Production and Characterization of Active Pectin Films with Olive or Guava Leaf Extract Used as Soluble Sachets for Chicken Stock Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
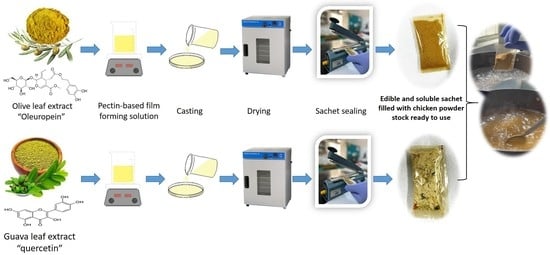
2.2. Preparation of Olive and Guava Leaves Extracts
2.3. Film Preparation with Olive and Guava Leaf Extracts
2.4. Film Characterization
2.5. Sachet Application Experiment
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Incorporation of OLE and GLE in Pectin-Based Films on Thickness and Opacity
3.2. Films’ Mechanical Properties
3.3. Antioxidant Activity
3.4. Water Content and Water Uptake
3.5. Effects of OLE and GLE on the Color of Pectin-Based Films
3.6. Permeability Properties
3.7. Film Application as a Soluble Sachet for Chicken Stock Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porta, R.; Sabbah, M.; Di Pierro, P. Bio-based materials for packaging. Int. J. Mol. Sci. 2022, 23, 3611. [Google Scholar] [CrossRef] [PubMed]
- Sing, N.; Ogunseitan, O.A.; Wong, M.H.; Tang, Y. Sustainable materials alternative to petrochemical plastics pollution: A review analysis. Sustain. Horiz. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Bauer, F.; Nielsen, T.D.; Nilsson, L.J.; Palm, E.; Ericsson, K.; Frane, A.; Cullen, J. Plastics and climate change—Breaking carbon lock-ins through three mitigation pathways. One Earth 2022, 5, 361–376. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2020; Plastics Europe: Brussels, Belgium, 2020. [Google Scholar]
- Dell’Olmo, E.; Gaglione, R.; Sabbah, M.; Schibeci, M.; Cesaro, A.; Di Girolamo, R.; Arciello, A. Host defense peptides identified in human apolipoprotein B as novel food biopreservatives and active coating components. Food Microbiol. 2021, 99, 103804. [Google Scholar] [CrossRef] [PubMed]
- Gerschenson, L.N.; Fissore, E.N.; Rojas, A.M.; Idrovo Encalada, A.M.; Zukowski, E.F.; Higuera Coelho, R.A. Pectins obtained by ultrasound from agroindustrial by-products. Food Hydrocoll. 2021, 118, 106799. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Rosseto, M.; Alessandretti, I.; de Oliveira, R.; Wohlmuth, D.A.R.; Menezes, J.F.; Loss, R.A.; Dettmer, A.; Pizzutti, I.R. Gelatin films from wastes: A review of production, characterization, and application trends in food preservation and agriculture. Food Res. Int. 2022, 162, 112114. [Google Scholar] [CrossRef]
- Yaseen, D.; Sabbah, M.; Al-Asmar, A.; Altamimi, M.; Famiglietti, M.; Giosafatto, C.V.L.; Mariniello, L. Functionality of films from Nigella sativa defatted seed cake proteins plasticized with grape juice: Use in wrapping sweet cherries. Coatings 2021, 11, 1383. [Google Scholar] [CrossRef]
- Villanueva, V.; Valdés, F.; Zúñiga, R.N.; Villamizar-Sarmiento, M.G.; Soto-Bustamante, E.; Romero-Hasler, P.; Riveros, A.L.; Tapia, J.; Lisoni, J.; Oyarzun-Ampuero, F.; et al. Development of biodegradable and vermicompostable films based on alginate and waste eggshells. Food Hydrocoll. 2023, 142, 108813. [Google Scholar] [CrossRef]
- Lahiri, A.; Daniel, S.; Kanthapazham, R.; Vanaraj, R.; Thambidurai, A.; Peter, L.S. A critical review on food waste management for the production of materials and biofuel. J. Hazard. Mater. Adv. 2023, 10, 100266. [Google Scholar] [CrossRef]
- Gerna, S.; D’Incecco, P.; Limbo, S.; Sindaco, M.; Pellegrino, L. Strategies for Exploiting Milk Protein Properties in Making Films and Coatings for Food Packaging: A Review. Foods 2023, 12, 1271. [Google Scholar] [CrossRef]
- Righetti, G.I.C.; Nasti, R.; Beretta, G.; Levi, M.; Turri, S.; Suriano, R. Unveiling the hidden properties of tomato peels: Cutin ester derivatives as bio-based plasticizers for polylactic acid. Polymers 2023, 15, 1848. [Google Scholar] [CrossRef]
- Cai, Z.; Haque, A.N.M.A.; Dhandapani, R.; Naebe, M. Sustainable Cotton Gin Waste/Polycaprolactone Bio-Plastic with Adjustable Biodegradation Rate: Scale-Up Production through Compression Moulding. Polymers 2023, 15, 1992. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Hejazi, S.; Zannini, D.; Giosafatto, C.V.L.; Di Pierro, P.; Cassese, E.; D’ambrosio, S.; Santagata, G.; Schiraldi, C.; Porta, R. Exploiting Potential Biotechnological Applications of Poly-γ-glutamic Acid Low Molecular Weight Fractions Obtained by Membrane-Based Ultra-Filtration. Polymers 2022, 14, 1190. [Google Scholar] [CrossRef]
- Avila, L.B.; Schnorr, C.; Silva, L.F.O.; Morais, M.M.; Moraes, C.C.; da Rosa, G.S.; Dotto, G.L.; Lima, É.C.; Naushad, M. Trends in Bioactive Multilayer Films: Perspectives in the Use of Polysaccharides, Proteins, and Carbohydrates with Natural Additives for Application in Food Packaging. Foods 2023, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Giosafatto, C.V.L.; Mirpoor, S.F.; Cammarota, M.; Hejazi, S.; Mariniello, L.; Schiraldi, C.; Porta, R. Sustainable exploitation of posidonia oceanica sea Balls (Egagropili): A Review. Int. J. Mol. Sci. 2023, 24, 7301. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Mariniello, L. Hydrocolloid-based coatings with nanoparticles and transglutaminase crosslinker as innovative strategy to produce healthier fried kobbah. Foods 2020, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Fusco, A.; Al-Asmar, A.; Mariniello, L. Microbial Transglutaminase as a tool to improve the features of hydrocolloid-based bioplastics. Int. J. Mol. Sci. 2020, 21, 3656. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Villalonga Santana, R.; Mariniello, L. Effect of mesoporous silica nanoparticles on the physicochemical properties of pectin packaging material for strawberry wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Góral, D.; Góral-Kowalczyk, M. Application of metal nanoparticles for production of self-sterilizing coatings. Coatings 2022, 12, 480. [Google Scholar] [CrossRef]
- Krivorotova, T.; Cirkovas, A.; Maciulyte, S.; Staneviciene, R.; Budriene, S.; Serviene, E.; Sereikaite, J. Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocoll. 2016, 54, 49–56. [Google Scholar] [CrossRef]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef] [Green Version]
- Sabbah, M.; Di Pierro, P.; Cammarota, M.; Dell’Olmo, E.; Arciello, A.; Porta, R. Development and properties of new chitosan-based films plasticized with spermidine and/or glycerol. Food Hydrocoll. 2019, 87, 245–252. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Sabbah, M.; Regalado-Gonzales, C.; Mariniello, L.; Kadivar, M.; Arabestani, A. Blend films of pectin and bitter vetch (Vicia ervilia) proteins: Properties and effect of transglutaminase. Innov. Food Sci. Emerg. Technol. 2016, 36, 245–251. [Google Scholar] [CrossRef]
- Sabbah, M.; Di Pierro, P.; Giosafatto, C.V.L.; Esposito, M.; Mariniello, L.; Regalado-Gonzales, C.; Porta, R. Plasticizing effects of polyamines in protein-based films. Int. J. Mol. Sci. 2017, 18, 1026. [Google Scholar] [CrossRef] [Green Version]
- Mierczyńska, J.; Cybulska, J.; Pieczywek, P.; Zdunek, A. Effect of storage on rheology of water-soluble, chelate-soluble and diluted alkali-soluble pectin in carrot cell walls. Food Bioprocess Technol. 2015, 8, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current advancements in pectin: Extraction, properties and multifunctional applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Sabbah, M.; Al-Asmar, A.; Esposito, M.; Sanchez, A.; Villalonga Santana, R.; Cammarota, M.; Mariniello, L.; Di Pierro, P.; Porta, R. Effect of mesoporous silica nanoparticles on glycerol-plasticized anionic and cationic polysaccharide edible films. Coatings 2019, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kumar Kalakandan, S. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2019, 61, 104812. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez, N.; Londoño, L.; Martinez, G.A.; Díaz, R.; Navarro, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal, M.; Ascacio, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263. [Google Scholar] [CrossRef] [Green Version]
- Tanhatan Naseri, A.; Thibault, J.-F.; Ralet-Renard, M.-C.; Tanhatan Nasseri, A.; Ralet, M.-C. Citrus Pectin: Structure and Application in Acid Dairy Drinks. In Tree and Forestry Science and Biotechnology; Global Science Books: Carrollton, GA, USA, 2008. [Google Scholar]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Nastasi, J.R.; Kontogiorgos, V.; Daygon, V.D.; Fitzgerald, M.A. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Y.; Lin, Y.; Shao, P. Facile fabrication of multifunctional citrus pectin aerogel fortified with cellulose nanofiber as controlled packaging of edible fungi. Food Chem. 2022, 374, 131763. [Google Scholar] [CrossRef] [PubMed]
- Moradi, L.T.; Sharifan, A.; Larijani, K. The Effect of multilayered chitosan–pectin–mentha piperita and lemon essential oil on oxidation effects and quality of rainbow trout fillet (Oncorhynchus Mykiss) during refrigeration at 4 ± 1 °C storage. Iran. J. Fish. Sci. 2020, 19, 2544–2559. [Google Scholar]
- Sumonsiri, N.; Danpongprasert, W.; Thaidech, K. Comparison of sweet orange (Citrus sinensis) and lemon (Citrus limonum) essential oils on qualities of fresh-cut apples during storage. Chem. Chem. Eng. Biotechnol. Food Ind. 2020, 21, 47–57. [Google Scholar]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control. 2020, 114, 107226. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Zhu, D.; Wang, Y.; Zhao, Y.; Li, J. Preparation of Soy Protein Isolate (SPI)-Pectin Complex Film Containing Cinnamon Oil and Its Effects on Microbial Growth of Dehydrated Soybean Curd (Dry Tofu). J. Food Process. Preserv. 2014, 38, 1371–1376. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Lime peel pectin integrated with coconut water and lime peel extract as a new bioactive film sachet to retard soybean oil oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef]
- Famiglietti, M.; Savastano, A.; Gaglione, R.; Arciello, A.; Naviglio, D.; Mariniello, L. Edible films made of dried olive leaf extract and chitosan: Characterization and applications. Foods 2022, 11, 2078. [Google Scholar] [CrossRef]
- Hayes, J.E.; Stepanyan, V.; Allen, P.; O’Grady, M.N.; Kerry, J.P. Evaluation of the effects of selected phytochemicals on quality indices and sensorial properties of raw and cooked pork stored in different packaging systems. Meat Sci. 2010, 85, 289–296. [Google Scholar] [CrossRef]
- Hayes, J.E.; Stepanyan, V.; Allen, P.; O’Grady, M.N.; Kerry, J.P. Evaluation of the effects of selected plant-derived nutraceuticals on the quality and shelf-life stability of raw and cooked pork sausages. Meat Sci. 2011, 44, 164–172. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Elsayed, N.; Hasanin, M.S.; Abdelraof, M. Utilization of olive leaves extract coating incorporated with zinc/selenium oxide nanocomposite to improve the postharvest quality of green beans pods. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100333. [Google Scholar] [CrossRef]
- Naseer, S.; Hussain, S.; Naeem, N.; Pervaiz, M.; Rahman, M. The phytochemistry and medicinal value of Psidium guajava (guava). Clin. Phytosci. 2018, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Phimolsiripol, Y.; Barba, F.J.; Sringarm, K.; Rachtanapun, P.; Jantanasakulwong, K.; Jantrawut, P.; Chittasupho, C.; et al. Guava (Psidium guajava L.) leaf extract as bioactive substances for anti-androgen and antioxidant Activities. Plants 2022, 11, 3514. [Google Scholar] [CrossRef] [PubMed]
- Nantitanon, W.; Yotsawimonwat, S.; Okonogi, S. Factors influencing antioxidant activities and total phenolic content of guava leaf extract. LWT Food Sci. Technol. 2010, 43, 1095–1103. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Anwar, L.N.; Mordi, M.N.; Ramanathan, S.; Mansor, S.M. Influence of sonication on the phenolic content and antioxidant activity of Terminalia catappa L. leaves. Pharm. Res. 2010, 2, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Tonyali, B.; Cikrikci, S.; Oztop, M.H. Physicochemical and microstructural characterization of gum tragacanth added whey protein based films. Food Res. Int. 2018, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Papadakis, S.E.; Abdul-Malek, S.; Kamdem, R.E.; Yam, K.L. A versatile and inexpensive technique for measuring colour of foods. Food Technol. 2000, 54, 48–51. [Google Scholar]
- ASTM F1249-13; Standard Test Method for Water Vapor Transmission Rate through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D3985-05; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Colorimetric Sensor. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM F2476-05; Standard Test Method for Carbon Dioxide Gas Transmission Rate through Barrier Materials Using an Infrared Detector. ASTM International: West Conshohocken, PA, USA, 2005.
- Chou, M.; Osako, K.; Lee, T.; Wang, M.; Lu, W.; Wu, W.; Huang, P.; Li, P.; Ho, J. Characterization and antibacterial properties of fish skin gelatin/guava leaf extract bio-composited films incorporated with catechin. LWT Food Sci. Technol. 2023, 178, 114568. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Unsalan, O.; Altunayar-Unsalan, C.; Xiong, S.; Manyande, A.; Chen, H. Development and characterization of fish myofibrillar protein/chitosan/ rosemary extract composite edible films and the improvement of lipid oxidation stability during the grass carp fillets storage. Int. J. Biol. Macromol. 2021, 184, 463–475. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharjee, S.K.; Mudenur, C.; Ghosh, T.; Goud, V.V.; Katiyar, V. Development of antioxidant-rich edible active films and coatings incorporated with de-oiled ethanolic green algae extract: A candidate for prolonging the shelf life of fresh produce. RSC Adv. 2022, 12, 13295–13313. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium alginate-based green packaging films functionalized by guava leaf extracts and their bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.; Rico, D.; McHugh, T.H. Antimicrobial olive leaf gelatin films for enhancing the quality of cold-smoked Salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- McHugh, T.H.; Avena-Bustillos, R.J.; Krochta, J.M. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Jiao, C.; Zhang, Z.; Shen, G.; Li, S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Roviello, V.; Sabbah, M. Tuning the functional properties of bitter vetch (Vicia ervilia) protein films grafted with spermidine. Int. J. Mol. Sci. 2017, 18, 2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Films | L* | a* | b* |
|---|---|---|---|
| PEC (control) | 83.44 ± 0.09 | −3.09 ± 0.04 | 17.41 ± 0.30 |
| PEC + OLE 0.1% | 85.02 ± 0.05 A | −1.50 ± 0.01 A | 11.13 ± 0.17 A |
| PEC + OLE 0.2% | 86.92 ± 0.14 A,B | 0.72 ± 0.04 A,B | 3.36 ± 0.29 A,B |
| PEC + GLE 0.1% | 79.83 ± 0.91 A,D | −2.01 ± 0.03 A,D | 21.76 ± 1.80 A,D |
| PEC + GLE 0.2% | 74.89 ± 0.38 A,C,D | −3.08 ± 0.11 C,D | 34.50 ± 0.43 A,C,D |
| Films | CO2 | O2 | WV |
|---|---|---|---|
| cm3·mm·m−2·day−1·kPa−1 | g·mm·m−2·day−1·kPa−1 | ||
| PEC * | 0.08 ± 0.03 | 0.01 ± 0.00 | 0.12 ± 0.01 |
| PEC + OLE 0.1% | 0.15 ± 0.02 A | 0.01 ± 0.00 | 3.87 ± 0.76 A |
| PEC + OLE 0.2% | 0.35 ± 0.01 A,B | 0.05 ± 0.00 A,B | 4.64 ± 0.02 A |
| PEC + GLE 0.1% | 0.31 ± 0.05 A | 0.05 ± 0.00 A | 4.64 ± 0.11 A |
| PEC + GLE 0.2% | 0.37 ± 0.01 A,B | 0.04 ± 0.00 A | 3.45 ± 0.79 A |
| Viscofan (NDX) ** | 3.71 ± 0.16 | 0.03 ± 0.01 | 0.08 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbah, M.; Al-Asmar, A.; Younis, D.; Al-Rimawi, F.; Famiglietti, M.; Mariniello, L. Production and Characterization of Active Pectin Films with Olive or Guava Leaf Extract Used as Soluble Sachets for Chicken Stock Powder. Coatings 2023, 13, 1253. https://doi.org/10.3390/coatings13071253
Sabbah M, Al-Asmar A, Younis D, Al-Rimawi F, Famiglietti M, Mariniello L. Production and Characterization of Active Pectin Films with Olive or Guava Leaf Extract Used as Soluble Sachets for Chicken Stock Powder. Coatings. 2023; 13(7):1253. https://doi.org/10.3390/coatings13071253
Chicago/Turabian StyleSabbah, Mohammed, Asmaa Al-Asmar, Duaa Younis, Fuad Al-Rimawi, Michela Famiglietti, and Loredana Mariniello. 2023. "Production and Characterization of Active Pectin Films with Olive or Guava Leaf Extract Used as Soluble Sachets for Chicken Stock Powder" Coatings 13, no. 7: 1253. https://doi.org/10.3390/coatings13071253