A Facile Preparation Method for Corrosion-Resistant Copper Superhydrophobic Surfaces with Ordered Microstructures by Etching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
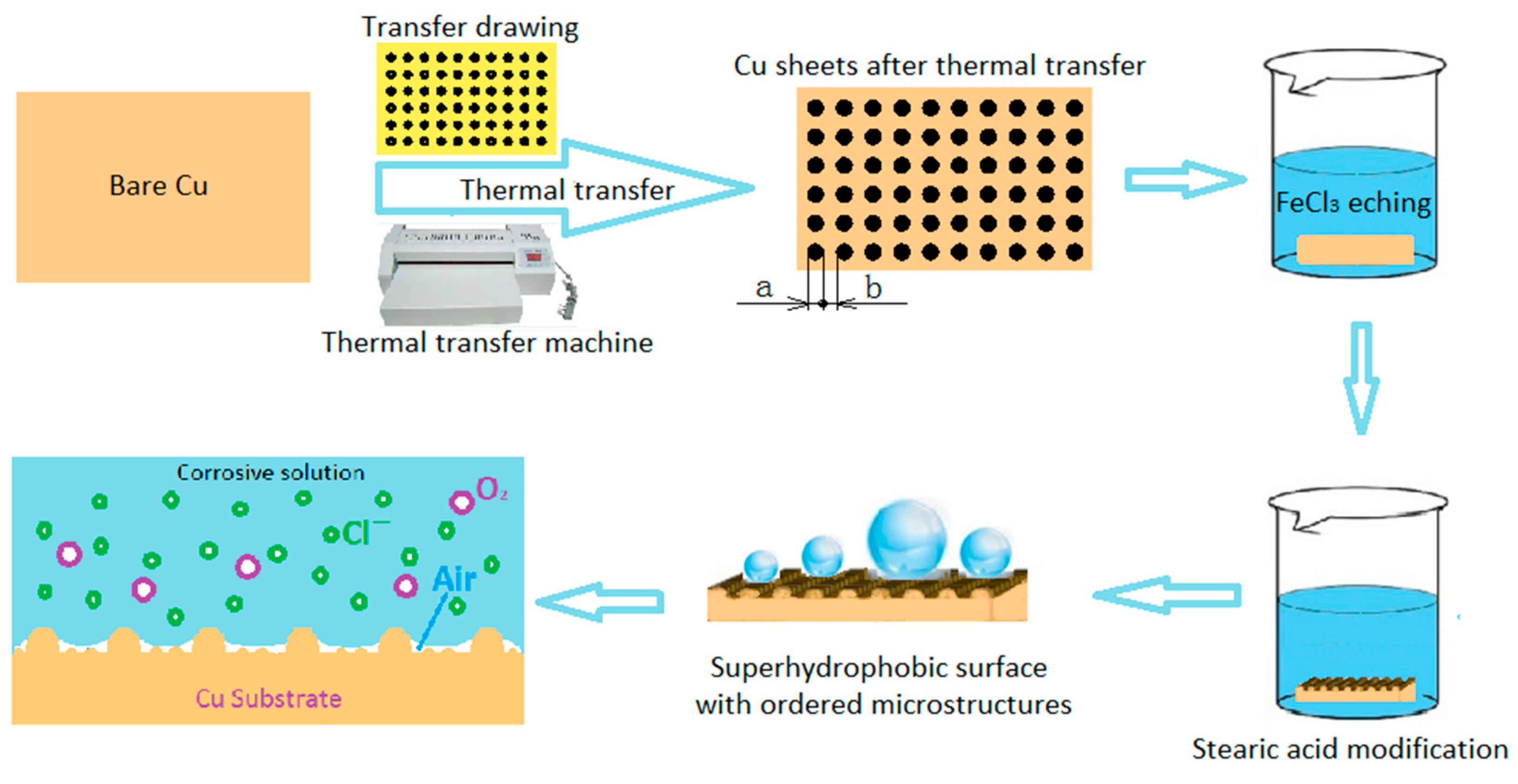
2.2. Preparation Method
2.2.1. Thermal Transfer
2.2.2. Chemical Treatment
2.3. Sample Characterization
3. Results and Discussion
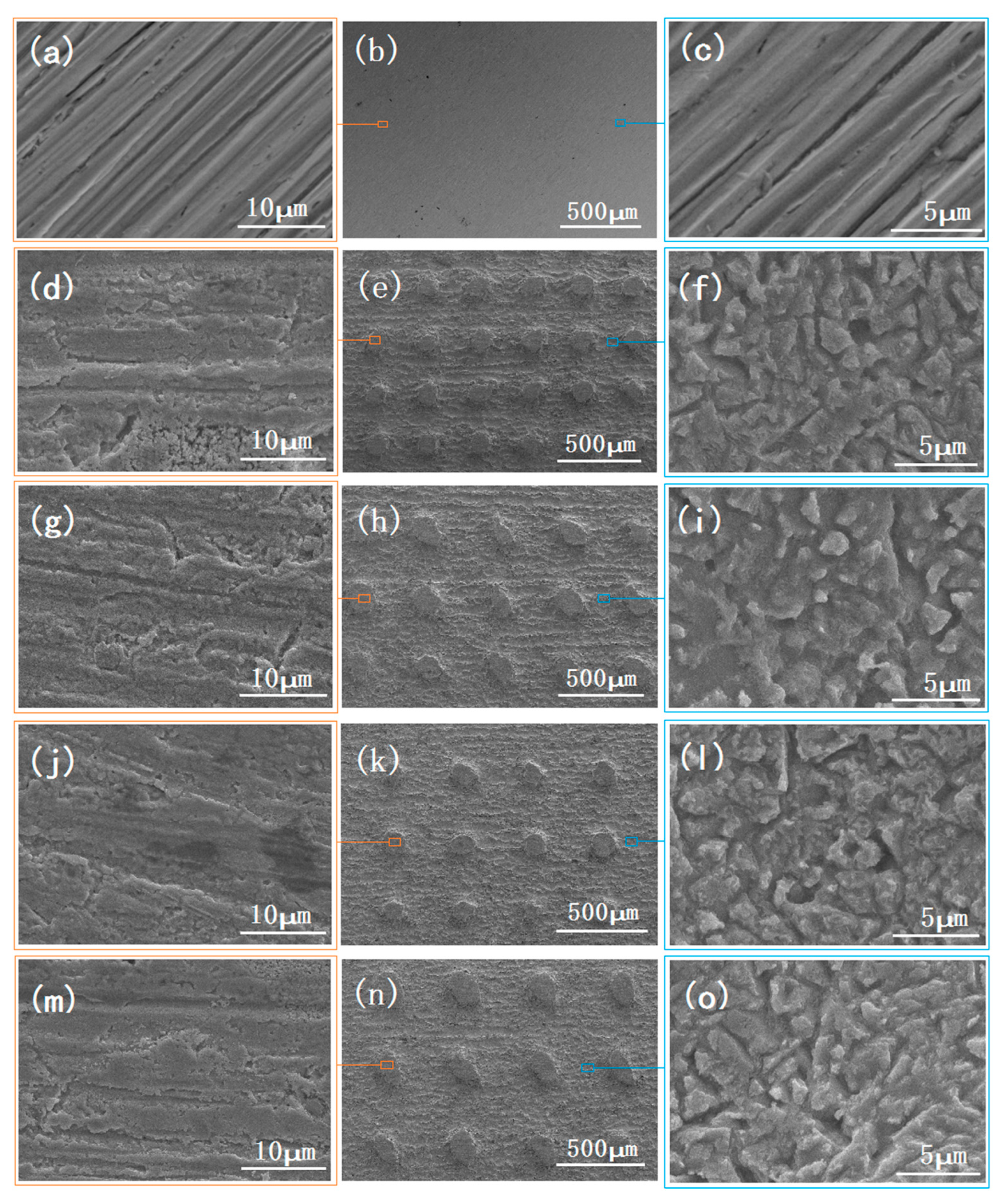
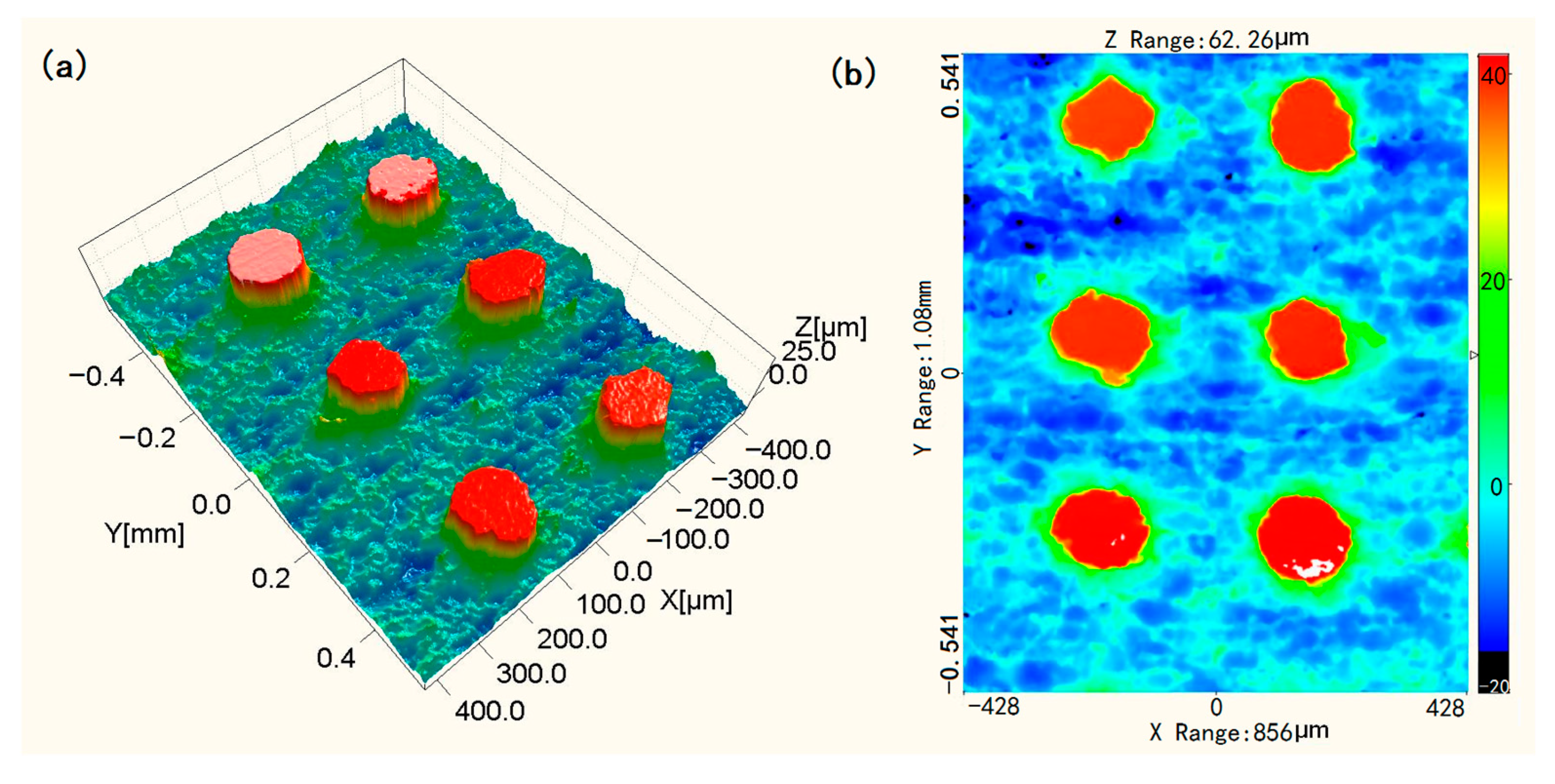
3.1. SEM Images
3.2. Surface Wetting Performance
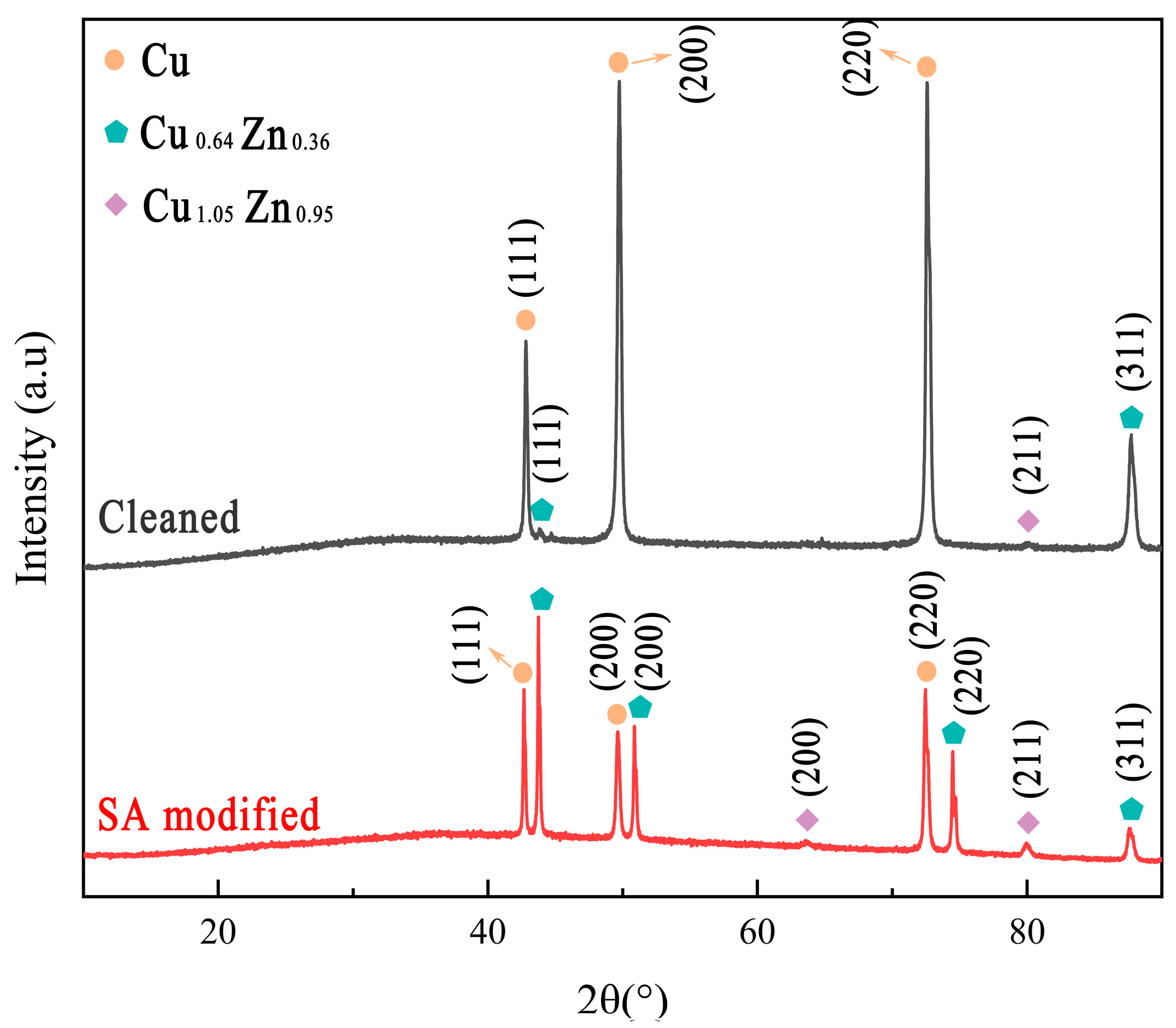
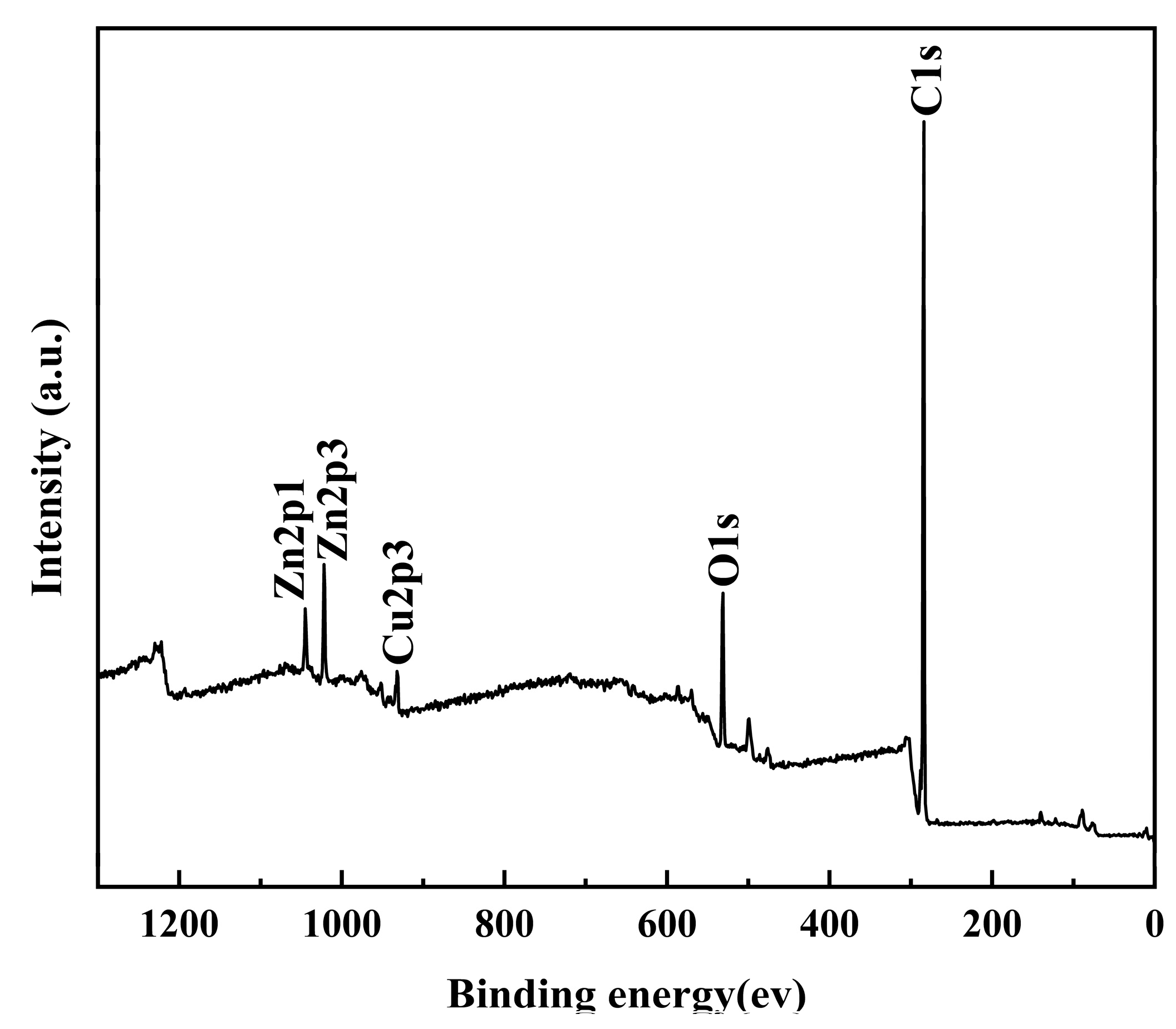
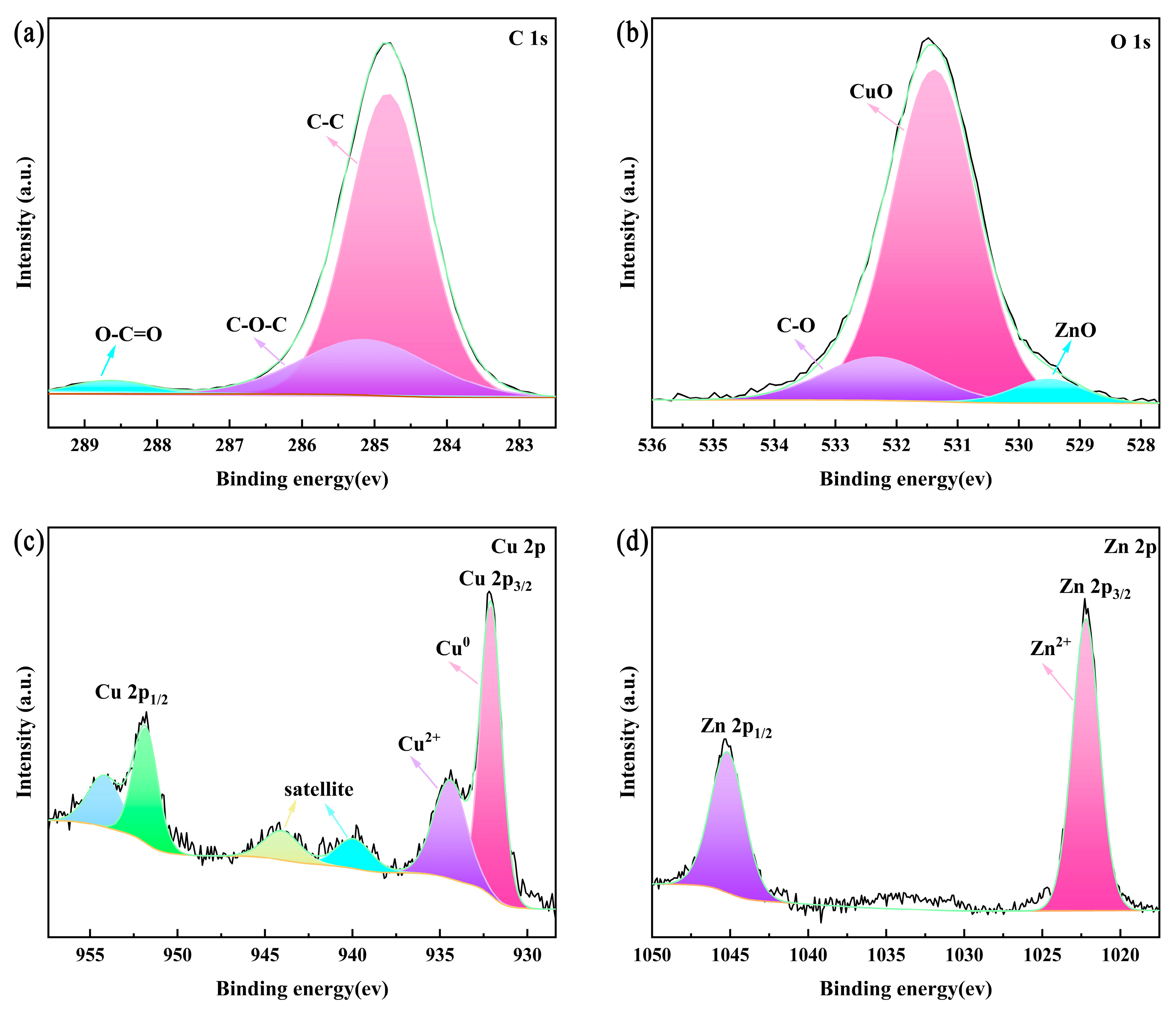
3.3. Surface Composition
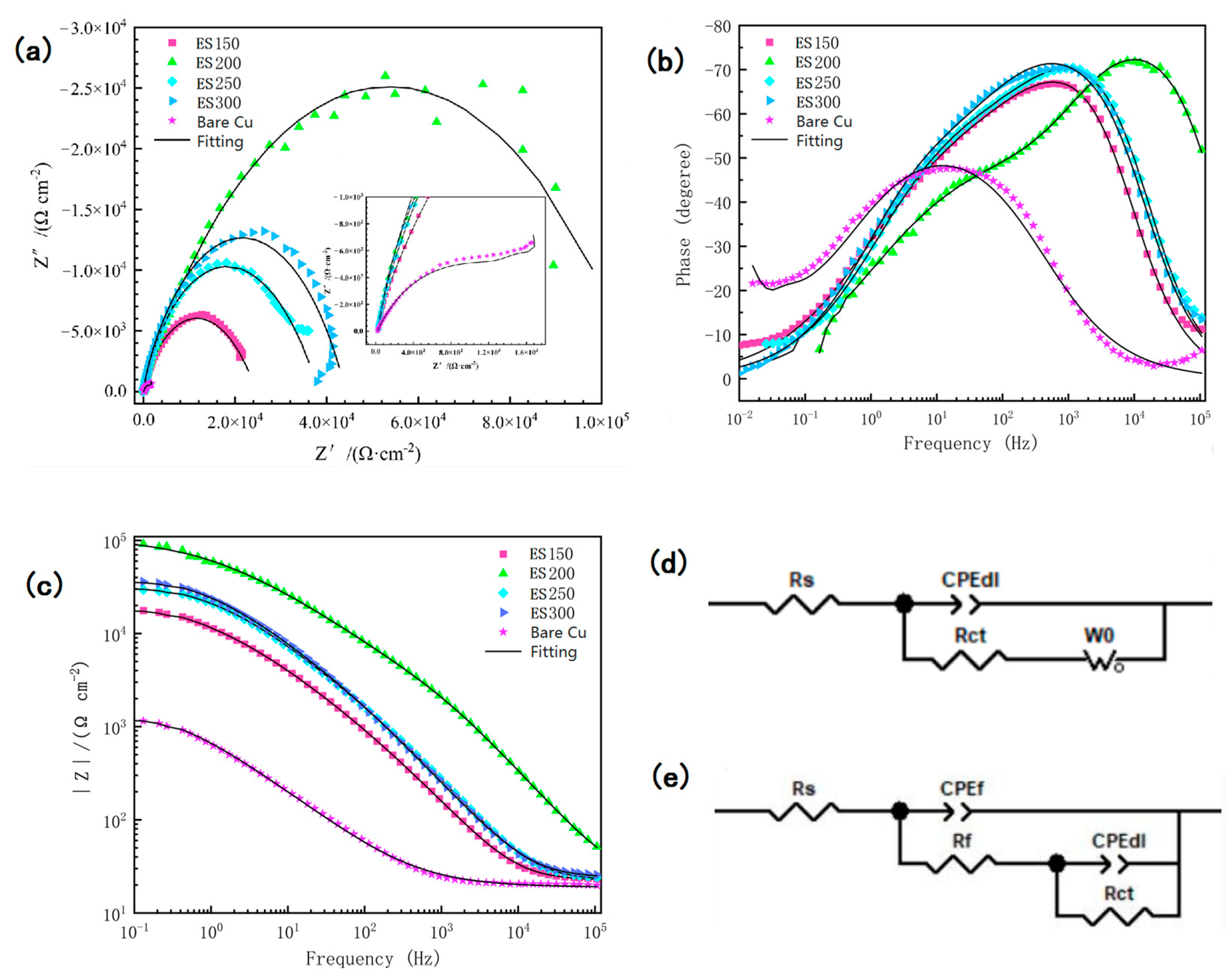
3.4. Surface Anti-Corrosion Performance
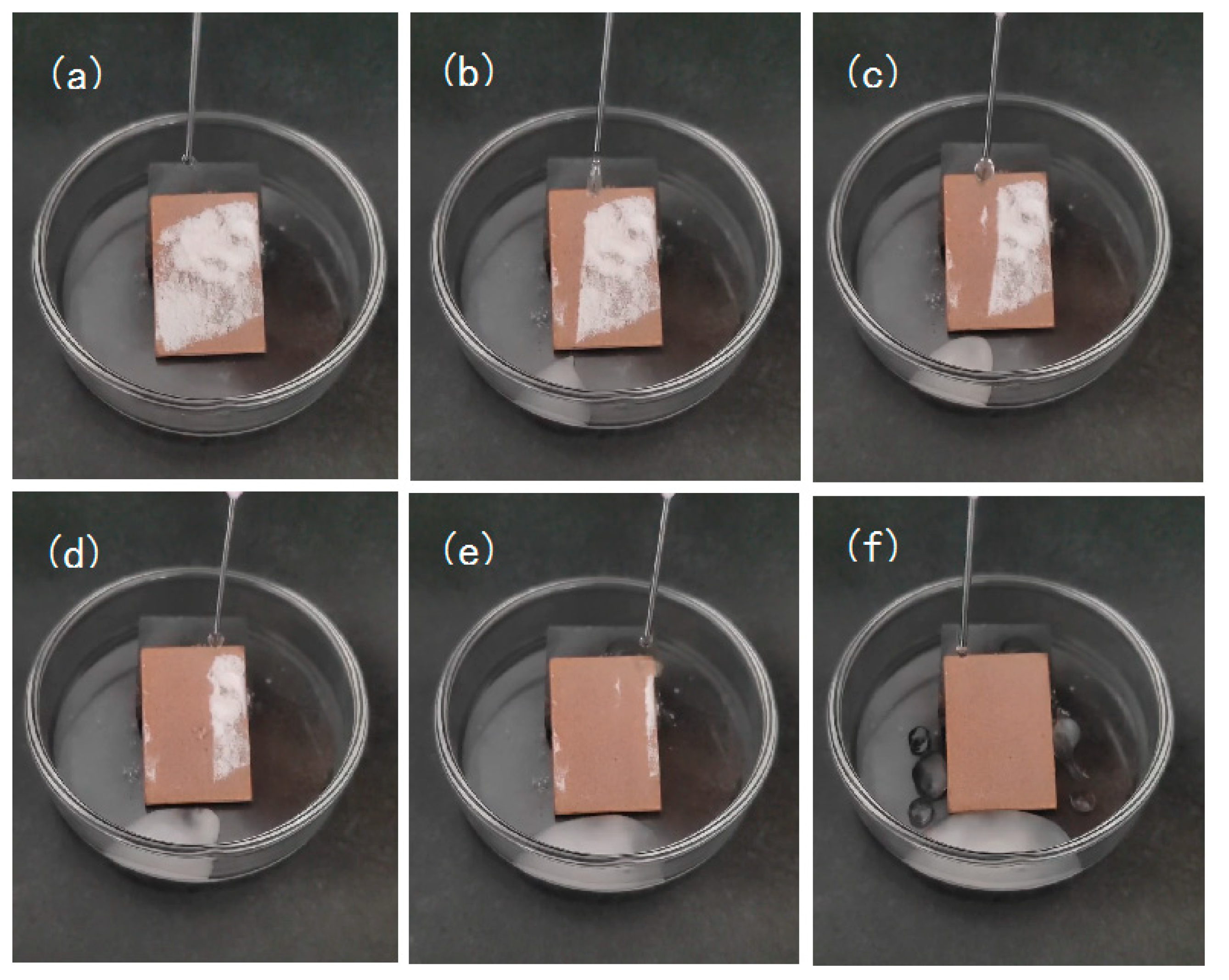
3.5. Self-Cleaning Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Zhang, D.; Qiu, R.; Wan, Y.; Wu, J. Green approach to fabrication of a super-hydrophobic film on copper and the consequent corrosion resistance. Corros. Sci. 2014, 80, 366–373. [Google Scholar] [CrossRef]
- Almomani, M.A.; Tyfour, W.R.; Nemrat, M.H. Effect of silicon carbide Addition on the corrosion behavior of powder metallurgy Cu-30Zn brass in a 3.5 wt.% NaCl solution. J. Alloys Compd. 2016, 679, 104–114. [Google Scholar] [CrossRef]
- Xu, W.; Hu, Y.; Bao, W.; Xie, X.; Liu, Y.; Song, A.; Hao, J. Superhydrophobic copper surfaces fabricated by fatty acid soaps in aqueous solution for excellent corrosion resistance. Appl. Surf. Sci. 2016, 399, 491–498. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, H.; Wang, Z.; Liu, Y. Superhydrophobic aluminum alloy surface: Fabrication, structure, and corrosion resistance. Colloid Surf. A-Physicochem. Eng. Asp. 2014, 441, 319–325. [Google Scholar] [CrossRef]
- Su, F.; Yao, K. Facile fabrication of superhydrophobic surface with excellent mechanical abrasion and corrosion resistance on copper substrate by a novel method. ACS Appl. Mater. Interfaces 2014, 6, 8762–8770. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Neinghuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Wang, Q.; Wang, N. Chemical fabrication strategies for achieving bioinspired superhydrophobic surfaces with micro and nanostructures: A review. Adv. Eng. Mater. 2021, 23, 2001083. [Google Scholar] [CrossRef]
- Ding, C.; Tai, Y.; Wang, D.; Tan, L.; Fu, J. Superhydrophobic composite coating with active corrosion resistance for AZ31B magnesium alloy protection. Chem. Eng. J. 2019, 357, 518–532. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Wu, J. Super-hydrophobic metal-complex film fabricated electrochemically on copper as a barrier to corrosive medium. Corros. Sci. 2014, 83, 317–326. [Google Scholar] [CrossRef]
- Ouyang, Y.; Qiu, R.; Xiao, Y.; Shi, Z.; Hu, S.; Zhang, Y.; Chen, M.; Wang, P. Magnetic fluid based on mussel inspired chemistry as corrosion-resistant coating of NdFeB magnetic material. Chem. Eng. J. 2019, 368, 331–339. [Google Scholar] [CrossRef]
- Dominic, J.; Perumal, G.; Grewal, H.S.; Arora, H.S. Facile fabrication of superhydrophobic brass surface for excellent corrosion resistance. Surf. Eng. 2020, 36, 660–664. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Aly, M.A.S.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.S.; Sadasivuni, K.K.; Liu, S. Recent advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust anti-icing performance of a flexible superhydrophobic surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Xu, W.; Liu, H.; Lu, Y. Inexpensive and non-fluorinated superhydrophobic concrete coating for anti-icing and anti-corrosion. J. Colloid. Interface. Sci. 2019, 541, 86–92. [Google Scholar] [CrossRef]
- Kefallinou, D.; Ellinas, K.; Speliotis, T.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Optimization of antibacterial properties of “hybrid” metal-sputtered superhydrophobic surfaces. Coatings 2020, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Durdu, S. Characterization, bioactivity and antibacterial properties of copper-based TiO2 bioceramic coatings fabricated on titanium. Coatings 2019, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Latthe, S.S.; Sutar, R.; Shinde, T.; Pawar, S.; Khot, T.; Bhosale, A.; Sadasivuni, K.K.; Xing, R.; Mao, L.; Liu, S. Superhydrophobic leaf mesh decorated with SiO2 nanoparticle-polystyrene nanocomposite for oil-water separation. ACS Appl. Nano Mater. 2019, 2, 799–805. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Li, J.; Zou, M.; Zheng, H. UV laser-produced copper micro-mesh with superhydrophobic-oleophilic surface for oil-water Separation. JMR&T 2021, 15, 5733–5745. [Google Scholar]
- Ma, L.; Wang, L.; Li, C.; Guo, J.; Shrotriya, P.; Deng, C.; Zhao, J. Hybrid nanosecond laser processing and heat treatment for rapid preparation of super-hydrophobic copper surface. Metals 2019, 9, 668. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Xu, Q.; Han, J.; Chen, X.; Min, Y. A novel combination approach for the preparation of superhydrophobic surface on copper and the consequent corrosion resistance. Corros. Sci. 2016, 110, 105–113. [Google Scholar] [CrossRef]
- Li, H.; Wei, H.; Zou, X.; Wang, C.; Gao, Q.; Li, Q.; Liu, Q.; Zhang, J. Copper-based nanoribbons fabricated on a copper substrate by a liquid-solid reaction and their corrosion performance. Mater. Chem. Phys. 2020, 246, 122839. [Google Scholar] [CrossRef]
- Mousavi, S.M.A.; Pitchumani, R. A study of corrosion on electrodeposited superhydrophobic copper surfaces. Corros. Sci. 2021, 186, 109420. [Google Scholar] [CrossRef]
- Chen, Z.; Li, F.; Hao, L.; Chen, A.; Kong, Y. One-step electrodeposition process to fabricate cathodic superhydrophobic Surface. Appl. Surf. Sci. 2011, 258, 1395–1398. [Google Scholar] [CrossRef]
- Chaitanya, B.; Gunjan, M.R.; Sarangi, R.; Raj, R.; Thakur, A.D. Per-fluorinated chemical free robust superhydrophobic copper surface using a scalable technique. Mater. Chem. Phys. 2022, 278, 125667. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, X.; Bing, J.; Peng, C.; Xing, S.; Wang, M.; Xiao, J.; Zeng, J.; Xie, Y.; Xiao, X.; et al. A novel fabrication of a superhydrophobic surface with highly similar hierarchical structure of the lotus leaf on a copper sheet. Appl. Surf. Sci. 2013, 285, 205–210. [Google Scholar] [CrossRef]
- Ta, D.V.; Dunn, A.; Wasley, T.J.; Kay, R.W.; Stringer, J.; Smith, P.J.; Connaughton, C.; Shephard, J.D. Nanosecond laser textured superhydrophobic metallic surfaces and their chemical sensing applications. Appl. Surf. Sci. 2015, 357, 248–254. [Google Scholar] [CrossRef] [Green Version]
- He, A.; Liu, W.; Xue, W.; Yang, H.; Cao, Y. Nanosecond laser ablated copper superhydrophobic surface with tunable ultrahigh adhesion and its renewability with low temperature Annealing. Appl. Surf. Sci. 2018, 434, 120–125. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, G.; Chen, K.; Chen, Y.; He, Z.; Ou, J. Preparation and anti-corrosion performances of grass-like microstructured superhydrophobic surface on copper via solution-immersion. Mater. Lett. 2022, 323, 132482. [Google Scholar] [CrossRef]
- Liu, L.; Xu, F.; Ma, L. Facile fabrication of a superhydrophobic Cu Surface via a selective etching of high-energy facets. J. Phys. Chem. C. 2012, 116, 18722–18727. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, M.; Liu, W.; Shen, X.; Min, Y.; Xu, Q. The research on preparation of superhydrophobic surfaces of pure copper by hydrothermal method and its corrosion Resistance. Electrochim. Acta 2018, 270, 310–318. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, C.; Liu, R.; Zhang, Q.; Gong, J.; Tao, D.; Ji, Z. Fabrication of superhydrophobic surface with enhanced corrosion resistance on H62 brass substrate. Colloid. Surf. A 2020, 589, 124475. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Elia, A.; Wael, K.D.; Dowsett, M.; Adriaens, A. Electrochemical deposition of a copper carboxylate layer on copper as potential corrosion inhibitor. J. Solid State Electron. 2012, 16, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Pehkonen, S.O.; Liang, B.; Ting, Y.P.; Neoh, K.G.; Kang, E.T. Superhydrophobic fluoropolymer-modifified copper surface via surface graft polymerisation for corrosion protection. Corros. Sci. 2011, 53, 2738–2747. [Google Scholar] [CrossRef]
- Shinato, K.W.; Zewde, A.A.; Jin, Y. Corrosion protection of copper and copper alloys in different corrosive medium using envionmentally friendly corrosion inhibitors. Corros. Rev. 2020, 38, 101–109. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, R.; Wang, J.; Yang, W.; Liu, L. One-step method for the fabrication of superhydrophobic surface on magnesium alloy and its corrosion protection, antifouling performance. Corros. Sci. 2014, 80, 177–183. [Google Scholar] [CrossRef]
- Rao, B.V.A.; Reddy, M.N. Formation, characterization and corrosion protection effificiency of self-assembled 1-octadecyl-1Himidazole fifilms on copper for corrosion protection. Arab. J. Chem. 2017, 10, S3270–S3283. [Google Scholar]


| Cu | Fe | Pb | Total Impurity | Zn |
|---|---|---|---|---|
| 63.0~68.5 | ≤0.07 | ≤0.09 | ≤0.45 | residuals |
| Sample | Ecorr (V vs. Ag/AgCl) | Icorr (A/cm2 ) | ba (mV dec.−1) | bc (mV dec.−1) | η (%) |
|---|---|---|---|---|---|
| Bare Cu | −0.3173 | 3.9105 × 10−5 | 425.26 | −526.27 | — |
| ES150 | −0.3060 | 8.2692 × 10−6 | 160 | −115.19 | 78.85 |
| ES200 | −0.2923 | 3.5421 × 10−6 | 205.66 | −110.36 | 90.94 |
| ES250 | −0.2845 | 6.266 × 10−6 | 155.21 | −133.82 | 83.98 |
| ES300 | −0.3136 | 6.8877 × 10−6 | 136.52 | −76.69 | 82.39 |
| Sample | Rs (Ω cm2) | Rf (Ω cm2) | CPEdl (μF/cm2) | CPEf (μF/cm2) | Rct (Ω cm2) | Zw (S cm–2 s0.5) |
|---|---|---|---|---|---|---|
| Bare Cu | 18.92 | ---- | 0.64363 | ---- | 1647 | 0.61571 |
| ES150 | 22.27 | 1049 | 0.51619 | 0.88168 | 23,919 | ---- |
| ES200 | 25.38 | 2946 | 0.52677 | 0.90579 | 112,420 | ---- |
| ES250 | 22.55 | 2091 | 0.56371 | 0.889 | 35,756 | ---- |
| ES300 | 24.32 | 1905 | 0.51605 | 0.89031 | 47,976 | ---- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Z.; Zhu, J. A Facile Preparation Method for Corrosion-Resistant Copper Superhydrophobic Surfaces with Ordered Microstructures by Etching. Coatings 2023, 13, 1151. https://doi.org/10.3390/coatings13071151
Bai Z, Zhu J. A Facile Preparation Method for Corrosion-Resistant Copper Superhydrophobic Surfaces with Ordered Microstructures by Etching. Coatings. 2023; 13(7):1151. https://doi.org/10.3390/coatings13071151
Chicago/Turabian StyleBai, Zigang, and Jiyuan Zhu. 2023. "A Facile Preparation Method for Corrosion-Resistant Copper Superhydrophobic Surfaces with Ordered Microstructures by Etching" Coatings 13, no. 7: 1151. https://doi.org/10.3390/coatings13071151





