Modification of Liquid Glasses Is a Key Factor in the Technology of Obtaining Hybrid Compositions and Coatings with Anticorrosive Properties
Abstract
:1. Introduction
| |
OH R3N:OH
~CH2N−CO−N−CH2OH + (HO)X SiY → ~CH2N−CO−N−CH2O SiY(OH)X−1
2. Materials and Methods
2.1. Obtaining of Chemically Modified Liquid Glass
2.2. Testing of Silicate Compositions and Cured Coatings
3. Results
Author’s Contribution to the Development of Anticorrosion Coatings on the Surface of Aluminum and Non-Ferrous Metals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semchenko, G.D.; Tishchenko, S.V.; Rodomanov, A.S.; Sergienko, Y.E. Composition for Making Acid Resistant Coatings. Inventor’s Sertificate 1143724 of USSR. 7 March 1985. [Google Scholar]
- Korneev, A.D.; Solomatov, V.I.; Vasilieva, G.M.; Zvyagintsev, Y.V.; Kozomazov, V.N.; Kretinin, V.I. Acid Resistant Composition. Inventor’s Sertificate 1134557 of USSR. 15 January 1985. [Google Scholar]
- Dibrov, G.D.; Karpukhina, A.A.; Drozd, A.P.; Martynenko, Y.A.; Ryndovsky, Y.N. Composition for Making Acid Resistant Coatings. Inventor’s Sertificate 1281547 of USSR. 7 January 1987. [Google Scholar]
- Kuznetsov, A.T. Increasing the Durability of Liquid Glass-Based Protective Compositions. Coll. Papers; Saratov Politech Institute: Saratov, Russia, 1974; pp. 69–72. [Google Scholar]
- Kondo, H.; Koshii, T. Method for the Production of An Aqueous Silicone Emulsion Composition. Inventor’s Application 60-96650 of Japan. 24 April 1985. [Google Scholar]
- O’ Malley, W.J.; Vaughn, H.A. Silicon Organic Coating Compositions with Improved Storage Stability. U.S. Patent 4539351, 3 September 1985. [Google Scholar]
- Tarasov, V.I. New colloid silicate solutions for restoration and conservation of stone facades. Rus. J. Appl. Chem. 2001, 74, 1985–1989. [Google Scholar] [CrossRef]
- Zheng, H.; Lehtinen, M.J.; Liu, G. Hydrophobic modification of sintered glass filters for the separation of organic solvents and gasoline from water as well as emulsified water. J. Environ. Chem. Eng. 2021, 9, 106449. [Google Scholar] [CrossRef]
- Mossety-Leszczak, B.; Pilch-Pitera, B.; Karaś, J.; Kisiel, M.; Zając, W.; Włodarska, M. The application of liquid crystalline epoxy resin for forming hybrid powder coatings. Prog. Org. Coat. 2022, 168, 106873. [Google Scholar] [CrossRef]
- Brand, M.C.; Greenwell, F.; Clowes, R.; Egleston, B.D.; Kai, A.; Cooper, A.I.; Bennett, T.D.; Greenaway, R.L. Melt-quenched porous organic cage glasses. J. Mater. Chem. A 2021, 9, 19807–19816. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V. Structural Changes in Metallic Glass-Forming Liquids on Cooling and Subsequent Vitrification in Relationship with Their Properties. Materials 2022, 15, 7285. [Google Scholar] [CrossRef]
- Chen, R.S.; Muhammad, Y.H.; Ahmad, S. Physical, mechanical and environmental stress cracking characteristics of epoxy/glass fiber composites: Effect of matrix/fiber modification and fiber loading. Polym. Test. 2021, 96, 107088. [Google Scholar] [CrossRef]
- Skachkova, V.K.; Grachev, A.V.; Shaulov, A.Y.; Berlin, A.A. Inorganic polymers based on sodium liquid glass silicates. Features of polycondensation of silicates. Chem. Phys. 2019, 38, 78–82. [Google Scholar] [CrossRef]
- Shaibadullina, A.V.; Yakovlev, G.I.; Grakhov, V.P.; Polyanskikh, I.S. Facade Cement Silicate Coating for Ceramic Bricks. Intellekt. Sist. Proizv. 2017, 15, 118. [Google Scholar] [CrossRef]
- Mori, I.; Kimura, H. Compositions for Protective Coatings. Inventor’s Application 60-79071 of Japan, 1985.
- Shestyorkina, N.F.; Paturoev, V.V.; Sergeeva, E.V.; Supran, Y.A. Polymersilicate Composition. Inventor’s Sertificate 1180363 of USSR, 1985.
- Yashchishin, I.N.; Vakhula, Y.I.; Semchuk, O.R. Changes in the structure and properties of colloidal silicate solutions during the sol-gel transition. Zhurn. Prikl. Khim. 2000, 73, 187–191. [Google Scholar]
- Kuznetsova, L.A.; Golubeva, T.Y.; Khashkovskii, S.V.; Belyustin, A.A. Enamel compositions based on gel-forming alkaline silicate solutions. Zhurn. Prikl. Khim. 1997, 70, 553–555. [Google Scholar]
- Lisovskiy, V.V.; Detsuk, V.S.; Zlotnikov, V.V. About stabilisation of silicate polymer systems. Zhurn. Prikl. Khim. 1990, 63, 917–919. [Google Scholar]
- Tadasi, G. Aqueous Polymer Coating Composition. Inventor’s Application 61-148273 of Japan, 1986.
- Kokin, A.A. Water resistance of polymer silicate compositions with acetone-formaldehyde resin additive. Coll. papers: Special concretes and buildigs. M. 1986; pp. 12–16.
- Primachenko, O.N.; Pavlyuchenko, V.N.; Gagarina, K.A.; Skrifvars, M.; Laamanen, H.; Passiniemi, P.; Ivanchev, S.S. Antistatic latex coatings based on amine-containing copolymers. Zhurn. Prikl. Khim. 2000, 73, 1713–1719. [Google Scholar]
- Primachenko, O.N.; Sorochinskaya, O.V.; Pavlyuchenko, V.N.; Ivanchev, S.S.; Skrifvars, M.; Koskinen, J.; Karna, T.; Laamanen, H. Latex compositions for antistatic coatings. Zhurn. Prikl. Khim. 2002, 75, 1739–1742. [Google Scholar]
- Ishchenko, S.S.; Novikova, T.I.; Pridatko, A.B.; Lebedev, E.V. Co-curing of aqueous sodium silicate solution with polyisocyanate and epoxy resin. Zhurn. Prikl. Khim. 1995, 68, 1198–1201. [Google Scholar]
- Ishchenko, S.S.; Pridatko, A.B.; Novikova, T.I.; Lebedev, E.V. Soluble silicates in reactions with isocyanates. Polymer Sci. 1995, 37, 1125–1129. [Google Scholar]
- Ishchenko, S.S.; Pridatko, A.B.; Novikova, T.I.; Lebedev, E.V. Interaction of isocyanates with aqueous solutions of alkali metal silicates. Polym. Sci. Ser. A 1996, 38, 485–490. [Google Scholar]
- Ishchenko, S.S.; Rosovitsky, V.F.; Pridatko, A.B.; Babkina, N.V. Effect of organic modifiers on the formation of organosilicate polymer compositions. Zhurn. Prikl. Khim. 1998, 71, 1929–1933. [Google Scholar]
- Ishchenko, S.S.; Novikova, T.I.; Veselovskiy, R.A. The role of adsorption processes in the formation of compositions of liquid glass and organic reagents. Zhurn. Prikl. Khim. 1991, 64, 842–845. [Google Scholar]
- Askadsky, A.A.; Afanasiev, E.S.; Bruyako, M.; Petunova, M.; Matseevich, T.; Safonova, E.; Pustovgar, A. Organo-mineral composite materials based on sodium liquid glass, 2,4-toluylene diisocyanate, epoxy oligomer and polyisocyanate. Rep. Acad. Sci. 2018, 481, 47–52. [Google Scholar] [CrossRef]
- Sangalov, Y.A.; Antonova, N.G.; Saburova, O.I. Adhesive composition of liquid glass and glycerine. Zhurn. Prikl. Khim. 1999, 72, 170. [Google Scholar]
- Eritsyan, M.L.; Gyurdzhyan, L.A.; Melkonyan, L.T.; Akopyan, G.V. Acrylic acid urea copolymers. Zhurn. Prikl. Khim. 2006, 79, 1686–1688. [Google Scholar] [CrossRef]
- Korneev, V.I.; Danilov, V.V.; Medvedeva, I.N.; Nuzhdina, N.I. The polymer state of silicon dioxide in liquid glasses and bonding based on them. Zhurn. Prikl. Khim. 1997, 70, 220–224. [Google Scholar]
- Razgovorov, P.B.; Ignatov, V.A.; Koifman, Z.T.; Terskaya, I.N. Study on the modification mechanism of liquid glasses with urea. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 1993, 36, 68–70. [Google Scholar]
- Razgovorov, P.B.; Ignatov, V.A. Building silicate paint based on modified sodium liquid glass. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 1995, 38, 183–185. [Google Scholar]
- Ignatov, V.A.; Razgovorov, P.B. Silicate paint based on potassium modified liquid glass. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 1994, 37, 170–172. [Google Scholar]
- Bogoyavlenskaya, G.A.; Denisova, M.V. Silicate adhesives based on modified liquid glass. Zhurn. Prikl. Khim. 1996, 69, 2075. [Google Scholar]
- Eremina, N.V.; Avvakumov, E.G.; Zelinskiy, V.Y. Properties of a flame retardant composition based on liquid glass and mechanically activated aluminium oxide. Zhurn. Prikl. Khim. 2005, 78, 1065–1069. [Google Scholar]
- Razgovorov, P.B. Scientific Bases of Creation of the Composite Materials from Technical and Natural Silicates. Extended Abstract of Dissertation for Doctor Degree on Technical Scienses; Ivanovo State University of Chemistry and Technology (ISUCT): Ivanovo, Russia, 2008; 32p. [Google Scholar]
- Markov, S.V.; Zavalishin, E.V.; Yunkevich, A.V. Physical and mechanical properties of composites based on liquid glass for buildings and structures. Vestnik. MGSU. 2015, 7, 69–78. [Google Scholar] [CrossRef]
- Danilov, V.V.; Korneev, V.I.; Morozova, E.V.; Agafonov, G.I.; Itsko, E.F. Classification of additives regulating the properties of liquid glass binders. Zhurn. Prikl. Khim. 1987, 60, 331–334. [Google Scholar]
- Lebedeva, O.V.; Sinev, A.E. Hybrid composites and their properties. Izvestiya vuzov. Appl. Chem. Biotechnol. 2015, 2, 7–11. [Google Scholar]
- Lambourne, R. (Ed.) Paint and Surface Coatings: Theory and Practice; Translated from English; Chemistry: St. Petersburg, Fl, USA, 1991. [Google Scholar]
- Fomin, G.S. Paint and varnish materials and coatings. In Encyclopedia of International Standards. M; Wm. B. Eerdmans Publishing Company: Grand Rapids, MI, USA, 2008. [Google Scholar]
- Shabanova, N.A.; Sarkisov, P.D. Basis of Sol-Gel Technology of Polydisperse Silica; ICC Academkniga: Moscow, Russia, 2004. [Google Scholar]
- Weinmann, K. Kunststoffdisperrsions und Silicatfarben füur den Alt und Neubau. Farbe Und Lack. 1979, 5, 361–364. [Google Scholar]
- Weinmann, K. Kunststoffdisperrsions und Silicatfarben für den Alt und Neubau. Farbe Und Lack. 1985, 9, 806–809. [Google Scholar]
- Friedemann, W.; Lauf, B. Adhesive and/or Paint Compositions Based on Alkali Metal Silicate Solutions. Inventor’s Application 3020864 of West Germany, 1981.
- Andrutskaya, O.M. BASF products for the Russian market. Paint. Varn. Mater. Appl. 2001, 12, 14–16. [Google Scholar]
- Leiko, V.V.; Stepanova, N.A.; Bazarova, N.V.; Kupriyanov, V.D. Study of the effect of the dispersed phosphor phase on the rheological behaviour of butadiene nitrile rubber solutions. Zhurn. Prikl. Khim. 1987, 68, 1928–1930. [Google Scholar]
- Batdorf, V.H. Curing Silicate Composition and Its Use. U.S. Patent 4347285, 31 August 1982. [Google Scholar]
- Terlikovsky, E.V.; Kruglitsky, N.N.; Skorobogach, L.P. Silicon Protection Coatings. Coll. Papers: Progressive Paint and Varnish Materials and Technology of Colouring; MDNTP: Moscow, Russia, 1985; pp. 32–36. [Google Scholar]
- Albertinsky, L.G.; Delgadilyo, H.E.; Agafonov, G.I.; Verkholantsev, V.V. Effect of surfactants on the properties of aqueous solutions of alkali metal silicates. Paint. Varn. Mater. Appl. 1988, 2, 13–15. [Google Scholar]
- Ovchiyan, V.N. On the passivating effect of silicate-alkali solutions on metals. Zhurn. Prikl. Khim. 2003, 76, 231–233. [Google Scholar]
- Katsanis, E.P.; Esmonde, W.B.; Spencer, R.W. Soluble silicate corrosion inhibitors in water systems. Mater. Perform. 1986, 25, 19–25. [Google Scholar]
- Denker, I.I.; Kuleshova, I.D. Protection of Parts Made of Aluminium and Its Alloys by Paint-and-Lacquer Coatings; Chemistry: Moscow, Russia, 1985. [Google Scholar]
- Perekhrest, A.; Pimenova, K.N.; Litovchenko, V.D. Formation of phosphate coatings on aluminium alloys. Zhurn. Prikl. Khim. 1992, 65, 1163–1166. [Google Scholar]
- Klejn, E.V.; Razgovorov, P.B.; Sitanov, S.V.; Gorshkov, V.K.; Simunova, S.S. Features of phosphate film formation on aluminium and its alloys. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 2006, 49, 45–48. [Google Scholar]
- Starikova, E.Y.; Feiler, L.A. Protective phosphate coatings of metals. Bull. Kuzbass State Tech. Univ. J. 2020, 46–50. [Google Scholar] [CrossRef]
- Popova, A.A. Methods of Protection from Corrosion; LAN: Saint Petersburg, Russia, 2014. [Google Scholar]
- Anufriev, N.G.; Goncharov, V.L.; Ivanov, A.M.; Akolzin, A.P. New production technology for zinc silicate paint binder. Paint. Varn. Mater. Appl. 2001, 4, 7–9. [Google Scholar]
- Sychev, M.M. Inorganic Adhesives; Chemistry: Moscow, Russia, 1986. [Google Scholar]
- Shaulov, A.Y.; Vladimirov, L.V.; Grachev, A.V.; Lalayan, V.M.; Nechvolodova, E.M.; Sakovich, R.A.; Berlin, A.A. Inorganic and hybrid polymers and composites. Chem. Phys. 2020, 39, 75–82. [Google Scholar] [CrossRef]
- Eselev, A.D.; Yakushin, F.S. PF-115 enamel, quality and pricing. Paint. Varn. Mater. Appl. 2001, 7–8, 3–12. [Google Scholar]
- Razgovorov, P.B.; Ignatov, V.A.; Alekseev, S.M.; Mesnik, O.M.; Krylova, T.A.; Pelevina, N.I. Composite Silicate Dye. Russian Federation Patent 2160753, 20 December 2000. [Google Scholar]
- Razgovorov, P.B.; Ignatov, V.A.; Alekseev, S.M.; Momot, V.S.; Shubniy, V.K.; Cherezov, Y.M.; Pelevina, N.I. Silicate Dye. Russian Federation Patent 2041900, 20 August 1995. [Google Scholar]

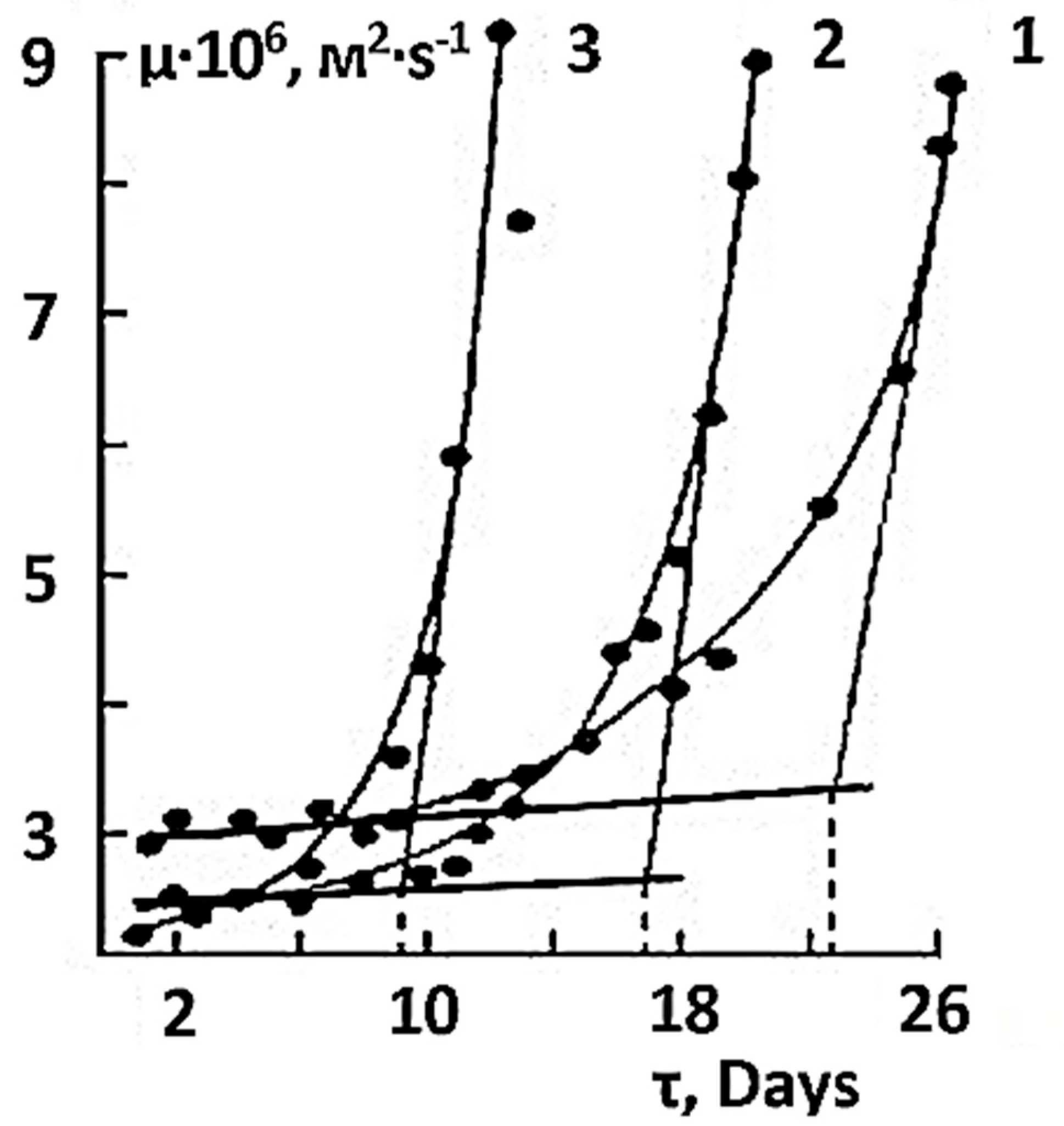

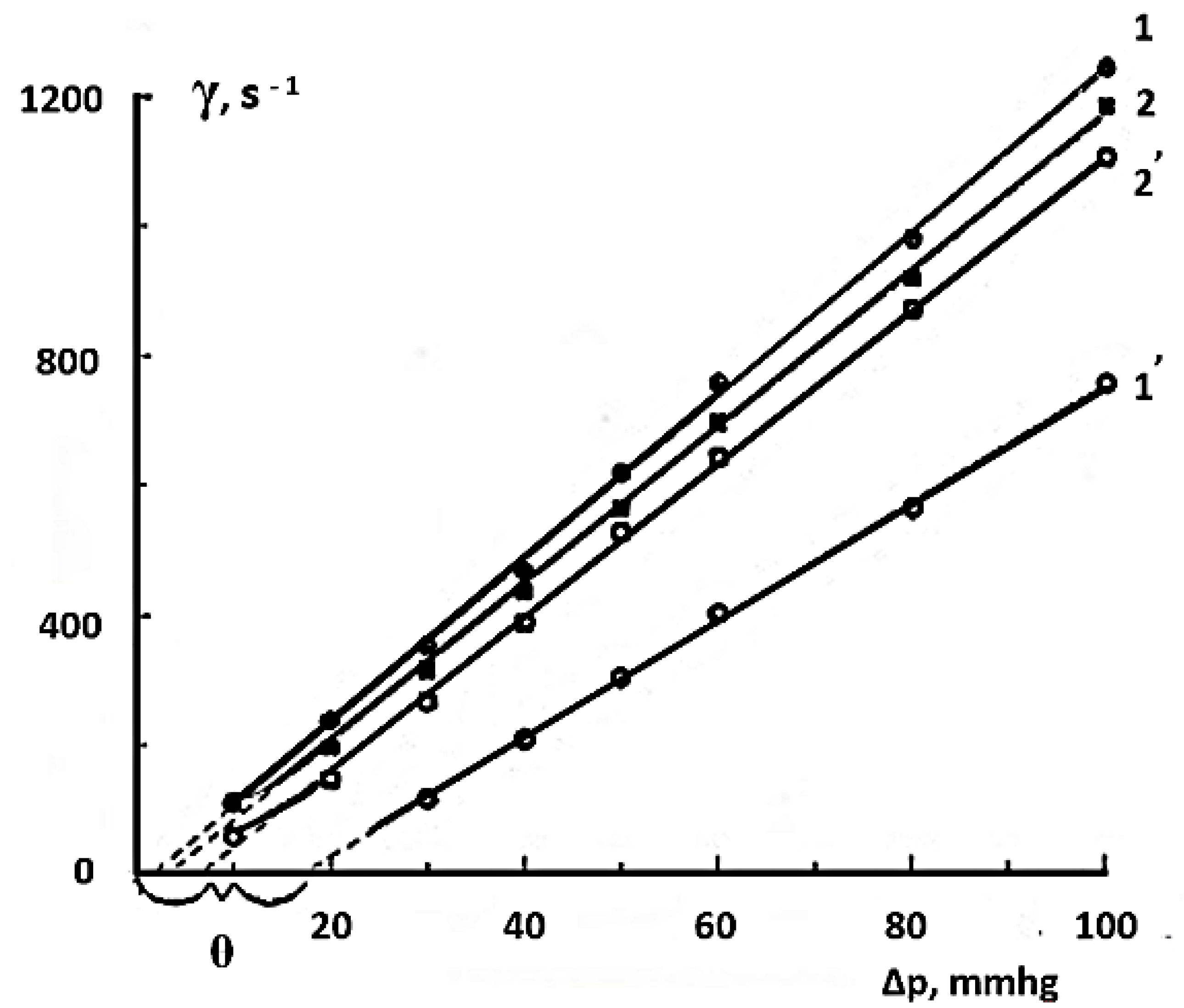
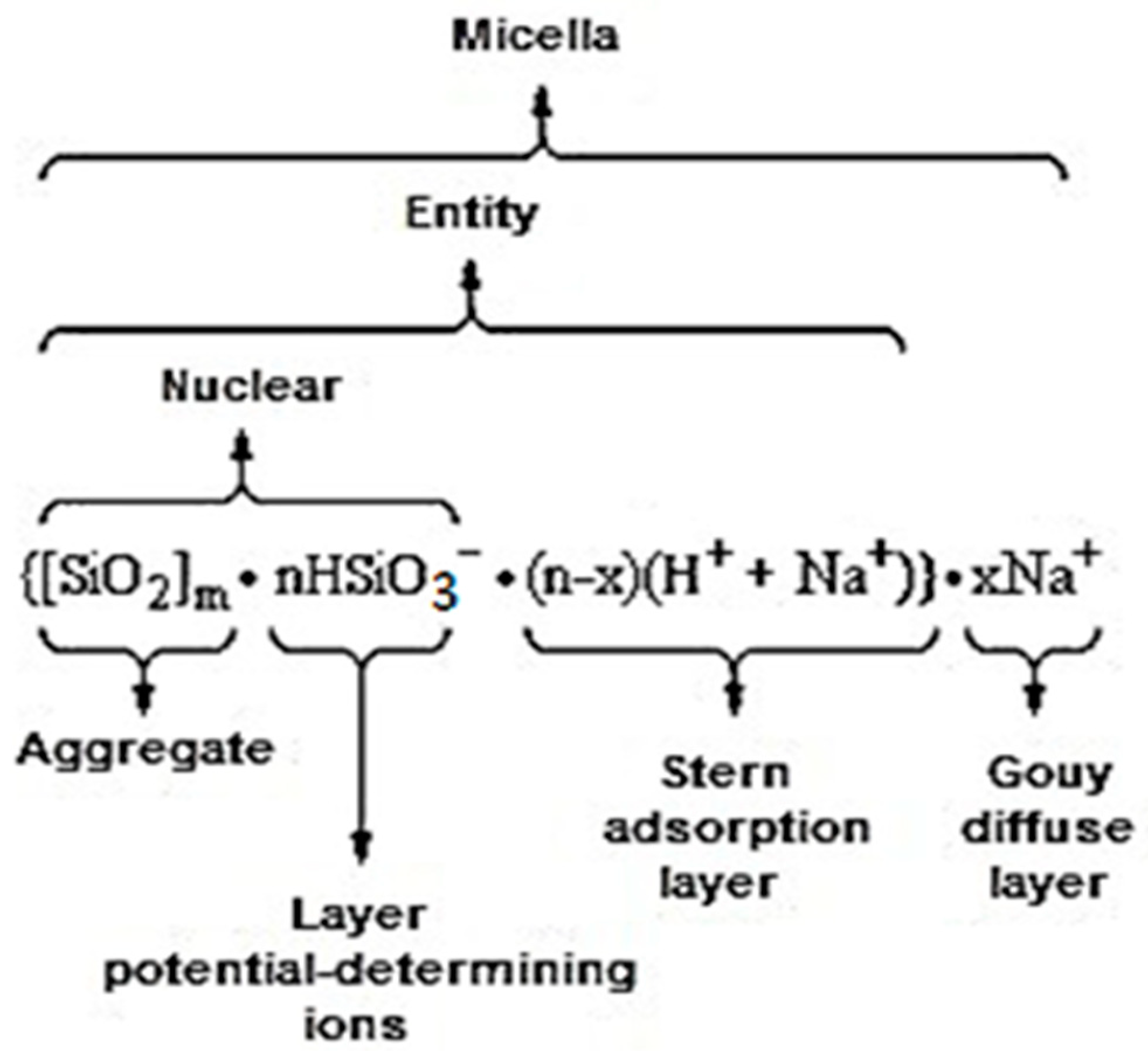
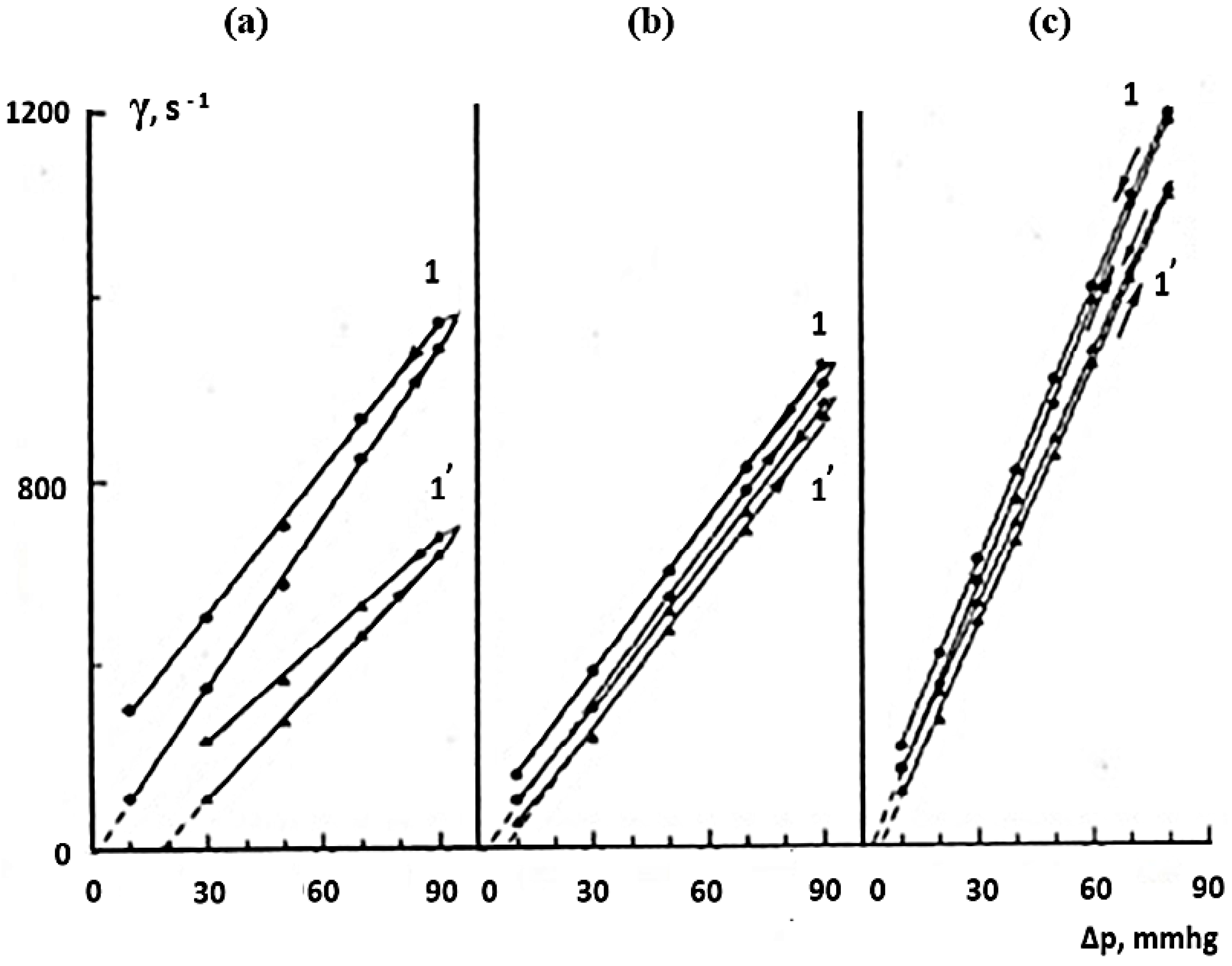
| Type of Solution | Unstored Solution, mV | Solution after 7 Days of Ageing with Modifier, mV |
|---|---|---|
| Sodium liquid glass solution | 3.2 ± 0.1 | 4.4 ± 0.1 |
| Liquid sodium glass—urea | 6.2 ± 0.2 | 14.6 ± 0.4 |
| Liquid sodium glass— auxiliary substance OP-10 | 26.0 ± 0.8 | 40.9 ± 1.1 |
| Parameter/Characteristic | Sodium and Potassium Based Liquid Glass Coatings | Liquid Glass Coatings Containing Urea and Butadiene Styrene Latex |
|---|---|---|
| Atmosphere resistance | Satisfactory | Good |
| Resistance in marine and tropical climates | Hydrophobization is required | Satisfactory |
| Water infiltration | From limited to high | Small |
| Water absorption W | W = 0.3–0.8 kg/m2 c0.5 | W = 0.2–0.5 kg/m2 c0.5 |
| Elasticity (bend test) | 50 mm | ≤15 mm |
| Resistant to detergents and abrasives | Relatively limited | Up to good |
| Application conditions | Do not apply in the rain | Resistant in the rain |
| Cost | More expensive | Less expensive |
| Route | Operation Material | Viscosity on Funnel VZ-4, s | Drying Mode | ||
|---|---|---|---|---|---|
| Air Sprayer KR-10 | Brush Application | Time Duration, Hours | Temperature, K | ||
| Degreasing | White spirit solvent | - | - | - | - |
| Sandblasting | Sand | - | - | - | - |
| Priming | Primer | 14–25 | 40–45 | 3 | 291–296 |
| Coating | Sodium liquid glass—aluminum powder | 20–25 | 25–30 | 7–8 | 291–296 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razgovorov, P.; Loginova, S.; Politaeva, N.; Velmozhina, K.; Shinkevich, P. Modification of Liquid Glasses Is a Key Factor in the Technology of Obtaining Hybrid Compositions and Coatings with Anticorrosive Properties. Coatings 2023, 13, 974. https://doi.org/10.3390/coatings13060974
Razgovorov P, Loginova S, Politaeva N, Velmozhina K, Shinkevich P. Modification of Liquid Glasses Is a Key Factor in the Technology of Obtaining Hybrid Compositions and Coatings with Anticorrosive Properties. Coatings. 2023; 13(6):974. https://doi.org/10.3390/coatings13060974
Chicago/Turabian StyleRazgovorov, Pavel, Svetlana Loginova, Natalia Politaeva, Ksenia Velmozhina, and Polina Shinkevich. 2023. "Modification of Liquid Glasses Is a Key Factor in the Technology of Obtaining Hybrid Compositions and Coatings with Anticorrosive Properties" Coatings 13, no. 6: 974. https://doi.org/10.3390/coatings13060974







