Study of Wear and Corrosion Resistance of Cold Sprayed TC4 Coating on the Surface of Mg-Li Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
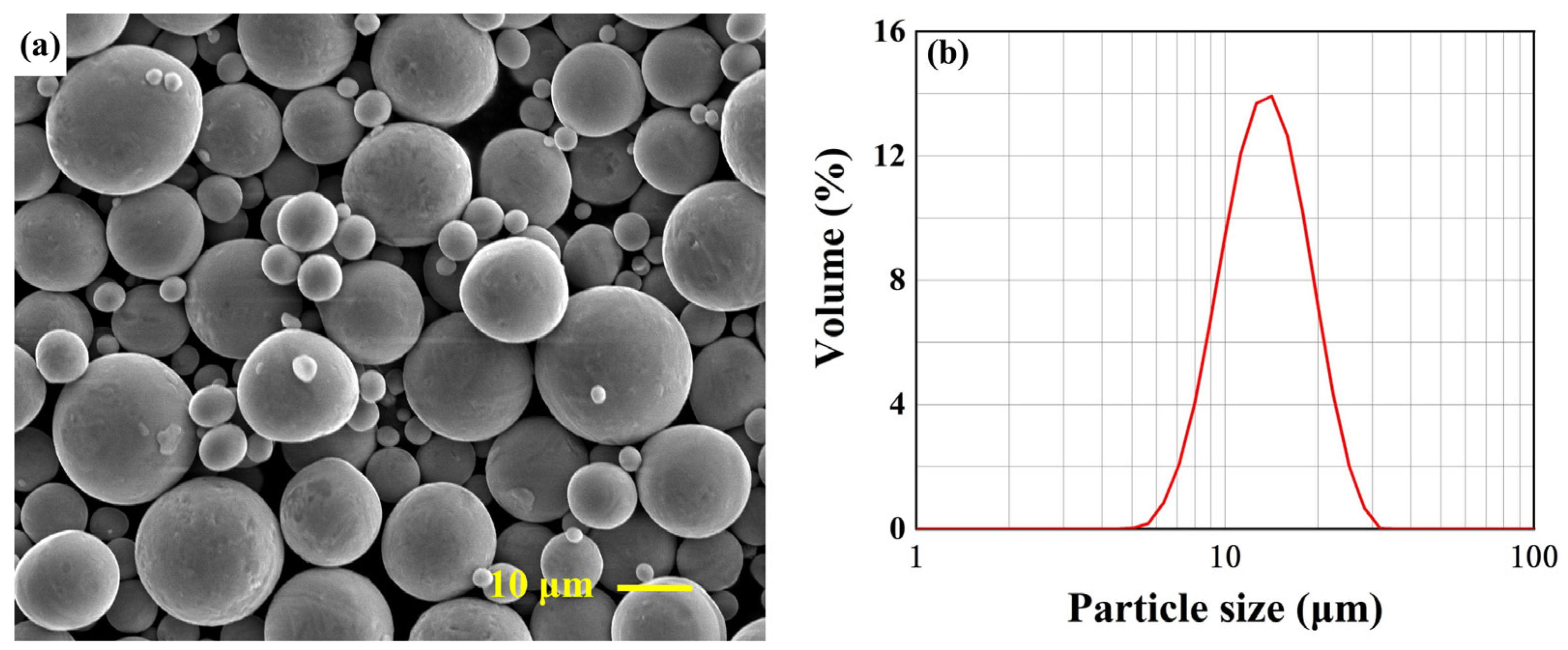
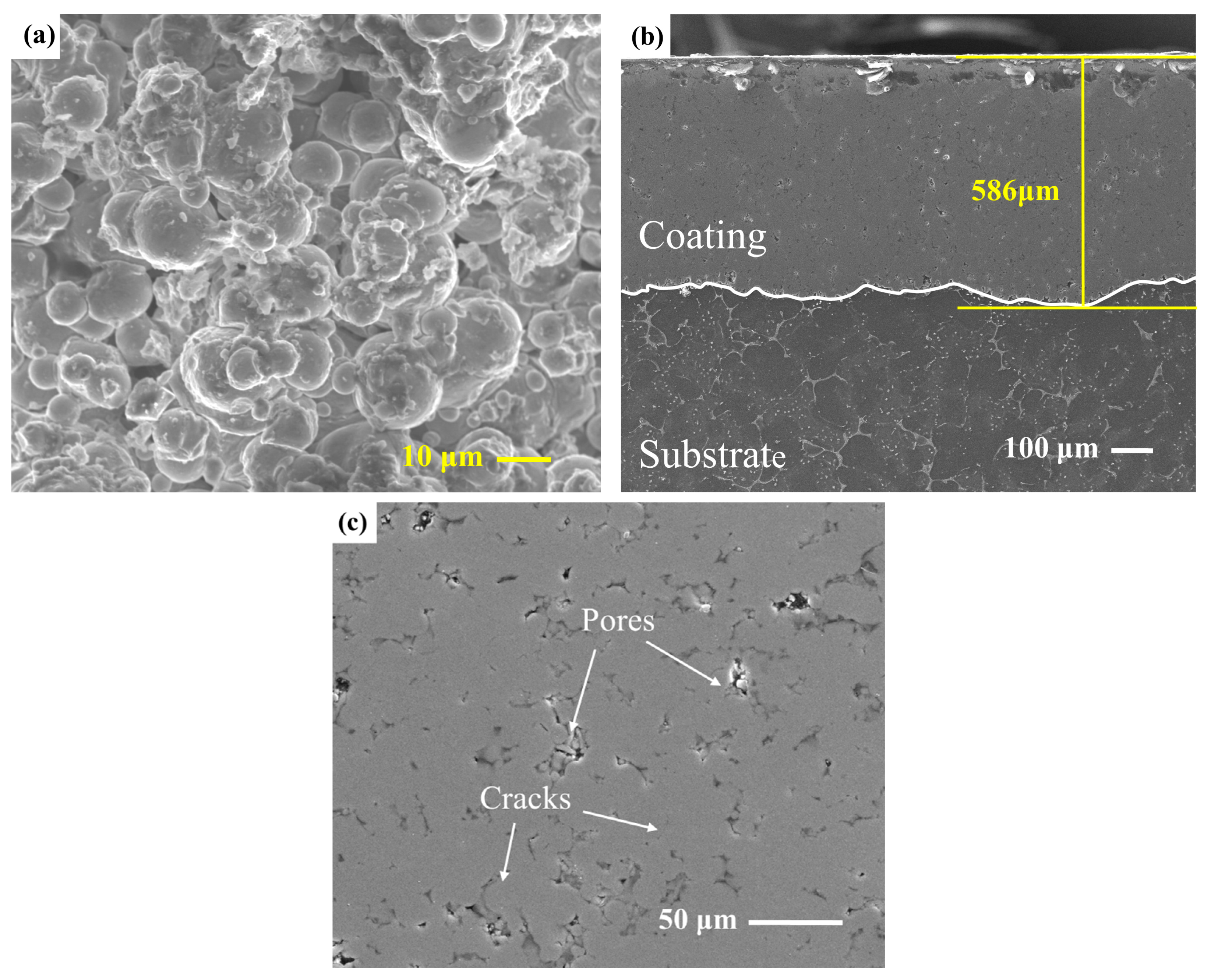
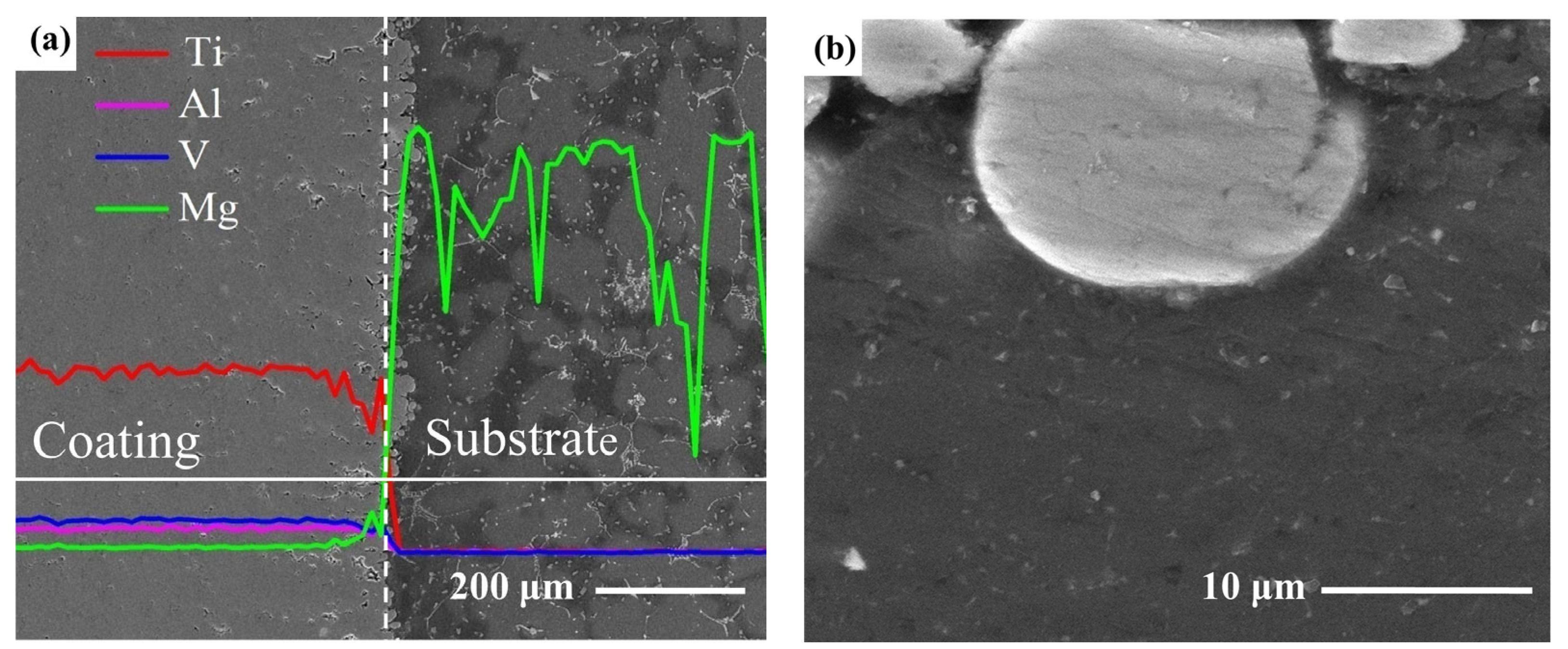
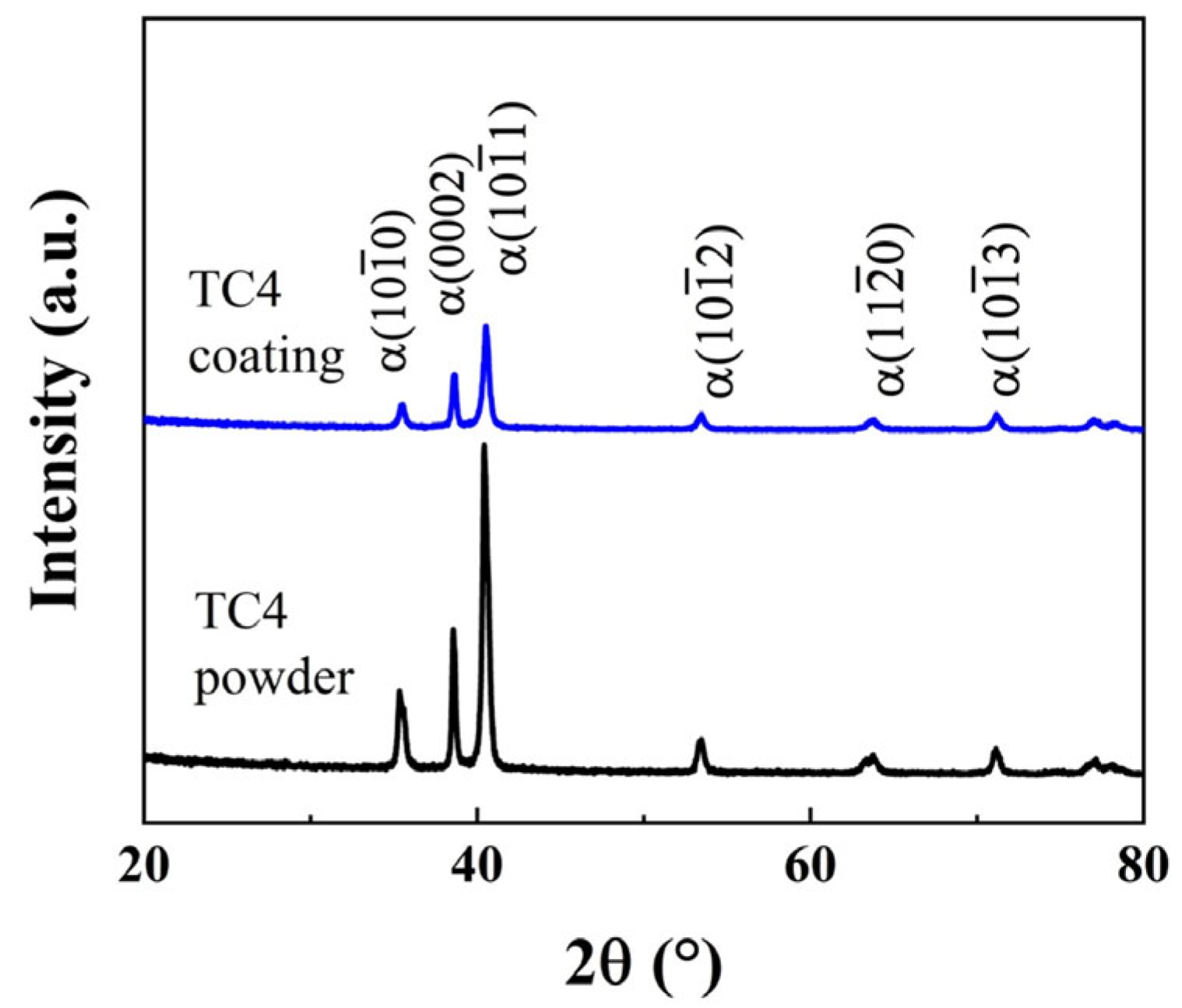
3.1. Surface Morphology and Microstructure
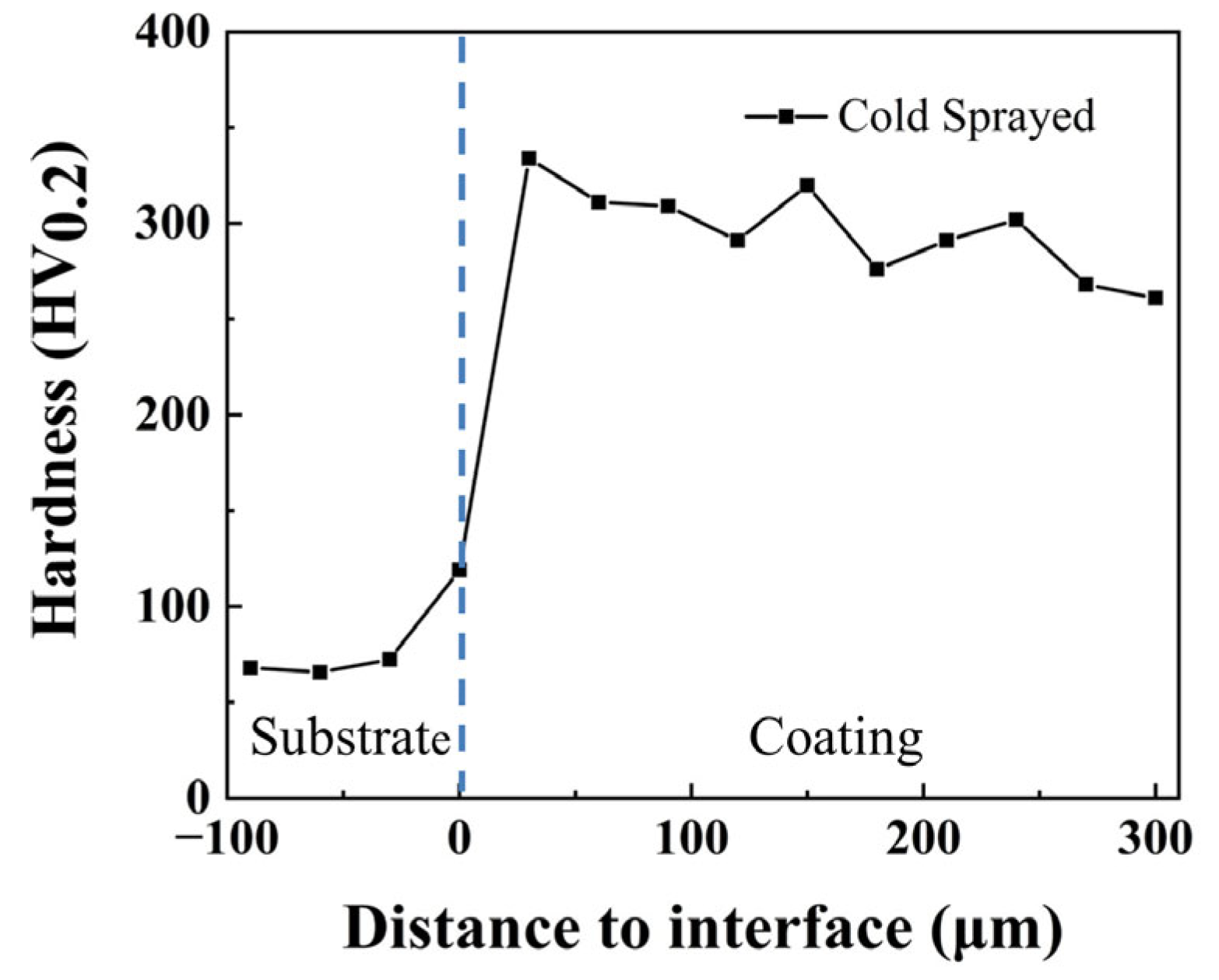
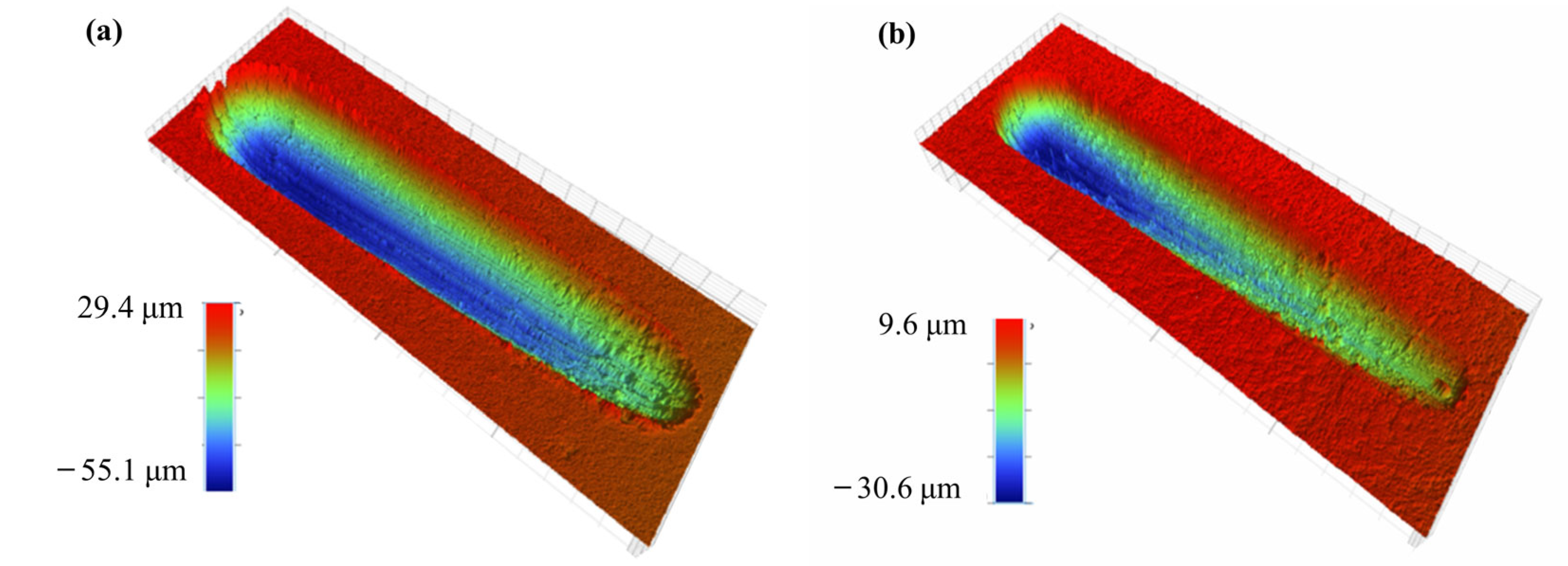
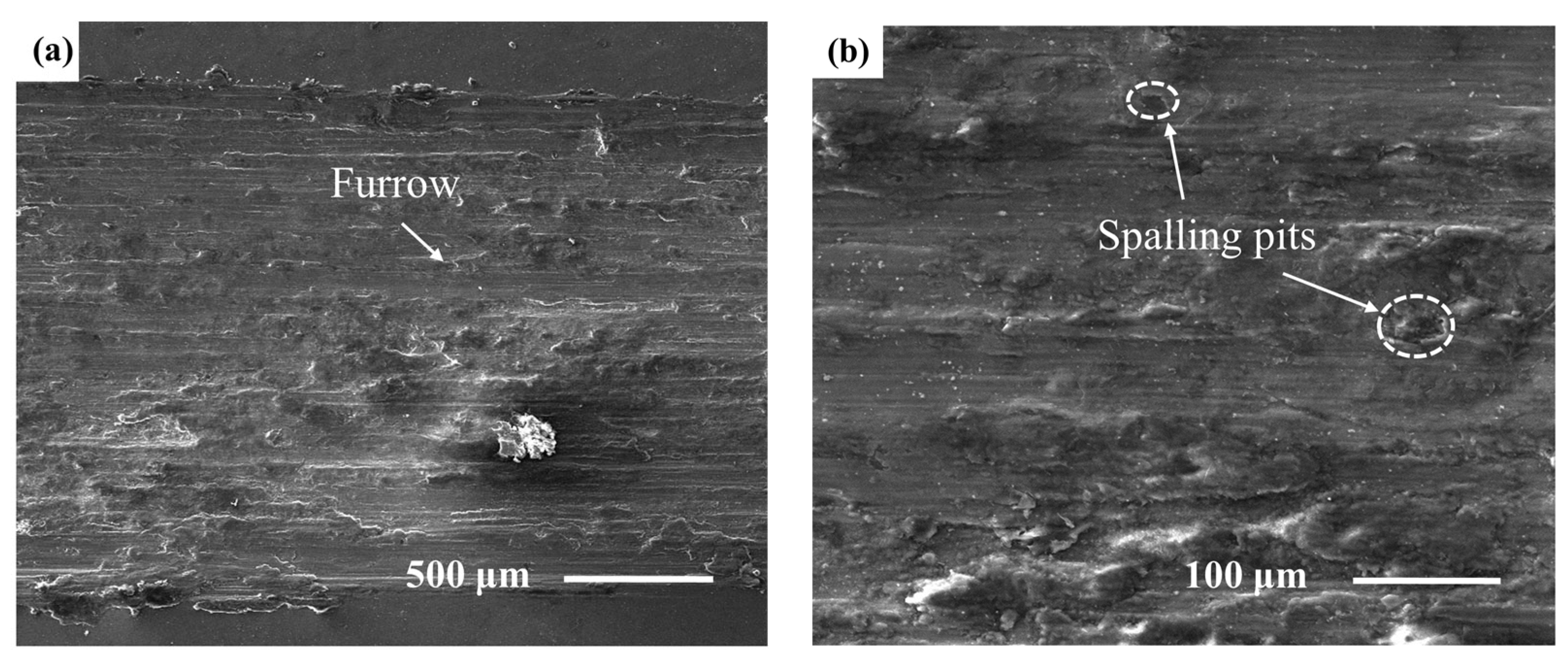
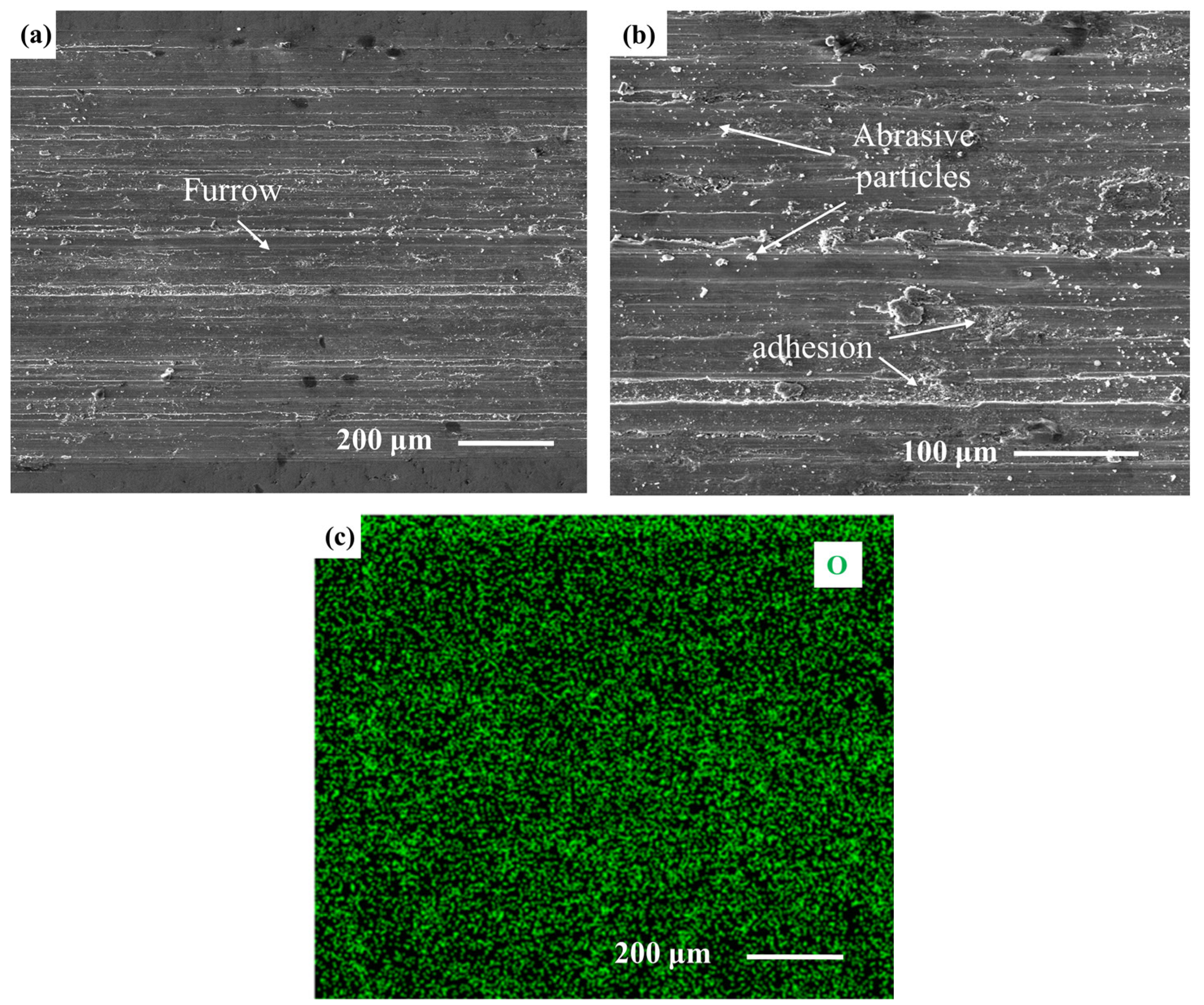
3.2. Microhardness and Tribological Properties
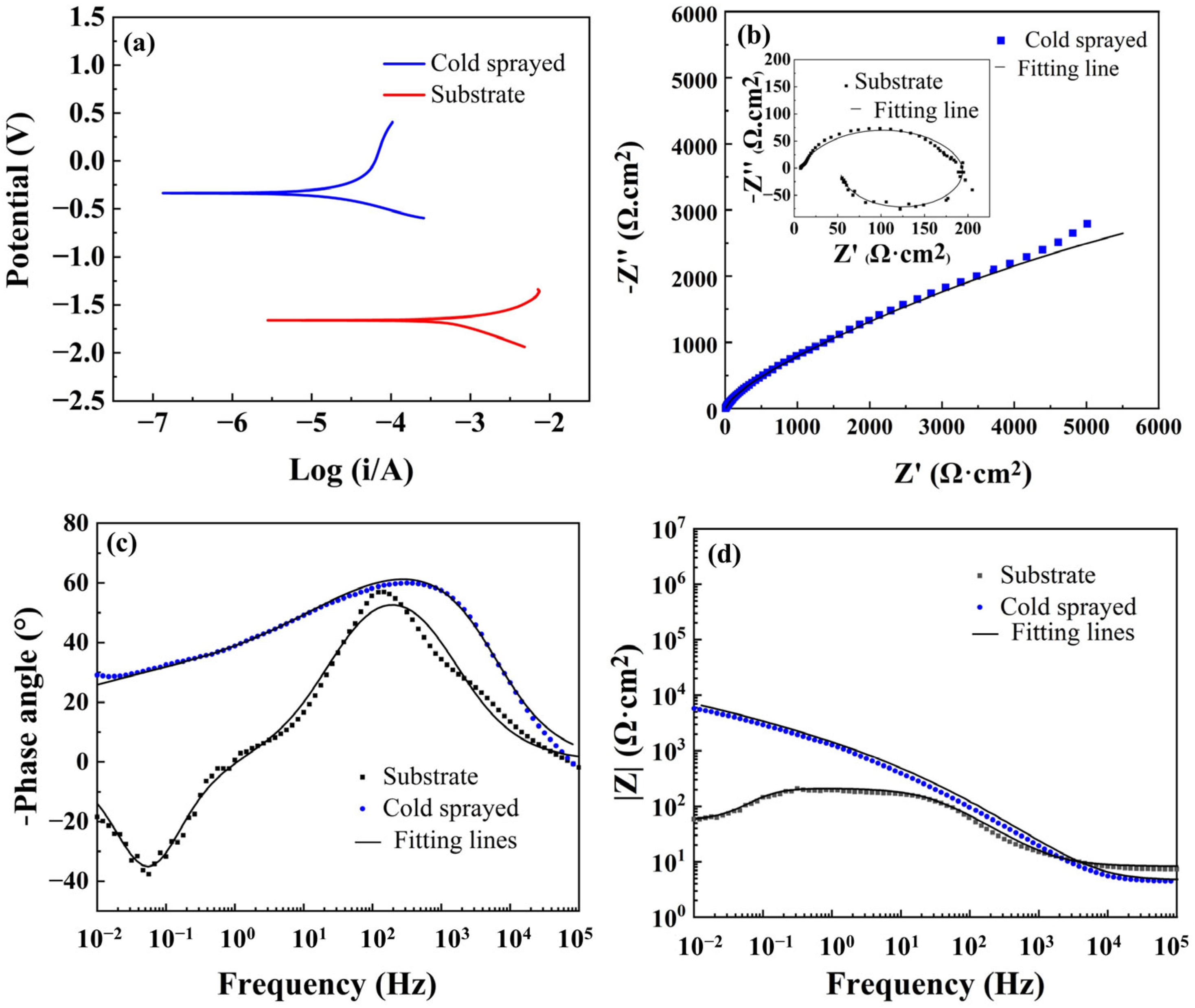
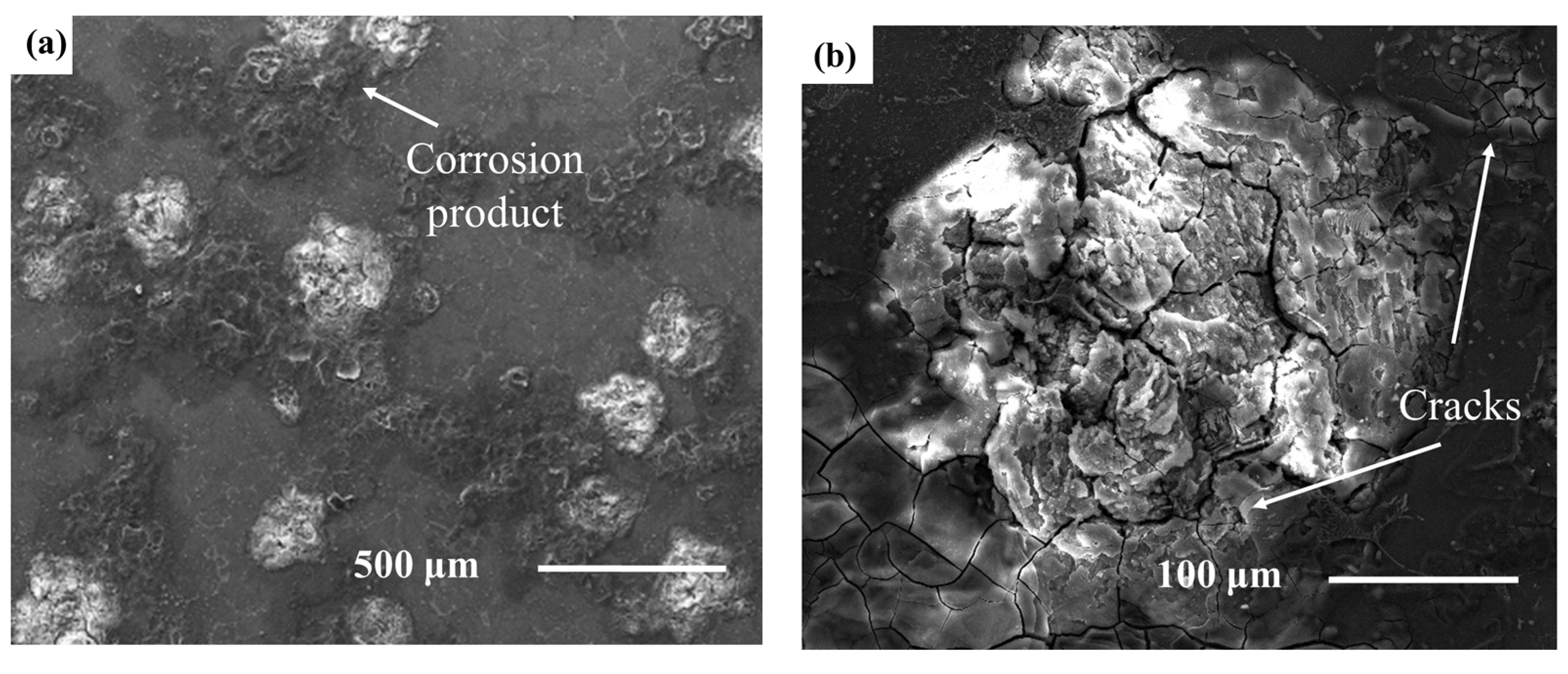
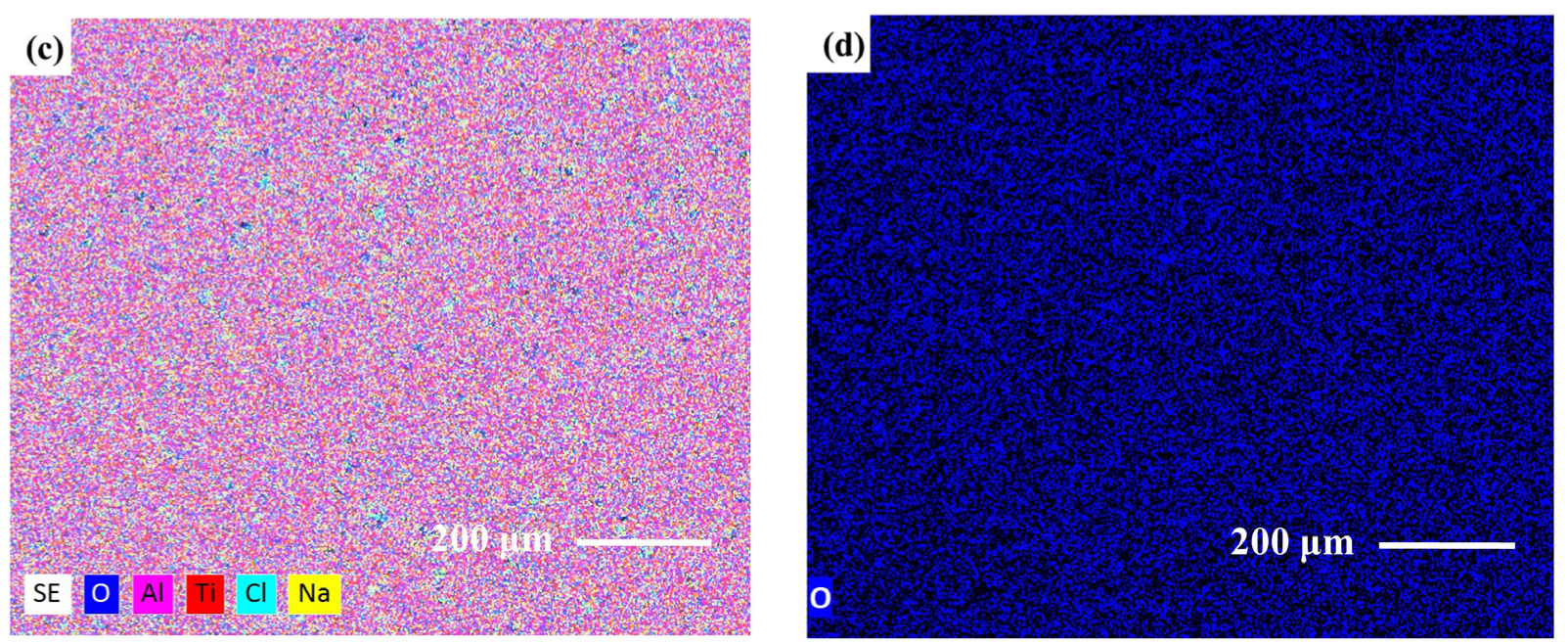
3.3. Corrosion Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, X.; Cui, X.; Jin, G.; Jiao, Y.; Fang, Y. A novel Ni2MnCuSnAl0.1 multi-principal element alloy coating to enhance the wear resistance and corrosion resistance of Mg-Li alloy. Opt. Laser Technol. 2021, 142, 107243. [Google Scholar] [CrossRef]
- Jiang, L.; Cui, X.; Jin, G.; Tian, H.; Tian, Z.; Zhang, X.; Wan, S. Synthesis and microstructure, properties characterization of Ni-Ti-Cu/Cu-Al functionally graded coating on Mg-Li alloy by laser cladding. Appl. Surf. Sci. 2021, 575, 151645. [Google Scholar] [CrossRef]
- Guo, J.; Guo, S.; Shen, Y.; Li, D. Hot Deformation Behavior and Microstructural Evolution Based on the Processing Map of Dual-Phase Mg-Li Based Alloy. Materials 2022, 15, 1022. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liu, S.H.; Wang, Q.L.; Li, Y. A Comparative Study on Mechanical and Corrosion Behaviours of α/(α + β) Mg-Li Al-loys Subjected to Ultrasonic Nanocrystal Surface Modification. Metals 2022, 12, 681. [Google Scholar] [CrossRef]
- Fathi, M.; Safavi, M.S.; Mahdavi, S.; Mirzazadeh, S.; Charkhesht, V.; Mardanifar, A.; Mehdipour, M. Co–P alloy matrix composite deposits reinforced by nano-MoS2 solid lubricant: An alternative tribological coating to hard chromium coatings. Tribol. Int. 2021, 159, 106956. [Google Scholar] [CrossRef]
- Safavi, M.S.; Fathi, M.; Ahadzadeh, I. Feasible strategies for promoting the mechano-corrosion performance of Ni-Co based coatings: Which one is better? Surf. Coat. Technol. 2021, 420, 127337. [Google Scholar] [CrossRef]
- Miyakita, N.; Tanigaki, N.; Morishige, T.; Takenaka, T. Effect of Voltage on Mg-Li-Al Alloy Anodic Oxide Film. Mater. Sci. Forum 2018, 941, 1194–1197. [Google Scholar] [CrossRef]
- Lee, S.-J.; Do, L.H.T. Effects of copper additive on micro-arc oxidation coating of LZ91 magnesium-lithium alloy. Surf. Coat. Technol. 2016, 307, 781–789. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Z.; Liu, S.; Chen, D.; Wang, G.; Wang, Y.; Zhang, M.; Chen, Y. Ultrasonic-Assisted Electroless Ni-P Plating on Dual Phase Mg-Li Alloy. J. Electrochem. Soc. 2015, 162, C64–C70. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, G.; Wang, S.; Liu, Y.; Luo, H. A chrome-free conversion coating for magnesium–lithium alloy by a phosphate–permanganate solution. Surf. Coat. Technol. 2008, 202, 1825–1830. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, T.; Fu, B.; Li, G.; Liu, Q.; Li, Y. Microstructure and Properties of Cold Sprayed NiCrAl Coating on AZ91D Magnesium Alloy. Coatings 2021, 11, 193. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 30, 369–395. [Google Scholar] [CrossRef]
- Sun, W.; Chu, X.; Lan, H.; Huang, R.; Huang, J.; Xie, Y.; Huang, J.; Huang, G. Current Implementation Status of Cold Spray Technology: A Short Review. J. Therm. Spray Technol. 2022, 31, 848–865. [Google Scholar] [CrossRef]
- Li, W.; Cao, C.; Yin, S. Solid-state cold spraying of Ti and its alloys: A literature review. Prog. Mater. Sci. 2020, 110, 100633. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Khan, M.U.F.; Saadeh, Y.; Kay, C.M.; Kasar, A.K.; Kumar, P.; Esteves, L.; Misra, M.; Menezes, P.; Kalvala, P.R.; et al. Modification of surface hardness, wear resistance and corrosion resistance of cold spray Al coated AZ31B Mg alloy using cold spray double layered Ta/ Ti coating in 3.5 wt % NaCl solution. Corros. Sci. 2020, 176, 109029. [Google Scholar] [CrossRef]
- Khun, N.W.; Tan, A.W.Y.; Sun, W.; Liu, E. Wear and Corrosion Resistance of Thick Ti-6Al-4V Coating Deposited on Ti-6Al-4V Substrate via High-Pressure Cold Spray. J. Therm. Spray Technol. 2017, 26, 1393–1407. [Google Scholar] [CrossRef]
- Sun, W.; Tan, A.W.Y.; Marinescu, I.; Toh, W.Q.; Liu, E. Adhesion, tribological and corrosion properties of cold-sprayed CoCrMo and Ti6Al4V coatings on 6061-T651 Al alloy. Surf. Coat. Technol. 2017, 326, 291–298. [Google Scholar] [CrossRef]
- Singh, S.; Raman, R.K.S.; Berndt, C.C.; Singh, H. Influence of Cold Spray Parameters on Bonding Mechanisms: A Review. Metals 2021, 11, 2016. [Google Scholar] [CrossRef]
- Yin, S.; Cavaliere, P.; Aldwell, B.; Jenkins, R.; Liao, H.; Li, W.; Lupoi, R. Cold spray additive manufacturing and repair: Fundamentals and applications. Addit. Manuf. 2018, 21, 628–650. [Google Scholar] [CrossRef]
- Tiamiyu, A.A.; Schuh, C.A. Particle flattening during cold spray: Mechanistic regimes revealed by single particle impact tests. Surf. Coat. Technol. 2020, 403, 126386–126395. [Google Scholar] [CrossRef]
- Luo, X.-T.; Wei, Y.-K.; Wang, Y.; Li, C.-J. Microstructure and mechanical property of Ti and Ti6Al4V prepared by an in-situ shot peening assisted cold spraying. Mater. Des. 2015, 85, 527–533. [Google Scholar] [CrossRef]
- Fan, J.; Yun, H.; Zhang, X.; Chang, D.; Chu, X.; Xie, Y.; Huang, G. High-Performance Pure Aluminum Coatings on Stainless Steels by Cold Spray. Coatings 2023, 13, 738. [Google Scholar] [CrossRef]
- Wang, H.J.; Wu, S.S.; Zhang, K.J.; Sun, W.; Huang, R.Z.; Yu, M.; Xie, Y.C. Microstructure and Properties of Cold Sprayed IN718 Coating. Surf. Technol. 2022, 51, 361–369. [Google Scholar]
- Jiang, X.; Overman, N.; Smith, C.; Ross, K. Microstructure, hardness and cavitation erosion resistance of different cold spray coatings on stainless steel 316 for hydropower applications. Mater. Today Commun. 2020, 25, 101305–101314. [Google Scholar] [CrossRef]
- Boruah, D.; Ahmad, B.; Lee, T.L.; Kabra, S.; Syed, A.K.; McNutt, P.; Dore, M.; Zhang, X. Evaluation of residual stresses induced by cold spraying of Ti-6Al-4V on Ti-6Al-4V substrates. Surf. Coat. Technol. 2019, 374, 591–602. [Google Scholar] [CrossRef]
- Liu, R.; Li, D.Y. Modification of Archard’s equation by taking account of elastic/pseudoelastic properties of materials. Wear 2001, 251, 956–964. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.H.; Zhu, L.; Wang, H.; Zhang, Y. Effect of Different Substrate Angles on Wear Resistance of Laser Cladding Ni25/WC Coating. Surf. Technol. 2022, 51, 371–379. [Google Scholar]
- Shen, M.; Zhu, X.; Han, B.; Ying, T.; Jia, J. Dry sliding wear behaviour of AZ31 magnesium alloy strengthened by nanoscale SiCp. J. Mater. Res. Technol. 2022, 16, 814–823. [Google Scholar] [CrossRef]
- Koricherla, M.; Torgerson, T.; Alidokht, S.; Munagala, V.; Chromik, R.; Scharf, T. High temperature sliding wear behavior and mechanisms of cold-sprayed Ti and Ti–TiC composite coatings. Wear 2021, 476, 203746. [Google Scholar] [CrossRef]
- Yang, L.H.; Lin, C.G.; Gao, H.P.; Xu, W.C.; Li, Y.T.; Hou, B.R.; Huang, Y.L. Corrosion Behaviour of AZ63 Magnesium Alloy in Natural Seawater and 3.5 wt. % NaCl Aqueous Solution. Int. J. Electrochem. Sci. 2018, 18, 8084–8093. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, B.G.; Wang, Y.F.; Dong, T.S.; Li, J.K.; Li, G.L.; Zhao, X.B.; Liu, J.H.; Zhang, G.X. Effect of Cu Content on the Precipitation Behaviors, Mechanicaland Corrosion Properties of As-Cast Ti-Cu Alloys. Materials 2022, 15, 1696. [Google Scholar] [CrossRef] [PubMed]
- Acquesta, A.; Sinagra, C.; Monetta, T. Electrochemical and Economic Analysis of No-Rinse, “True-Cr-Free” Surface Conver-sion Coatings for a Green, Cost-Saving, Corrosion Protection of 8079 Aluminium Alloy. Appl. Sci. 2023, 13, 1560. [Google Scholar] [CrossRef]
- Xie, C.; Li, H.; Zhou, X.; Sun, C. Corrosion behavior of cold sprayed pure zinc coating on magnesium. Surf. Coat. Technol. 2019, 374, 797–806. [Google Scholar] [CrossRef]
- Li, R.; Fu, B.; Wang, Y.; Li, J.; Dong, T.; Li, G.; Zhang, G.; Liu, J. Effect of Cold-Rolling Reduction on Recrystallization Microstructure, Texture and Corrosion Properties of the X2CrNi12 Ferritic Stainless Steel. Materials 2022, 15, 6914. [Google Scholar] [CrossRef] [PubMed]
- Bijalwan, P.; Singh, C.; Kumar, A.; Sarkar, K.; Rani, N.; Laha, T.; Banerjee, A.; Mondal, K. Corrosion behaviour of plasma sprayed Fe based metallic glass (Fe73Cr2Si11B11C3 (at%) coatings in 3.5% NaCl solution. J. Non-Cryst. Solids 2021, 567, 120913–120925. [Google Scholar] [CrossRef]





| Composition | Li | Zn | Y | Mg | V | Al | Ti |
|---|---|---|---|---|---|---|---|
| Mg-Li alloy | 8.03. | 4.94 | 0.73 | Bal | - | - | - |
| TC4 powder | - | - | - | - | 4 | 6.09 | Bal |
| Specimens | icorr/A·cm−2 | Ecorr/VSCE |
|---|---|---|
| Mg-Li alloy | 1.008 × 10−3 | −1.659 |
| TC4 alloy coating | 1.426 × 10−5 | −0.334 |
| Specimen | Rs(Ω·cm2) | Qc–Y0(Ω−1cm−2s−n) | Qc−n | Rc(Ω·cm2) | Qt−Y0 (Ω−1cm−2s−n) | Qt−n | Rt(Ω·cm2) | L(H·cm2) | Rl(Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|
| Substrate | 7.63 | 8.49 × 10−5 | - | - | - | 8.07 × 10−1 | 1.90 × 102 | 3.62× 102 | 5.90 × 101 |
| TC4 coating | 3.82 | 3.42 × 10−4 | 3.66 × 10−1 | 5.01 × 10−1 | 3.50 × 10−5 | 8.34 × 10−1 | 2.36 × 104 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Fu, B.; Jiao, Y.; Dong, T.; Li, J.; Li, G. Study of Wear and Corrosion Resistance of Cold Sprayed TC4 Coating on the Surface of Mg-Li Alloy. Coatings 2023, 13, 988. https://doi.org/10.3390/coatings13060988
Bao Y, Fu B, Jiao Y, Dong T, Li J, Li G. Study of Wear and Corrosion Resistance of Cold Sprayed TC4 Coating on the Surface of Mg-Li Alloy. Coatings. 2023; 13(6):988. https://doi.org/10.3390/coatings13060988
Chicago/Turabian StyleBao, Yongtao, Binguo Fu, Yunlei Jiao, Tianshun Dong, Jingkun Li, and Guolu Li. 2023. "Study of Wear and Corrosion Resistance of Cold Sprayed TC4 Coating on the Surface of Mg-Li Alloy" Coatings 13, no. 6: 988. https://doi.org/10.3390/coatings13060988
APA StyleBao, Y., Fu, B., Jiao, Y., Dong, T., Li, J., & Li, G. (2023). Study of Wear and Corrosion Resistance of Cold Sprayed TC4 Coating on the Surface of Mg-Li Alloy. Coatings, 13(6), 988. https://doi.org/10.3390/coatings13060988





