Coatings Prepared by Electro-Spark Alloying with SHS Electrode Materials Based on Ti-B-Fe-AlN
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objects of Study
2.2. ESA Coating
2.3. Sample Preparation
2.4. Methods
2.5. Tribological Tests
3. Results and Discussion
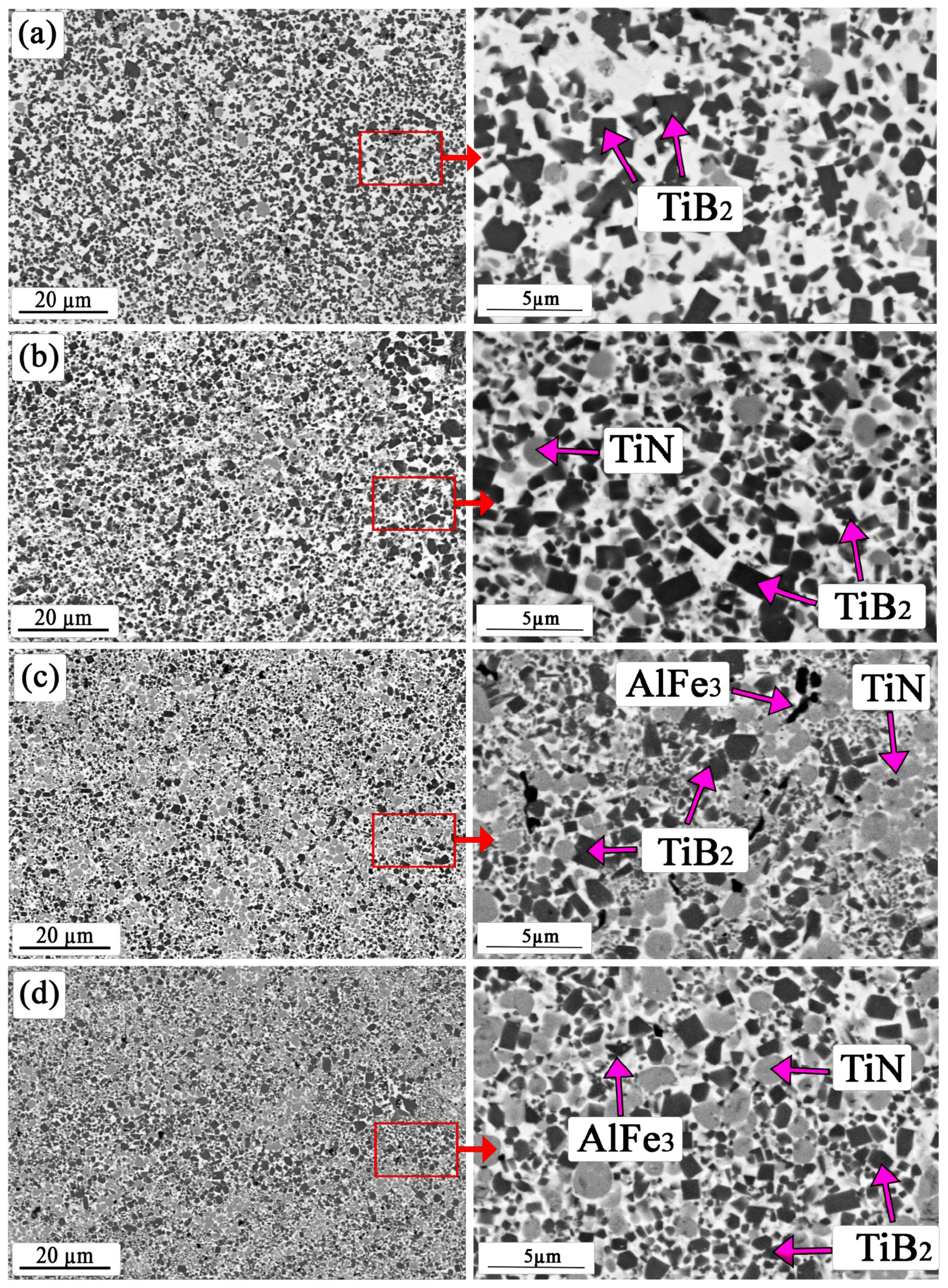
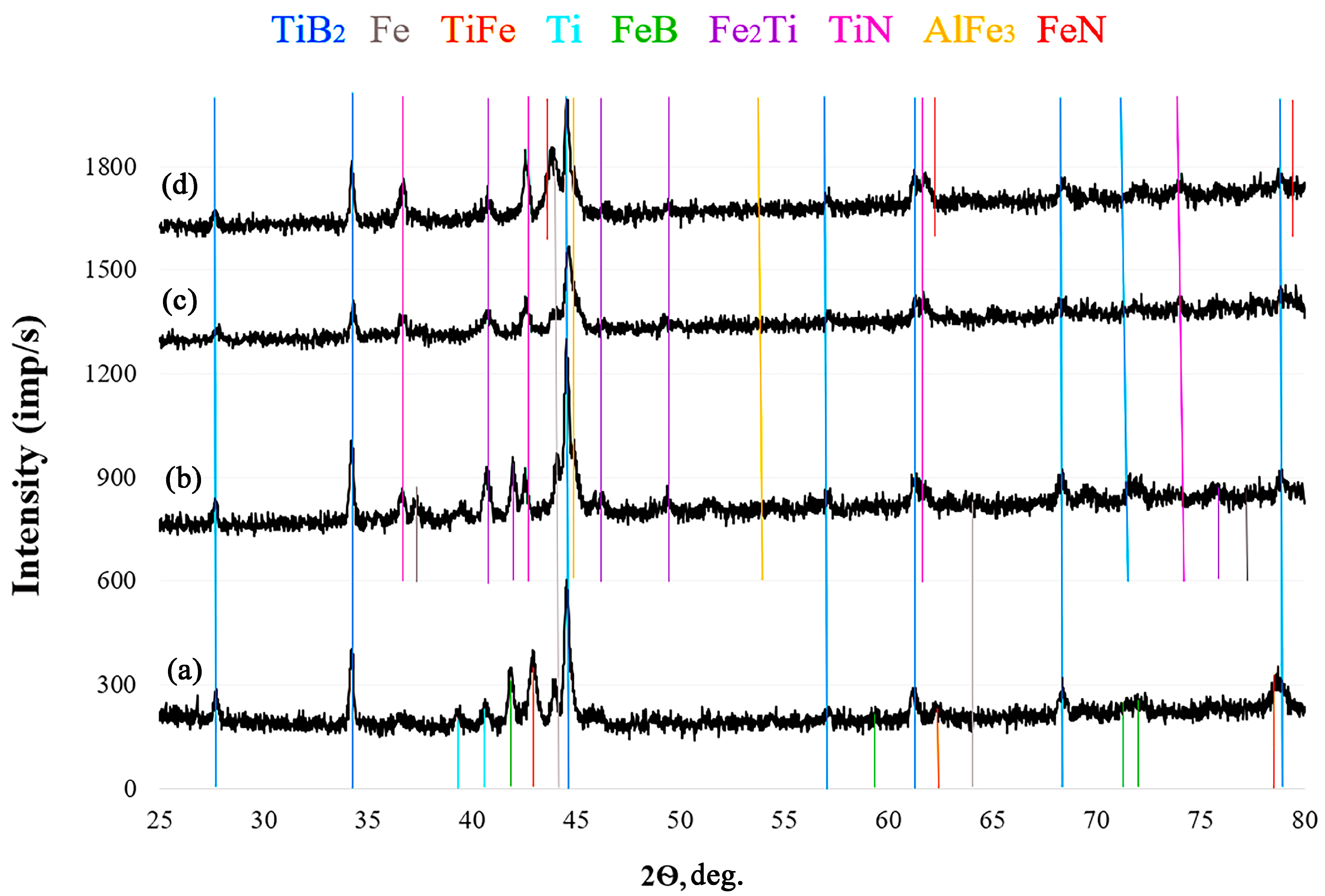
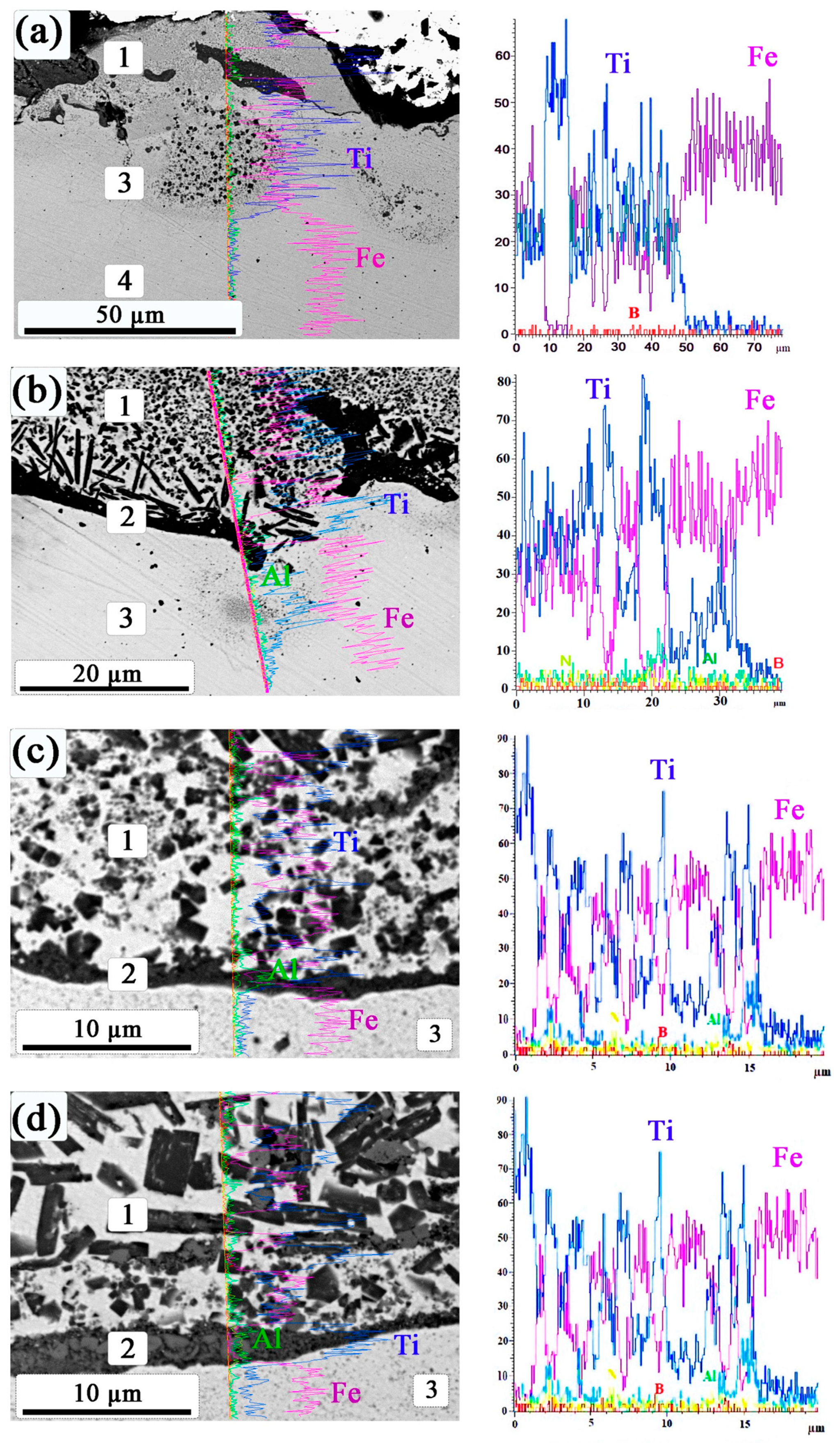
3.1. SEM and Energy-Dispersive X-ray Spectroscopy Data of Coatings
3.2. Distribution of Microhardness in the Coating
3.3. Tribological Test Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, K.N.; Syed, I. Surface texturing and laser shock peening processes on high-speed steel tool for sustainable machining. Arab. J. Sci. Eng. 2022, 47, 8589–8600. [Google Scholar] [CrossRef]
- Okokpujie, I.P.; Bolu, C.A.; Ohunakin, O.S.; Akinlabi, E.T. Experimental study of the effect of TiN–Zn coated high-speed steel cutting tool on surface morphology of AL1060 alloy during machining operation. In Trends in Manufacturing and Engineering Management; Vijayan, S., Subramanian, N., Sankaranarayanasamy, K., Eds.; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2021; pp. 637–647. [Google Scholar] [CrossRef]
- Qi, Y.; Nguyen, V.; Melkote, S.; Varenberg, M. Wear of mechanochemically treated high-speed steel cutting tools. J. Manuf. Sci. Eng. 2022, 144, 094505. [Google Scholar] [CrossRef]
- Yasin, J.; Selvakumar, S.; Mathan, K.P.; Sundaresan, R.; Arunraja, K.M. Experimental study of TiN, TiAlN and TiSiN coated high speed steel tool. Mater. Today Proc. 2022, 64, 1707–1710. [Google Scholar] [CrossRef]
- Aditharajan, A.; Radhika, N.; Saleh, B. Recent advances and challenges associated with thin film coatings of cutting tools: A critical review. Trans. IMF 2023, 101, 205–221. [Google Scholar] [CrossRef]
- Vardanyan, E.L.; Ramazanov, K.N.; Nagimov, R.S.; Nazarov, A.Y. Properties of intermetallic Ti-Al based coatings deposited on ultrafine grained martensitic steel. Surf. Coat. Technol. 2020, 389, 125657. [Google Scholar] [CrossRef]
- Ramazanov, K.N.; Vardanyan, E.L.; Mukhamadeev, V.R.; Mukhamadeev, I.R.; Maslov, A.A. Change in the chemical composition of a carbide tool with Ti-Al-N coating surface layers during machining. J. Surf. Investig. 2022, 16, 412–415. [Google Scholar] [CrossRef]
- Khusainov, Y.G.; Nikolaev, A.A.; Agzamov, R.D.; Ramazanov, K.N.; Tagirov, A.F. Influence of hydrogen content on ion nitriding of coarse-grained and ultrafine-grained VT6 titanium alloys. J. Phys. Conf. Ser. 2019, 1393, 012095. [Google Scholar] [CrossRef]
- Suhova, N.A.; Shekhtman, S.R.; Migranov, M.S. Synthesis of nanostructured composite coatings in arc discharge plasma. Lect. Notes Mech. Eng. 2019, 28, 1393–1399. [Google Scholar] [CrossRef]
- Migranov, M.S.; Migranov, A.M.; Minigaleev, S.M.; Shehtman, S.R. Tribological properties of multilayer coatings for cutting tool. J. Frict. Wear. 2018, 39, 245–250. [Google Scholar] [CrossRef]
- Novikov, V.Y.; Beresnev, V.M.; Kolesnikov, D.A.; Ivanov, O.N.; Lytovchenko, S.V.; Glukhov, O.V.; Gorokh, D.V.; Kozachenko, A.O.; Krytsyna, E.V.; Sirota, V.V. Structure and physicomechanical properties of superhard multicomponent multilayer (TiAlCrY/Zr)/(TiAlCrYN/ZrN) coatings with double modulation period of the structure. J. Nano-Electron. Phys. 2019, 11, 02027. [Google Scholar] [CrossRef]
- Sirota, V.; Zaitsev, S.; Prokhorenkov, D.; Limarenko, M.; Skiba, A.; Kovaleva, M. NiB-CrC coatings prepared by magnetron sputtering using composite ceramic NiCr-BC target produced by detonation spray coating. Nanomaterials 2022, 12, 3584. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, X.; Lu, J.; Geng, X.; Wan, Y.; Wu, M.; Yang, P. Substrate effects on the CVD growth of MoS2 and WS2. J. Mater. Sci. 2020, 55, 990–996. [Google Scholar] [CrossRef]
- Sasikumar, Y.; Indira, K.; Rajendran, N. Surface modification methods for titanium and its alloys and their corrosion behavior in biological environment: A review. J. Bio—Tribo-Corros. 2019, 5, 36. [Google Scholar] [CrossRef]
- Khlyustova, A.; Cheng, Y.; Yang, R. Vapor-deposited functional polymer thin films in biological applications. J. Mater. Chem. B 2020, 8, 6588–6609. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, T.; Wang, D.; Liang, Z.; Yang, X.; Peng, Z.; Liu, Y.; Liang, Y.; Zeng, Z.; Oliveira, J.P. A novel heterogeneous multi-wire indirect arc directed energy deposition for in-situ synthesis Al-Zn-Mg-Cu alloy: Process, microstructure and mechanical properties. Addit. Manuf. 2023, 72, 103639. [Google Scholar] [CrossRef]
- Youqiang, X.; Cheng, L.; Mingyu, Z.; Yanhua, Z.; Kornel, E.; Ze, W.; Lei, L. Assessment of self-lubricating coated cutting tools fabricated by laser additive manufacturing technology for friction-reduction. J. Mater. Process. Technol. 2023, 318, 118010. [Google Scholar] [CrossRef]
- Yassmin, S.A.; Veldhuis, S.C. Enhancement of carbide tool performance during dry machining through a combination of laser surface texturing and tungsten disulfide soft coatings. Surf. Coat. Technol. 2021, 428, 127849. [Google Scholar] [CrossRef]
- Bazhin, P.M.; Titov, N.V.; Zhidovich, A.O.; Avdeeva, V.V.; Kolomeichenko, A.V.; Stolin, A.M. Features of the carbo-vibroarc surfacing in the development of multicomponent cermet wear-resistant coatings. Surf. Coat. Technol. 2022, 429, 127952. [Google Scholar] [CrossRef]
- Frank, S.; Maximilian, S.; Alexander, K.; Thomas, L. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Martsynkovskyy, V.; Tarelnyk, V.B.; Konoplianchenko, I.; Gaponova, O.P.; Antoszewski, B.; Kundera, C.; Dyadyura, K.; Tarelnyk, N.; Sarzhanov, B.A.; Mikulina, M.; et al. New process for forming multicomponent wear-resistant nanostructures by electrospark Alloying method. In Microstructure and Properties of Micro- and Nanoscale Materials, Films, and Coatings (NAP 2019); Pogrebnjak, A., Bondar, O., Eds.; Springer Proceedings in Physics; Springer: Singapore, 2019; Volume 240, pp. 135–149. [Google Scholar] [CrossRef]
- Koreshkov, A.V.; Boitsov, A.G.; Kuritsyna, V.V.; Siluyanova, M.V. Multicomponent and multilayer electrospark alloying of airplane parts. Russ. Eng. Res. 2021, 41, 653–656. [Google Scholar] [CrossRef]
- Tarelnyk, V.B.; Gaponova, O.P.; Loboda, V.B.; Konoplyanchenko, E.V.; Martsinkovskii, V.S.; Semirnenko, Y.I.; Tarelnyk, N.V.; Mikulina, M.A.; Sarzhanov, B.A. Improving ecological safety when forming wear-resistant coatings on the surfaces of rotation body parts of 12Kh18N10T steel using a combined technology based on electrospark alloying. Surf. Eng. Appl. Electrochem. 2021, 57, 173–184. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.; Sytchenko, A.; Sheveyko, A.; Moskovskikh, D.; Vorotylo, S. Two-Layer nanocomposite TiC-based coatings produced by a combination of pulsed cathodic arc evaporation and vacuum electro-spark alloying. Materials 2020, 13, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiryukhantsev-Korneev, P.V.; Sytchenko, A.D.; Levashov, E.A. Comparative study of coatings formed by electrospark alloying using TiC-NiCr and TiC-NiCr-Eu2O3 electrodes. Russ. J. Non-Ferr. Met. 2019, 60, 662–672. [Google Scholar] [CrossRef]
- Burkov, A.A. Tribotechnical and corrosion characteristics of electrospark Fe–Al aluminide coatings on AISI 304 stainless steel. J. Frict. Wear. 2022, 43, 236–242. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.V.; Sytchenko, A.D.; Gorshkov, V.A.; Loginov, P.A.; Sheveyko, A.N.; Nozhkina, A.V.; Levashov, E.A. Complex study of protective Cr3C2–NiAl coatings deposited by vacuum electro-spark alloying, pulsed cathodic arc evaporation, magnetron sputtering, and hybrid technology. Ceram. Int. 2022, 48, 10921–10931. [Google Scholar] [CrossRef]
- Kandeva, M.; Kostadinov, G.; Penyashki, T.; Kamburov, V.; Dimitrova, R.; Valcanov, S.; Petrzhik, M. Abrasive wear resistance of electrospark coatings on titanium alloys. Tribol. Ind. 2022, 44, 132. [Google Scholar] [CrossRef]
- Barile, C.; Casavola, C.; Pappalettera, G.; Renna, G. Advancements in electrospark deposition (ESD) technique: A short review. Coatings 2022, 12, 1536. [Google Scholar] [CrossRef]
- Stolin, A.M.; Bazhin, P.M.; Mikheyev, M.V.; Saguidollayev, A.S.; Kylyshbaev, K.T. Deposition of protective coatings by electric arc cladding with SHS electrodes. Weld. Int. 2015, 29, 657–660. [Google Scholar] [CrossRef]
- Panteleenko, F.I.; Sarantsev, V.V.; Stolin, A.M.; Bazhin, P.M.; Azarenko, E.L. Formation of composite coatings based on titanium carbide via electrospark alloying. Surf. Eng. Appl. Electrochem. 2011, 47, 328–337. [Google Scholar] [CrossRef]
- Farhad, A.; Hossein, A.; Shahin, K.A. Evaluation of the corrosion resistance of AlCoCrFeMnNi high entropy alloy hard coating applied by electro spark deposition. Surf. Coat. Technol. 2023, 454, 129156. [Google Scholar] [CrossRef]
- Chandrakant; Reddy, N.S.; Bharat, B.P. Electro spark coating of AlCoCrFeNi high entropy alloy on AISI410 stainless steel. Mater. Lett. 2021, 304, 130580. [Google Scholar] [CrossRef]
- Sheveyko, A.N.; Kuptsov, K.A.; Antonyuk, M.N.; Bazlov, A.I.; Shtansky, D.V. Electro-spark deposition of amorphous Fe-based coatings in vacuum and in argon controlled by surface wettability. Mater. Lett. 2022, 318, 132195. [Google Scholar] [CrossRef]
- Kefeng, L.; Jian, Z.; Wenqing, G.; Xidong, H. Progress on New Preparation Methods, Microstructures, and Protective Properties of High-Entropy Alloy Coatings. Coatings 2022, 12, 1472. [Google Scholar] [CrossRef]
- Chizhikov, A.P.; Konstantinov, A.S.; Bazhin, P.M. Self-propagating high-temperature synthesis of ceramic material based on aluminum-magnesium spinel and titanium diboride. Russ. J. Inorg. Chem. 2021, 66, 1115. [Google Scholar] [CrossRef]
- Bazhin, P.M.; Konstantinov, A.S.; Chizhikov, A.P.; Pazniak, A.I.; Kostitsyna, E.V.; Prokopets, A.D.; Stolin, A.M. Laminated cermet composite materials: The main production methods, structural features and properties (review). Ceram. Int. 2021, 47, 1513–1525. [Google Scholar] [CrossRef]
- Antipov, M.S.; Bazhin, P.M.; Chizhikov, A.P.; Konstantinov, A.S.; Stolin, A.M.; Khomenko, N.Y. Formability, phase composition, and microstructure of TiC-(5–50 wt %) NiCr–based materials obtained by free SHS compression. Russ. J. Inorg. Chem. 2022, 67, 1658–1664. [Google Scholar] [CrossRef]
- Bazhin, P.; Chizhikov, A.; Stolin, A.; Antipov, M.; Konstantinov, A. Long-sized rods of Al2O3–SiC–TiB2 ceramic composite material obtained by SHS-extrusion: Microstructure, X-ray analysis and properties. Ceram. Int. 2021, 47, 28444–28448. [Google Scholar] [CrossRef]
- Bolotskaya, A.V.; Mikheev, M.V.; Bazhin, P.M.; Stolin, A.M. Preparation by SHS-extrusion method of compact ceramic materials based on the Ti–B system modified with nanosize Si3N4 particles. Refract. Ind. Ceram. 2021, 62, 305–308. [Google Scholar] [CrossRef]
- Bazhin, P.M.; Kostitsyna, E.V.; Stolin, A.M.; Chizhikov, A.M.; Bychkova, M.Y.; Pazniak, A. Nanostructured ceramic composite rods: Synthesis, properties and application. Ceram. Int. 2019, 45, 9297–9301. [Google Scholar] [CrossRef]
- Bazhin, P.M.; Stolin, A.M.; Shcherbakov, V.A.; Zamyatkina, E.V. Ceramic nanocomposite produced by SHS extrusion. Dokl. Chem. 2010, 430, 58–61. [Google Scholar] [CrossRef]
- Kirakosyan, H. Cu-Mo nanocomposite preparation by combining solution combustion synthesis and self propagating high temperature synthesis. Inorg. Nano-Met. Chem. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y. Simultaneously enhanced reaction temperature and velocity of self-propagating high-temperature synthesis via Joule-heat induced multi-channel heat flow. J. Appl. Phys. 2021, 129, 165304. [Google Scholar] [CrossRef]
- Shi, H.; Dou, Z.; Meng, Y.; Zhang, T. Effects of reactants proportions on features of in-situ magnesiothermic self-propagating high temperature synthesized boron carbide powder. Ceram. Int. 2022, 48, 33400–33411. [Google Scholar] [CrossRef]
- Tang, L.; Lin, B.; Zhang, B.; Zhang, D.; Gong, Y.; Xiong, N. Research status of titanium diboride high temperature ceramics. J. Phys. Conf. Ser. 2022, 2200, 012024. [Google Scholar] [CrossRef]
- Lark, A.; Du, J.; Chandran, K.S.R. Material design and processing of a new class of titanium boride cermets with tough metallic phases and mechanical properties. J. Mater. Res. 2018, 33, 4296–4306. [Google Scholar] [CrossRef]
- Grigorenko, S.G.; Grigorenko, G.M.; Zadorozhnyuk, O.M. Intermetallics of titanium. Peculiar features, properties, application (Review). Electrometall. Today 2017, 3, 51–58. [Google Scholar] [CrossRef]
- Morsi, K. Titanium–titanium boride composites. J. Mater. Sci. 2019, 54, 6753–6771. [Google Scholar] [CrossRef]
- Saurabh, A.; Meghana, C.M.; Singh, P.K.; Verma, P.C. Titanium-based materials: Synthesis, properties, and applications. Mater. Today Proc. 2022, 56, 412–419. [Google Scholar] [CrossRef]
- Bolotskaia, A.V.; Mikheev, M.V.; Bazhin, P.M.; Stolin, A.M. The effect of aluminum nitride nanoparticles on the structure, phase composition and properties of materials of the Ti-B-Fe system obtained by SHS-extrusion. Lett. Mater. 2020, 10, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Bolotskaya, A.V.; Mikheev, M.V. Preparation by SHS-extrusion method of compact ceramic electrode materials based on Ti-B-Fe system modified with nanosized AlN particles. Refract. Ind. Ceram. 2020, 61, 336–340. [Google Scholar] [CrossRef]
- Bolotskaya, A.V.; Mikheev, M.V. Extraction of compact ceramic electrode materials based on the Ti–B–Fe system modified by nanosized AlN particles by SHS extrusion. Novye Ogneupory (New Refract.) 2020, 6, 51–55. [Google Scholar] [CrossRef]
- ASTM G99-05; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus, Book of ASTM Standards. American Soc. for Metals: Detroit, MI, USA, 2010; Volume 03.02, p. 6.
- Markelova, O.A.; Taran, V.M.; Fomin, A.A. Electro-plasma coating of coatings with a porous structure filled with liquid substances. Vopr. Elektrotekhn. 2020, 2, 10–19. [Google Scholar]
- Proskuryakov, V.; Rodionov, I.; Borodina, S. The effect of graphite coating on the composition, structure and microhardness of the surface of structural chromium nickel steel during laser pulse processing. J. Phys. Conf. Ser. 2020, 1695, 012069. [Google Scholar] [CrossRef]
- Jiang, P.F.; Zhang, C.H.; Zhang, S.; Zhang, J.B.; Chen, J.; Liu, Y. Fabrication and wear behavior of TiC reinforced FeCoCrAlCu-based high entropy alloy coatings by laser surface alloying. Mater. Chem. Phys. 2020, 255, 123571. [Google Scholar] [CrossRef]


| Chemical Element/Characteristics | SHS Electrode | |||
|---|---|---|---|---|
| Composition (Ti-B-Fe)xAlN, wt % | ||||
| 0 | 5 | 10 | 15 | |
| Ti | 0.57 | 0.55 | 0.51 | 0.49 |
| B | 0.13 | 0.12 | 0.12 | 0.11 |
| Fe | 0.3 | 0.28 | 0.27 | 0.25 |
| AlN | 0 | 0.05 | 0.1 | 0.15 |
| Wear-resistant component | TiB2 | TiB2, TiN | TiB2, TiN | TiB2, TiN |
| Density, g/cm3 | 5.25 | 5.22 | 4.94 | 4.93 |
| Porosity, % | 3 | 5 | 3 | 4 |
| Electrical resistivity ρ, Ohm m (×10–7) | 11.2 | 4.7 | 4.0 | 4.9 |
| Conductivity ϭ, S/m (×10) | 0.9 | 2.1 | 2.5 | 2 |
| Vickers hardness (HV10/30) GPa | 10.7 | 14.7 | 12.6 | 12.4 |
| Element | W | Mo | V | Cr | C | Si | Mn | Fe |
|---|---|---|---|---|---|---|---|---|
| Content, wt % | 5.5–6.5 | 4.8–5.3 | 1.7–2.1 | 3.8–4.4 | 0.82–0.9 | 0.2–0.5 | 0.2–0.5 | base |
| Content x(AlN), wt % | ||||
|---|---|---|---|---|
| Phase composition | 0 | 5 | 10 | 15 |
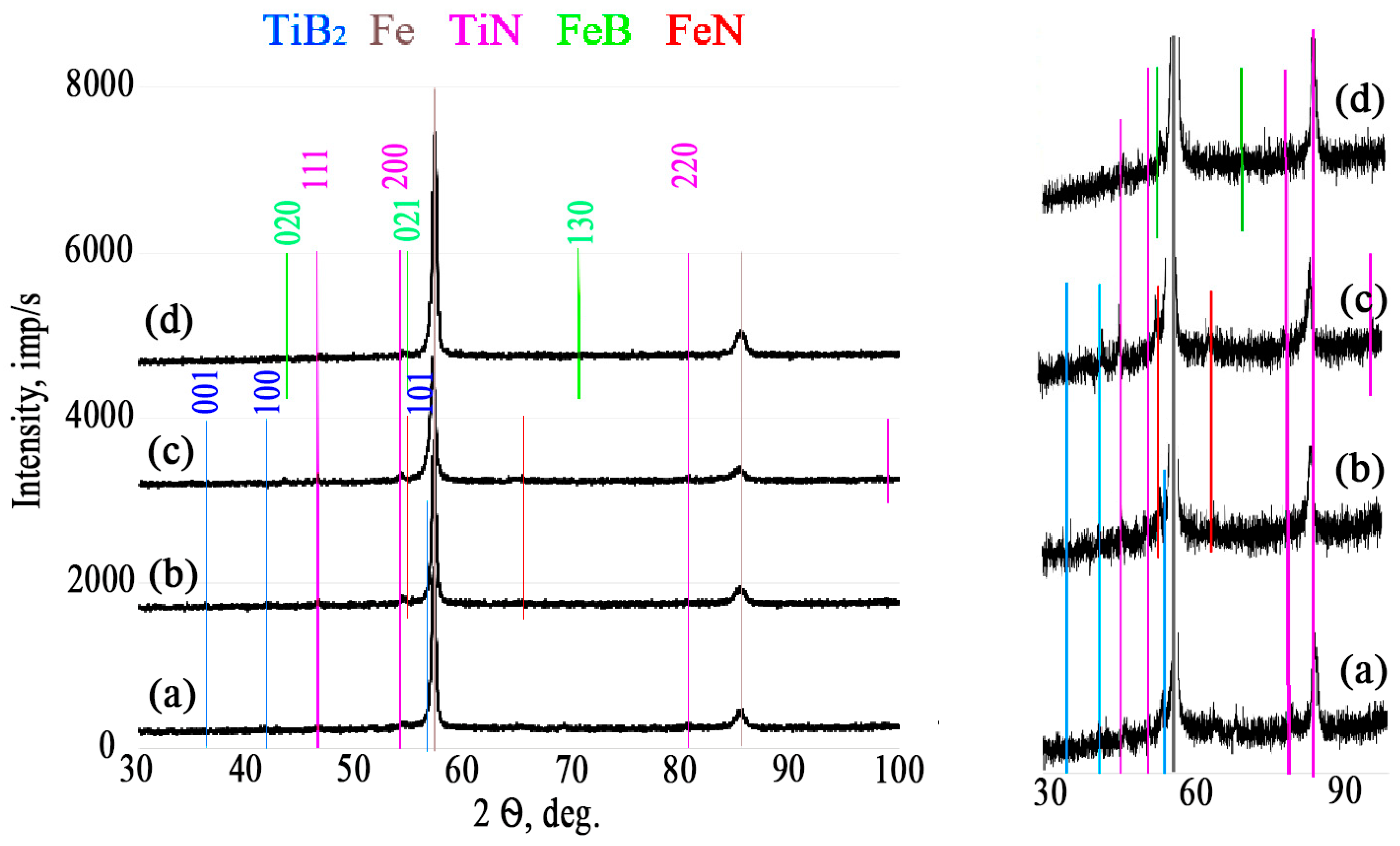
| TiB2 | TiB2 | TiB2 | TiB2 | |
| TiFe | Fe2Ti | TiN | TiN | |
| Fe | Fe | Fe | Fe | |
| Ti | AlFe | Fe2Ti | Fe2Ti | |
| FeB | TiN | AlFe3 | AlFe3 | |
| - | - | - | FeN | |
| Content x (AlN), wt % | ||||
|---|---|---|---|---|
| Phase composition | 0 | 5 | 10 | 15 |
| TiB2 | TiB2 | TiB2 | TiB2 | |
| TiN | TiN | TiN | TiN | |
| Fe | Fe | Fe | FeB | |
| - | FeN (probably) | FeN (probably) | - | |
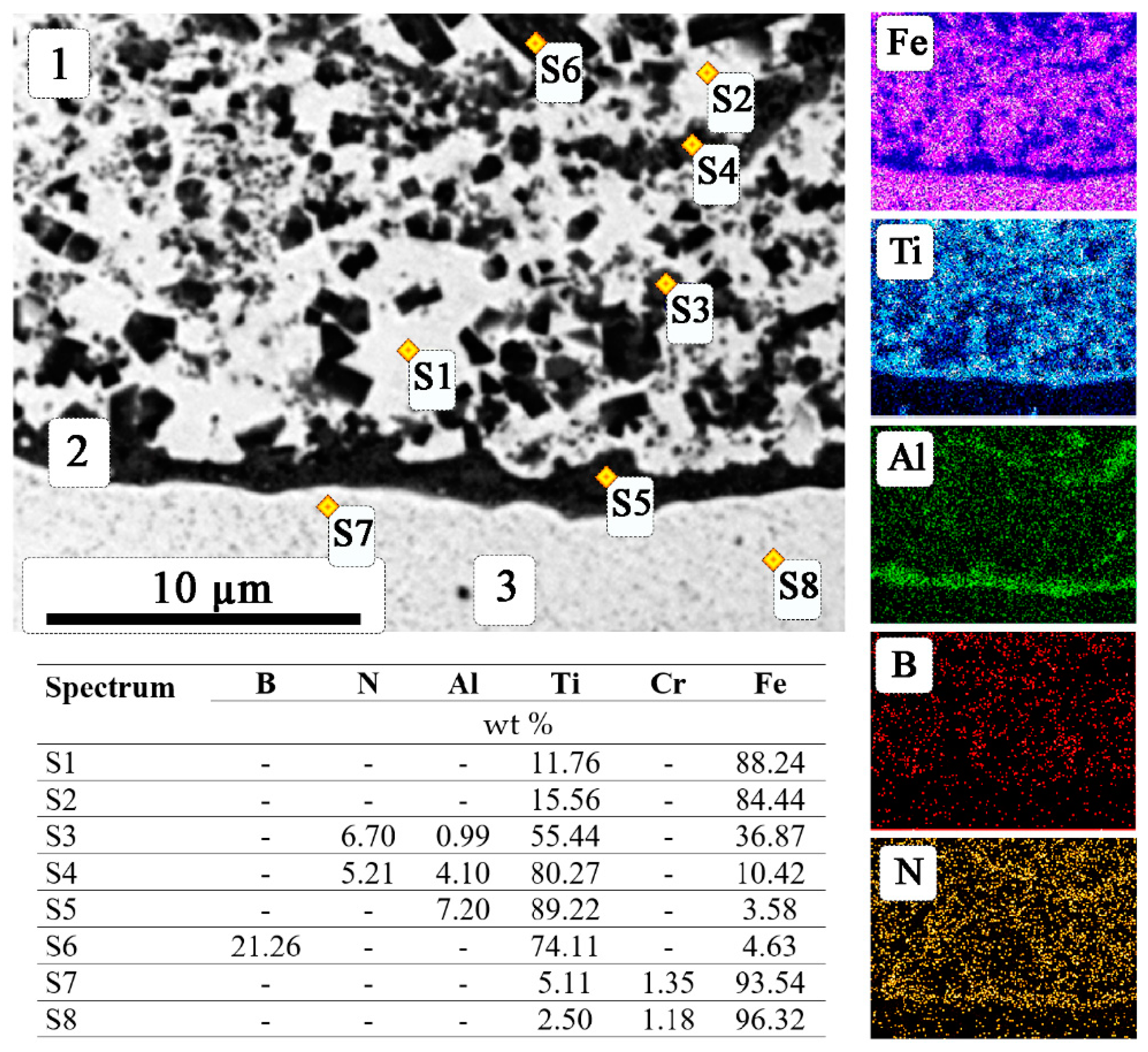
| Sample | Specific Wear Rate (×10–5), mm3 m−1 N−1 | Surface Microhardness, GPa | |
|---|---|---|---|
| Specimen | Counter Body | ||
| High-speed steel R6M5 (without coating) | 12.67 | 0.089 | 2.2 |
| Coating obtained by SHS electrodes (Ti-B-Fe) | 1.894 | 0.099 | 9.8 |
| Coating obtained by SHS electrodes (Ti-B-Fe)xAlN | |||
| x = 5 | 1.951 | 0.129 | 11.9 |
| x = 10 | 3.360 | 0.158 | 10.6 |
| x = 15 | 2.916 | 0.093 | 10.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolotskaia, A.; Avdeeva, V.; Bazhin, P.; Mikheev, M.; Stolin, A.; Novikov, V.; Kovaleva, M.; Sirota, V. Coatings Prepared by Electro-Spark Alloying with SHS Electrode Materials Based on Ti-B-Fe-AlN. Coatings 2023, 13, 1264. https://doi.org/10.3390/coatings13071264
Bolotskaia A, Avdeeva V, Bazhin P, Mikheev M, Stolin A, Novikov V, Kovaleva M, Sirota V. Coatings Prepared by Electro-Spark Alloying with SHS Electrode Materials Based on Ti-B-Fe-AlN. Coatings. 2023; 13(7):1264. https://doi.org/10.3390/coatings13071264
Chicago/Turabian StyleBolotskaia, Anastasia, Varvara Avdeeva, Pavel Bazhin, Maksim Mikheev, Alexander Stolin, Vseslav Novikov, Marina Kovaleva, and Viacheslav Sirota. 2023. "Coatings Prepared by Electro-Spark Alloying with SHS Electrode Materials Based on Ti-B-Fe-AlN" Coatings 13, no. 7: 1264. https://doi.org/10.3390/coatings13071264
APA StyleBolotskaia, A., Avdeeva, V., Bazhin, P., Mikheev, M., Stolin, A., Novikov, V., Kovaleva, M., & Sirota, V. (2023). Coatings Prepared by Electro-Spark Alloying with SHS Electrode Materials Based on Ti-B-Fe-AlN. Coatings, 13(7), 1264. https://doi.org/10.3390/coatings13071264







