovskite Solar Cells to Attain High Stabilized Efficiency 14.81%. Nano Energy 2018, 52, 408–415. [Google Scholar] [CrossRef]
- Ye, T.; Sun, X.; Zhang, X.; Hao, S. Recent Advances of Cu-Based Hole Transport Materials and Their Interface Engineering Concerning Different Processing Methods in Perovskite Solar Cells. J. Energy Chem. 2021, 62, 459–476. [Google Scholar] [CrossRef]
- Sánchez, S.; Vallés-Pelarda, M.; Alberola-Borràs, J.-A.; Vidal, R.; Jerónimo-Rendón, J.J.; Saliba, M.; Boix, P.P.; Mora-Seró, I. Flash Infrared Annealing as a Cost-Effective and Low Environmental Impact Processing Method for Planar Perovskite Solar Cells. Mater. Today 2019, 31, 39–46. [Google Scholar] [CrossRef]
- Li, X.; Bi, D.; Yi, C.; Décoppet, J.-D.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. A Vacuum Flash–Assisted Solution Process for High-Efficiency Large-Area Perovskite Solar Cells. Science 2016, 353, 58–62. [Google Scholar] [CrossRef]
- Swartwout, R.; Hoerantner, M.T.; Bulović, V. Scalable Deposition Methods for Large-area Production of Perovskite Thin Films. Energy Environ. Mater. 2019, 2, 119–145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wei, D.; Xie, B.; Yue, X.; Li, M.; Song, D.; Li, Y. High Reproducibility of Perovskite Solar Cells via a Complete Spin-Coating Sequential Solution Deposition Process. Sol. Energy 2015, 122, 97–103. [Google Scholar] [CrossRef]
- Bishop, J.E.; Smith, J.A.; Lidzey, D.G. Development of Spray-Coated Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 48237–48245. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, H.; Yan, K.; Yang, S. Inkjet Printing and Instant Chemical Transformation of a CH3NH3PbI3/Nanocarbon Electrode and Interface for Planar Perovskite Solar Cells. Angew. Chem. Int. Ed. 2014, 53, 13239–13243. [Google Scholar] [CrossRef]
- Yang, Z.; Chueh, C.-C.; Zuo, F.; Kim, J.H.; Liang, P.-W.; Jen, A.K.-Y. High-Performance Fully Printable Perovskite Solar Cells via Blade-Coating Technique under the Ambient Condition. Adv. Energy Mater. 2015, 5, 1500328. [Google Scholar] [CrossRef]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; Vulpoi, A.; David, L.; Astilean, S.; Botiz, I. Enhancing Photoluminescence Quenching in Donor–Acceptor PCE11:PPCBMB Films through the Optimization of Film Microstructure. Nanomaterials 2019, 9, 1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; David, L.; Astilean, S.; Botiz, I. Control of Microstructure in Polymer: Fullerene Active Films by Convective Self-Assembly. Thin Solid Film. 2020, 697, 137780. [Google Scholar] [CrossRef]
- Botiz, I.; Codescu, M.-A.; Farcau, C.; Leordean, C.; Astilean, S.; Silva, C.; Stingelin, N. Convective Self-Assembly of π-Conjugated Oligomers and Polymers. J. Mater. Chem. C 2017, 5, 2513–2518. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.-R.; Yang, F.; Yang, J.-E.; Song, J.; Li, M.; Li, Z.; Tsoi, W.C.; Chinweokwu Eze, M.; Liu, Z.-Y.; et al. π-Conjugated Small Molecules Enable Efficient Perovskite Growth and Charge-Extraction for High-Performance Photovoltaic Devices. J. Power Sources 2020, 448, 227420. [Google Scholar] [CrossRef]
- Song, D.; Heo, J.H.; Han, H.J.; You, M.S.; Im, S.H. Reproducible Formation of Uniform CH3NH3PbI3-xClx Mixed Halide Perovskite Film by Separation of the Powder Formation and Spin-Coating Process. J. Power Sources 2016, 310, 130–136. [Google Scholar] [CrossRef]
- Tanaka, H.; Ohishi, Y.; Oku, T. Fabrication and Characterization of the Copper Bromides-Added CH3NH3PbI3-xClx Perovskite Solar Cells. Synth. Met. 2018, 244, 128–133. [Google Scholar] [CrossRef]
- Thankaraj Salammal, S.; Panneerselvam, V.; Chinnakutti, K.K.; Manidurai, P.; Parasuraman, K. Highly Crystalline Methylammonium Lead Iodide Films: Phase Transition from Tetragonal to Cubic Structure by Thermal Annealing. J. Vac. Sci. Technol. A 2021, 39, 022801. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological Control for High Performance, Solution-Processed Planar Heterojunction Perovskite Solar Cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Barrows, A.T.; Lilliu, S.; Pearson, A.J.; Babonneau, D.; Dunbar, A.D.F.; Lidzey, D.G. Monitoring the Formation of a CH3NH3PbI3-xClx Perovskite during Thermal Annealing Using X-ray Scattering. Adv. Funct. Mater. 2016, 26, 4934–4942. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.-K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovai, I.; Todor-Boer, O.; Vulpoi, A.; David, L.; Botiz, I. Generation of Hybrid Lead Halide CH3NH3PbI3-xClx Perovskite Crystals via Convective Self-Assembly. Coatings 2023, 13, 1130. https://doi.org/10.3390/coatings13061130
Petrovai I, Todor-Boer O, Vulpoi A, David L, Botiz I. Generation of Hybrid Lead Halide CH3NH3PbI3-xClx Perovskite Crystals via Convective Self-Assembly. Coatings. 2023; 13(6):1130. https://doi.org/10.3390/coatings13061130
Chicago/Turabian StylePetrovai, Ioan, Otto Todor-Boer, Adriana Vulpoi, Leontin David, and Ioan Botiz. 2023. "Generation of Hybrid Lead Halide CH3NH3PbI3-xClx Perovskite Crystals via Convective Self-Assembly" Coatings 13, no. 6: 1130. https://doi.org/10.3390/coatings13061130
APA StylePetrovai, I., Todor-Boer, O., Vulpoi, A., David, L., & Botiz, I. (2023). Generation of Hybrid Lead Halide CH3NH3PbI3-xClx Perovskite Crystals via Convective Self-Assembly. Coatings, 13(6), 1130. https://doi.org/10.3390/coatings13061130







