Damage Mechanism of Trivalent Chromium Coatings under Tensile Stress
Abstract
:1. Introduction
- -
- Developing and implementing new processes involving a “product/process” technological breakthrough. This is particularly the case for dry processes such as physical/chemical vapor deposition (PVD-CVD) and high-velocity oxygen fuel (HVOF).
- -
- The implementation of processes without a tangible technological breakthrough. This is the case for electrolytic processes, which are intended to meet the need to treat parts with complex geometries, mainly where an internal surface is difficult to access.
2. Materials and Methods
2.1. Coating Deposition
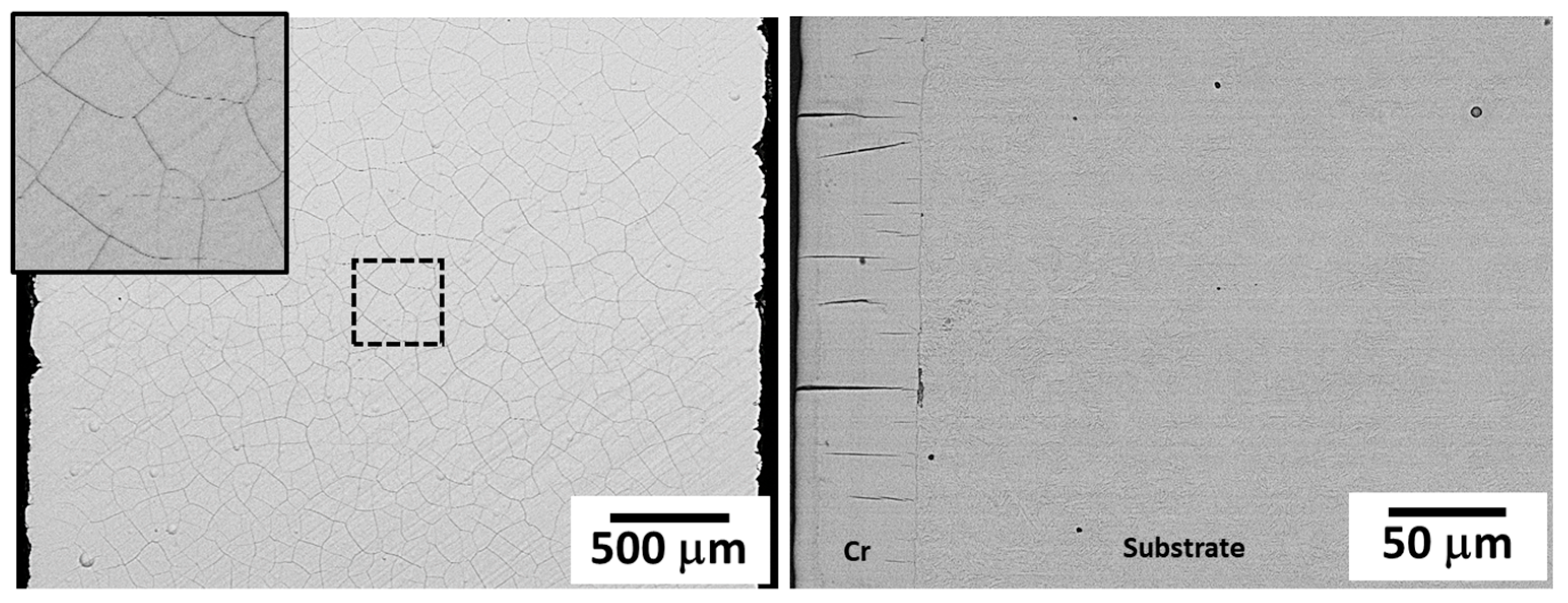
2.2. Coating Characterisation
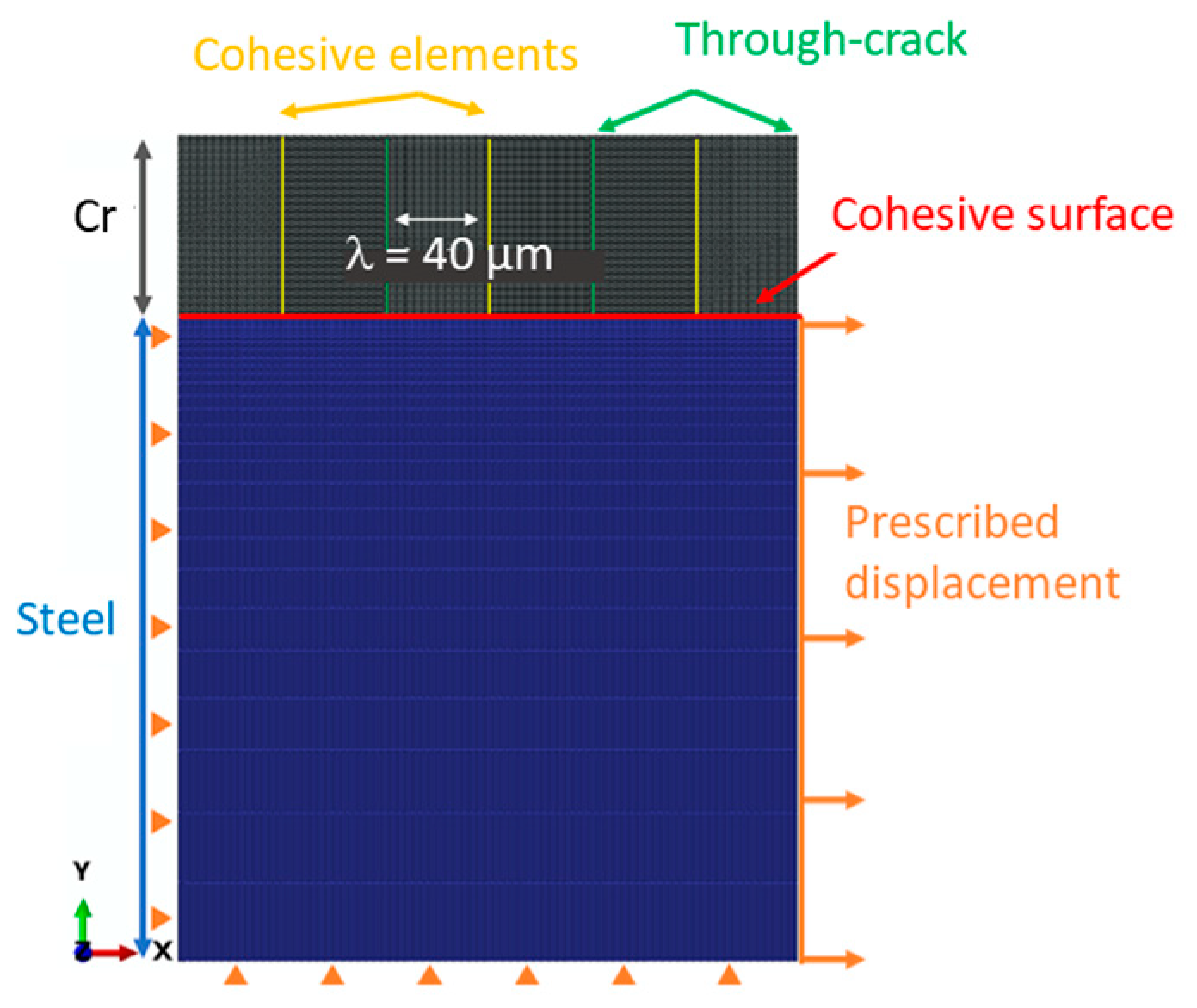
2.3. Methodology for the Numerical Simulation of the Tensile Adhesion Test
3. Results
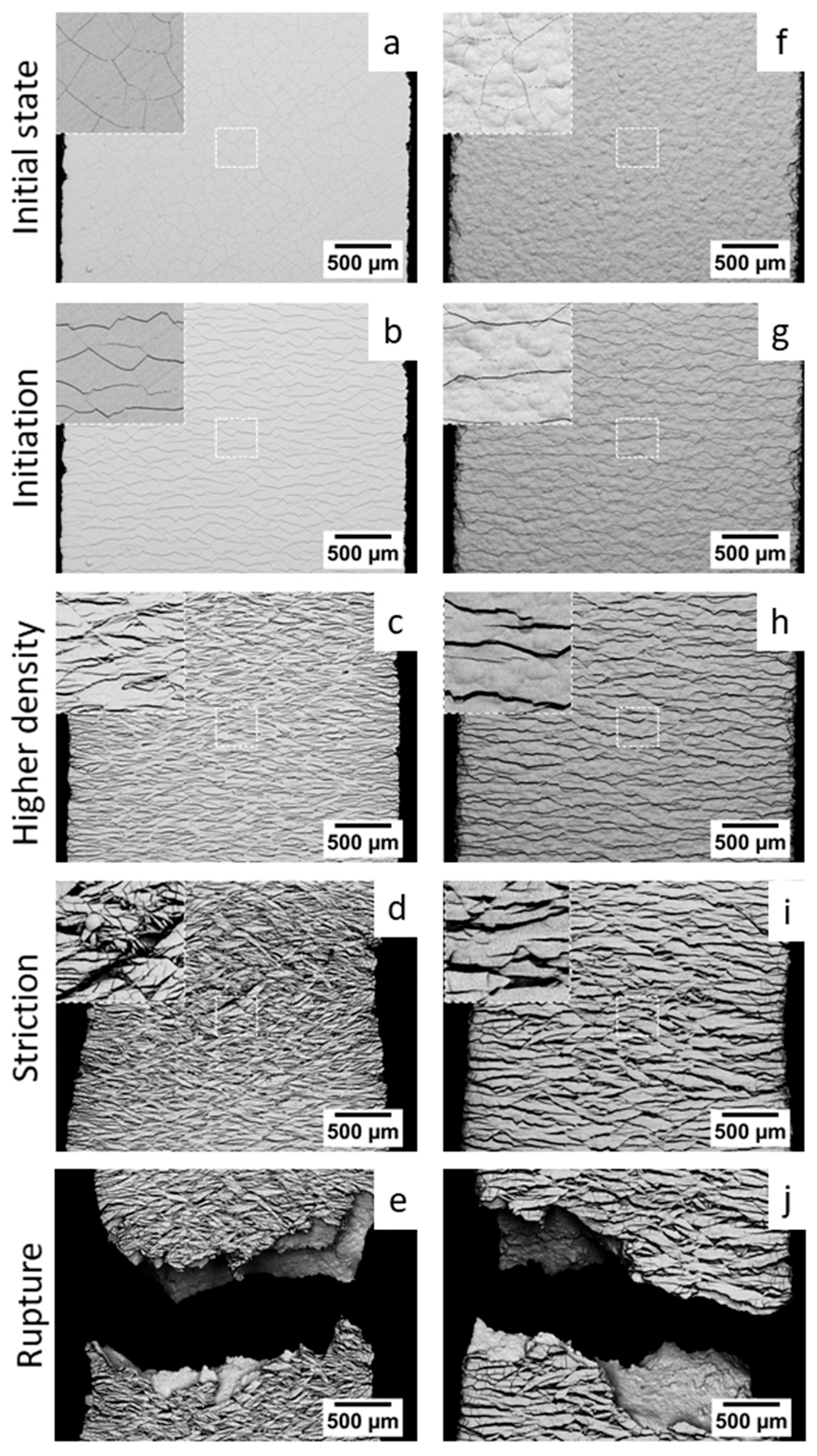
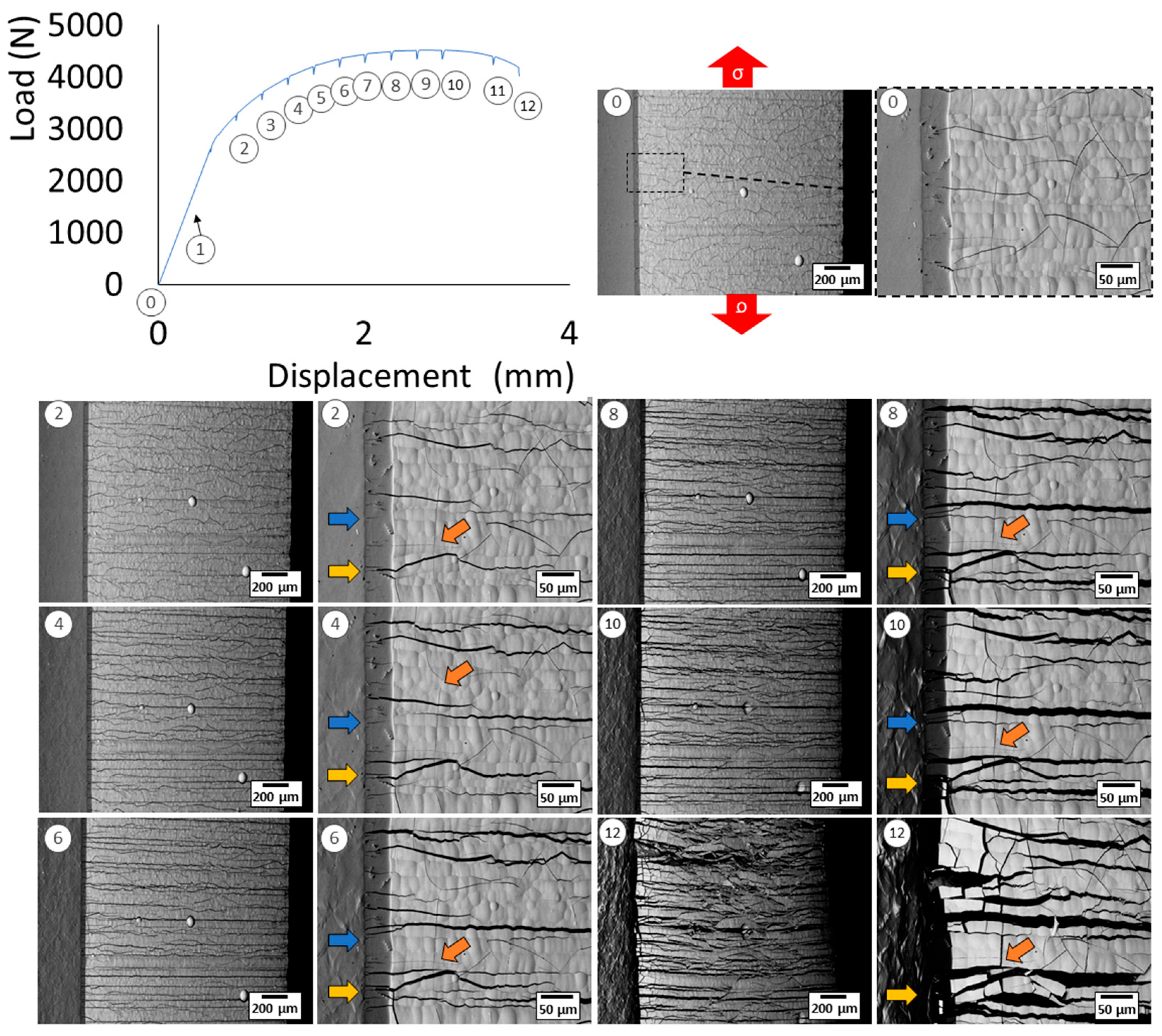
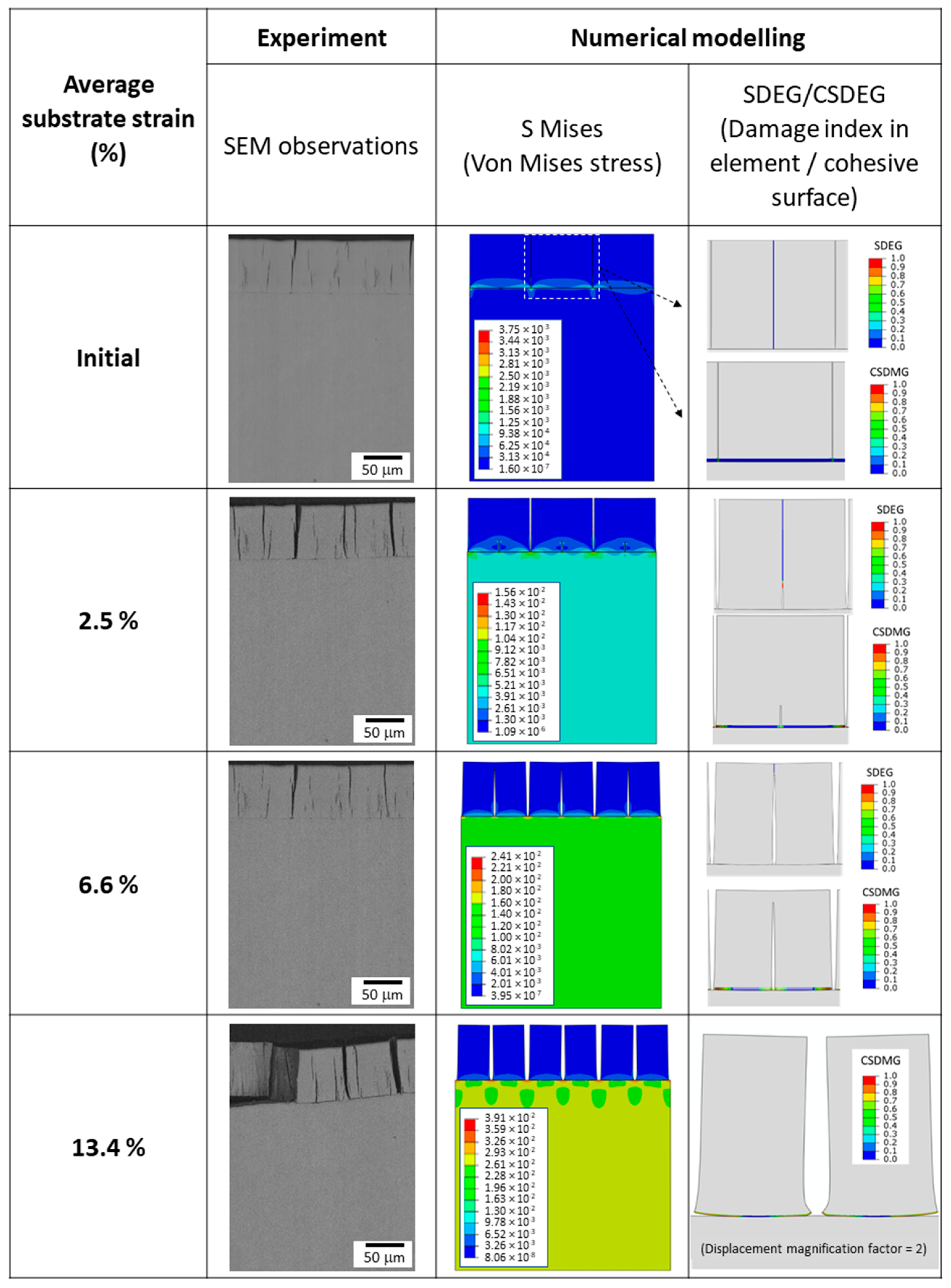
3.1. Damage Mechanism of Hexavalent Chromium Reference Coatings
- -
- The initial state, corresponding to the density of cracks in the coating before loading;
- -
- Initiation, corresponding to the appearance of additional cracks perpendicular to the tensile axis;
- -
- Crack propagation, corresponding to the evolution of the crack rate during the test (always perpendicular to the tensile axis);
- -
- Stabilisation, which indicates the end of the crack density evolution.
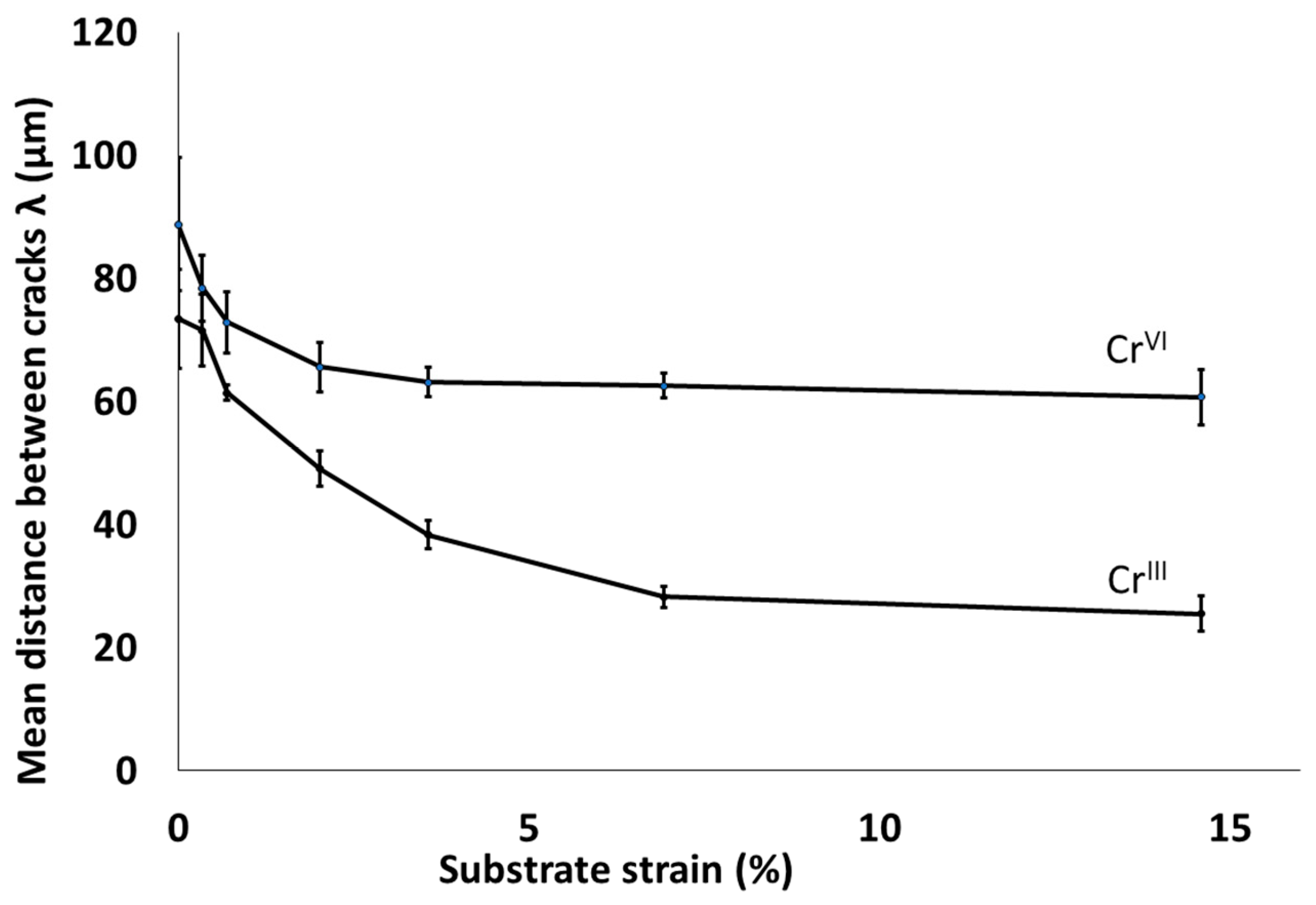
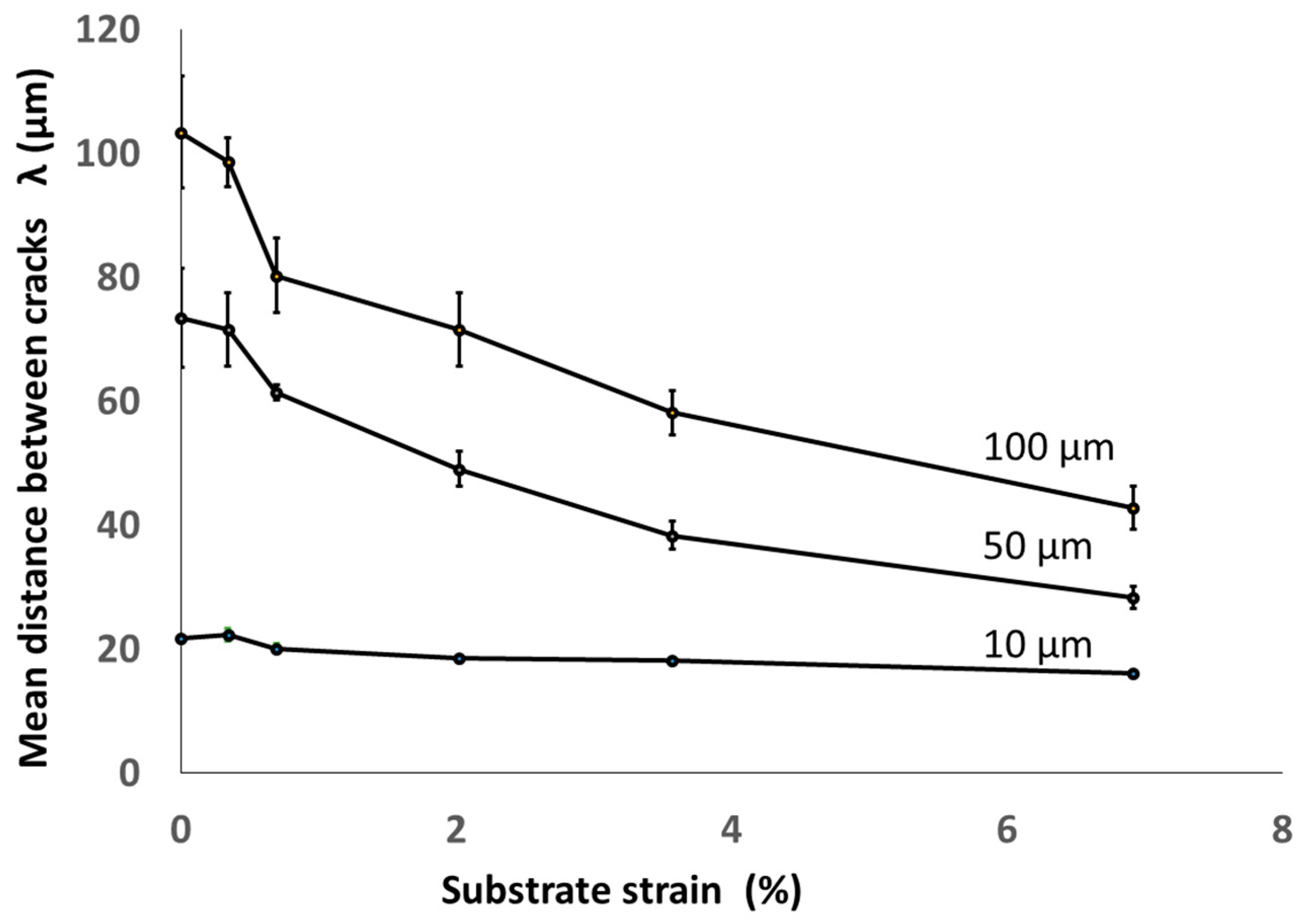
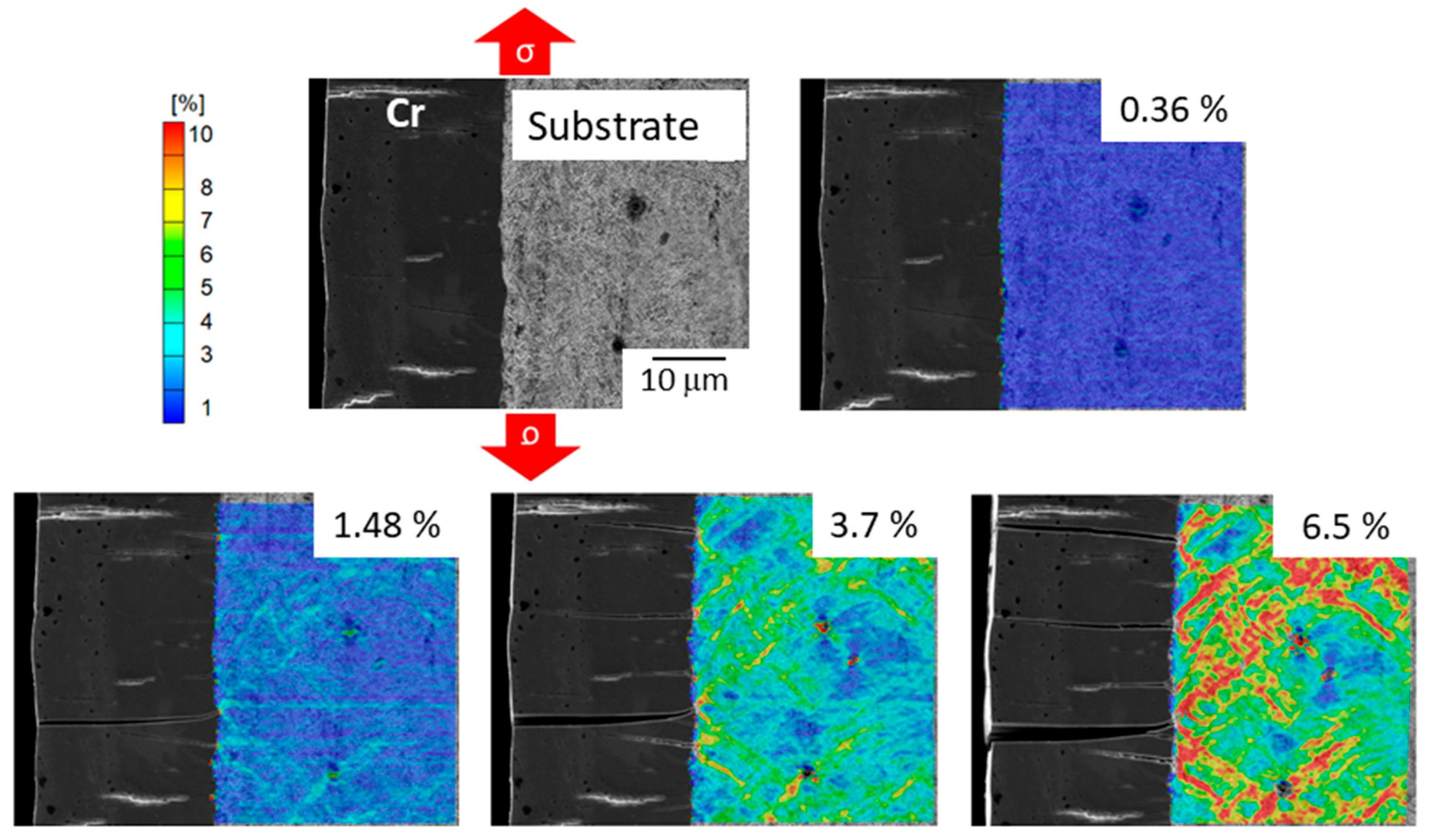
3.2. Damage Mechanism of Trivalent Chromium Coatings
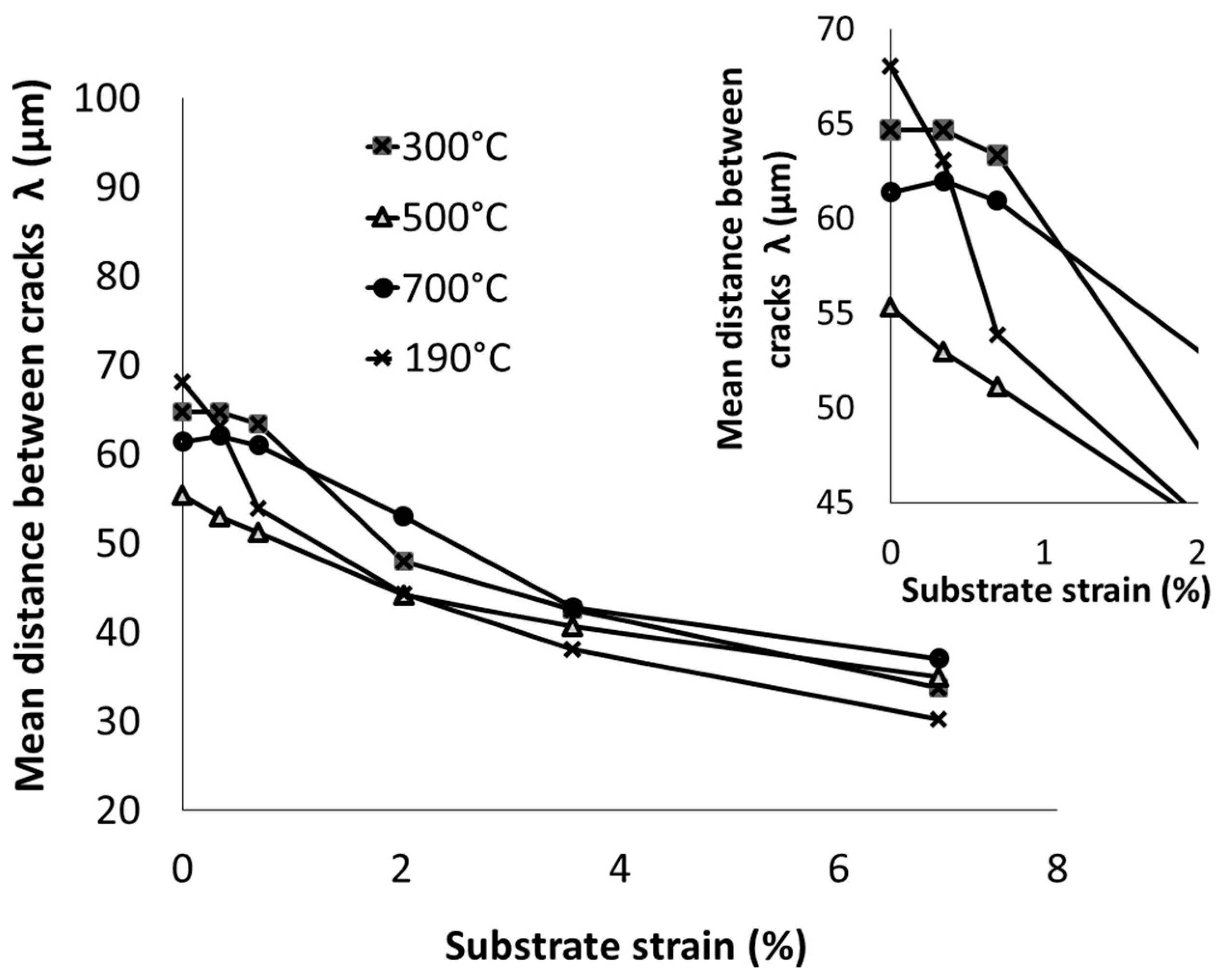
3.3. Damage Mechanism of Trivalent Chromium Coatings Post-Heat Treatment
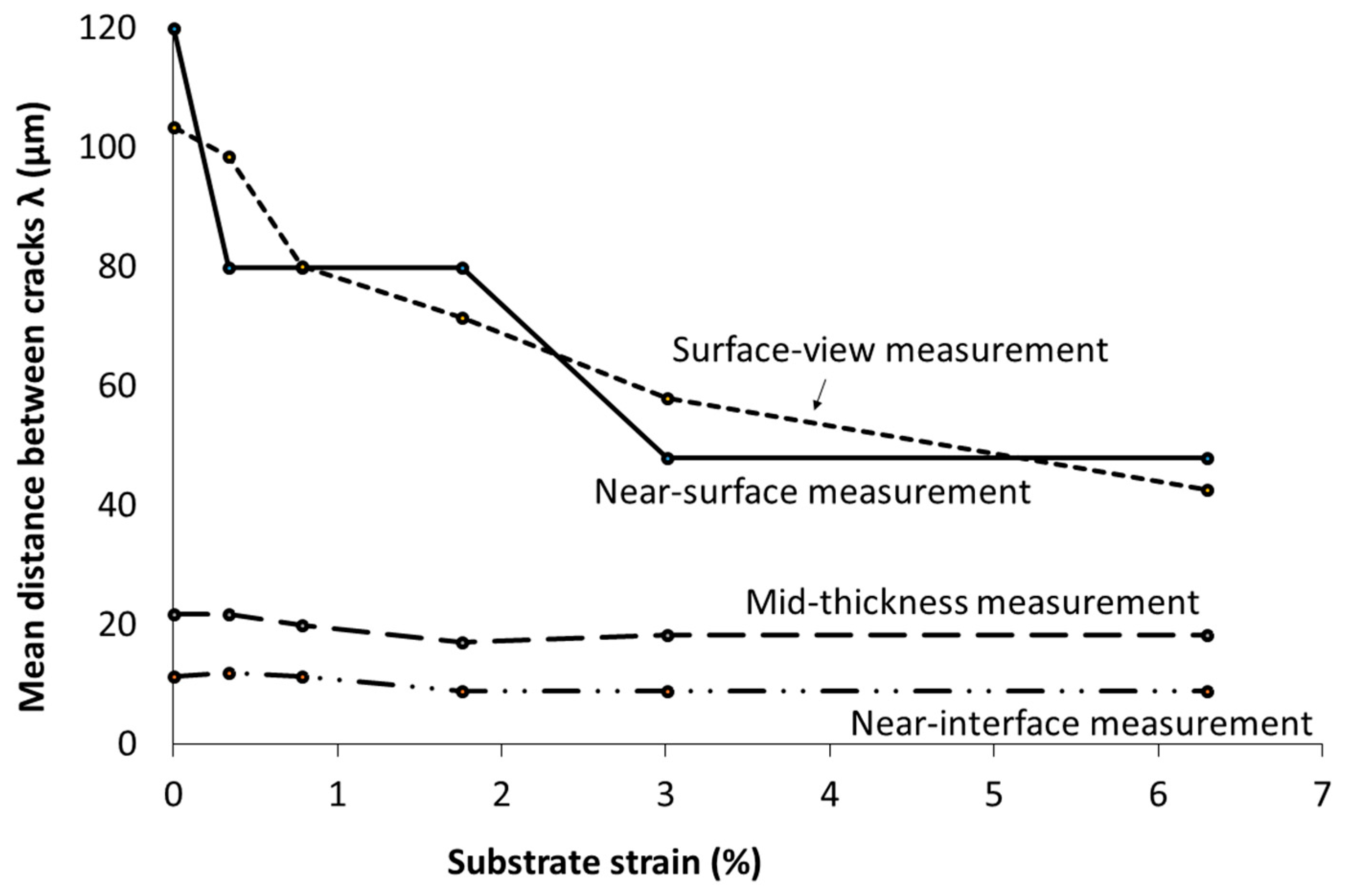
3.4. Study of the Evolution of Cracking in the Thickness of Coatings
3.5. Estimation of a Numerical Adhesion Criterion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohan, S.; Saravanan, G.; Renganathan, N.G. Comparison of Chromium Coatings and Electrochemical Behaviour with Direct Current and Pulse Current Deposition in Trivalent Chromium Formate Urea Bath as Alternative to Conventional Cr Coatings. Surf. Eng. 2011, 27, 775–783. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Danilov, F.I. Chromium Electroplating from Trivalent Chromium Baths as an Environmentally Friendly Alternative to Hazardous Hexavalent Chromium Baths: Comparative Study on Advantages and Disadvantages. Clean Techn Env. Policy 2014, 16, 1201–1206. [Google Scholar] [CrossRef]
- Wang, S.; Ma, C.; Walsh, F.C. Alternative Tribological Coatings to Electrodeposited Hard Chromium: A Critical Review. Trans. IMF 2020, 98, 173–185. [Google Scholar] [CrossRef]
- Hordienko, V.O.; Protsenko, V.S.; Kwon, S.C.; Lee, J.-Y.; Danilov, F.I. Electrodeposition of Chromium Coatings from Sulfate–Carbamide Electrolytes Based on Cr(III) Compounds. Mater. Sci. 2011, 46, 647–652. [Google Scholar] [CrossRef]
- Mohan, S.; Vijayakumar, J.; Saravanan, G. Influence of CH3SO3H and AlCl3 in Direct and Pulse Current Electrodeposition of Trivalent Chromium. Surf. Eng. 2009, 25, 570–576. [Google Scholar] [CrossRef]
- Protsenko, V.; Danilov, F. Kinetics and Mechanism of Chromium Electrodeposition from Formate and Oxalate Solutions of Cr(III) Compounds. Electrochim. Acta 2009, 54, 5666–5672. [Google Scholar] [CrossRef]
- Danilov, F.I.; Protsenko, V.S.; Gordiienko, V.O.; Kwon, S.C.; Lee, J.Y.; Kim, M. Nanocrystalline Hard Chromium Electrodeposition from Trivalent Chromium Bath Containing Carbamide and Formic Acid: Structure, Composition, Electrochemical Corrosion Behavior, Hardness and Wear Characteristics of Deposits. Appl. Surf. Sci. 2011, 257, 8048–8053. [Google Scholar] [CrossRef]
- Hoare, J.P. A Voltammetric Study of the Reduction of Chromic Acid on Bright Platinum. J. Electrochem. Soc. 1983, 130, 1475. [Google Scholar] [CrossRef]
- Hoare, J.P. On the Mechanisms of Chromium Electrodeposition. J. Electrochem. Soc. 1979, 126, 190–199. [Google Scholar] [CrossRef]
- Lu, C.-E.; Pu, N.-W.; Hou, K.-H.; Tseng, C.-C.; Ger, M.-D. The Effect of Formic Acid Concentration on the Conductivity and Corrosion Resistance of Chromium Carbide Coatings Electroplated with Trivalent Chromium. Appl. Surf. Sci. 2013, 282, 544–551. [Google Scholar] [CrossRef]
- Zeng, Z.; Sun, Y.; Zhang, J. The Electrochemical Reduction Mechanism of Trivalent Chromium in the Presence of Formic Acid. Electrochem. Commun. 2009, 11, 331–334. [Google Scholar] [CrossRef]
- Del Pianta, D.; Frayret, J.; Gleyzes, C.; Cugnet, C.; Dupin, J.C.; Le Hecho, I. Determination of the Chromium(III) Reduction Mechanism during Chromium Electroplating. Electrochim. Acta 2018, 284, 234–241. [Google Scholar] [CrossRef]
- Baral, A.; Engelken, R. Modeling, Optimization, and Comparative Analysis of Trivalent Chromium Electrodeposition from Aqueous Glycine and Formic Acid Baths. J. Electrochem. Soc. 2005, 152, C504. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Gordiienko, V.O.; Danilov, F.I.; Kwon, S.C. Thick Chromium Electrodeposition from Trivalent Chromium Bath Containing Carbamide and Formic Acid. Metal Finishing 2011, 109, 33–37. [Google Scholar] [CrossRef]
- Ferreira, E.S.C.; Pereira, C.M.; Silva, A.F. Electrochemical Studies of Metallic Chromium Electrodeposition from a Cr(III) Bath. J. Electroanal. Chem. 2013, 707, 52–58. [Google Scholar] [CrossRef]
- Ghaziof, S.; Golozar, M.A.; Raeissi, K. Characterization of As-Deposited and Annealed Cr–C Alloy Coatings Produced from a Trivalent Chromium Bath. J. Alloys Compd. 2010, 496, 164–168. [Google Scholar] [CrossRef]
- Ghaziof, S.; Raeissi, K.; Golozar, M.A. Improving the Corrosion Performance of Cr–C Amorphous Coatings on Steel Substrate by Modifying the Steel Surface Preparation. Surf. Coat. Technol. 2010, 205, 2174–2183. [Google Scholar] [CrossRef]
- Kwon, S.C.; Kim, M.; Park, S.U.; Kim, D.Y.; Kim, D.; Nam, K.S.; Choi, Y. Characterization of Intermediate Cr-C Layer Fabricated by Electrodeposition in Hexavalent and Trivalent Chromium Baths. Surf. Coat. Technol. 2004, 183, 151–156. [Google Scholar] [CrossRef]
- Nam, K.-S.; Lee, K.-H.; Kwon, S.-C.; Lee, D.Y.; Song, Y.-S. Improved Wear and Corrosion Resistance of Chromium(III) Plating by Oxynitrocarburising and Steam Oxidation. Mater. Lett. 2004, 58, 3540–3544. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, L.; Liang, A.; Chen, L.; Zhang, J. Fabrication of a Nanocrystalline Cr–C Layer with Excellent Anti-Wear Performance. Mater. Lett. 2007, 61, 4107–4109. [Google Scholar] [CrossRef]
- Bikulčius, G.; Češūnienė, A.; Matijošius, T.; Selskienė, A.; Pakštas, V. Investigation of the Properties of Cr Coatings Deposited in an Improved Cr(III) Electrolyte. Chemija 2019, 30, 69–77. [Google Scholar] [CrossRef]
- Tsai, R.; Wu, S. Phase Stability of Chromium Plating from Chromic Acid Electrolyte Containing Formic Acid. J. Electrochem. Soc. 1990, 137, 3057–3060. [Google Scholar] [CrossRef]
- Liang, A.; Liu, Q.; Zhang, B.; Ni, L.; Zhang, J. Preparation of Crystalline Chromium Coating on Cu Substrate Directly by DC Electrodepositing from Wholly Environmentally Acceptable Cr(III) Electrolyte. Mater. Lett. 2014, 119, 131–134. [Google Scholar] [CrossRef]
- Mahdavi, S.; Allahkaram, S.R.; Heidarzadeh, A. Characteristics and Properties of Cr Coatings Electrodeposited from Cr(III) Baths. Mater. Res. Express 2018, 6, 026403. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, J. Electrodeposition and Tribological Behavior of Amorphous Chromium-Alumina Composite Coatings. Surf. Coat. Technol. 2008, 202, 2725–2730. [Google Scholar] [CrossRef]
- Gabe, D.R. The Role of Hydrogen in Metal Electrodeposition Processes. J. Appl. Electrochem. 1997, 27, 908–915. [Google Scholar] [CrossRef]
- Bouaziz, H.; Brinza, O.; Haddar, N.; Gasperini, M.; Feki, M. In-Situ SEM Study of Crack Initiation, Propagation and Interfacial Debonding of Ni-P Coating during Tensile Tests: Heat Treatment Effect. Mater. Charact. 2017, 123, 106–114. [Google Scholar] [CrossRef]
- Chen, B.F.; Hwang, J.; Chen, I.F.; Yu, G.P.; Huang, J.-H. A Tensile-Film-Cracking Model for Evaluating Interfacial Shear Strength of Elastic Film on Ductile Substrate. Surf. Coat. Technol. 2000, 126, 91–95. [Google Scholar] [CrossRef]
- Xie, C.; Tong, W. Cracking and Decohesion of a Thin Al2O3 Film on a Ductile Al–5%Mg Substrate. Acta Mater. 2005, 53, 477–485. [Google Scholar] [CrossRef]
- Ohmura, T.; Matsuoka, S. Evaluation of Mechanical Properties of Ceramic Coatings on a Metal Substrate. Surf. Coat. Technol. 2003, 169–170, 728–731. [Google Scholar] [CrossRef]
- Azevedo, J.C.S.; Campilho, R.D.S.G.; Da Silva, F.J.G.; Faneco, T.M.S.; Lopes, R.M. Cohesive Law Estimation of Adhesive Joints in Mode II Condition. Theor. Appl. Fract. Mech. 2015, 80, 143–154. [Google Scholar] [CrossRef]
- Campilho, R.D.S.G.; Banea, M.D.; Neto, J.A.B.P.; Da Silva, L.F.M. Modelling Adhesive Joints with Cohesive Zone Models: Effect of the Cohesive Law Shape of the Adhesive Layer. Int. J. Adhes. Adhes. 2013, 44, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Fukumasu, N.K.; Souza, R.M. Numerical Evaluation of Cohesive and Adhesive Failure Modes during the Indentation of Coated Systems with Compliant Substrates. Surf. Coat. Technol. 2014, 260, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Chao, Y.; Zhang, W.; Chao, Y.; Wu, C. Study on the Effect of Post Curing on the Mode II Fracture Energy of Structural Adhesive Using a Parameter Identification Approach. Int. J. Adhes. Adhes. 2019, 95, 102398. [Google Scholar] [CrossRef]
- Liao, L.; Huang, C.; Sawa, T. Effect of Adhesive Thickness, Adhesive Type and Scarf Angle on the Mechanical Properties of Scarf Adhesive Joints. Int. J. Solids Struct. 2013, 50, 4333–4340. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.V.M.; Akhavan-Safar, A.; Carbas, R.; Marques, E.A.S.; Goyal, R.; El-zein, M.; Da Silva, L.F.M. Numerical Analysis of Mixed-Mode Fatigue Crack Growth of Adhesive Joints Using CZM. Theor. Appl. Fract. Mech. 2020, 106, 102493. [Google Scholar] [CrossRef]
- Stuparu, F.A.; Apostol, D.A.; Constantinescu, D.M.; Picu, C.R.; Sandu, M.; Sorohan, S. Cohesive and XFEM Evaluation of Adhesive Failure for Dissimilar Single-Lap Joints. Procedia Struct. Integr. 2016, 2, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Watson, B.; Worswick, M.J.; Cronin, D.S. Quantification of Mixed Mode Loading and Bond Line Thickness on Adhesive Joint Strength Using Novel Test Specimen Geometry. Int. J. Adhes. Adhes. 2020, 102, 102682. [Google Scholar] [CrossRef]
- Jiang, J.; Zhai, H.; Gong, P.; Zhang, W.; He, X.; Ma, X.; Wang, B. In-Situ Study on the Tensile Behavior of Cr-Coated Zircaloy for Accident Tolerant Fuel Claddings. Surf. Coat. Technol. 2020, 394, 125747. [Google Scholar] [CrossRef]
- Dalverny, O.; Tongne, A.; Ho, C.; Alexis, J.; Châtel, S.; Faure, B. Numerical Simulation of Interfacial Delamination between SiO2 Thin Film and Polymeric Substrate. In Proceedings of the AIP Conference Proceedings 2196, Ischia, Italy, 12 December 2019; p. 020014. [Google Scholar]
- Fan, X.L.; Xu, R.; Zhang, W.X.; Wang, T.J. Effect of Periodic Surface Cracks on the Interfacial Fracture of Thermal Barrier Coating System. Appl. Surf. Sci. 2012, 258, 9816–9823. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, L.; Guo, J.W.; Zhou, Y.C.; Lu, C. Numerical Study on Interaction of Surface Cracking and Interfacial Delamination in Thermal Barrier Coatings under Tension. Appl. Surf. Sci. 2014, 315, 292–298. [Google Scholar] [CrossRef]
- Guillon, R.; Dalverny, O.; Fori, B.; Gazeau, C.; Alexis, J. Mechanical Behaviour of Hard Chromium Deposited from a Trivalent Chromium Bath. Coatings 2022, 12, 354. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, L.; Guo, J.W.; Zhou, Y.C.; Lu, C. Determination of Interfacial Adhesion Energies of Thermal Barrier Coatings by Compression Test Combined with a Cohesive Zone Finite Element Model. Int. J. Plast. 2015, 64, 76–87. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Qi, H.; Song, J.; Yang, X.; Shi, D. Experimental Study and Numerical Modeling of the Damage Evolution of Thermal Barrier Coating Systems under Tension. Sci. China Technol. Sci. 2018, 61, 1882–1888. [Google Scholar] [CrossRef]
- Benzeggagh, M.L.; Kenane, M. Measurement of Mixed-Mode Delamination Fracture Toughness of Unidirectional Glass/Epoxy Composites with Mixed-Mode Bending Apparatus. Compos. Sci. Technol. 1996, 56, 439–449. [Google Scholar] [CrossRef]
- Sebastiani, M.; Johanns, K.E.; Herbert, E.G.; Carassiti, F.; Pharr, G.M. A Novel Pillar Indentation Splitting Test for Measuring Fracture Toughness of Thin Ceramic Coatings. Philos. Mag. 2015, 95, 1928–1944. [Google Scholar] [CrossRef]
- Guillon, R.; Stéphan, C.; Balcaen, Y.; Josse, C.; Fori, B.; Dalverny, O.; Alexis, J. An Estimation of Local Residual Stresses in Amorphous and Crystallized Trivalent Chromium Coatings. Coatings 2023, 13, 124. [Google Scholar] [CrossRef]
- Guillon, R. Amélioration de L’adhérence de Revêtements Électrolytiques de Chrome: Étude Expérimentale et Numérique. Ph.D. Thesis, University of Toulouse, Toulouse, France, 2022. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillon, R.; Balcaen, Y.; Fori, B.; Gazeau, C.; Dalverny, O.; Alexis, J. Damage Mechanism of Trivalent Chromium Coatings under Tensile Stress. Coatings 2023, 13, 1194. https://doi.org/10.3390/coatings13071194
Guillon R, Balcaen Y, Fori B, Gazeau C, Dalverny O, Alexis J. Damage Mechanism of Trivalent Chromium Coatings under Tensile Stress. Coatings. 2023; 13(7):1194. https://doi.org/10.3390/coatings13071194
Chicago/Turabian StyleGuillon, Robin, Yannick Balcaen, Benoit Fori, Celine Gazeau, Olivier Dalverny, and Joel Alexis. 2023. "Damage Mechanism of Trivalent Chromium Coatings under Tensile Stress" Coatings 13, no. 7: 1194. https://doi.org/10.3390/coatings13071194




