Comparative Analysis of the Advantages and Disadvantages of Utilizing Spirulina-Derived Pigment as a Bio-Based Colorant for Wood Impregnator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Production
2.3. Characterization
- 4 h at +50 °C and relative humidity < 30%
- 4 h at −20 °C
- 16 h at room temperature.
3. Results
3.1. Fillers and Coatings Morphology
3.2. Endurance of the Samples in Severe Surroundings
3.2.1. UV-B Exposure
3.2.2. Climatic Chamber Exposure
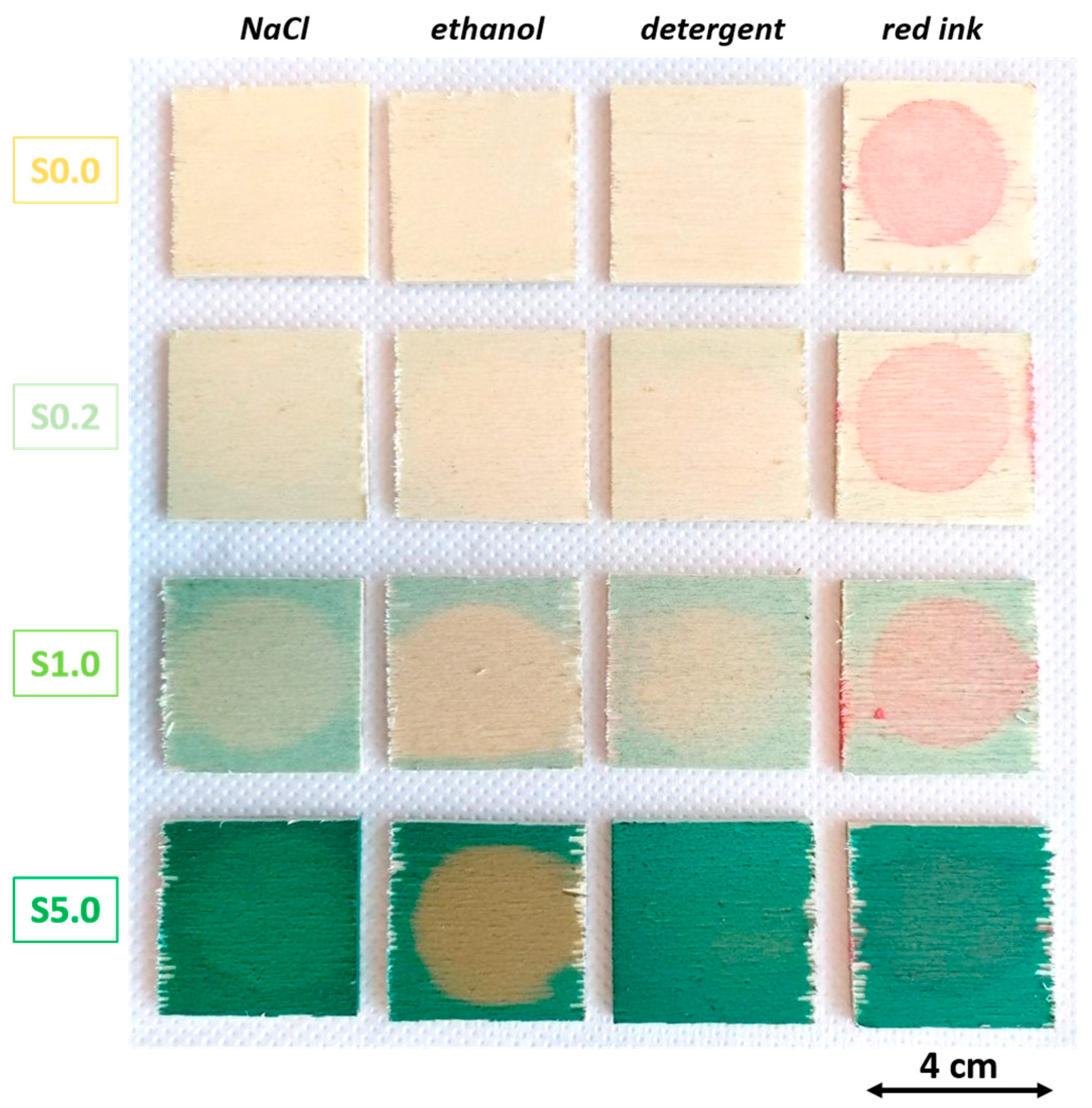
3.3. Coating Liquid Resistance
3.4. Coating Abrasion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radkau, J. Wood: A History; Polity Press: Cambridge, UK, 2012. [Google Scholar]
- Hoadley, R.B. Chemical and physical properties of wood. In The Structural Conservation of Panel Paintings: Proceedings. Part 1: Wood Science and Technology; The Getty Conservation Institute: Los Angeles, CA, USA, 1998; pp. 2–20. [Google Scholar]
- Hao, J.; Wu, X.; Oporto, G.; Liu, W.; Wang, J. Structural analysis and strength-to-weight optimization of wood-based sandwich composite with honeycomb core under three-point flexural test. Eur. J. Wood Wood Prod. 2020, 78, 1195–1207. [Google Scholar] [CrossRef]
- Kim, J.K.; Pal, K. Recent Advances in the Processing of Wood-Plastic Composites; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Janin, G.; Gonçalez, J.C.; Ananías, R.A.; Charrier, B.; Silva, G.F.d.; Dilem, A. Aesthetics appreciation of wood colour and patterns by colorimetry. Part 1. Colorimetry theory for the CIELab system. Maderas Cienc. Tecnol. 2001, 3, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Broman, N.O. Aesthetic properties in knotty wood surfaces and their connection with people’s preferences. J. Wood Sci. 2001, 47, 192–198. [Google Scholar] [CrossRef]
- Lowden, L.A.; Hull, T.R. Flammability behaviour of wood and a review of the methods for its reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Tolvaj, L.; Popescu, C.-M.; Molnar, Z.; Preklet, E. Effects of air relative humidity and temperature on photodegradation processes in beech and spruce wood. BioResources 2016, 11, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Mattonai, M.; Watanabe, A.; Shiono, A.; Ribechini, E. Degradation of wood by UV light: A study by EGA-MS and Py-GC/MS with on line irradiation system. J. Anal. Appl. Pyrolysis 2019, 139, 224–232. [Google Scholar] [CrossRef]
- Hon, D.N.S.; Chang, S.T. Surface degradation of wood by ultraviolet light. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 2227–2241. [Google Scholar] [CrossRef]
- Bansal, R.; Nair, S.; Pandey, K.K. UV resistant wood coating based on zinc oxide and cerium oxide dispersed linseed oil nano-emulsion. Mater. Today Commun. 2022, 30, 103177. [Google Scholar] [CrossRef]
- Varganici, C.-D.; Rosu, L.; Rosu, D.; Mustata, F.; Rusu, T. Sustainable wood coatings made of epoxidized vegetable oils for ultraviolet protection. Environ. Chem. Lett. 2021, 19, 307–328. [Google Scholar] [CrossRef]
- Jirouš-Rajković, V.; Miklečić, J. Enhancing weathering resistance of wood—A review. Polymers 2021, 13, 1980. [Google Scholar] [CrossRef]
- Hochmańska-Kaniewska, P.; Janiszewska, D.; Oleszek, T. Enhancement of the properties of acrylic wood coatings with the use of biopolymers. Prog. Org. Coat. 2022, 162, 106522. [Google Scholar] [CrossRef]
- Veigel, S.; Grüll, G.; Pinkl, S.; Obersriebnig, M.; Müller, U.; Gindl-Altmutter, W. Improving the mechanical resistance of waterborne wood coatings by adding cellulose nanofibres. React. Funct. Polym. 2014, 85, 214–220. [Google Scholar] [CrossRef]
- Šimůnková, K.; Hýsek, Š.; Reinprecht, L.; Šobotník, J.; Lišková, T.; Pánek, M. Lavender oil as eco-friendly alternative to protect wood against termites without negative effect on wood properties. Sci. Rep. 2022, 12, 1909. [Google Scholar] [CrossRef] [PubMed]
- Miri Tari, S.M.; Tarmian, A.; Azadfallah, M. Improving fungal decay resistance of solvent and waterborne polyurethane-coated wood by free and microencapsulated thyme essential oil. J. Coat. Technol. Res. 2022, 19, 959–966. [Google Scholar] [CrossRef]
- Rosu, L.; Mustata, F.; Rosu, D.; Varganici, C.-D.; Rosca, I.; Rusu, T. Bio-based coatings from epoxy resins crosslinked with a rosin acid derivative for wood thermal and anti–fungal protection. Prog. Org. Coat. 2021, 151, 106008. [Google Scholar] [CrossRef]
- Mustata, F.; Rosu, D.; Varganici, C.-D.; Rosu, L.; Rosca, I.; Tudorachi, N. Assessing the thermal and fungal behavior of eco-friendly epoxy thermosets derived from vegetable oils for wood protective coatings. Prog. Org. Coat. 2022, 163, 106612. [Google Scholar] [CrossRef]
- Nikolic, M.; Lawther, J.M.; Sanadi, A.R. Use of nanofillers in wood coatings: A scientific review. J. Coat. Technol. Res. 2015, 12, 445–461. [Google Scholar] [CrossRef]
- Pacheco, C.M.; Cecilia, B.A.; Reyes, G.; Oviedo, C.; Fernández-Pérez, A.; Elso, M.; Rojas, O.J. Nanocomposite additive of SiO2/TiO2/nanocellulose on waterborne coating formulations for mechanical and aesthetic properties stability on wood. Mater. Today Commun. 2021, 29, 102990. [Google Scholar] [CrossRef]
- Salla, J.; Pandey, K.; Srinivasa, K. Improvement of UV resistance of wood surfaces by using ZnO nanoparticles. Polym. Degrad. Stab. 2012, 97, 592–596. [Google Scholar] [CrossRef]
- Jalili, M.M.; Moradian, S.; Dastmalchian, H.; Karbasi, A. Investigating the variations in properties of 2-pack polyurethane clear coat through separate incorporation of hydrophilic and hydrophobic nano-silica. Prog. Org. Coat. 2007, 59, 81–87. [Google Scholar] [CrossRef]
- Janesch, J.; Czabany, I.; Hansmann, C.; Mautner, A.; Rosenau, T.; Gindl-Altmutter, W. Transparent layer-by-layer coatings based on biopolymers and CeO2 to protect wood from UV light. Prog. Org. Coat. 2020, 138, 105409. [Google Scholar] [CrossRef]
- Cheumani Yona, A.M.; Žigon, J.; Ngueteu Kamlo, A.; Pavlič, M.; Dahle, S.; Petrič, M. Preparation, Surface Characterization, and Water Resistance of Silicate and Sol-Silicate Inorganic–Organic Hybrid Dispersion Coatings for Wood. Materials 2021, 14, 3559. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Yi, Z.; Liao, M.; Qin, Z. Stable superhydrophobic wood surface constracting by KH580 and nano-Al2O3 on polydopamine coating with two process methods. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128219. [Google Scholar] [CrossRef]
- Nkeuwa, W.N.; Riedl, B.; Landry, V. Transparent UV-cured clay/UV-based nanocomposite coatings on wood substrates: Surface roughness and effect of relative humidity on optical properties. J. Coat. Technol. Res. 2017, 14, 555–569. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liu, S.; Huang, E.; Shen, X.; Wang, Z.; Li, S.; Jin, C. Superhydrophobic and antibacterial wood enabled by polydopamine-assisted decoration of copper nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125145. [Google Scholar] [CrossRef]
- Qian, L.; Long, L.; Xu, J.F. Surface modification of nano-titanium and its effect on the antibacterial property of waterborne wood coating. In Key Engineering Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2014; pp. 88–93. [Google Scholar]
- Gaylarde, C.; Morton, L.; Loh, K.; Shirakawa, M. Biodeterioration of external architectural paint films—A review. Int. Biodeterior. Biodegrad. 2011, 65, 1189–1198. [Google Scholar] [CrossRef]
- Cheng, L.; Ren, S.; Lu, X. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Calovi, M.; Coroneo, V.; Palanti, S.; Rossi, S. Colloidal silver as innovative multifunctional pigment: The effect of Ag concentration on the durability and biocidal activity of wood paints. Prog. Org. Coat. 2023, 175, 107354. [Google Scholar] [CrossRef]
- Wiemann, M.C. Characteristics and availability of commercially important woods. In Wood Handbook: Wood as an Engineering Material; United States Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010. [Google Scholar]
- Yan, X.; Chang, Y.; Qian, X. Effect of the concentration of pigment slurry on the film performances of waterborne wood coatings. Coatings 2019, 9, 635. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Wang, L.; Qian, X. Influence of thermochromic pigment powder on properties of waterborne primer film for Chinese fir. Coatings 2019, 9, 742. [Google Scholar] [CrossRef] [Green Version]
- Kaestner, D.; Petutschnigg, A.; Schnabel, T.; Illy, A.; Taylor, A. Influence of wood surface color on the performance of luminescent pigments. For. Prod. J. 2016, 66, 211–213. [Google Scholar] [CrossRef]
- Reinprecht, L.; Panek, M. Effect of pigments in paints on the natural and accelerated ageing of spruce wood surfaces. Acta Fac. Xylologiae 2013, 55, 71–84. [Google Scholar]
- Reinprecht, L.; Pánek, M. Effects of wood roughness, light pigments, and water repellent on the color stability of painted spruce subjected to natural and accelerated weathering. BioResources 2015, 10, 7203–7219. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-M.; Du, H.; Wang, W.-H.; Wang, Q.-W. Property changes of wood-fiber/HDPE composites colored by iron oxide pigments after accelerated UV weathering. J. For. Res. 2010, 21, 59–62. [Google Scholar] [CrossRef]
- Binoj, J.; Raj, R.E.; Daniel, B. Comprehensive characterization of industrially discarded fruit fiber, Tamarindus indica L. as a potential eco-friendly bio-reinforcement for polymer composite. J. Clean. Prod. 2017, 142, 1321–1331. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Sanjay, M.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and properties of natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
- Mustapha, R.; Rahmat, A.R.; Abdul Majid, R.; Mustapha, S.N.H. Vegetable oil-based epoxy resins and their composites with bio-based hardener: A short review. Polym. Plast. Technol. Mater. 2019, 58, 1311–1326. [Google Scholar] [CrossRef]
- Richard, S.; Rajadurai, J.S.; Manikandan, V. Influence of particle size and particle loading on mechanical and dielectric properties of biochar particulate-reinforced polymer nanocomposites. J. Polym. Anal. Charact. 2016, 21, 462–477. [Google Scholar] [CrossRef]
- Sansonetti, E.; Cīrule, D.; Kuka, E.; Andersone, I.; Andersons, B. Investigation of Linseed Oil Based Wood Coatings: Effect of Artificial Weathering. In Key Engineering Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2019; pp. 223–227. [Google Scholar]
- Calovi, M.; Rossi, S. From wood waste to wood protection: New application of black bio renewable water-based dispersions as pigment for bio-based wood paint. Prog. Org. Coat. 2023, 180, 107577. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Zhang, Y.; Wang, H. Microbial dyeing for inoculation and pigment used in wood processing: Opportunities and challenges. Dyes Pigm. 2021, 186, 109021. [Google Scholar] [CrossRef]
- Vega Gutierrez, S.M.; Stone, D.W.; He, R.; Vega Gutierrez, P.T.; Walsh, Z.M.; Robinson, S.C. Potential Use of the Pigments from Scytalidium cuboideum and Chlorociboria aeruginosa to Prevent ‘Greying’ Decking and Other Outdoor Wood Products. Coatings 2021, 11, 511. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Impact of High Concentrations of Cellulose Fibers on the Morphology, Durability and Protective Properties of Wood Paint. Coatings 2023, 13, 721. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Christaki, E.; Bonos, E.; Florou-Paneri, P. Innovative microalgae pigments as functional ingredients in nutrition. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–243. [Google Scholar]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Wan, D.; Wu, Q.; Kuča, K. Spirulina. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 959–974. [Google Scholar]
- De Morais, M.G.; de Morais, E.G.; Silva Vaz, B.D.; Goncalves, C.F.; Lisboa, C.; Vieira Costa, J.A. Nanoencapsulation of the bioactive compounds of Spirulina with a microalgal biopolymer coating. J. Nanosci. Nanotechnol. 2016, 16, 81–91. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem. 2019, 82, 189–198. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Synergistic contribution of bio-based additives in wood paint: The combined effect of pigment deriving from spirulina and multifunctional filler based on carnauba wax. Prog. Org. Coat. 2023, 182, 107713. [Google Scholar] [CrossRef]
- ASTM G154:16; Standard Practice for Operating Fluorescent Ultraviolet (UV) Lamp Apparatus for Exposure of Nonmetallic Materials. ASTM International: West Conshohocken, PA, USA, 2016; pp. 1–10.
- UNI 9429:2022; Finiture del Legno e dei Mobili—Determinazione della Resistenza delle Superfici Agli Sbalzi di Temperatura. UNI—Ente Nazionale Italiano di Unificazione: Milan, Italy, 2022; pp. 1–11.
- GB/T1733-93GB/T1733-93; Determination of Resistance to Water of Films. Standardization Administration of the People’s Republic of China: Beijing, China, 1993; pp. 1–11.
- EN927-5; Paints and Varnishes—Coating Materials and Coating Systems for Exterior Wood—Part 5: Assessment of the Liquid Water Permeability. European Standard: Brussels, Belgium, 2005; pp. 1–18.
- ISO 11998:2006; Paints and Varnishes: Determination of Wet-Scrub Resistance and Cleanability of Coatings. BSI British Standards: London, UK, 2006; pp. 1–11.
- ASTM-E308-18; Standard Practice for Computing the Colors of Objectives by Using the CIE System. ASTM International: West Conshohocken, PA, USA, 2018; pp. 1–45.
- Mokrzycki, W.; Tatol, M. Colour difference Δ E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Calovi, M.; Russo, F.; Rossi, S. Synergic behavior of graphene-based filler and thermochromic pigments in cataphoretic coatings. Prog. Org. Coat. 2021, 150, 105978. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Olive pit powder as multifunctional pigment for waterborne paint: Influence of the bio-based filler on the aesthetics, durability and mechanical features of the polymer matrix. Ind. Crops Prod. 2023, 194, 116326. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Ghavidel, A.; Hosseinpourpia, R.; Gelbrich, J.; Bak, M.; Sandu, I. Microstructural and chemical characteristics of archaeological white elm (Ulmus laevis P.) and poplar (Populus spp.). Appl. Sci. 2021, 11, 10271. [Google Scholar] [CrossRef]
- Ghavidel, A.; Bak, M.; Hofmann, T.; Vasilache, V.; Sandu, I. Evaluation of some wood-water relations and chemometric characteristics of recent oak and archaeological oak wood (Quercus robur) with archaeometric value. J. Cult. Herit. 2021, 51, 21–28. [Google Scholar] [CrossRef]
- Gu, Y.; Bian, H.; Wei, L.; Wang, R. Enhancement of hydrotropic fractionation of poplar wood using autohydrolysis and disk refining pretreatment: Morphology and overall chemical characterization. Polymers 2019, 11, 685. [Google Scholar] [CrossRef] [Green Version]
- Bian, H.; Li, G.; Jiao, L.; Yu, Z.; Dai, H. Enzyme-assisted mechanical fibrillation of bleached spruce kraft pulp for producing well-dispersed and uniform-sized cellulose nanofibrils. Bioresources 2016, 11, 10483–10496. [Google Scholar] [CrossRef] [Green Version]
- Lazzari, M.; Chiantore, O. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- Balanuca, B.; Stan, R.; Hanganu, A.; Iovu, H. Novel linseed oil-based monomers: Synthesis and characterization. Sci. Bull. B Chem. Mater. Sci. UPB 2014, 76, 129–140. [Google Scholar]
- Schmitt, T.; Rosi, F.; Mosconi, E.; Shull, K.; Fantacci, S.; Miliani, C.; Gray, K. New insights into the deterioration of TiO2 based oil paints: The effects of illumination conditions and surface interactions. Herit. Sci. 2022, 10, 99. [Google Scholar] [CrossRef]
- Rajagopal, S.; Murthy, S.; Mohanty, P. Effect of ultraviolet-B radiation on intact cells of the cyanobacterium Spirulina platensis: Characterization of the alterations in the thylakoid membranes. J. Photochem. Photobiol. B Biol. 2000, 54, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Kumar, A.; Tyagi, M.; Hader, D. Ultraviolet-B-induced destruction of phycobiliproteins in cyanobacteria. Physiol. Mol. Biol. Plants 2005, 11, 313. [Google Scholar]
- Antelo, F.S.; Costa, J.A.; Kalil, S.J. Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochem. Eng. J. 2008, 41, 43–47. [Google Scholar] [CrossRef]
- Böcker, L.; Ortmann, S.; Surber, J.; Leeb, E.; Reineke, K.; Mathys, A. Biphasic short time heat degradation of the blue microalgae protein phycocyanin from Arthrospira platensis. Innov. Food Sci. Emerg. Technol. 2019, 52, 116–121. [Google Scholar] [CrossRef]
- Patel, A.; Pawar, R.; Mishra, S.; Sonawane, S.; Ghosh, P. Kinetic studies on thermal denaturation of C-phycocyanin. Indian J. Biochem. Biophys. 2004, 41, 254–257. [Google Scholar] [PubMed]
- Aoki, J.; Sasaki, D.; Asayama, M. Development of a method for phycocyanin recovery from filamentous cyanobacteria and evaluation of its stability and antioxidant capacity. BMC Biotechnol. 2021, 21, 40. [Google Scholar] [CrossRef]
- Yan, X.; Qian, X.; Chang, Y.; Lu, R.; Miyakoshi, T. The effect of glass fiber powder on the properties of waterborne coatings with thermochromic ink on a Chinese Fir surface. Polymers 2019, 11, 1733. [Google Scholar] [CrossRef] [Green Version]
- GB/T11186.3-90; Method of Measurement of Coating Color. Part III: Calculation of Chromatic Aberration. Standardization Administration of the People’s Republic of China: Beijing, China, 1990; pp. 1–12.
- Yuan, B.; Li, Z.; Shan, H.; Dashnyam, B.; Xu, X.; McClements, D.J.; Zhang, B.; Tan, M.; Wang, Z.; Cao, C. A review of recent strategies to improve the physical stability of phycocyanin. Curr. Res. Food Sci. 2022, 5, 2329–2337. [Google Scholar] [CrossRef]
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical degradation of phycocyanin and means to improve its stability: A short review. J. Pharm. Anal. 2022, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Liang, Z.-P.; Chang, X.-Y.; Li, F.-T.; Wang, X.-Q.; Lian, X.-J. Progress of microencapsulated phycocyanin in food and pharma industries: A review. Molecules 2022, 27, 5854. [Google Scholar] [CrossRef] [PubMed]
- Kymäläinen, M.; Dömény, J.; Rautkari, L. Moisture Sorption of Wood Surfaces Modified by One-Sided Carbonization as an Alternative to Traditional Façade Coatings. Coatings 2022, 12, 1273. [Google Scholar] [CrossRef]












| Sample Nomenclature | Spirulina Granules (wt%) |
|---|---|
| S0.0 | 0.0 |
| S0.2 | 0.2 |
| S1.0 | 1.0 |
| S5.0 | 5.0 |
| Class | Fractures | Fading |
|---|---|---|
| 0 | Free of defects | No whitening |
| 1 | Cracks discernible with 4× optical setup | Light fading |
| 2 | Evident and easily observable fractures | High whitening |
| Level | Extent of Color Fading | ΔE |
|---|---|---|
| 0 | no alteration in color | ≤1.5 |
| 1 | extremely minimal discoloration | 1.6–3.0 |
| 2 | minor change in color | 3.1–6.0 |
| 3 | noticeable discoloration | 6.1–9.0 |
| 4 | significant change in color | 9.1–12.0 |
| 5 | total discoloration | >12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calovi, M.; Rossi, S. Comparative Analysis of the Advantages and Disadvantages of Utilizing Spirulina-Derived Pigment as a Bio-Based Colorant for Wood Impregnator. Coatings 2023, 13, 1158. https://doi.org/10.3390/coatings13071158
Calovi M, Rossi S. Comparative Analysis of the Advantages and Disadvantages of Utilizing Spirulina-Derived Pigment as a Bio-Based Colorant for Wood Impregnator. Coatings. 2023; 13(7):1158. https://doi.org/10.3390/coatings13071158
Chicago/Turabian StyleCalovi, Massimo, and Stefano Rossi. 2023. "Comparative Analysis of the Advantages and Disadvantages of Utilizing Spirulina-Derived Pigment as a Bio-Based Colorant for Wood Impregnator" Coatings 13, no. 7: 1158. https://doi.org/10.3390/coatings13071158







