Experimental Investigation of the Initial Stage of the Oxidation Mechanism of Co Coating for Solid Oxide Fuel Cell Interconnects at 650 °C
Abstract
:1. Introduction
2. Materials and Methods
3. Results
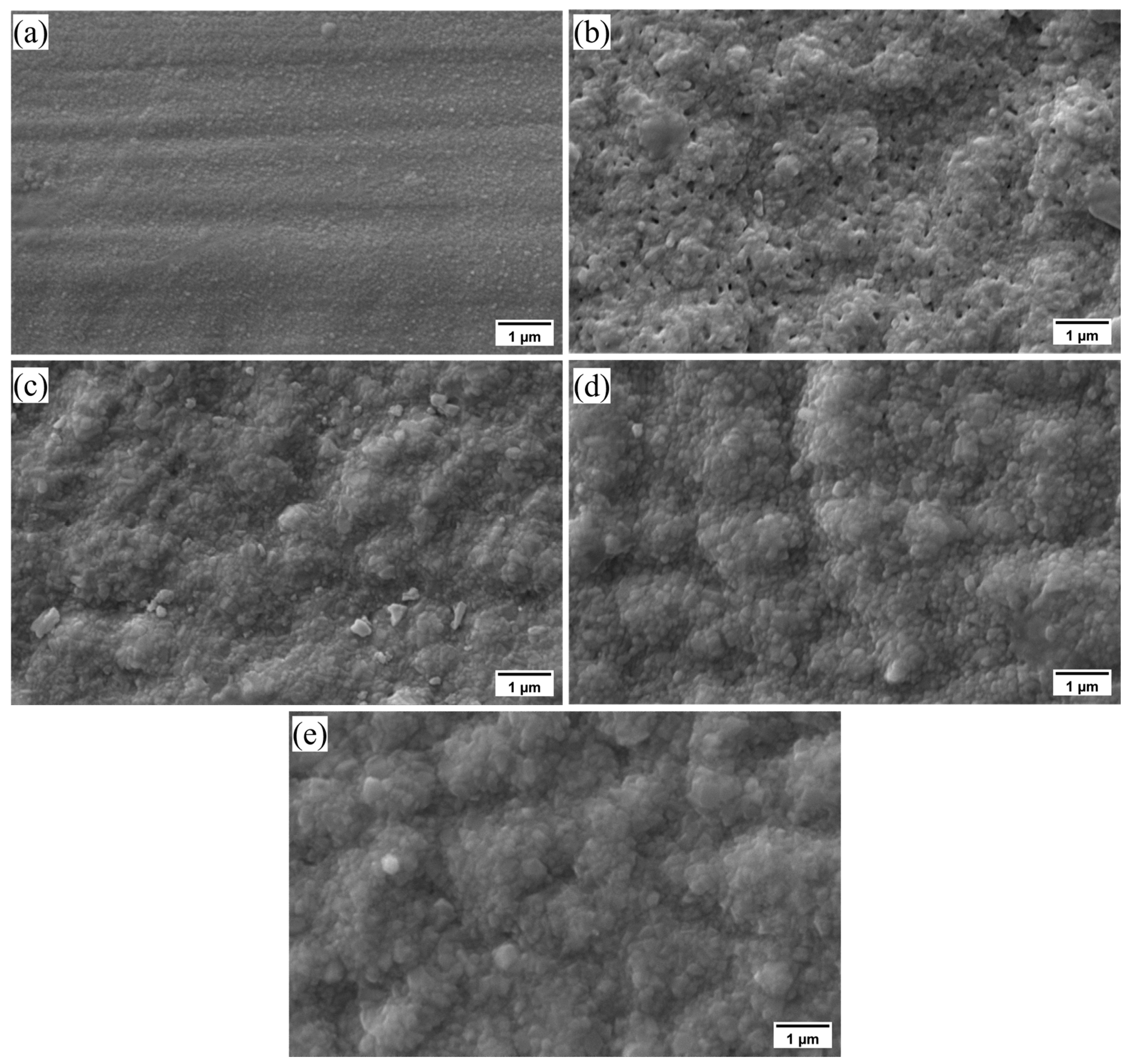
3.1. Oxidation Mass Gain
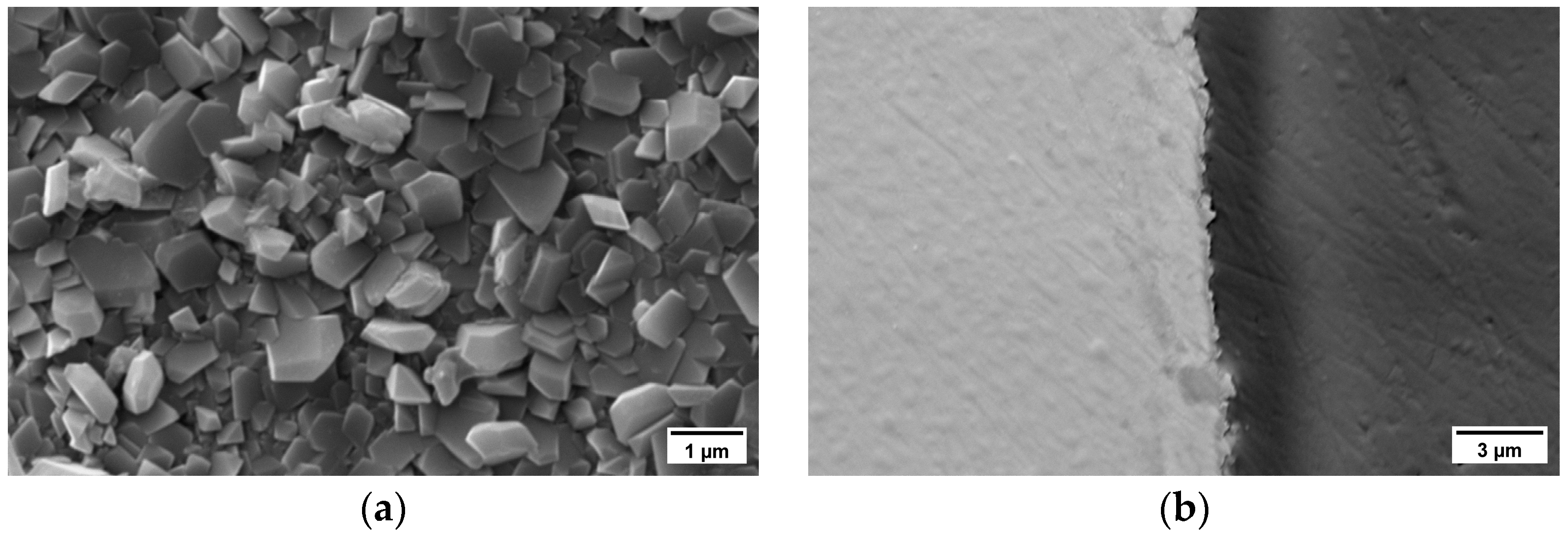
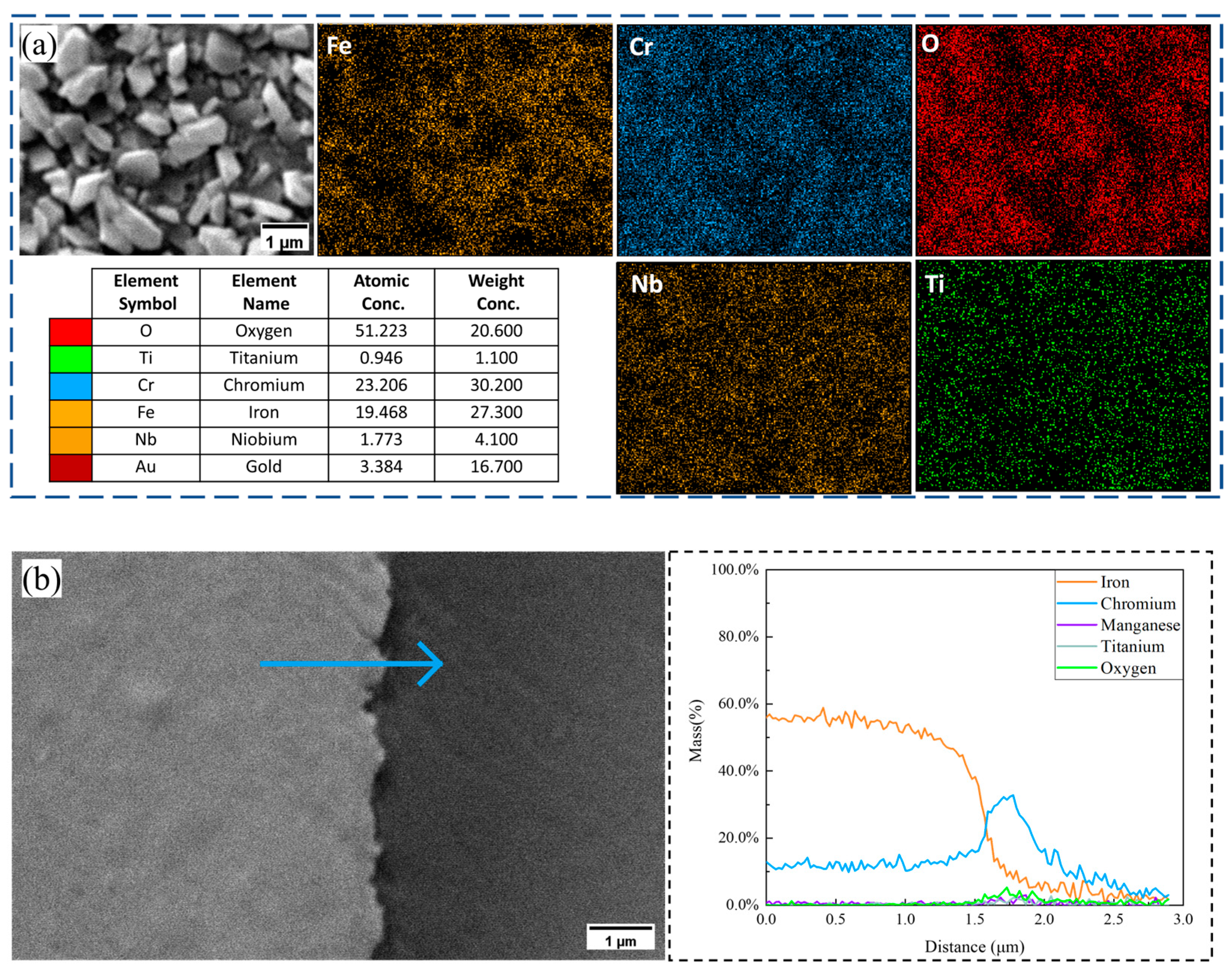
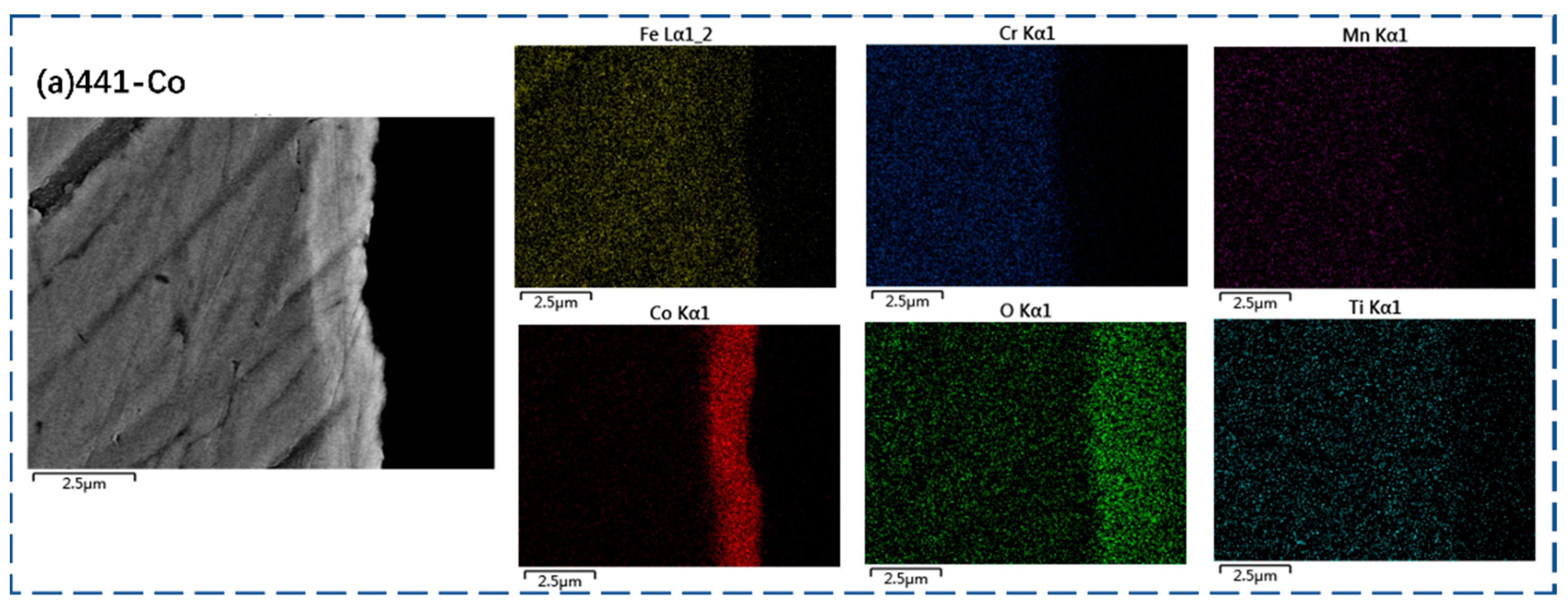
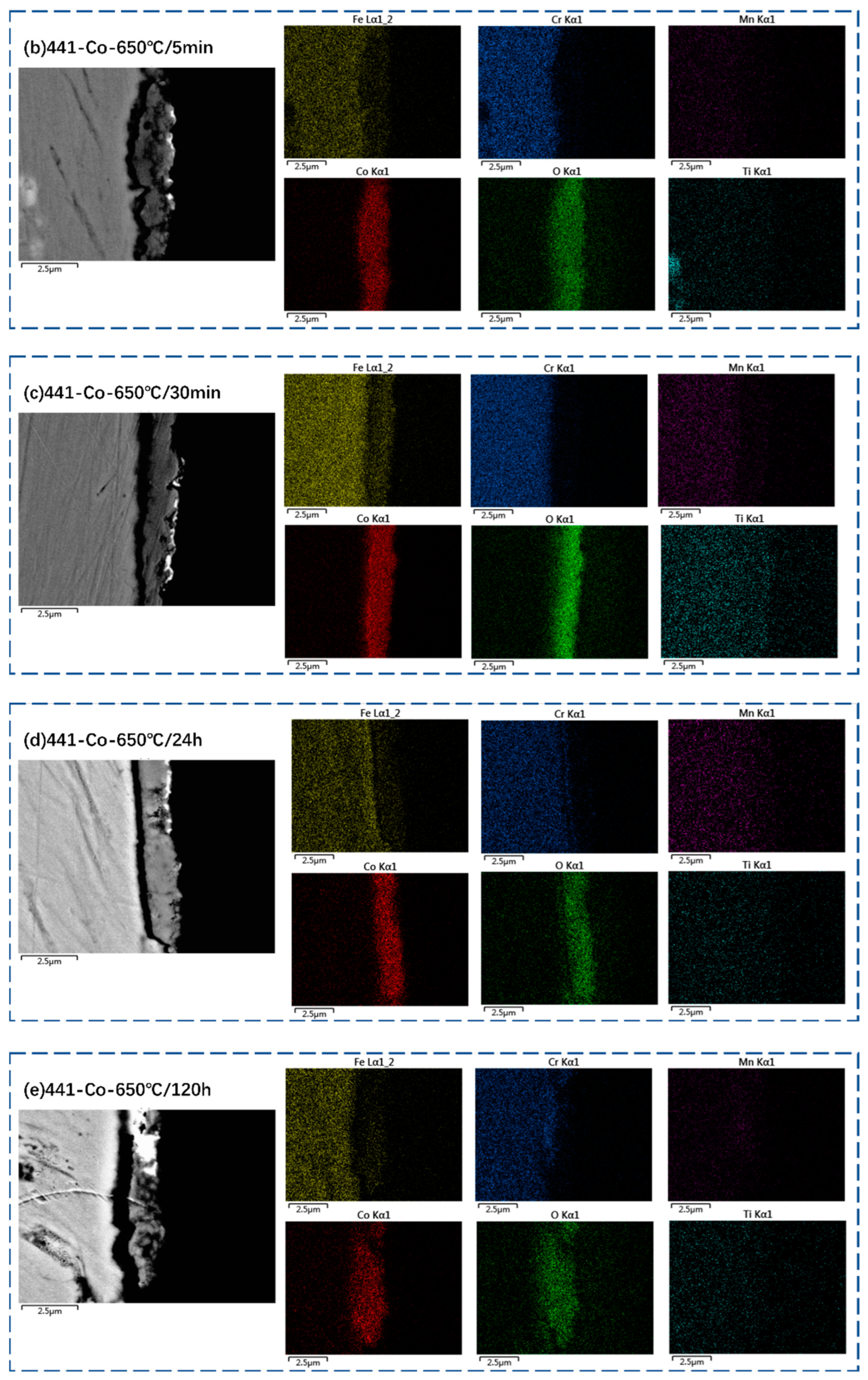
3.2. Oxide Scale Characterization
3.3. Electrical Property
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Wang, E.; Mao, J.; Wang, H.; Ouyang, M.; Hu, H. Performance analysis of a metal-supported intermediate-temperature solid oxide electrolysis cell. Front. Energy Res. 2022, 10, 3389. [Google Scholar] [CrossRef]
- Hilpert, K.; Das, D.; Miller, M.; Peck, D.H.; Weiß, R. Chromium Vapor Species Over Solid Oxide Fuel Cell Interconnect Materials and Their Potential for Degradation Processes. J. Electrochem. Soc. 1996, 143, 3642–3647. [Google Scholar] [CrossRef]
- Zarabi Golkhatmi, S.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools. Renew. Sustain. Energ. Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Mizusaki, J.; Singhal, S.C. Solid Oxide Fuel Cells IX: (SOFC-IX): Proceedings of the International Symposium; Pennington, N.J., Ed.; Electrochemical Society: Philadelphia, PA, USA, 2005; pp. 1866–1873. [Google Scholar]
- Whittemore, A.S.; Harris, R.; Itnyre, J.; Halpern, J. Recent development in precoating of stainless steel strips for interconnects at Sandvik materials technology. In Proceedings of the 10th European SOFC Forum, Lucerne, Switzerland, 26–29 June 2012; Volume 10, pp. 6–11. [Google Scholar]
- Hong, J.E.; Lim, T.H.; Lee, S.B.; Shin, D.R.; Song, R.H. Characteristics of a Protective Coating on Metal Interconnect for SOFCs. ECS Trans. 2009, 25, 1393–1396. [Google Scholar] [CrossRef]
- Garcia, E.M. The electrochemical behavior of cobalt electrodeposits on 430 stainless steel as solid oxide fuel cell interconnect. Surf. Coat. Technol. 2013, 235, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Xu, S.; Shi, J.; Teng, F.; Yu, X.; Sun, J. Impact of Different Atmospheres on Oxidation and Electrical Performance of a Solid Oxide Fuel Cell Interconnect with Co-Containing Protective Coating. Energy Fuels 2020, 34, 8864–8871. [Google Scholar] [CrossRef]
- Ebrahimifar, H. Microstructure and Oxidation Behavior of Cobalt Diffusional Coating Fabricated on AISI 429 Stainless Steel. Oxid. Met. 2019, 91, 417–435. [Google Scholar] [CrossRef]
- Robert, B.; Lundberg, M.W.; Westlinder, J.; Holmberg, H. Self Healing of Precoated AISI 441 for Solid Oxide Fuel Cell Interconnects. ECS Trans. 2015, 68, 1649–1655. [Google Scholar]
- Tomas, M.; Asokan, V.; Puranen, J.; Svensson, J.E.; Froitzheim, J. Efficiencies of cobalt- and copper-based coatings applied by different deposition processes for applications in intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2022, 47, 32628–32640. [Google Scholar] [CrossRef]
- Goebel, C.; Berger, R.; Bernuy-López, C.; Westlinder, J.; Svensson, J.E.; Froitzheim, J. Long-term (4 year) degradation behavior of coated stainless steel 441 used for solid oxide fuel cell interconnect applications. J. Power. Sources 2020, 449, 227480. [Google Scholar] [CrossRef]
- Young, D.J.; Zurek, J.; Singheiser, L.; Quadakkers, W.J. Temperature dependence of oxide scale formation on high Cr ferritic steels in Ar-H2-H2O. Corros. Sci. 2011, 53, 2131–2141. [Google Scholar] [CrossRef]
- Alnegren, P.; Sattari, M.; Svensson, J.E.; Froitzheim, J. Temperature dependence of corrosion of ferritic stainless steel in dual atmosphere at 600–800 °C. J. Power. Sources 2018, 392, 129–138. [Google Scholar] [CrossRef]
- Spotorno, R.; Paravidino, D.; Delsante, S.; Piccardo, P. Volatilization of chromium from AISI 441 stainless steel: Time and temperature dependence. Surf. Coat. Technol. 2022, 433, 128125. [Google Scholar] [CrossRef]
- Velraj, S.; Zhu, J.H.; Painter, A.S.; Du, S.W.; Li, Y.T. Impedance spectroscopy of the oxide films formed during high temperature oxidation of a cobalt-plated ferritic alloy. J. Power. Sources 2014, 247, 314–321. [Google Scholar] [CrossRef]
- Saeidpour, F.; Zandrahimi, M.; Ebrahimifar, H. Evaluation of pulse electroplated cobalt/yttrium oxide composite coating on the Crofer 22 APU stainless steel interconnect. Int. J. Hydrogen Energy 2019, 44, 3157–3169. [Google Scholar] [CrossRef]
- Saeidpour, F.; Zandrahimi, M.; Ebrahimifar, H. Oxidation and electrical behaviour of direct and pulse current electroplated cobalt coatings on Crofer22APU stainless steel interconnect. Mater. High. Temp. 2020, 37, 89–100. [Google Scholar] [CrossRef]
- Sachitanand, R.; Sattari, M.; Svensson, J.-E.; Froitzheim, J. Evaluation of the oxidation and Cr evaporation properties of selected FeCr alloys used as SOFC interconnects. Int. J. Hydrogen Energy 2013, 38, 15328–15334. [Google Scholar] [CrossRef] [Green Version]
- Falk-Windisch, H.; Claquesin, J.; Svensson, J.E.; Froitzheim, J. The effect of metallic Co-coating thickness on ferritic stainless steels intended for use as interconnect material in intermediate temperature solid oxide fuel cells. Oxid. Met. 2018, 89, 233–250. [Google Scholar] [CrossRef] [Green Version]
- Falk-Windisch, H.; Claquesin, J.; Sattari, M.; Svensson, J.E.; Froitzheim, J. Co- and Ce/Co-coated ferritic stainless steel as interconnect material for intermediate temperature solid oxide fuel cells. J. Power. Sources 2017, 343, 1–10. [Google Scholar] [CrossRef]
- Berger, R.; Lundberg, M.W.; Westlinder, J. Self-Healing of Precoated AISI 441 for Solid Oxide Fuel Cell Interconnects at Intermediate Temperatures. ECS Trans. 2017, 78, 1599–1605. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Bexell, U.; Stenstrom, M.; Norrby, N.; Westlinder, J. The Time for Industrialization Has Come: A Pre-Coated Solution for the GW Scale. ECS Trans. 2021, 103, 1803–1808. [Google Scholar] [CrossRef]
- Zhang, Y.; Javed, A.; Zhou, M.; Liang, S.; Xiao, P. Fabrication of Mn–Co Spinel Coatings on Crofer 22 APU Stainless Steel by Electrophoretic Deposition for Interconnect Applications in Solid Oxide Fuel Cells. Int. J. Appl. Ceram. Technol. 2014, 11, 332–341. [Google Scholar] [CrossRef]
- Piccardo, P.; Anelli, S.; Bongiorno, V.; Spotorno, R.; Repetto, L.; Girardon, P. K44M ferritic stainless steel as possible interconnect material for SOFC stack operating at 600 °C: Characterization of the oxidation behaviour at early working stages. Int. J. Hydrogen Energy 2015, 40, 3726–3738. [Google Scholar] [CrossRef]
- Zhang, M.; An, L.; Wang, E.; Wang, H.; Ouyang, M.; Hu, H. Effects of Sintering Parameters on the Low-Temperature Densification of GDC Electrolyte Based on an Orthogonal Experiment. Catalysts 2022, 12, 831. [Google Scholar] [CrossRef]
- Wagner, C. Theoretical analysis of the diffusion processes determining the oxidation rate of alloys. J. Electrochem. Soc. 1952, 99, 369–380. [Google Scholar] [CrossRef]
- Bexell, U.; Mikael, O.; Lundberg, M.W. High Temperature Oxidation of Plastically Deformed Ferritic Interconnect Steel. ECS Trans. 2011, 35, 2463–2470. [Google Scholar] [CrossRef]
- Bi, Z.H.; Zhu, J.H.; Du, S.W.; Li, Y.T. Effect of alloy composition on the oxide scale formation and electrical conductivity behavior of Co-plated ferritic alloys. Surf. Coat. Technol. 2013, 228, 124–131. [Google Scholar] [CrossRef]
- Kruk, A.; Adamczyk, A.; Gil, A.; Kąc, S.; Dąbek, J.; Ziąbka, M.; Brylewski, T. Effect of Co deposition on oxidation behavior and electrical properties of ferritic steel for solid oxide fuel cell interconnects. Thin Solid Films 2015, 590, 184–192. [Google Scholar] [CrossRef]
- Petric, A.; Ling, H. Electrical Conductivity and Thermal Expansion of Spinels at Elevated Temperatures. J. Am. Ceram. Soc. 2007, 90, 1515–1520. [Google Scholar] [CrossRef]
- Froitzheim, J.; Canovic, S.; Nikumaa, M.; Sachitanand, R.; Johansson, L.G.; Svensson, J.E. Long term study of Cr evaporation and high temperature corrosion behaviour of Co coated ferritic steel for solid oxide fuel cell interconnects. J. Power. Sources 2012, 220, 217–227. [Google Scholar] [CrossRef]
- Poitel, S.; Wang, Z.; Willinger, M.; Van Herle, J.; Hébert, C. In-Situ Observation of Co-Ce Coated Metallic Interconnect Oxidation Combined with High-Resolution Post Exposure Analysis. ECS Trans. 2017, 78, 1615. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, L.S.; Lundberg, M.W.; Berger, R.; Westlinder, J. A Time Resolved Study of Precoated AISI 441 for SOFC Interconnects Using STEM-EELS-Part I. ECS Meet. Abstr. 2015, 68, 1657. [Google Scholar]
- Karlsson, L.S.; Lundberg, M.W.; Berger, R.; Westlinder, J. A Time Resolved Study of Precoated AISI 441 for SOFC Interconnects Using STEM-EELS-Part II. ECS Trans. 2015, 68, 1667–1679. [Google Scholar] [CrossRef]
- Macauley, C.; Gannon, P.; Deibert, M.; White, P. The influence of pre-treatment on the oxidation behavior of Co coated SOFC interconnects. Int. J. Hydrogen Energy 2011, 36, 4540–4548. [Google Scholar] [CrossRef]
- Setiawan, A.R.; Ramelan, A.; Suratman, R.; Qizwini, A.M. The influence of Titanium alloying and Co3O4 coating on the oxidation behavior of Fe-20Cr ferritic stainless steels for SOFC interconnects. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012115. [Google Scholar] [CrossRef]




| FSS | Fe | Cr | Mn | Si | C | P | S | Nb | Ti |
|---|---|---|---|---|---|---|---|---|---|
| 441 | Bal. | 17.9 | 0.10 | 0.27 | 0.01 | 0.021 | 0.001 | 0.43 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Wang, E.; Wang, H.; Ouyang, M.; Hu, H.; Lu, L.; Ren, D.; Liu, Y. Experimental Investigation of the Initial Stage of the Oxidation Mechanism of Co Coating for Solid Oxide Fuel Cell Interconnects at 650 °C. Coatings 2023, 13, 1144. https://doi.org/10.3390/coatings13071144
Mao J, Wang E, Wang H, Ouyang M, Hu H, Lu L, Ren D, Liu Y. Experimental Investigation of the Initial Stage of the Oxidation Mechanism of Co Coating for Solid Oxide Fuel Cell Interconnects at 650 °C. Coatings. 2023; 13(7):1144. https://doi.org/10.3390/coatings13071144
Chicago/Turabian StyleMao, Jingwen, Enhua Wang, Hewu Wang, Minggao Ouyang, Haoran Hu, Languang Lu, Dongsheng Ren, and Yadi Liu. 2023. "Experimental Investigation of the Initial Stage of the Oxidation Mechanism of Co Coating for Solid Oxide Fuel Cell Interconnects at 650 °C" Coatings 13, no. 7: 1144. https://doi.org/10.3390/coatings13071144





