Preparation, Characterization, and Release Kinetics of Zanthoxylum bungeanum Leaf Polyphenol–Chitosan Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of ZMP
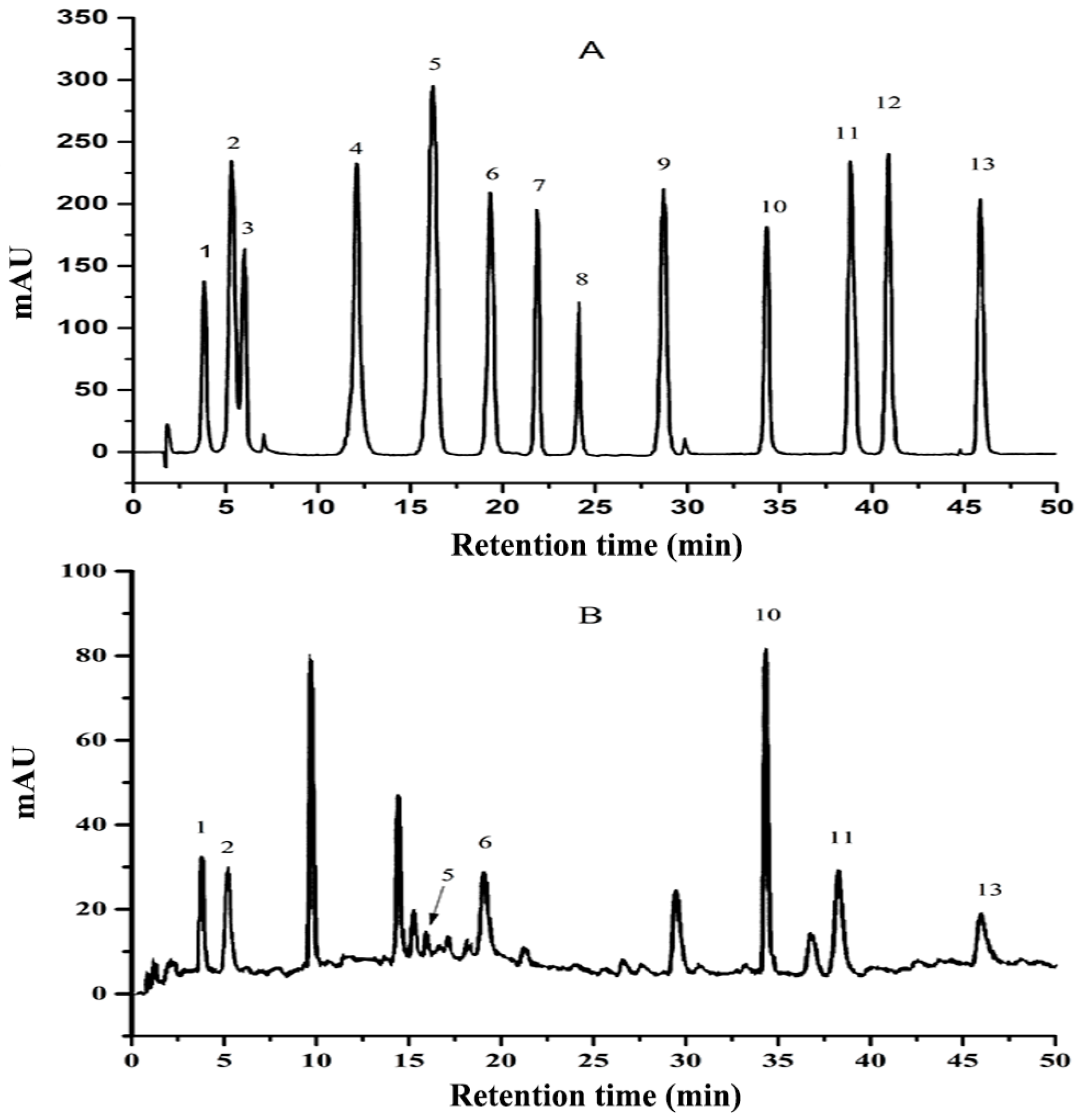
2.2.2. Purification and Analysis of ZMP
2.2.3. Preparation of Slow—Release Film
2.2.4. Performance Characterization of ZMP/C-film
Thickness, Opacity, and Solubility
Mechanical Properties
Structural Characterization
2.2.5. Kinetic Analysis of Polyphenol Release from ZMP/C-Film
2.2.6. Mathematical Modeling of Polyphenol Release
2.2.7. ZMP/C-Film Antioxidant Capacity
2.2.8. Practical Application of Film on Fresh Strawberry Fruit
2.3. Data Analysis
3. Results and Analysis
3.1. ZMP Composition Analysis
3.2. Physical Properties of ZMP/C-Film
3.2.1. Thickness, Opacity, Solubility, Tensile Strength, and Elongation at Break of the Film
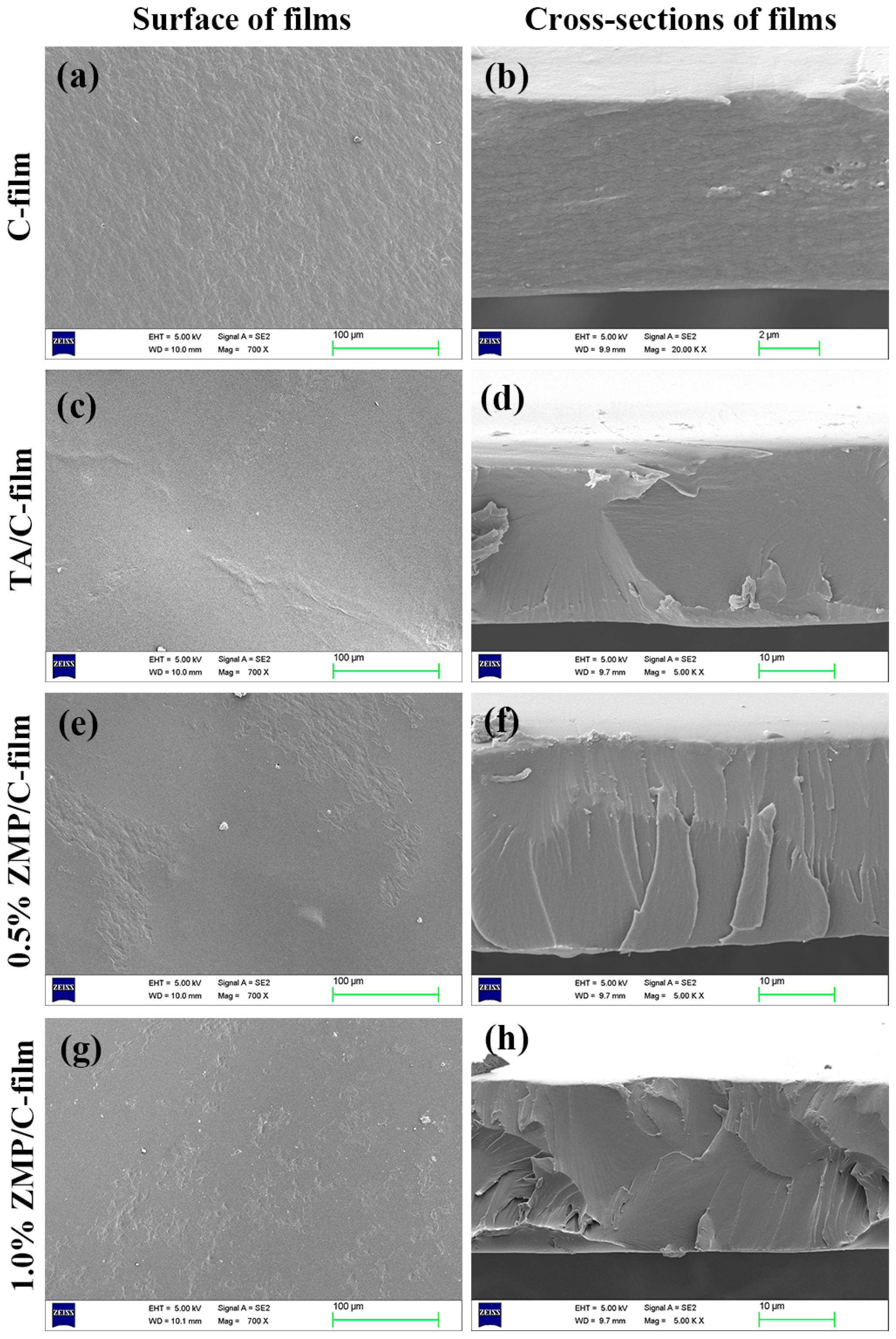
3.2.2. Scanning Electron Microscopy (SEM)
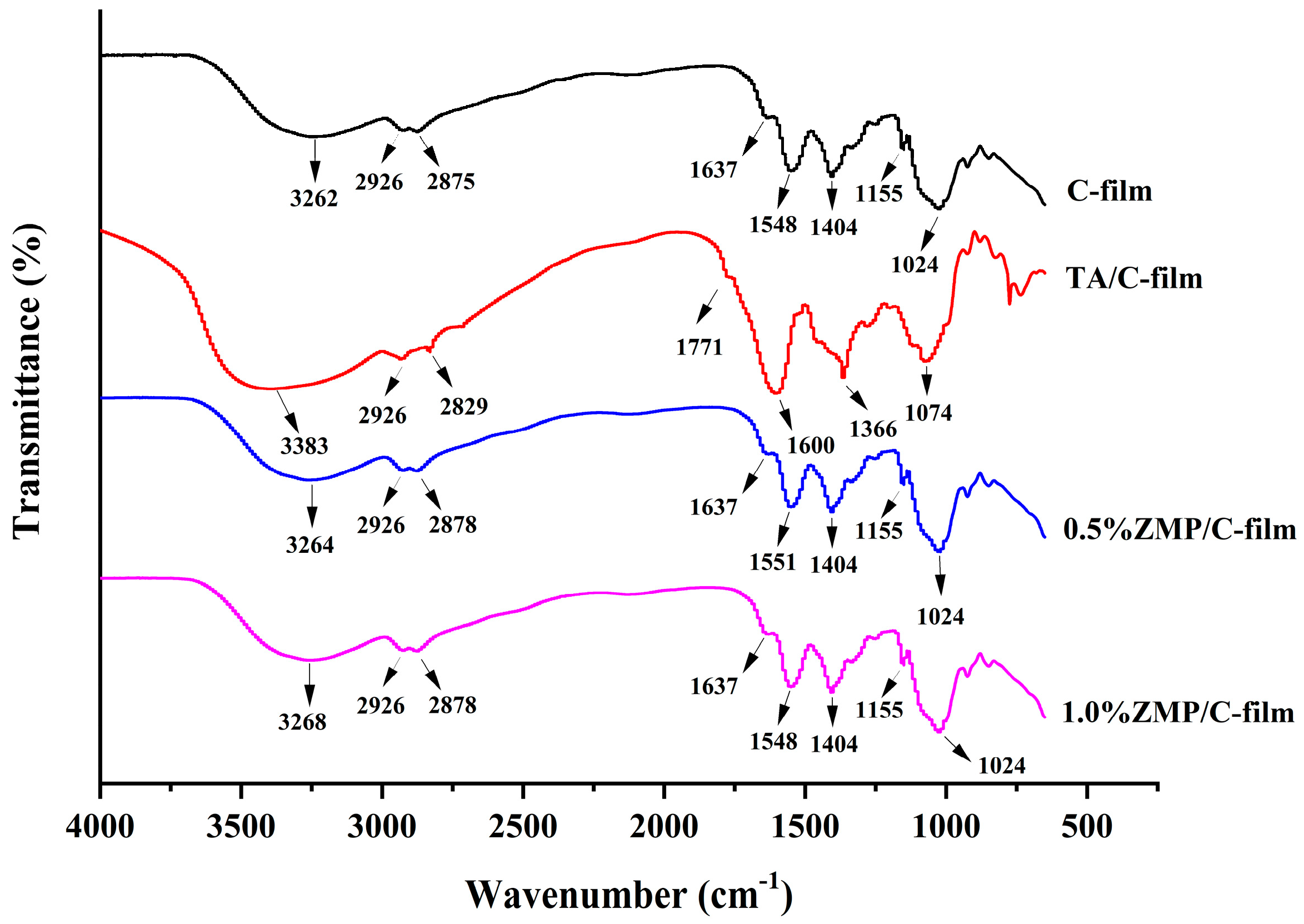
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.4. X-ray Diffraction Spectroscopy
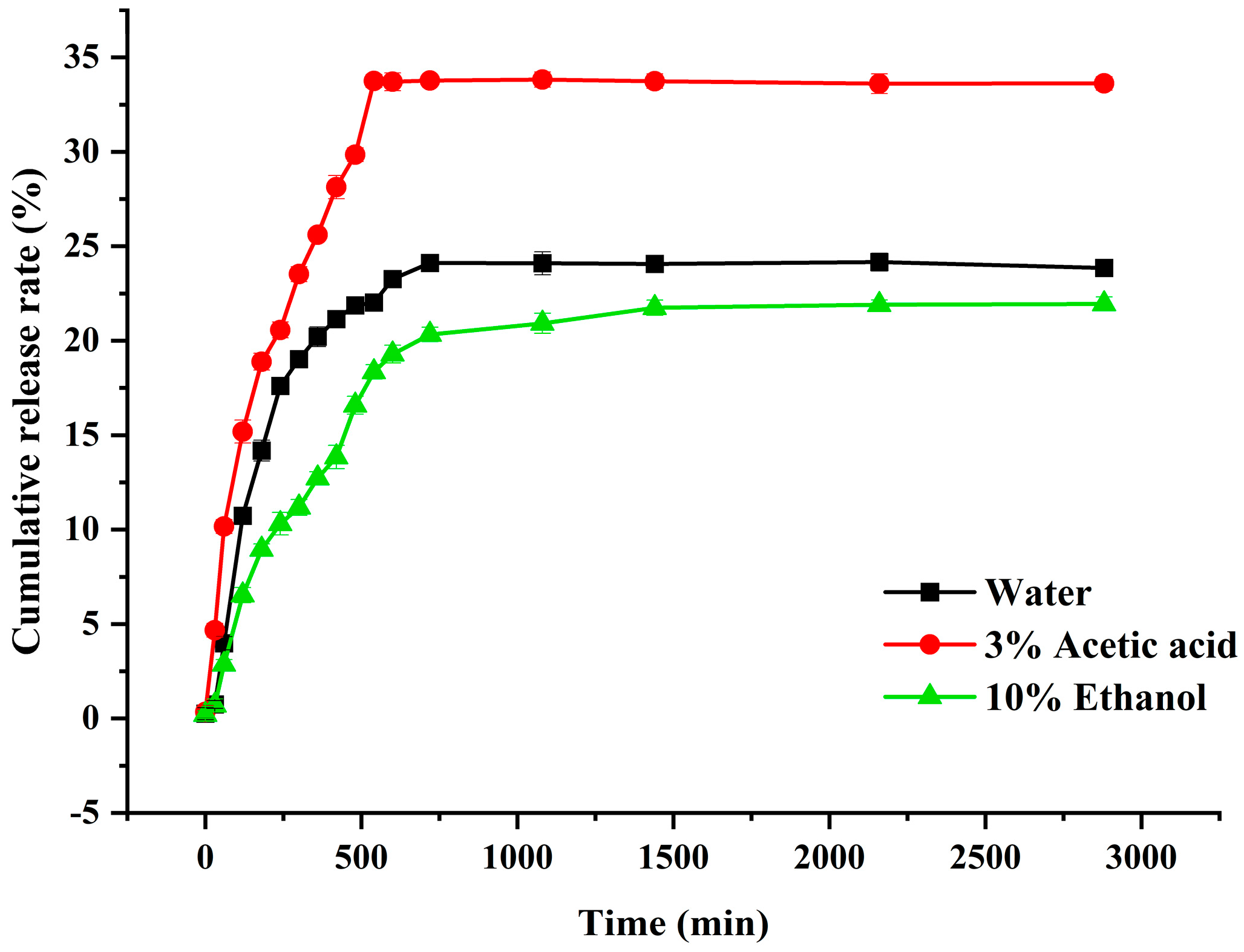
3.3. Kinetics of Polyphenol Release
3.4. Anti-Oxidant Capacity
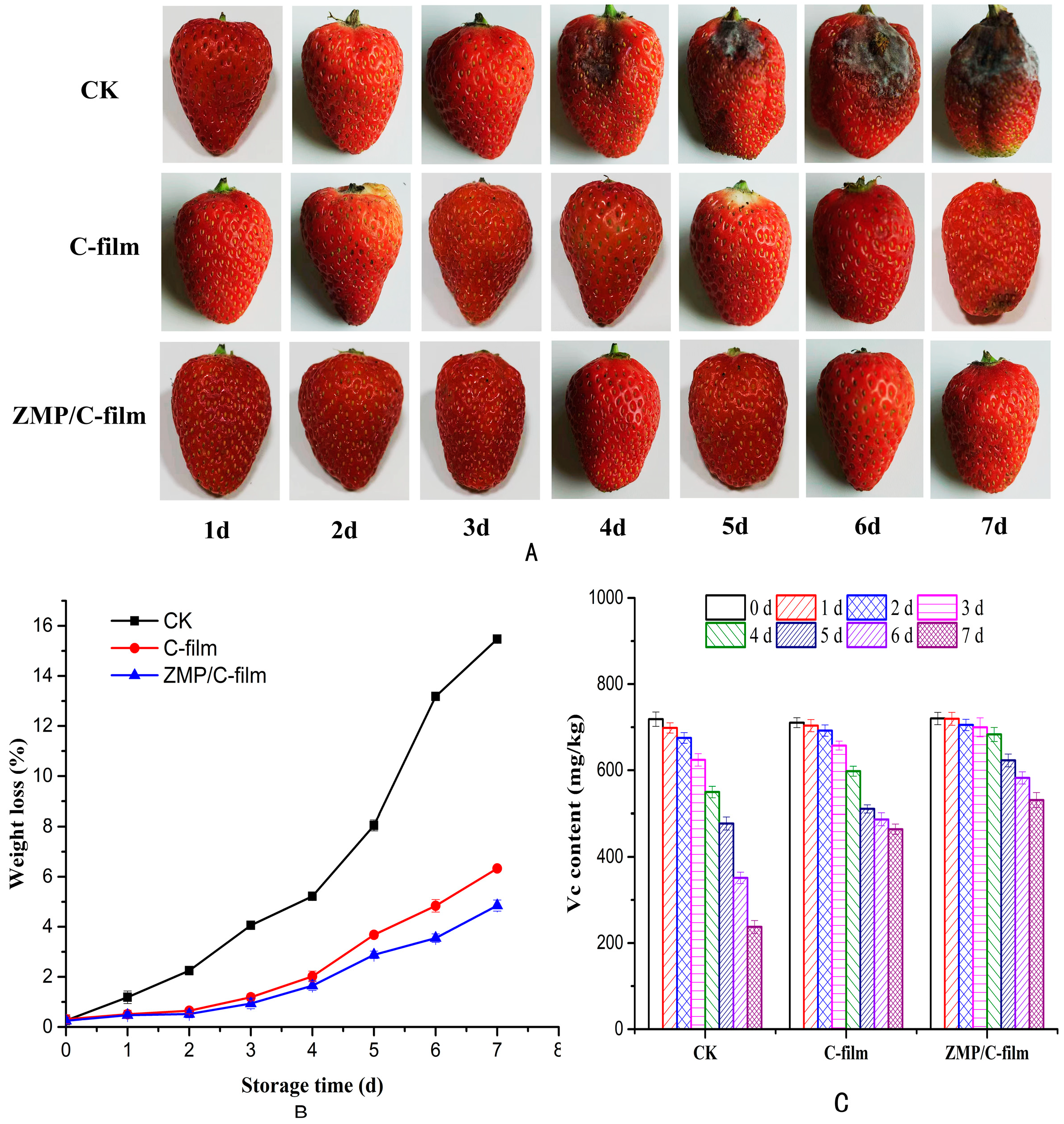
3.5. Strawberry Preservation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muley, A.B.; Kedia, P.; Pegu, K.; Kausley, S.B.; Rai, B. Analyzing the physical and biochemical changes in strawberries during storage at different temperatures and the development of kinetic models. J. Food Meas. Charact. 2022, 16, 222–247. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Moschovas, D.; Kollia, E.; Georgopoulos, S.; Gioti, C.; Leontiou, A.; Avgeropoulos, A.; Kopsacheili, A.; Avdylaj, L.; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels 2022, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Bureš, M.S.; Vlahoviček-Kahlina, K.; Stracenski, K.S.; Fruk, G.; Jalšenjak, N.; Bandić, L.M. Chitosan-based layer-by-layer edible coatings application for the preservation of mandarin fruit bioactive compounds and organic acids. Food Chem. X 2023, 17, 100575. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Huang, C.; Su, H.; Huang, H.; Luo, W.; An, J.; Zhao, H.; Xu, Y.; Wang, S. Chlorine dioxide gas slow—Release film for strawberry preservation. LWT 2023, 177, 114516. [Google Scholar]
- Thanh Huong, Q.T.; Hoai Nam, N.T.; Duy, B.T.; An, H.; Hai, N.D.; Kim Ngan, H.T.; Ngan, L.T.; Le Hoai Nhi, T.; Yen Linh, D.T.; Khanh, T.N.; et al. Structurally natural chitosan films decorated with Andrographis paniculata extract and selenium nanoparticles: Properties and strawberry preservation. Food Biosci. 2023, 53, 102647. [Google Scholar] [CrossRef]
- Hu, J. Preparation of Thermo-Sensitive Chitosan-Based Drug Carriers and Its Study of Sustained Release Property. Master’s Thesis, South China University of Technology, Guangzhou, China, 2000. [Google Scholar]
- Chen, W.B.; Yan, W.J.; Xu, X.L.; Zhang, J.H. Preparation, characterization and in vitro sustained antioxidant activity of α-tocopherol-loaded chitosan nanoparticles. Food Sci. 2017, 38, 216–223. [Google Scholar]
- Yang, L.-C.; Li, R.; Tan, J.; Jiang, Z.-T. Polyphenolics Composition of the Leaves of Zanthoxylum bungeanum Maxim. Grown in Hebei, China, and Their Radical Scavenging Activities. J. Agric. Food. Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Li, S.W.; Wang, Y.; Zhao, Y.Y.; Zhou, G.H.; Zhang, W.G. Study on the effect of pepper extract on the quality of conditioned pork patties during refrigeration. Sci. Technol. Food Ind. 2018, 39, 285–291+297. [Google Scholar]
- Luo, A.G. Separation, Purification, Biological Activity of Arthrospira plantensis Polysaccharide and Its Application in Preservation of Meat Products. Ph.D. Thesis, Shanxi University, Taiyuan, China, 2018. [Google Scholar]
- Quiñones, J.P.; Gothelf, K.V.; Kjems, J.; Yang, C.; Caballero, A.M.H.; Schmidt, C.; Covas, C.P. Self-assembled nanoparticles of modified-chitosan conjugates for the sustained release of dl-α-tocopherol. Carbohydr. Polym. 2013, 92, 856–864. [Google Scholar] [CrossRef]
- Gomaa, M.; Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M. Use of seaweed and filamentous fungus derived polysaccharides in the development of alginate-chitosan edible films containing fucoidan: Study of moisture sorption, polyphenol release and antioxidant properties. Food Hydrocoll. 2018, 82, 239–247. [Google Scholar] [CrossRef]
- Luo, A.G.; Zhao, Q.; Ma, J.H.; Yang, Y.J.; Hu, B.F. Preparation and characterization of phycocyanin-chitosan composite films. Sci. Technol. Food Ind. 2020, 41, 25–29+36. [Google Scholar]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Delivery Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Guo, C.H.; Zhu, Y.; Ma, X.Y.; Shao, M.M.; Yu, M.Q.; Wang, Y.G. Optimization of the Extraction Process of Polyphenols from Zanthoxylum schinifolium Sieb. et Zucc and Research on the Determination and Antioxidant Properties of Polyphenols. China Condiment. 2021, 46, 1–6. [Google Scholar]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Indumathi, M.P.; Saral Sarojini, K.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Gao, H.-X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.-C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, M.; Liu, X.; Chu, R.; Qiao, Z.; Li, C.; Adhikari, B. Physicochemical properties of chitosan/zein/essential oil emulsion-based active films functionalized by polyphenols. Future Foods 2021, 3, 100033. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Zhu, X.; Hou, X.; Ma, B.; Xu, H.; Yang, Y. Chitosan/gallnut tannins composite fiber with improved tensile, antibacterial and fluorescence properties. Carbohydr. Polym. 2019, 226, 115311. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasa, P.C.; Ramesh, M.N.; Kumar, K.R.; Tharanathan, R.N. Properties of chitosan films prepared under different drying conditions. J. Food Eng. 2004, 63, 79–85. [Google Scholar] [CrossRef]
- Shi, X.N. Synthesis, Preparation and Application of Functionalized Modified Nanocellulose System as a Dual Stimulus Responsive Drug Delivery System. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Kadam, D.; Shah, N.; Palamthodi, S.; Lele, S.S. An investigation on the effect of polyphenolic extracts of Nigella sativa seedcake on physicochemical properties of chitosan-based films. Carbohydr. Polym. 2018, 192, 347–355. [Google Scholar] [CrossRef] [PubMed]




| Sample | Thickness (μm) | Opacity (mm−1) | Solubility (%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| C-film | 43.95 ± 0.11 a | 3.81 ± 0.07 c | 17.62 ± 0.21 b | 47.66 ± 5.05 a | 21.11 ± 2.59 b |
| TA/C-film | 41.47 ± 0.10 b | 11.92 ± 0.04 b | 22.65 ± 0.39 a | 43.14 ± 3.21 a | 30.15 ± 1.28 c |
| 0.5%ZMP/C-film | 41.19 ± 0.18 b | 12.07 ± 0.11 ab | 21.98 ± 0.27 a | 40.30 ± 2.19 b | 32.64 ± 1.90 a |
| 1.0%ZMP/C-film | 39.07 ± 0.23 c | 12.53 ± 0.06 a | 22.44 ± 0.13 a | 32.15 ± 1.30 c | 36.37 ± 2.35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, A.; Chen, J.; Hu, B. Preparation, Characterization, and Release Kinetics of Zanthoxylum bungeanum Leaf Polyphenol–Chitosan Films. Coatings 2023, 13, 1138. https://doi.org/10.3390/coatings13071138
Luo A, Chen J, Hu B. Preparation, Characterization, and Release Kinetics of Zanthoxylum bungeanum Leaf Polyphenol–Chitosan Films. Coatings. 2023; 13(7):1138. https://doi.org/10.3390/coatings13071138
Chicago/Turabian StyleLuo, Aiguo, Jing Chen, and Bianfang Hu. 2023. "Preparation, Characterization, and Release Kinetics of Zanthoxylum bungeanum Leaf Polyphenol–Chitosan Films" Coatings 13, no. 7: 1138. https://doi.org/10.3390/coatings13071138





