Effects of UV Radiation on the Carbonation of Cement-Based Materials with Supplementary Cementitious Materials
Abstract
1. Introduction
2. Experimental
2.1. Raw Materials and Mixing Ratios
2.2. UV-Irradiation Test
2.3. Material Characterisation
3. Results and Discussion
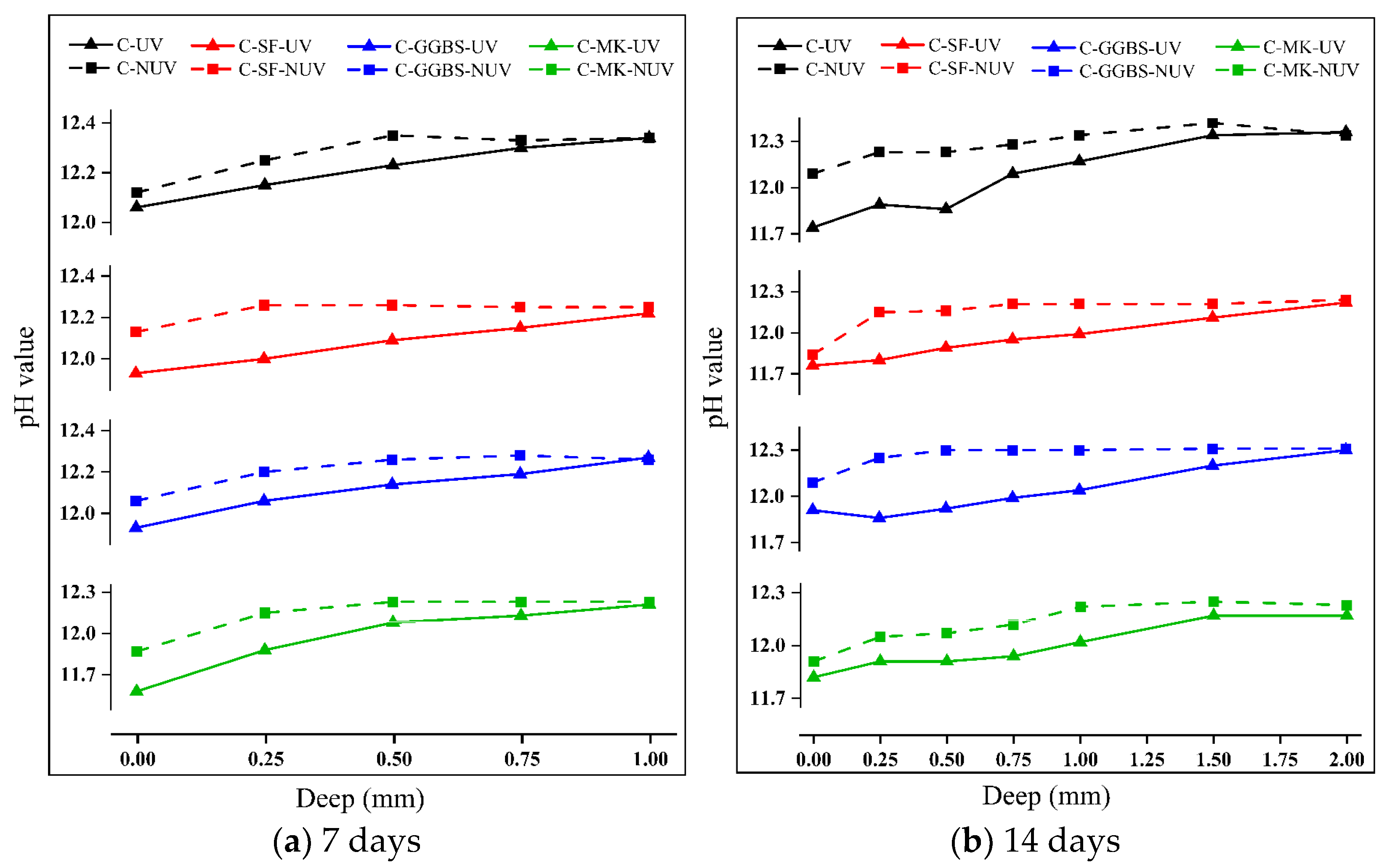
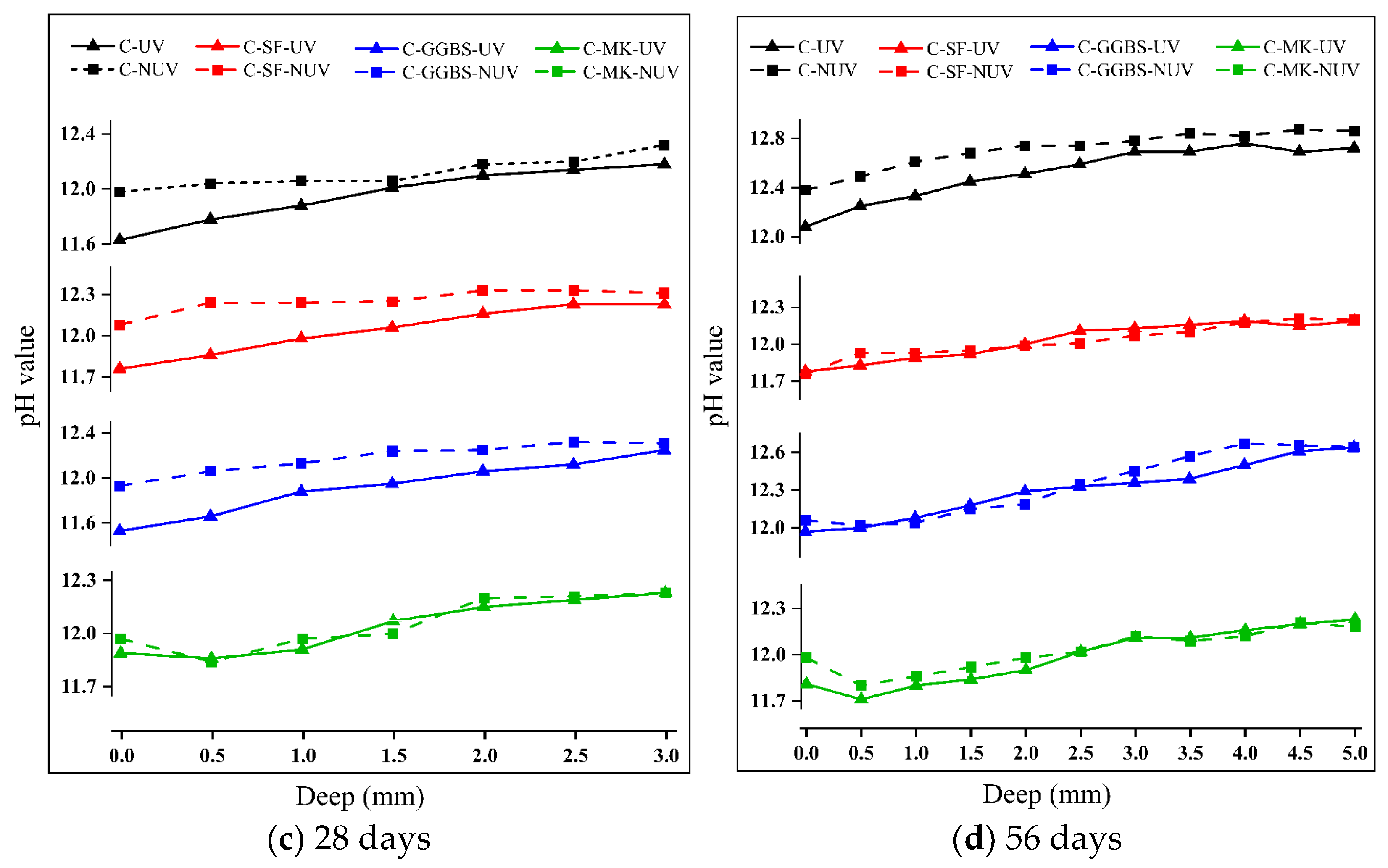
3.1. Change in the pH of Cement-Based Materials during Their Curing
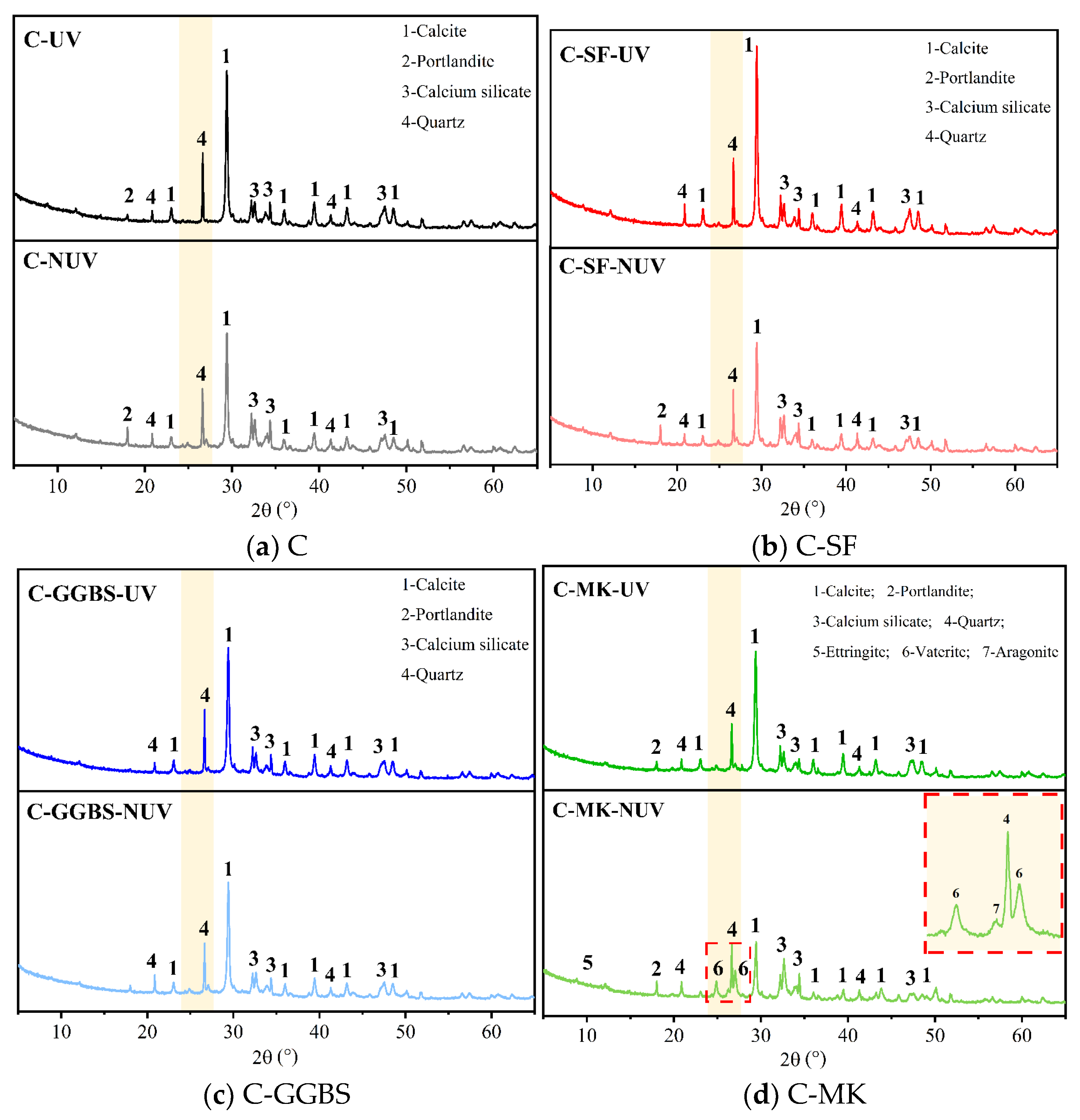
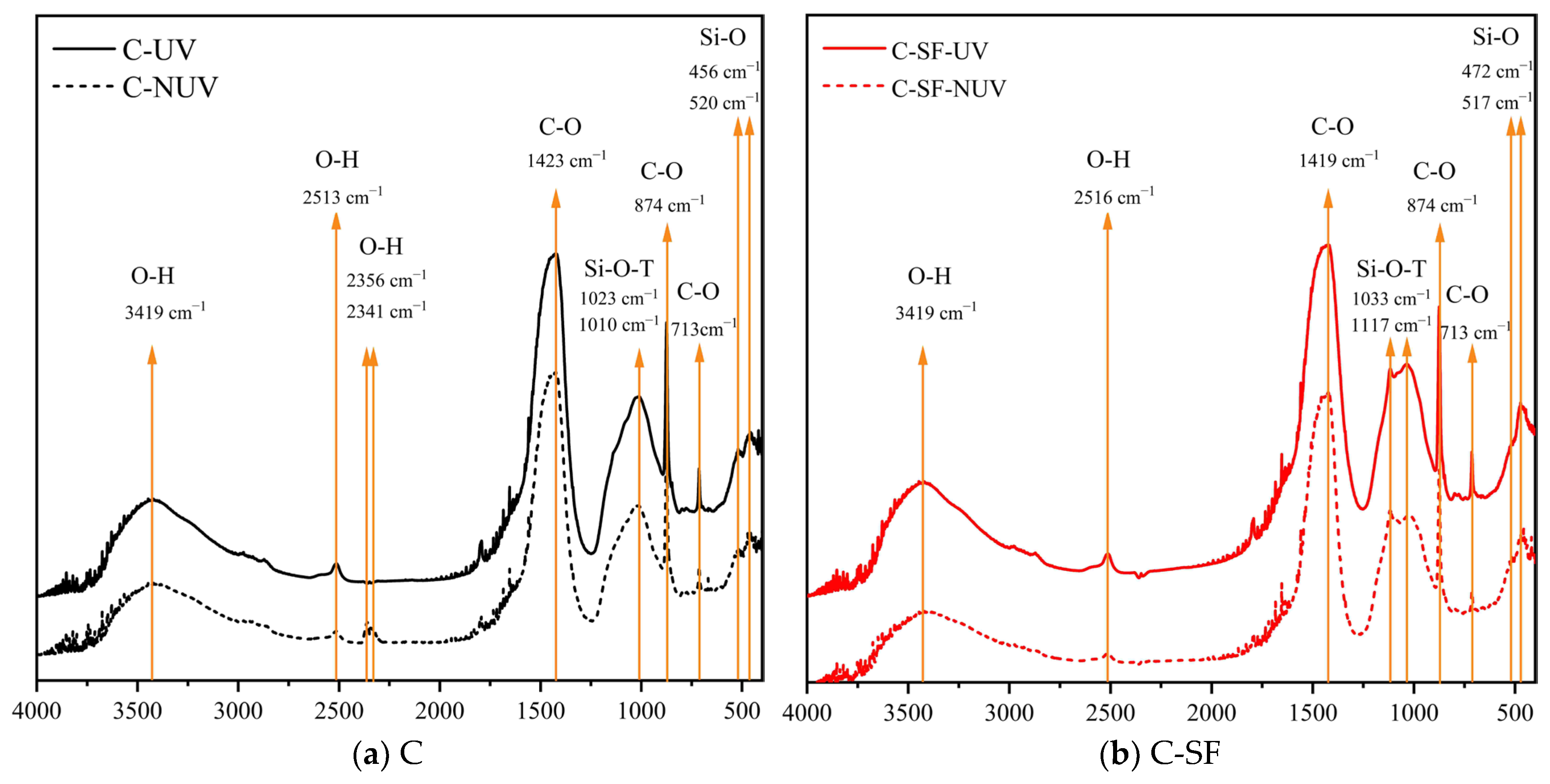
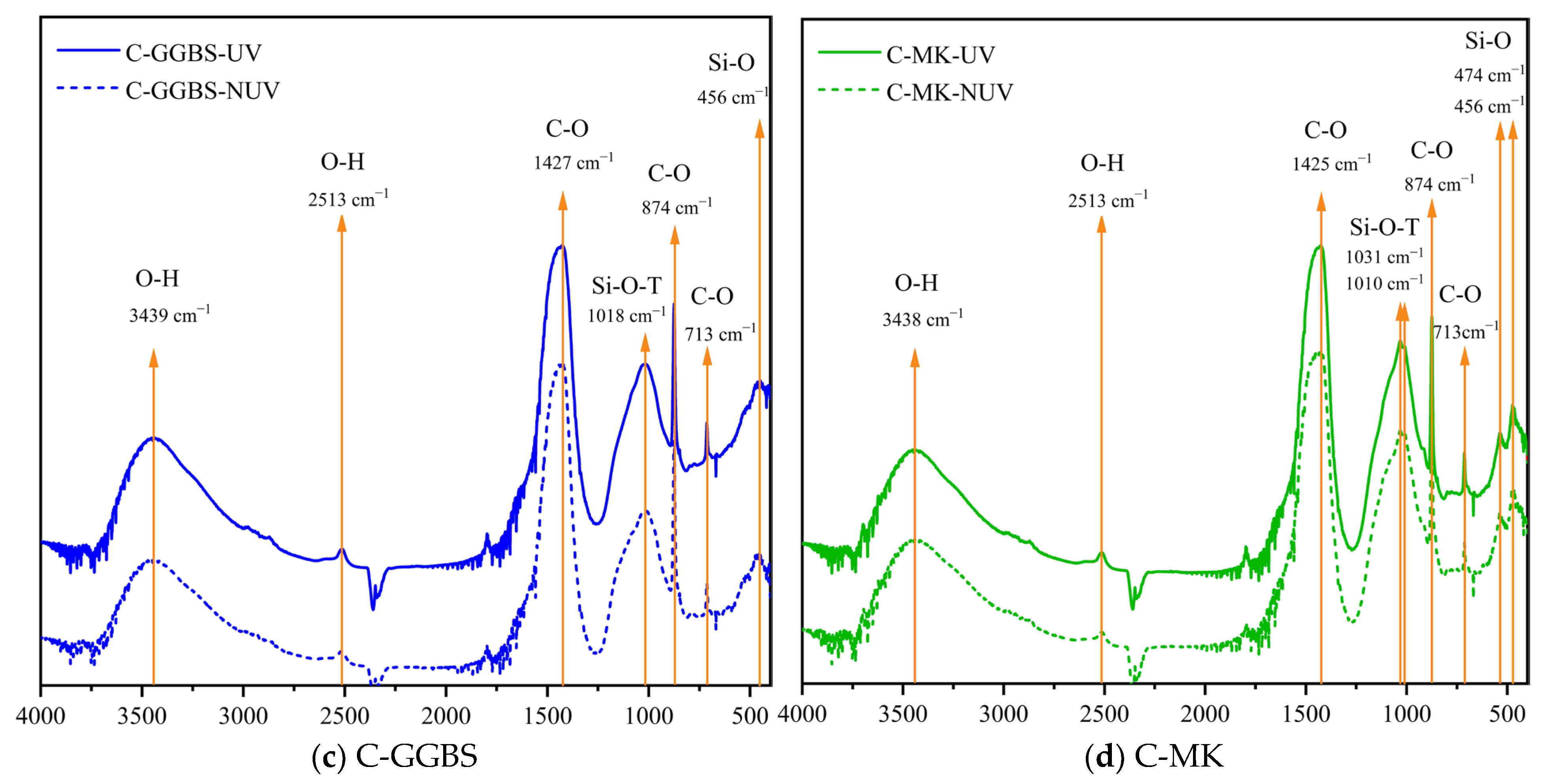
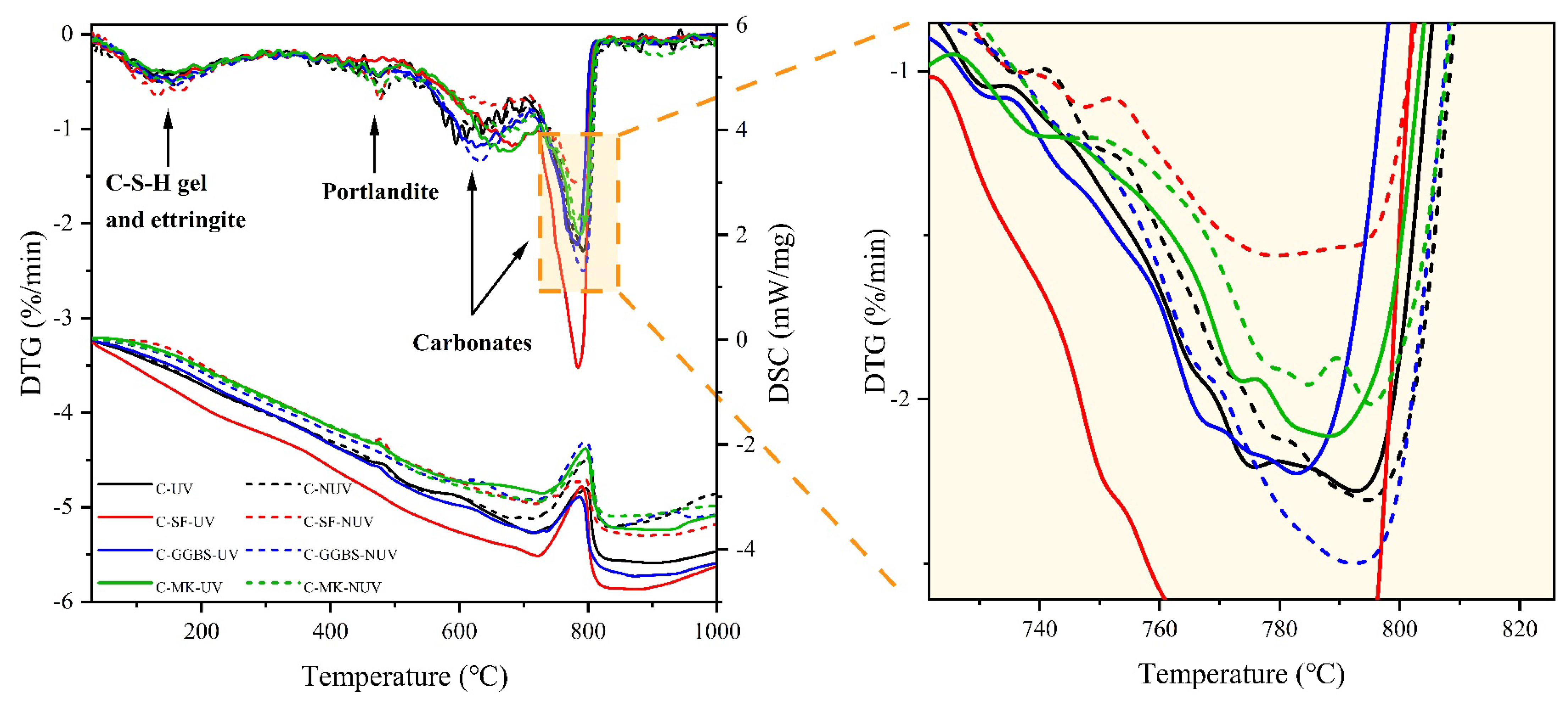
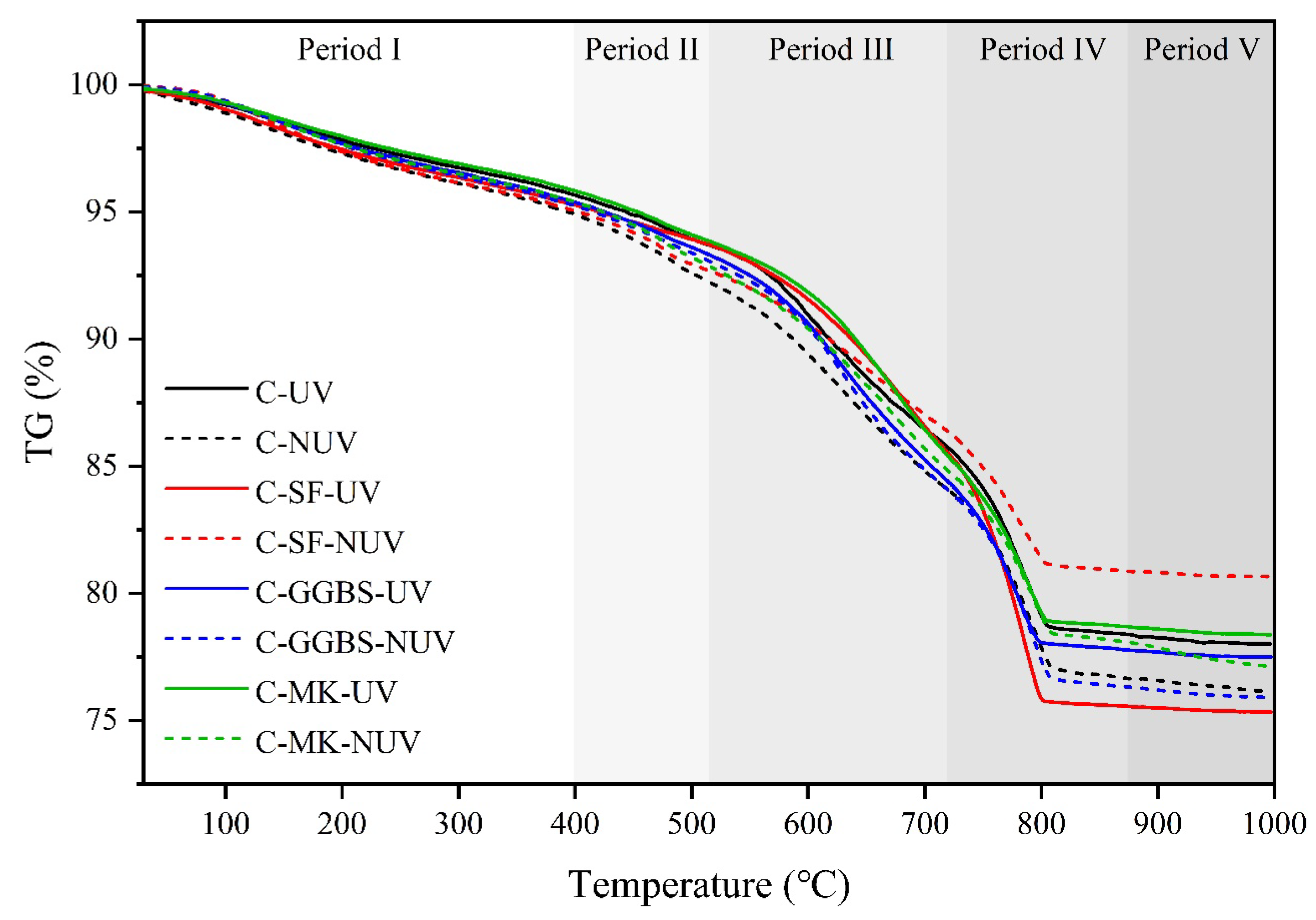
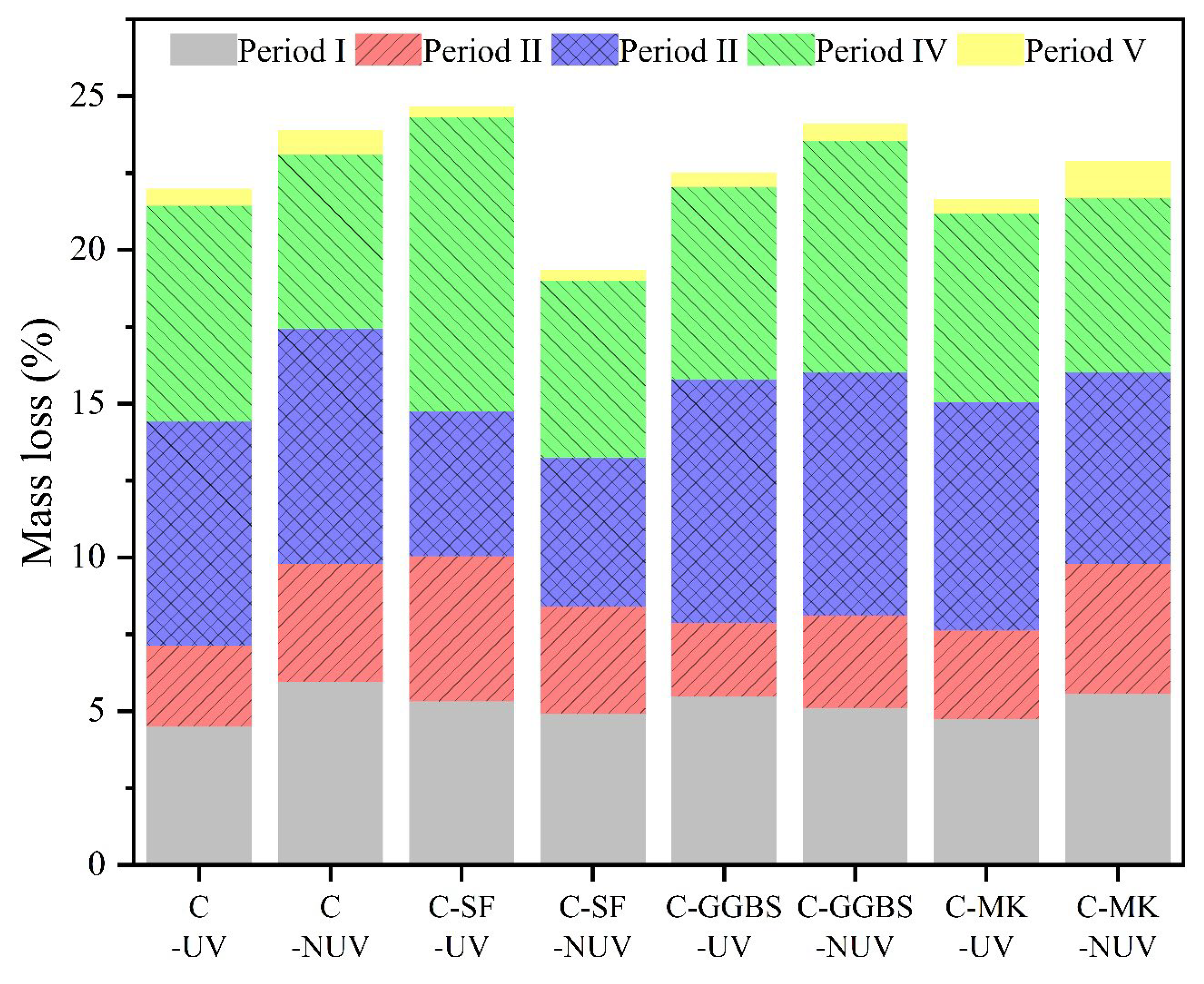
3.2. Hydration Phase Assemblage
3.2.1. Chemical Composition
3.2.2. Phase Content
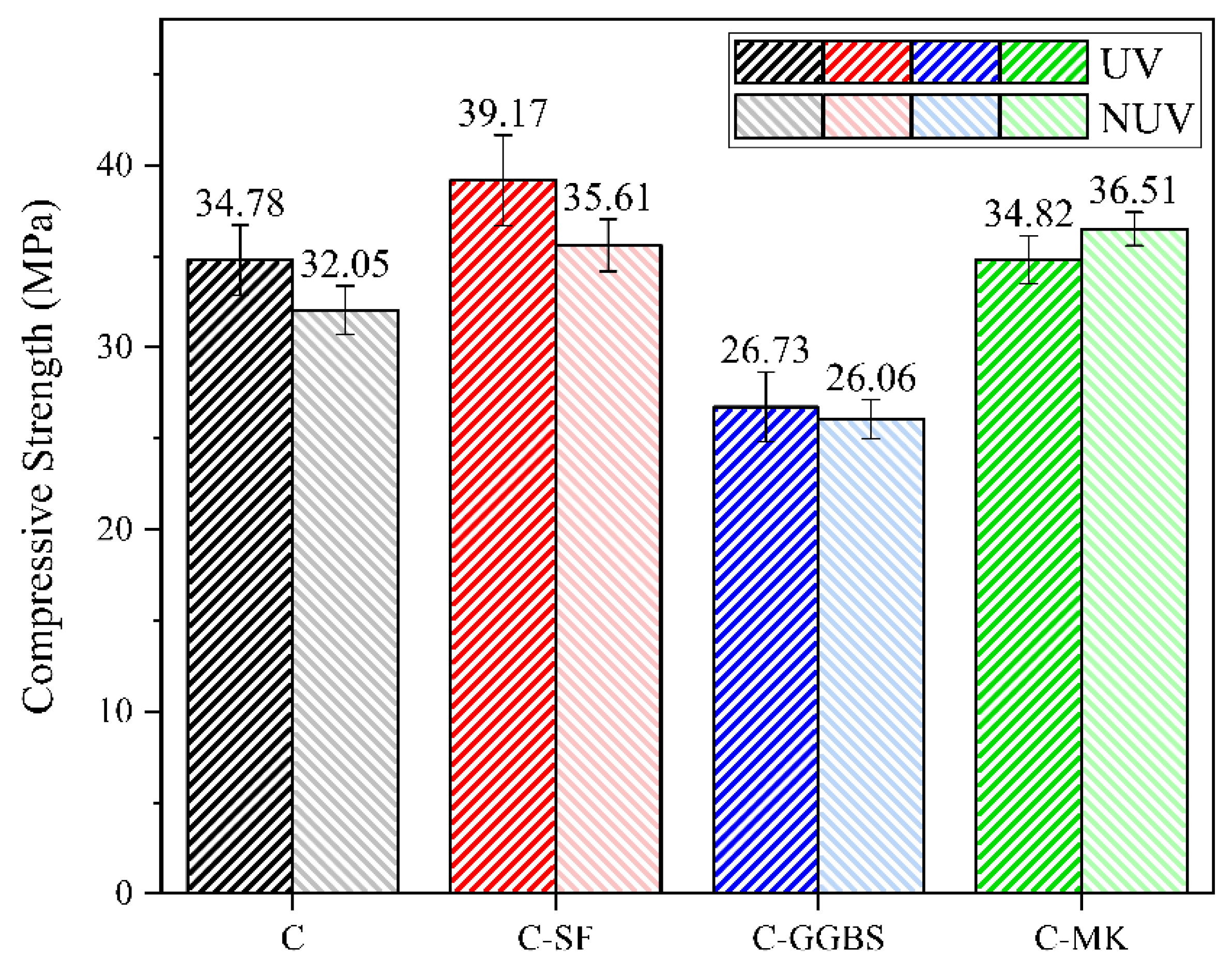
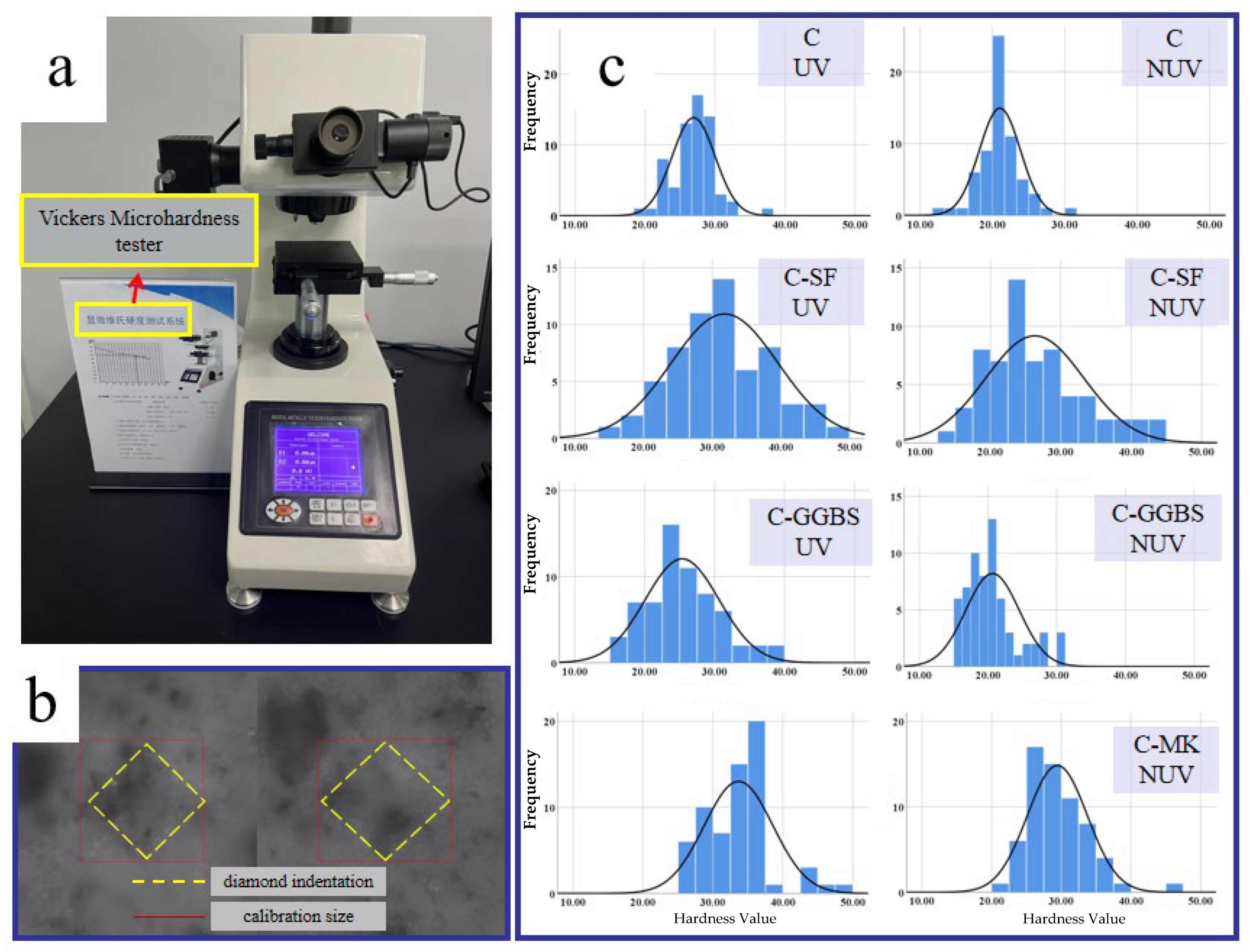
3.3. Mechanical Properties
4. Conclusions
- Under UV radiation, the pH of the cement-based materials decreased significantly due to the carbonation reaction. This effect decreases with depth and increases with time in the early stage of curing, but the difference gradually decreases after 28 days of curing.
- The action of UV radiation on the surface of the cement-based material promoted the carbonation reaction, leading to the formation of stable calcite crystallites with excellent micromechanical properties. This structural change improved the macroscopic and micromechanical properties of the cement-based material.
- The incorporation of SF led to a significant increase in the carbonation of the cement-based material under UV radiation, with a 53.3% increase in the amount of calcium carbonate coverage on the surface layer, whereas the addition of GGBS resulted in a lower effect of UV radiation. In practical engineering applications, the mechanical properties and carbon sequestration ability of cement-based materials can be improved by adding SF.
- Without UV radiation, the MK-incorporated cement-based material tended to produce vaterite and aragonite phases, whereas it tended to transform into calcite under UV radiation. This transformation was accompanied by the deterioration of the macroscopic mechanical properties because UV radiation facilitated the crystallographic transformation process. Therefore, in strong UV radiation, the admixture of MK should be controlled reasonably to prevent deterioration of the macroscopic mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Y.; Li, Y. Estimated spatiotemporal variability of total, direct and diffuse solar radiation across China during 1958–2016. Int. J. Climatol. 2018, 38, 4395–4404. [Google Scholar] [CrossRef]
- Gómez, C.; Costela, A.; García-Moreno, I.; Sastre, R. Comparative study between IR and UV laser radiation applied to the removal of graffitis on urban buildings. Appl. Surf. Sci. 2006, 252, 2782–2793. [Google Scholar] [CrossRef]
- Peng, Y.; Qiang, S. Analytical Solution to Temperature Variations in Highway Concrete Bridges Due to Solar Radiation. Am. Soc. Civ. Eng. 2012, 207, 1536–1541. [Google Scholar]
- Ibragimov, R.; Korolev, E.; Deberdeev, T.; Dolbin, I. Influence of electromagnetic radiation on the degradation of reinforced concrete structures—Review. Case Stud. Constr. Mater. 2022, 17, e01454. [Google Scholar] [CrossRef]
- Shui, Z.; Yu, J.; Yu, R.; Song, Q.; Zhu, S. Effects of Ultraviolet Radiation on the Surface of Hardened Cement Paste of the Physical and Chemical Properties. J. Wuhan Univ. Technol. 2018, 40, 14–18. [Google Scholar]
- Lan, C.; Liu, D.; Zhang, Y.; Lu, G. Study on the effect of ultraviolet irradiation on the performance of concrete. New Build. Mater. 2020, 47, 12–14. [Google Scholar]
- Wang, J.; Zhu, Q.; Yuan, Q.; Cao, H.; Wang, J.; Wang, D. Research on Influence of Ultraviolet Radiation on Carbonation of Fly Ash Concrete. Water Resour. Power 2022, 40, 163–166+174. [Google Scholar]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Song, H.-W.; Kwon, S.-J. Permeability characteristics of carbonated concrete considering capillary pore structure. Cem. Concr. Res. 2007, 37, 909–915. [Google Scholar] [CrossRef]
- Pu, Q.; Jiang, L.; Xu, J.; Chu, H.; Xu, Y.; Zhang, Y. Evolution of pH and chemical composition of pore solution in carbonated concrete. Constr. Build. Mater. 2012, 28, 519–524. [Google Scholar] [CrossRef]
- Meng, D.; Unluer, C.; Yang, E.-H.; Qian, S. Carbon sequestration and utilization in cement-based materials and potential impacts on durability of structural concrete. Constr. Build. Mater. 2022, 361, 129610. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Fardis, M.; Vayenas, C.G. Effect of composition, environmental factors and cement-lime mortar coating on concrete carbonation. Mater. Struct. 1992, 25, 293–304. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental Modeling and Experimental Investigation of Concrete Carbonation. Mater. J. 1991, 88, 363–373. [Google Scholar]
- Pihlajavaara, S.E. Some results of the effect of carbonation on the porosity and pore size distribution of cement paste. Matériaux Constr. 1968, 1, 521–527. [Google Scholar] [CrossRef]
- Bier, T.A.; Ludirdja, D.; Young, J.F.; Berger, R.L. The Effect of Pore Structure and Cracking on the Permeability of Concrete. MRS Online Proceedings Library (OPL); Cambridge University Press: Cambridge, MA, USA, 1988; Volume 137, p. 235. [Google Scholar]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Rathnarajan, S.; Dhanya, B.; Pillai, R.G.; Gettu, R.; Santhanam, M. Carbonation model for concretes with fly ash, slag, and limestone calcined clay—Using accelerated and five-year natural exposure data. Cem. Concr. Compos. 2022, 126, 104329. [Google Scholar] [CrossRef]
- Bastidas-Arteaga, E.; Rianna, G.; Gervasio, H.; Nogal, M. Multi-region lifetime assessment of reinforced concrete structures subjected to carbonation and climate change. Structures 2022, 45, 886–899. [Google Scholar] [CrossRef]
- Golewski, G.L.; Gil, D.M. Studies of Fracture Toughness in Concretes Containing Fly Ash and Silica Fume in the First 28 Days of Curing. Mater. Multidiscip. Digit. Publ. Inst. 2021, 14, 319. [Google Scholar] [CrossRef]
- Alaloul, W.S.; Musarat, M.A.; Haruna, S.; Law, K.; Tayeh, B.A.; Rafiq, W.; Ayub, S. Mechanical Properties of Silica Fume Modified High-Volume Fly Ash Rubberized Self-Compacting Concrete. Sustainability 2021, 13, 5571. [Google Scholar] [CrossRef]
- Güneyisi, E.; Gesoglu, M.; Akoi, A.O.M.; Mermerdaş, K. Combined effect of steel fiber and metakaolin incorporation on mechanical properties of concrete. Compos. Part B Eng. 2014, 56, 83–91. [Google Scholar] [CrossRef]
- Yalçınkaya, Ç.; Beglarigale, A.; Yazıcı, H. The effect of metakaolin and end type of steel fiber on fiber-SIFCON matrix bond characteristics. Usak Univ. J. Mater. Sci. 2014, 3, 97–105. [Google Scholar] [CrossRef]
- Ahmad, J.; Majdi, A.; Arbili, M.M.; Deifalla, A.F.; Naqash, M.T. Mechanical, Durability and Microstructure Analysis Overview of Concrete Made with Metakaolin (MTK). Buildings 2022, 12, 1401. [Google Scholar] [CrossRef]
- Suga, S.; Blattner, P.; Cordo, O.; Cornell, G.; Francis, A.; Habte, A.; Jung, J.; Kita, H.; Myers, D.R.; Regan, J.; et al. Recommended Reference Solar Spectra for Industrial Applications; CIE—International Commission on Illumination: Vienna, Austria, 2020; ISBN 978-3-902842-90-9. [Google Scholar]
- McPolin, D.O.; Basheer, P.A.; Long, A.E.; Grattan, K.T. New Test Method to Obtain pH Profiles due to Carbonation of Concretes Containing Supplementary Cementitious Materials. J. Mater. Civ. Eng. 2007, 19, 936–946. [Google Scholar] [CrossRef]
- Ai, H.; Clavier, K.A.; Watts, B.E.; Gale, S.A.; Townsend, T.G. The efficacy of pH-dependent leaching tests to provide a reasonable estimate of post-carbonation leaching. J. Hazard. Mater. 2019, 373, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Otieno, M.; Ikotun, J.; Ballim, Y. Experimental investigations on the effect of concrete quality, exposure conditions and duration of initial moist curing on carbonation rate in concretes exposed to urban, inland environment. Constr. Build. Mater. 2020, 246, 118443. [Google Scholar] [CrossRef]
- Liu, P.; Yu, Z.; Chen, Y. Carbonation depth model and carbonated acceleration rate of concrete under different environment. Cem. Concr. Compos. 2020, 114, 103736. [Google Scholar] [CrossRef]
- Wang, Z.; Shui, Z.; Sun, T.; Li, X.; Zhang, M. Recycling utilization of phosphogypsum in eco excess-sulphate cement: Synergistic effects of metakaolin and slag additives on hydration, strength and microstructure. J. Clean. Prod. 2022, 358, 131901. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Xu, D.; Du, P.; Zhou, Z.; Yuan, L.; Cheng, X. Accelerated carbonation of hardened cement pastes: Influence of porosity. Constr. Build. Mater. 2019, 225, 159–169. [Google Scholar] [CrossRef]
- Khalil, E.A.B.; Anwar, M. Carbonation of ternary cementitious concrete systems containing fly ash and silica fume. Water Sci. 2015, 29, 36–44. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Estévez, E.; Argiz, C.; del Barrio, D. Effect of curing time on granulated blast-furnace slag cement mortars carbonation. Cem. Concr. Compos. 2018, 90, 257–265. [Google Scholar] [CrossRef]
- Bhojaraju, C.; Mousavi, S.S.; Ouellet-Plamondon, C.M. Influence of GGBFS on corrosion resistance of cementitious composites containing graphene and graphene oxide. Cem. Concr. Compos. 2023, 135, 104836. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: Potential of utilizing low-lime calcium silicates in cement-based materials. J. Mater. Sci. 2016, 51, 6173–6191. [Google Scholar] [CrossRef]
- Saillio, M.; Baroghel-Bouny, V.; Pradelle, S.; Bertin, M.; Vincent, J.; de Lacaillerie, J.-B.D. Effect of supplementary cementitious materials on carbonation of cement pastes. Cem. Concr. Res. 2021, 142, 106358. [Google Scholar] [CrossRef]
- Drouet, E.; Poyet, S.; Le Bescop, P.; Torrenti, J.-M.; Bourbon, X. Carbonation of hardened cement pastes: Influence of temperature. Cem. Concr. Res. 2019, 115, 445–459. [Google Scholar] [CrossRef]
- Auroy, M.; Poyet, S.; Le Bescop, P.; Torrenti, J.-M.; Charpentier, T.; Moskura, M.; Bourbon, X. Comparison between natural and accelerated carbonation (3% CO2): Impact on mineralogy, microstructure, water retention and cracking. Cem. Concr. Res. 2018, 109, 64–80. [Google Scholar] [CrossRef]
- Vanoutrive, H.; Minne, P.; Van de Voorde, I.; Cizer, Ö.; Gruyaert, E. Carbonation of cement paste with GGBFS: Effect of curing duration, replacement level and CO2 concentration on the reaction products and CO2 buffer capacity. Cem. Concr. Compos. 2022, 129, 104449. [Google Scholar] [CrossRef]
- Steiner, S.; Lothenbach, B.; Proske, T.; Borgschulte, A.; Winnefeld, F. Effect of relative humidity on the carbonation rate of portlandite, calcium silicate hydrates and ettringite. Cem. Concr. Res. 2020, 135, 106116. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Goracci, G.; Dolado, J.; Blanco-Varela, M. Mineralogical and microstructural alterations in a portland cement paste after an accelerated decalcification process. Cem. Concr. Res. 2021, 140, 106312. [Google Scholar] [CrossRef]
- Hu, L.; Jia, Y.; Chen, Z.; Yao, Y.; Sun, J.; Xie, Q.; Yang, H. An insight of carbonation-hydration kinetics and microstructure characterization of cement paste under accelerated carbonation at early age. Cem. Concr. Compos. 2022, 134, 104763. [Google Scholar] [CrossRef]
- Shah, V.; Parashar, A.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Kulakowski, M.P.; Pereira, F.M.; Molin DC, C.D. Carbonation-induced reinforcement corrosion in silica fume concrete. Constr. Build. Mater. 2009, 23, 1189–1195. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Nie, S.; Wu, S.; Liu, Q.; Li, C.; Shu, B.; Li, C.; Song, W.; Zou, Y.; et al. Negative impacts of environmental factors (UV radiation, water and different solutions) on bitumen and its mechanism. Constr. Build. Mater. 2020, 265, 120288. [Google Scholar] [CrossRef]
- Ho, L.S.; Nakarai, K.; Ogawa, Y.; Sasaki, T.; Morioka, M. Effect of internal water content on carbonation progress in cement-treated sand and effect of carbonation on compressive strength. Cem. Concr. Compos. 2018, 85, 9–21. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of early carbonation curing on chloride penetration and weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar]
- Igarashi, S.; Bentur, A.; Mindess, S. Microhardness testing of cementitious materials. Adv. Cem. Based Mater. 1996, 4, 48–57. [Google Scholar]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Scrivener, K.L. Multi-scale investigation on mechanical behavior and microstructural alteration of C-S-H in carbonated Alite paste. Cem. Concr. Res. 2021, 144, 106448. [Google Scholar] [CrossRef]
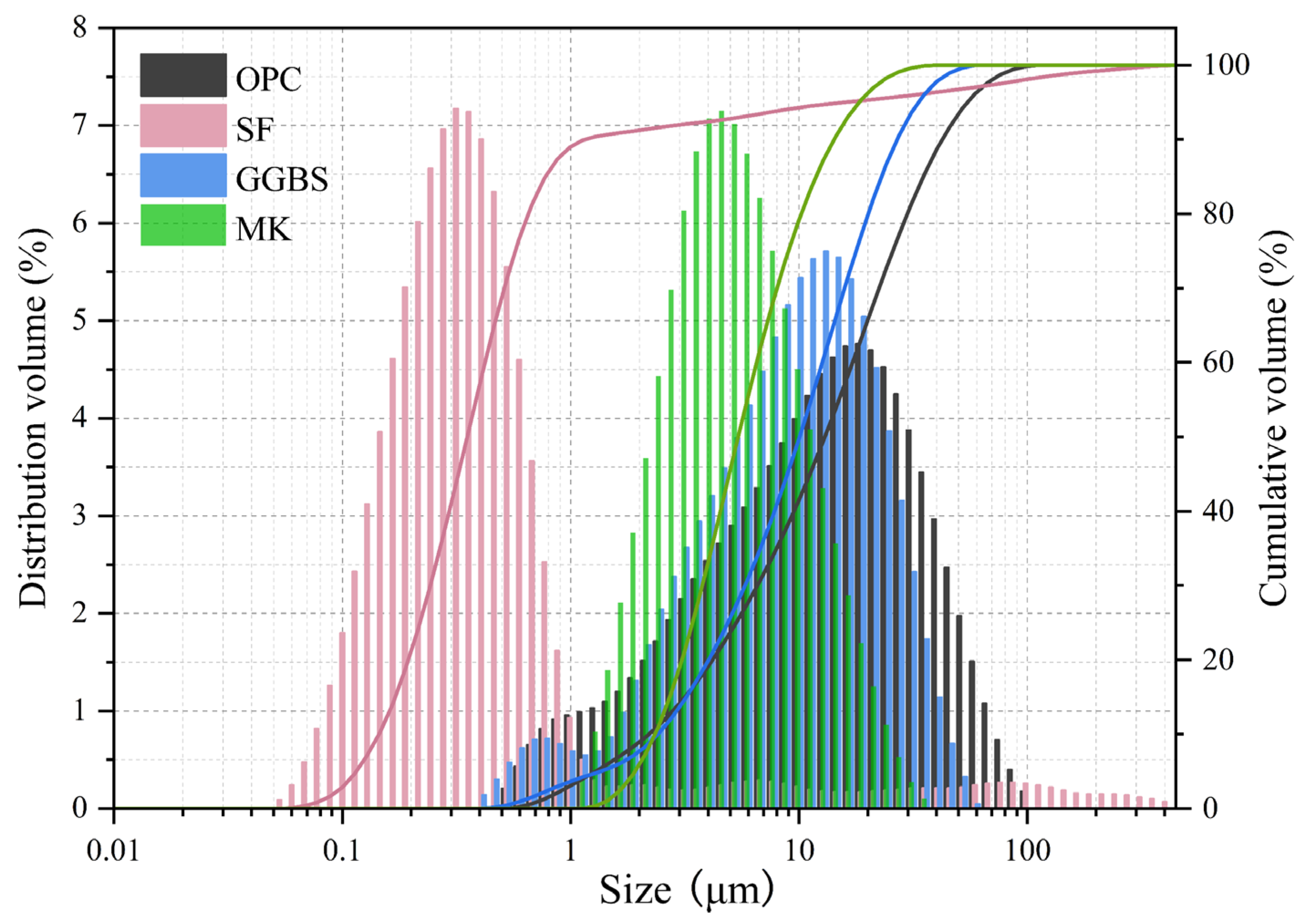
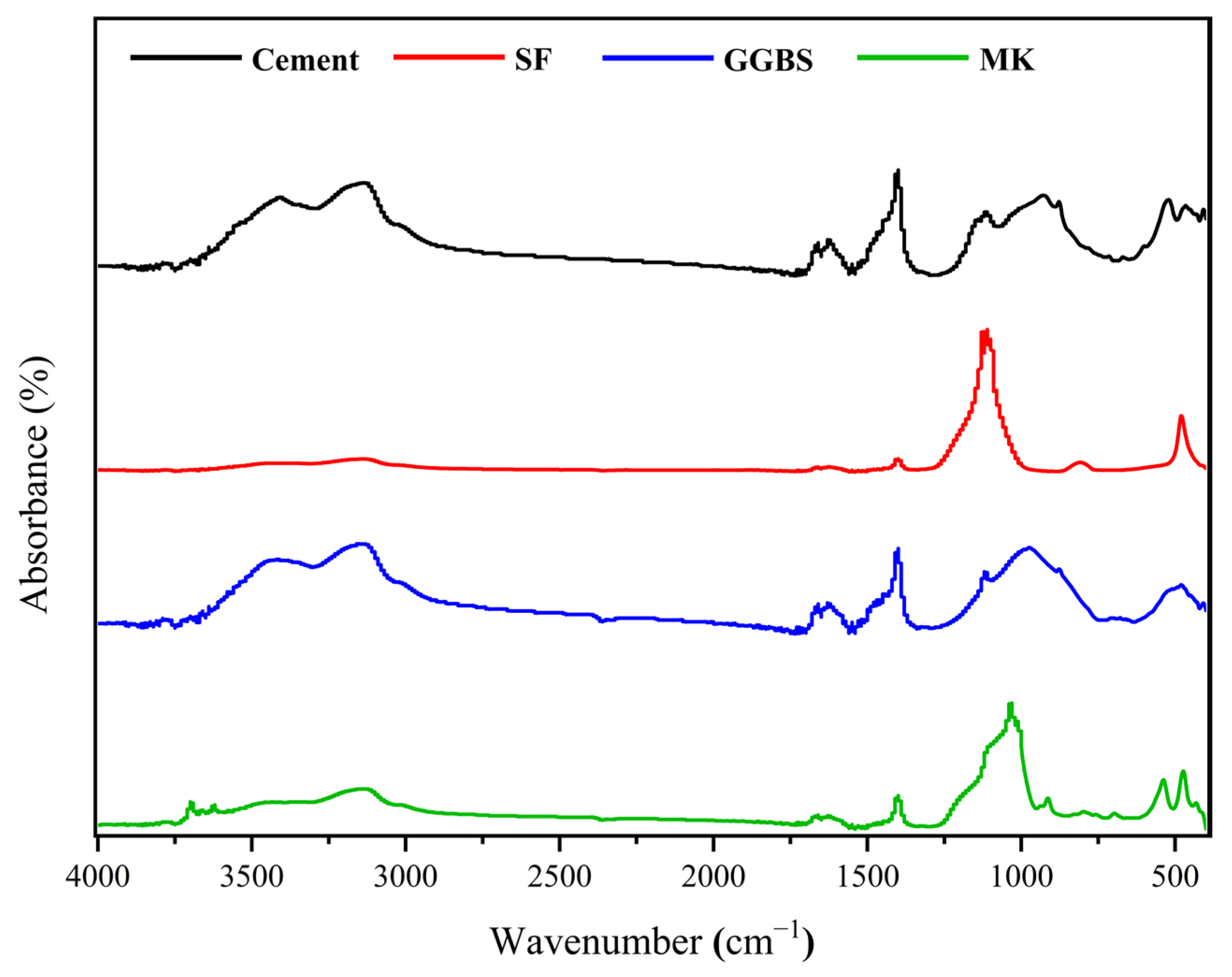

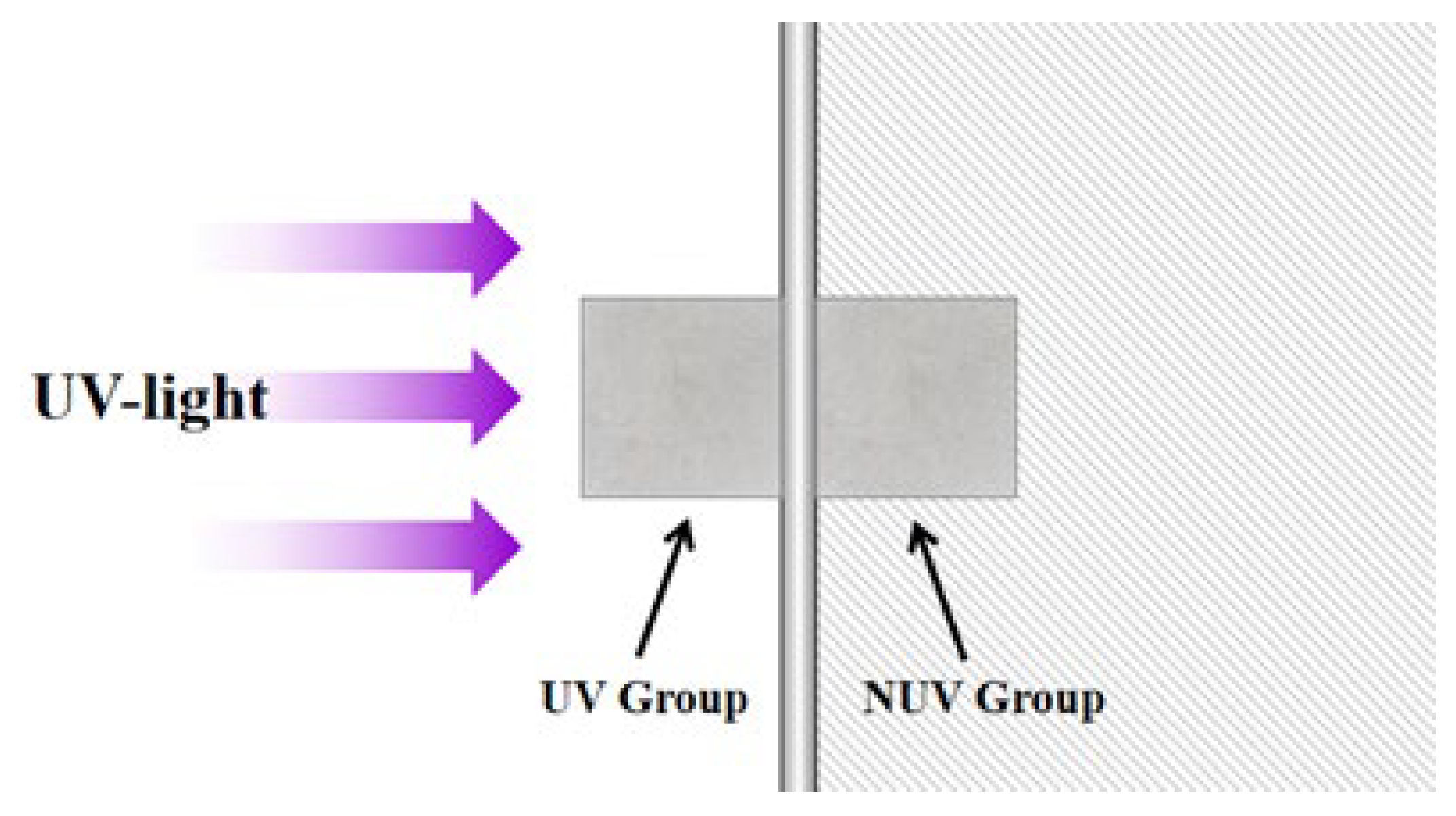
| Raw Materials | Chemical Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Fe2O3 | SO3 | MgO | Na2O | K2O | Others | |
| OPC | 25.15 | 54.65 | 7.42 | 3.07 | 2.68 | 1.53 | 0.18 | 0.98 | 4.33 |
| SF | 96.50 | 0.05 | 0.40 | 0.00 | 0.46 | 0.11 | 0.04 | 0.08 | 2.37 |
| GGBS | 32.31 | 42.66 | 12.61 | 0.32 | 2.50 | 6.88 | 0.49 | 0.42 | 1.80 |
| MK | 48.82 | 0.83 | 42.27 | 0.00 | 0.15 | 0.26 | 0.07 | 0.05 | 7.56 |
| Sample Code | Cementitious Materials (wt%) | w/b Ratio | |||
|---|---|---|---|---|---|
| OPC | SF | GGBS | MK | ||
| C | 100 | - | - | - | 0.45 |
| C-SF | 90 | 10 | - | - | |
| C-GGBS | 90 | - | 10 | - | |
| C-MK | 90 | - | - | 10 | |
| C | C-SF | C-GGBS | C-MK | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| UV | 26.9 | 3.05 | 31.8 | 7.72 | 25.4 | 5.26 | 33.7 | 4.87 |
| NUV | 21.0 | 2.83 | 26.3 | 6.9 | 20.7 | 3.85 | 29.4 | 4.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Shui, Z.; Wang, Z.; Xiao, X. Effects of UV Radiation on the Carbonation of Cement-Based Materials with Supplementary Cementitious Materials. Coatings 2023, 13, 994. https://doi.org/10.3390/coatings13060994
Li H, Shui Z, Wang Z, Xiao X. Effects of UV Radiation on the Carbonation of Cement-Based Materials with Supplementary Cementitious Materials. Coatings. 2023; 13(6):994. https://doi.org/10.3390/coatings13060994
Chicago/Turabian StyleLi, Haoyuan, Zhonghe Shui, Ziyan Wang, and Xunguang Xiao. 2023. "Effects of UV Radiation on the Carbonation of Cement-Based Materials with Supplementary Cementitious Materials" Coatings 13, no. 6: 994. https://doi.org/10.3390/coatings13060994
APA StyleLi, H., Shui, Z., Wang, Z., & Xiao, X. (2023). Effects of UV Radiation on the Carbonation of Cement-Based Materials with Supplementary Cementitious Materials. Coatings, 13(6), 994. https://doi.org/10.3390/coatings13060994





