Microstructure and Corrosion Behavior of the Modified Layers Grown In Situ by Plasma Nitriding Technology on the Surface of Zr Metal
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Influence of Nitriding Current on Temperature
3.2. Phase, Composition and Microstructure
3.3. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, J.; Xu, F.; Peng, Q.; Yang, Z.; Li, Z.; Qiu, S. Research on the existence and stability of interfacial tetragonal zirconia formed on zirconium alloys. J. Nucl. Mater. 2020, 528, 151846. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, H.; Chai, L.; Zhao, X.; Ma, P.; Zhang, R.; Wang, P. Assessment of microstructure, mechanical and tribological properties of Zr-4 alloy after pre-oxidation and ion irradiation. Appl. Surf. Sci. 2023, 610, 155444. [Google Scholar] [CrossRef]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Purandare, Y.P.; Ehiasarian, A.P.; Hovsepian, P.E. Structure and properties of ZrN coatings deposited by high power impulse magnetron sputtering technology. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 011004. [Google Scholar] [CrossRef]
- Maksakova, O.V.; Webster, R.F.; Tilley, R.D.; Ivashchenko, V.I.; Postolnyi, B.O.; Bondar, O.V.; Takeda, Y.; Rogoz, V.M.; Sakenova, R.E.; Zukowski, P.V.; et al. Nanoscale architecture of (CrN/ZrN)/(Cr/Zr) nanocomposite coatings: Microstructure, composition, mechanical properties and first-principles calculations. J. Alloys Compd. 2020, 831, 154808. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Sobol’, O.V.; Beresnev, V.M.; Pshyk, O. Phase Composition, Thermal Stability, Physical and Mechanical Properties of Superhard on Base Zr-Ti-Si-N Nanocomposite Coatings. Ceram. Eng. Sci. Proc. 2010, 31, 127–138. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, C.; Huo, K.; Tang, G.; Tian, X.; Chu, P.K. Corrosion behavior of ZrN/Zr coated biomedical AZ91 magnesium alloy. Surf. Coat. Technol. 2009, 203, 2554–2557. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.-h.; Cao, F.; Yang, D.-w. Barrier capability of Zr–N films with different density and crystalline structure in Cu/Si contact systems. Mater. Lett. 2008, 62, 3761–3763. [Google Scholar] [CrossRef]
- Spillmann, H.; Willmott, P.R.; Morstein, M.; Uggowitzer, P.J. ZrN, ZrxAlyN and ZrxGayN thin films—Novel materials for hard coatings grown using pulsed laser deposition. Appl. Phys. A 2014, 73, 441–450. [Google Scholar] [CrossRef]
- Qi, Z.B.; Wu, Z.T.; Liang, H.F.; Zhang, D.F.; Wang, J.H.; Wang, Z.C. In situ and ex situ studies of microstructure evolution during high-temperature oxidation of ZrN hard coating. Scr. Mater. 2015, 97, 9–12. [Google Scholar] [CrossRef]
- Egeland, G.W.; Wheeler, K.; Peralta, P.; McClellan, K.J.; Maloy, S.A.; Bond, G.M. Plastic deformation in zirconium nitride observed by nanoindentation and TEM. J. Nucl. Mater. 2011, 416, 253–261. [Google Scholar] [CrossRef]
- Craciun, D.; Vasile, B.S.; Lambers, E.; Makino, H.; Craciun, V. Microstructural investigations of 800 keV Ar ions irradiated nanocrystalline ZrN thin films. Surf. Eng. 2019, 36, 326–333. [Google Scholar] [CrossRef]
- Kim, W.; Jung, K.S.; Choi, B.H.; Kwon, H.S.; Lee, N.J.; Han, J.G.; Guseva, M.I.; Atamanov, M.V. Corrosion behavior of nitrogen-implanted Zircaloy. Surf. Coat. Technol. 1995, 76–77, 595–599. [Google Scholar] [CrossRef]
- Singh, A.; Kuppusami, P.; Khan, S.; Sudha, C.; Thirumurugesan, R.; Ramaseshan, R.; Divakar, R.; Mohandas, E.; Dash, S. Influence of nitrogen flow rate on microstructural and nanomechanical properties of Zr–N thin films prepared by pulsed DC magnetron sputtering. Appl. Surf. Sci. 2013, 280, 117–123. [Google Scholar] [CrossRef]
- Benia, H.M.; Guemmaz, M.; Schmerber, G.; Mosser, A.; Parlebas, J.-C. Investigation on non-stoichiometric zirconium nitrides. Appl. Surf. Sci. 2002, 200, 231–238. [Google Scholar] [CrossRef]
- Chou, W.-J.; Yu, G.-P.; Huang, J.-H. Bias effect of ion-plated zirconium nitride film on Si(100). Thin Solid Film. 2002, 405, 162–169. [Google Scholar] [CrossRef]
- Huang, J.-H.; Yang, H.-C.; Guo, X.-J.; Yu, G.-P. Effect of film thickness on the structure and properties of nanocrystalline ZrN thin films produced by ion plating. Surf. Coat. Technol. 2005, 195, 204–213. [Google Scholar] [CrossRef]
- Larijani, M.M.; Norouzian, S.; Afzalzadeh, R.; Balashabadi, P.; Dibaji, H. Effects of post annealing on micro and nanostructural properties of ZrN films prepared by ion beam sputtering technique on SS304. Surf. Coat. Technol. 2009, 203, 2486–2489. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, K.; Hu, Y.; Ling, Y.; Wang, D.; Peng, H.; Xie, Z.; Yang, R.; Zhang, Z. Microstructure and Young’s modulus of ZrN thin film prepared by dual ion beam sputtering deposition. Surf. Coat. Technol. 2019, 374, 997–1005. [Google Scholar] [CrossRef]
- Larijani, M.M.; Tabrizi, N.; Norouzian, S.; Jafari, A.; Lahouti, S.; Haj Hosseini, H.; Afshari, N. Structural and mechanical properties of ZrN films prepared by ion beam sputtering with varying N2/Ar ratio and substrate temperature. Vacuum 2006, 81, 550–555. [Google Scholar] [CrossRef]
- Kiahosseini, S.R.; Mojtahedzadeh Larijani, M. Effects of nitrogen gas ratio on the structural and corrosion properties of ZrN thin films grown on biodegradable magnesium alloy by ion-beam sputtering. Appl. Phys. A 2017, 123, 759. [Google Scholar] [CrossRef]
- Larijani, M.M.; Elmi, M.; Yari, M.; Ghoranneviss, M.; Balashabadi, P.; Shokouhy, A. Nitrogen effect on corrosion resistance of ion beam sputtered nanocrystalline zirconium nitride films. Surf. Coat. Technol. 2009, 203, 2591–2594. [Google Scholar] [CrossRef]
- Menghani, J.; Pai, K.B.; Totlani, M.K. Corrosion and wear behaviour of ZrN thin films. Tribol.—Mater. Surf. Interfaces 2013, 5, 122–128. [Google Scholar] [CrossRef]
- Huang, J.-H.; Hsu, C.-Y.; Chen, S.-S.; Yu, G.-P. Effect of substrate bias on the structure and properties of ion-plated ZrN on Si and stainless steel substrates. Mater. Chem. Phys. 2002, 77, 14–21. [Google Scholar] [CrossRef]
- Kavitha, A.; Subramanian, N.S.; Loganathan, S.; Kannan, R. Deposition and characterization of zirconium nitride (ZrN) thin films by reactive magnetron sputtering with linear gas ion source and bias voltage. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; pp. 1705–1707. [Google Scholar]
- Ju, H.; Yu, D.; Yu, L.; Ding, N.; Xu, J.; Zhang, X.; Zheng, Y.; Yang, L.; He, X. The influence of Ag contents on the microstructure, mechanical and tribological properties of ZrN-Ag films. Vacuum 2018, 148, 54–61. [Google Scholar] [CrossRef]
- Zhou, Q.G.; Bai, X.D.; Xue, X.Y.; Ling, Y.H.; Chen, X.W.; Xu, J.; Wang, D.R. The influence of Y ion implantation on the oxidation behaviour of ZrN coating. Vacuum 2004, 76, 517–521. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zu, X.T.; Qiu, S.Y.; Li, C.; Ma, W.G.; Huang, X.Q. Surface characteristics and oxidation behavior of nitrogen ion-implanted Zr–Sn–Nb alloy. Surf. Coat. Technol. 2006, 200, 5631–5635. [Google Scholar] [CrossRef]
- Han, J.G.; Lee, J.S.; Choi, B.H.; Kim, W.; Tang, G. Wear and fretting wear behaviour of ion-implanted Zircaloy-4. Surf. Coat. Technol. 1996, 83, 307–312. [Google Scholar] [CrossRef]
- Han, J.G.; Lee, J.S.; Kim, W.; Sun, D.S.; Chung, K.H. Zirconium oxide formation and surface hardening behavior by nitrogen implantation under oxygen atmosphere in Zircaloy-4. Surf. Coat. Technol. 1997, 97, 492–498. [Google Scholar] [CrossRef]
- Han, J.G.; Choi, B.H.; Kim, W.; Guseva, M. Tribological hard layer synthesis by duplex ion beam treatment. Nucl. Instrum. Methods Phys. Res. B 1997, 121, 79–83. [Google Scholar] [CrossRef]
- Reger, N.C.; Balla, V.K.; Das, M.; Bhargava, A.K. Wear and corrosion properties of in-situ grown zirconium nitride layers for implant applications. Surf. Coat. Technol. 2018, 334, 357–364. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Dong, D.; Wang, J.; Zhou, L. Improving wear resistance of Zr-2.5Nb alloy by formation of microtextured nitride layer produced via laser surface texturing/plasma nitriding technology. Surf. Interfaces 2020, 20, 100638. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Q.; Song, T.; Chen, B.; Liu, S.; Li, Q. Corrosion behavior and wear resistance of Zr-2.5Nb alloy after thermal oxy-nitriding treatment. Surf. Coat. Technol. 2022, 446, 128756. [Google Scholar] [CrossRef]
- Pei, C.; Deng, L.; Liu, H.; He, Z.; Xiang, C.; Zhang, S.; Sun, D. Corrosion inhibition behaviors of ZrNx thin films with varied N vacancy concentration. Vacuum 2019, 162, 28–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Cheng, L.; Zhao, Y.; Peng, S.; Jiang, S. Exploring cavitation erosion resistance of ZrN nanocrystalline coating prepared by double-cathode glow discharge plasma technique. Vacuum 2020, 182, 109697. [Google Scholar] [CrossRef]
- Ghemras, I.; Abdelli-Messaci, S.; Alili, B.; González-Elipe, A.R.; Rico, V.J.; Izerrouken, M.; Khereddine, A.Y.; Hadj-Larbi, F. Characterizing the physicochemical and mechanical properties of ZrN thin films deposited on Zr substrates by pulsed laser technique. Eur. Phys. J. Appl. Phys. 2021, 95, 10301. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zu, X.T.; Zhu, S.; Wang, L.M. Phase formation and corrosion behavior of nitrogen implanted Zr–Sn–Nb alloy in alkaline environment. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2006, 246, 345–350. [Google Scholar] [CrossRef]
- Lee, S.J.; Kwon, H.S.; Kim, W.; Choi, B.H. Effects of compositional and structural change on the corrosion behaviour of nitrogen implanted Zircaloy-4. Mater. Sci. Eng. A 1999, 263, 23–31. [Google Scholar] [CrossRef]
- Tang, G.; Choi, B.H.; Kim, W.; Jung, K.-S.; Kwon, H.S.; Lee, S.J.; Lee, J.H.; Song, T.Y.; Shon, D.H.; Han, J.G. The characteristics of surface oxidation and corrosion resistance of nitrogen implanted zircaloy-4. Surf. Coat. Technol. 1997, 89, 252–257. [Google Scholar] [CrossRef]
- Khan, I.A.; Hassan, M.; Ahmad, R.; Qayyum, A.; Murtaza, G.; Zakaullah, M.; Rawat, R.S. Nitridation of zirconium using energetic ions from plasma focus device. Thin Solid Film. 2008, 516, 8255–8263. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Liu, Y.; Sun, W.; Dong, C. Preparation and characterization of CuN-based ternary alloy films using Cr or Zr for stabilizing N. J. Mater. Res. 2017, 32, 1333–1342. [Google Scholar] [CrossRef]
- Roman, D.; Bernardi, J.; Amorim, C.L.G.d.; de Souza, F.S.; Spinelli, A.; Giacomelli, C.; Figueroa, C.A.; Baumvol, I.J.R.; Basso, R.L.O. Effect of deposition temperature on microstructure and corrosion resistance of ZrN thin films deposited by DC reactive magnetron sputtering. Mater. Chem. Phys. 2011, 130, 147–153. [Google Scholar] [CrossRef]
- Han, K.-C.; Lin, G.-Q.; Dong, C.; Tai, K.-P.; Jiang, X. Influence of Nitrogen Vacancy Concentration on Mechanical and Electrical Properties of Rocksalt Zirconium Nitride Films. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, M.; Isotani, S.; Sucasaire, W.; Kuratani, N.; Ogata, K. X-ray photoelectron spectroscopy analysis of zirconium nitride-like films prepared on Si(100) substrates by ion beam assisted deposition. Surf. Coat. Technol. 2008, 202, 3129–3135. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, Z. Phase-dependent and defect-driven d0 ferromagnetism in undoped ZrO2 thin films. RSC Adv. 2015, 5, 3636–3641. [Google Scholar] [CrossRef]
- Baer, D.R.; Artyushkova, K.; Brundle, C.R.; Castle, J.E.; Engelhard, M.H.; Gaskell, K.J.; Grant, J.T.; Haasch, R.T.; Linford, M.R.; Powell, C.J.; et al. Practical Guides for X-ray Photoelectron Spectroscopy (XPS): First Steps in planning, conducting and reporting XPS measurements. J. Vac. Sci. Technol. A 2019, 37, 031401. [Google Scholar] [CrossRef]
- Tao, N.R.; Wang, Z.B.; Tong, W.P.; Sui, M.L.; Lu, J.; Lu, K. An investigation of surface nanocrystallization mechanism in Fe induced by surface mechanical attrition treatment. Acta Mater. 2002, 50, 4603–4616. [Google Scholar] [CrossRef]
- Subramanian, B.; Swaminathan, V.; Jayachandran, M. Microstructural, tribological and electrochemical corrosion studies on reactive DC magnetron sputtered zirconium nitride films with Zr interlayer on steel. Met. Mater. Int. 2012, 18, 957–964. [Google Scholar] [CrossRef]
- Feng, L.; Xingsheng, S.; Zhaoxing, M.Y. Microstructure, Hardness and Corrosion Resistance of ZrN Films Prepared by Inductively Coupled Plasma Enhanced RF Magnetron Sputtering. Plasma Sci. Technol. 2008, 10, 170–175. [Google Scholar] [CrossRef]
- Yi, P.; Zhu, L.; Dong, C.; Xiao, K. Corrosion and interfacial contact resistance of 316L stainless steel coated with magnetron sputtered ZrN and TiN in the simulated cathodic environment of a proton-exchange membrane fuel cell. Surf. Coat. Technol. 2019, 363, 198–202. [Google Scholar] [CrossRef]
- Ningshen, S.; Gupta, R.K.; Kamal, S.; Chawla, V.; Chandra, R.; Mudali, U.K. Corrosion study of ZrN deposited on 304L stainless steel. Surf. Eng. 2013, 29, 264–270. [Google Scholar] [CrossRef]
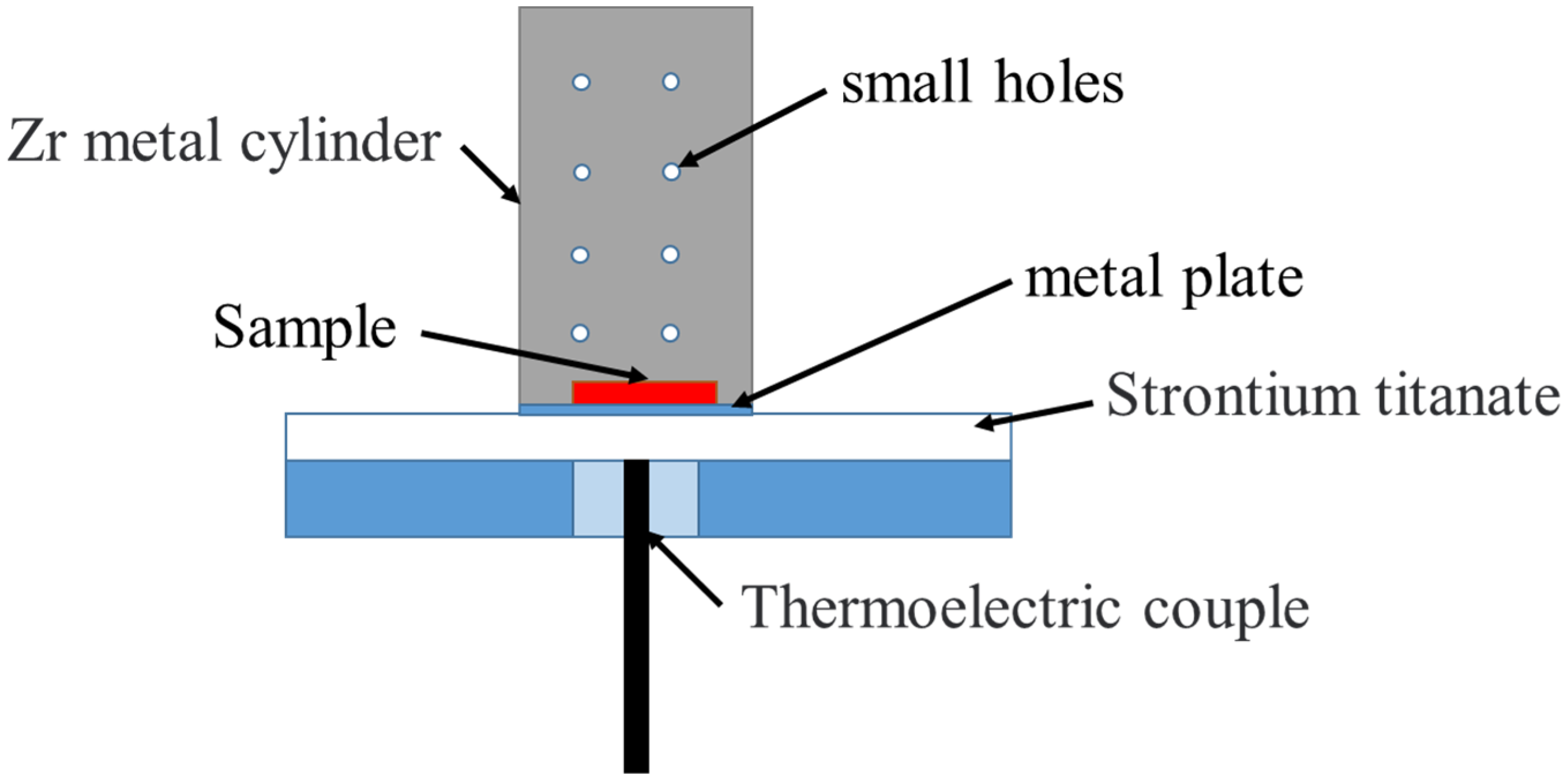

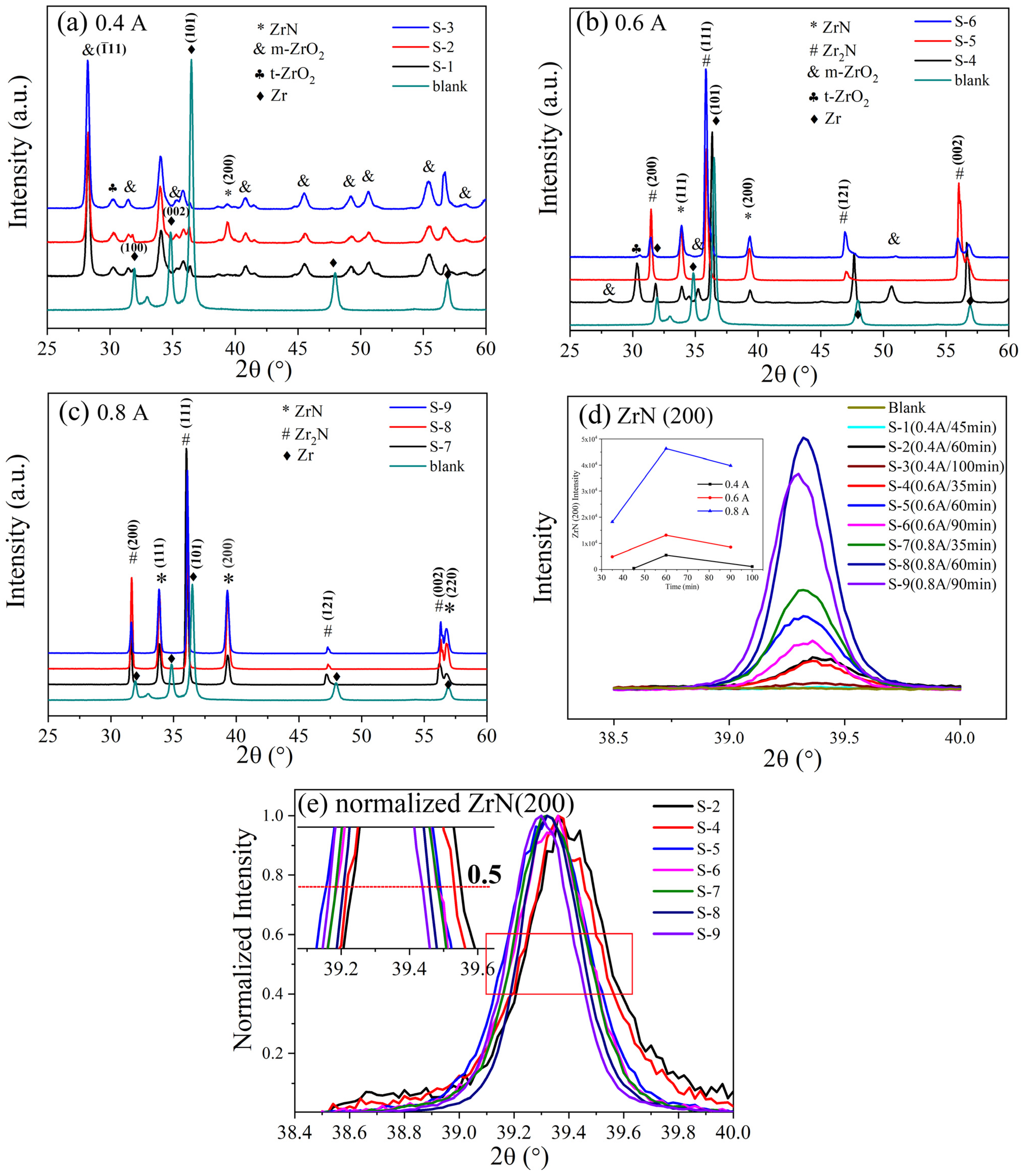

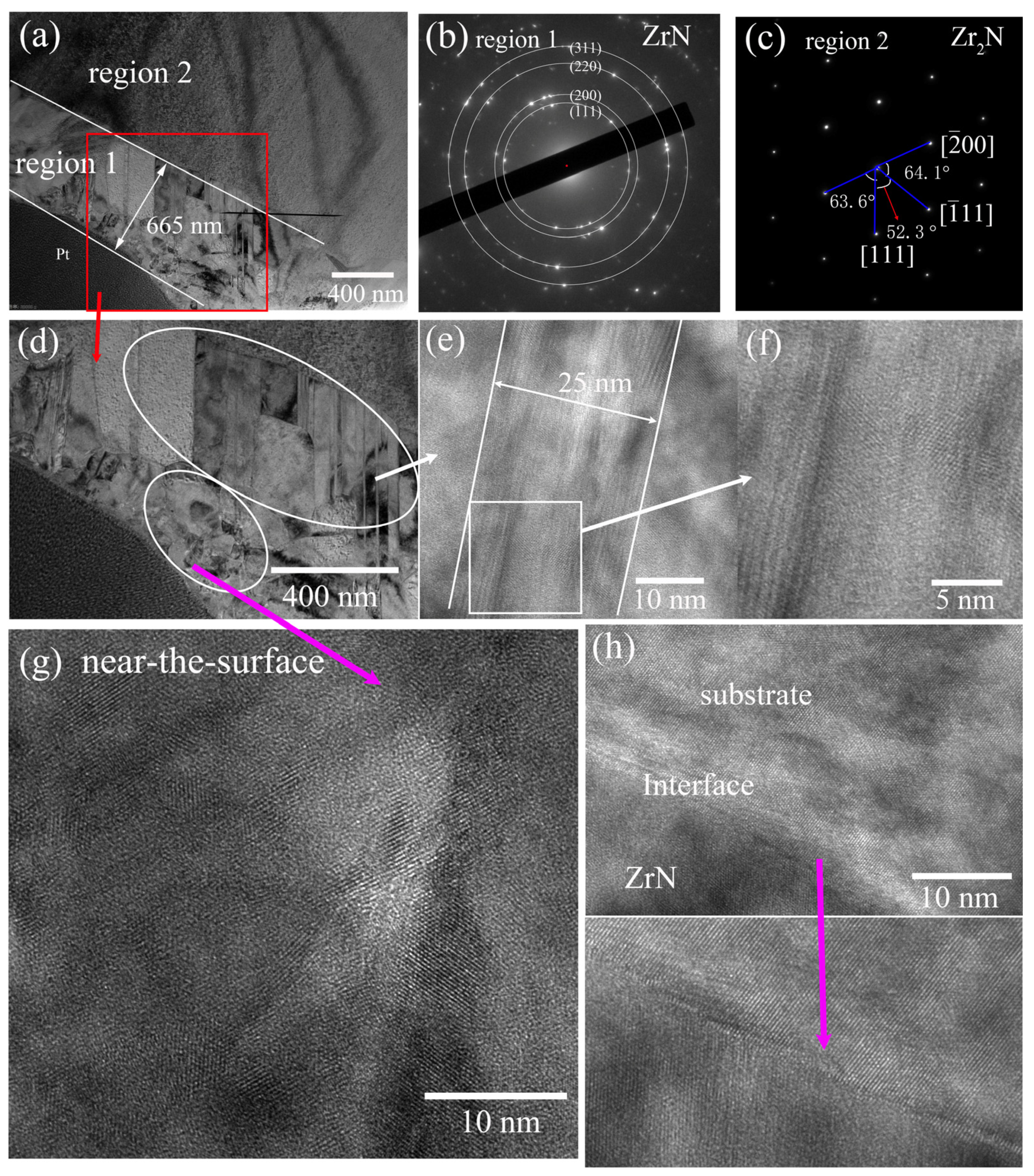
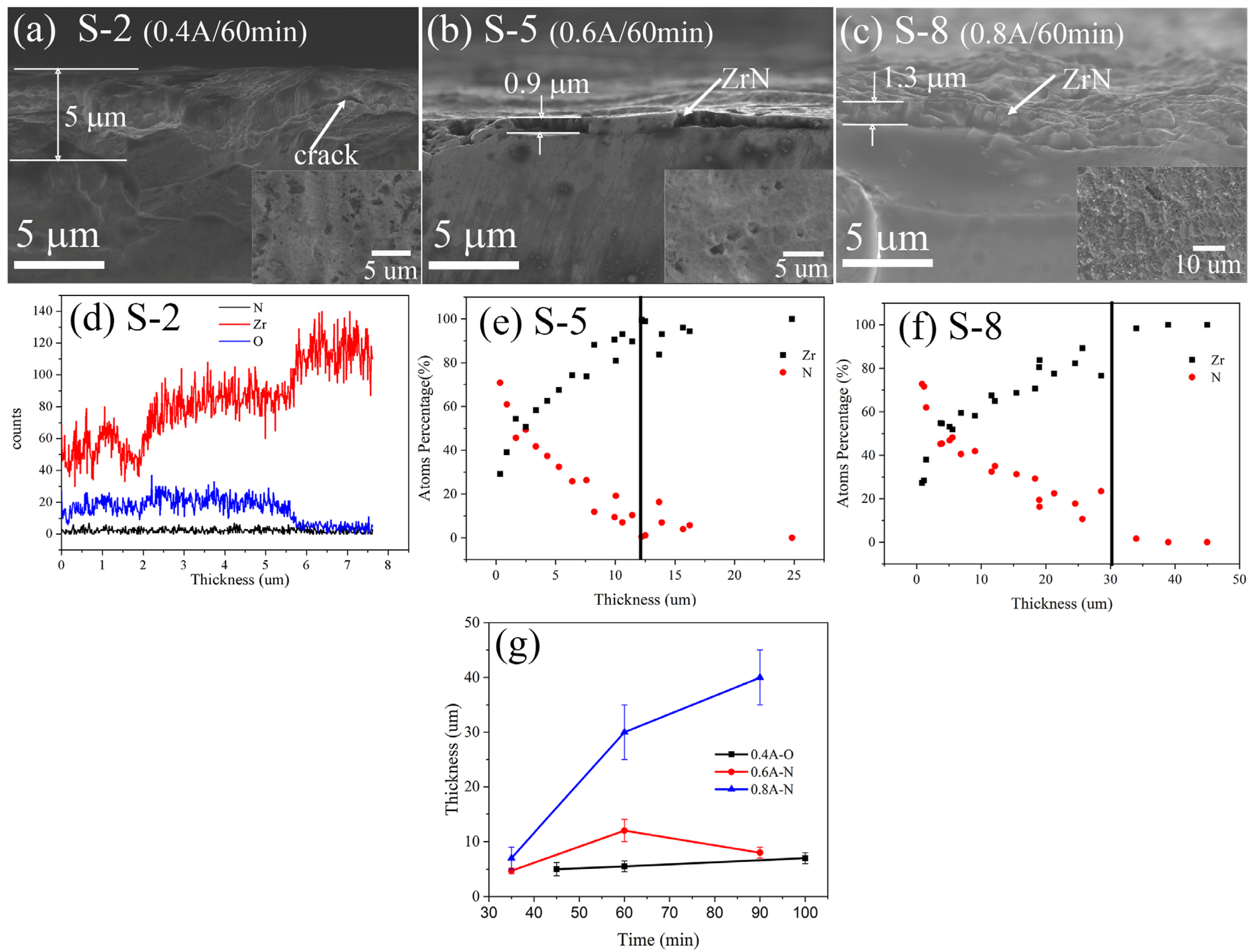

| Number of Samples | Nitriding Current (A) | Nitriding Time (min) | Duty Cycle (%) |
|---|---|---|---|
| S-1 | 0.4 | 45 | ~20 |
| S-2 | 0.4 | 60 | ~21 |
| S-3 | 0.4 | 100 | ~21 |
| S-4 | 0.6 | 35 | ~25 |
| S-5 | 0.6 | 60 | ~36 |
| S-6 | 0.6 | 90 | ~27 |
| S-7 | 0.8 | 35 | ~43 |
| S-8 | 0.8 | 60 | ~44 |
| S-9 | 0.8 | 90 | ~43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Zhang, W.; Zhu, K.; Hu, Y.; Ma, X.; Zhang, Q.; Song, L. Microstructure and Corrosion Behavior of the Modified Layers Grown In Situ by Plasma Nitriding Technology on the Surface of Zr Metal. Coatings 2023, 13, 1160. https://doi.org/10.3390/coatings13071160
Zhu F, Zhang W, Zhu K, Hu Y, Ma X, Zhang Q, Song L. Microstructure and Corrosion Behavior of the Modified Layers Grown In Situ by Plasma Nitriding Technology on the Surface of Zr Metal. Coatings. 2023; 13(7):1160. https://doi.org/10.3390/coatings13071160
Chicago/Turabian StyleZhu, Fei, Wenqing Zhang, Kangwei Zhu, Yin Hu, Xianfeng Ma, Qiang Zhang, and Ligang Song. 2023. "Microstructure and Corrosion Behavior of the Modified Layers Grown In Situ by Plasma Nitriding Technology on the Surface of Zr Metal" Coatings 13, no. 7: 1160. https://doi.org/10.3390/coatings13071160





