A Comparative Study on Characterization and High-Temperature Wear Behaviors of Thermochemical Coatings Applied to Cobalt-Based Haynes 25 Superalloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procedure of Thermochemical Coatings
2.2. Characterization
3. Results and Discussion
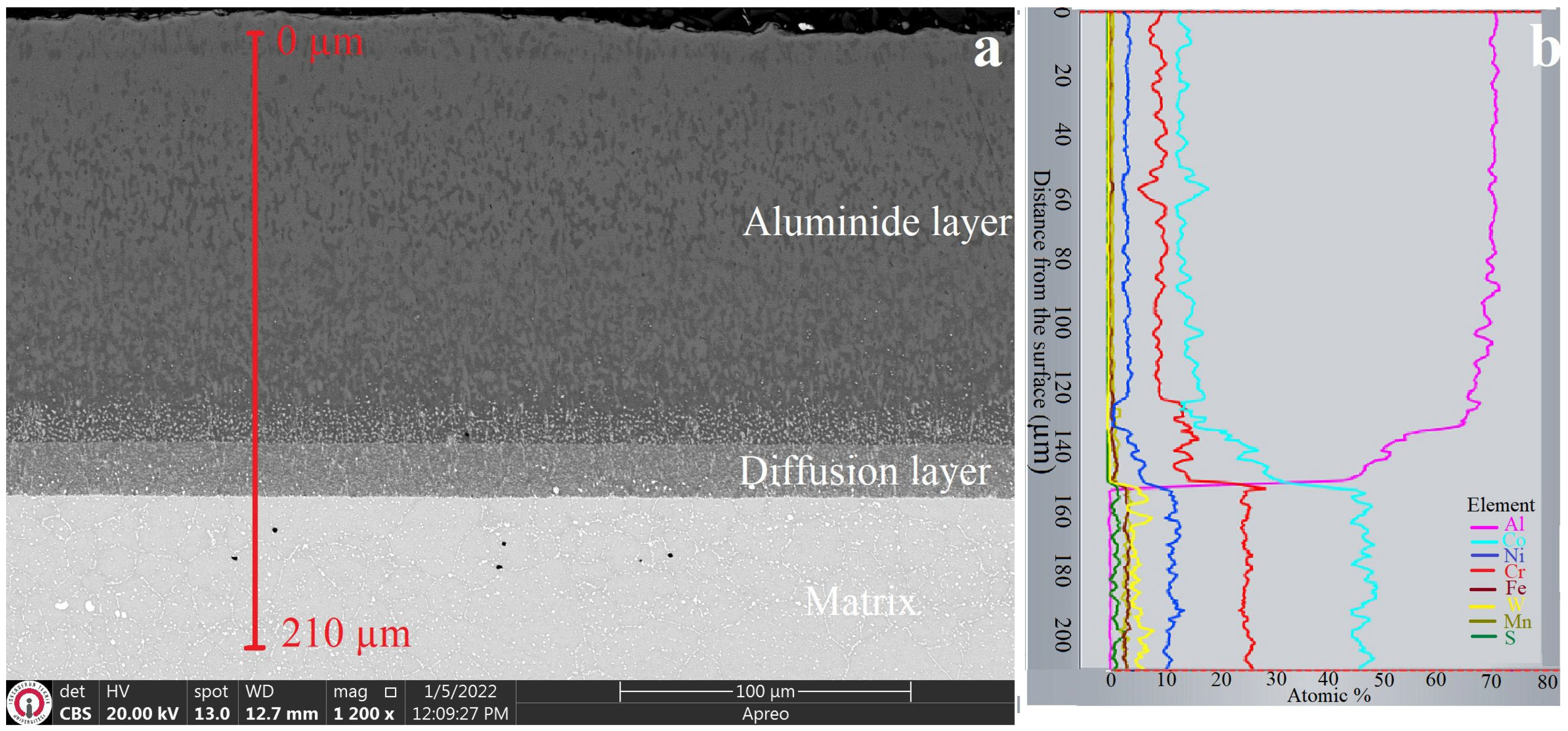
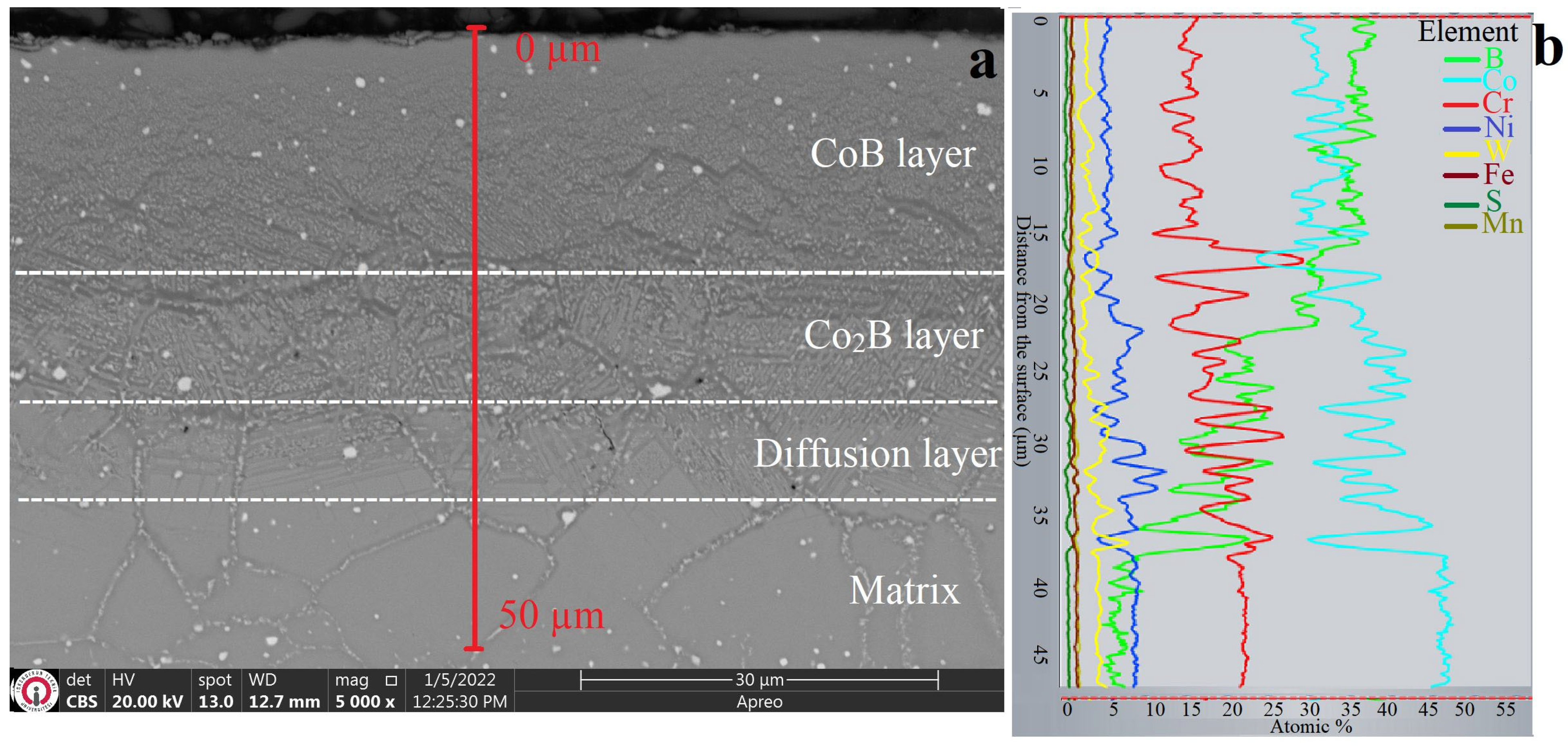
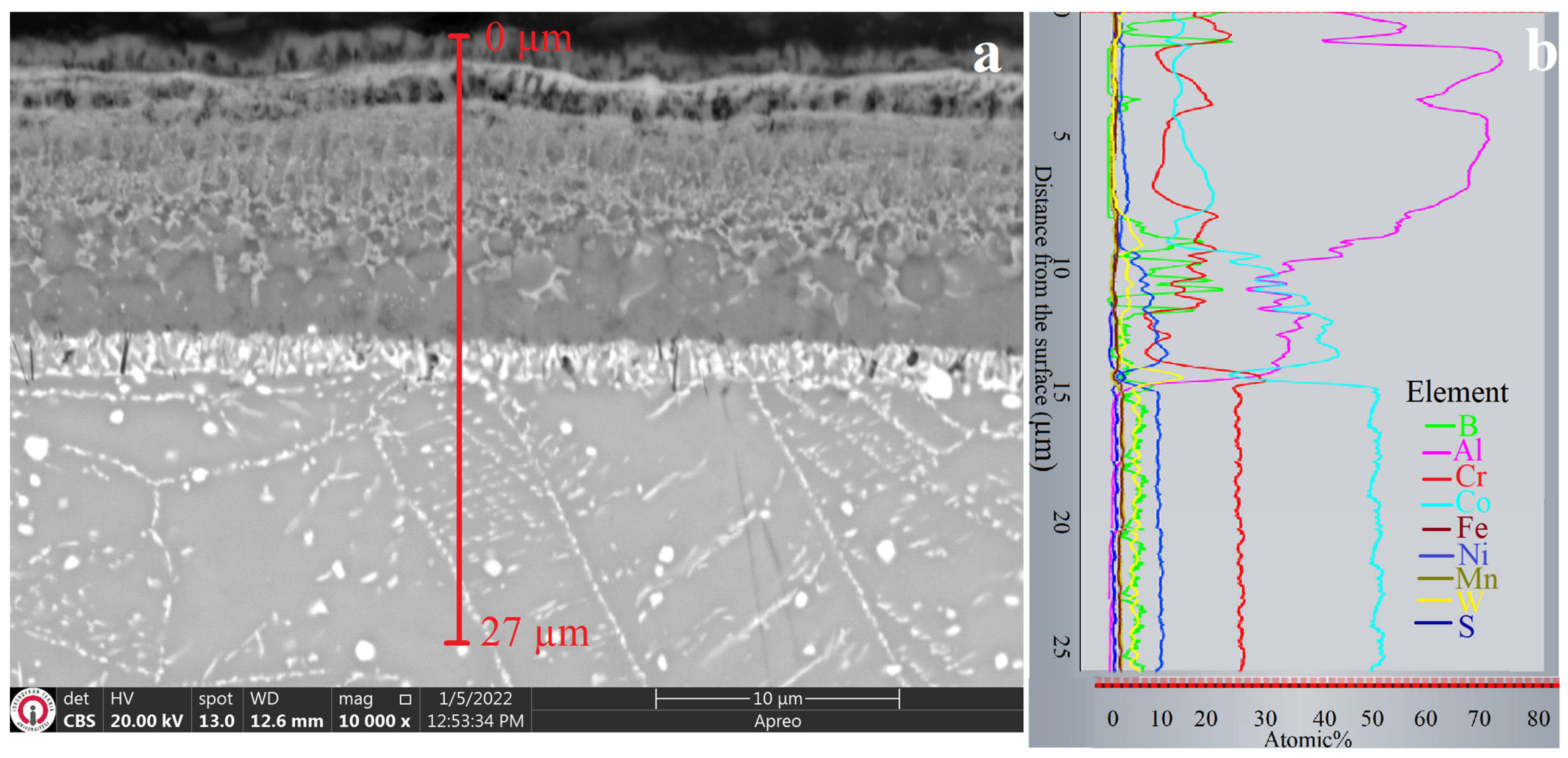
3.1. XRD and Microstructure Analyses
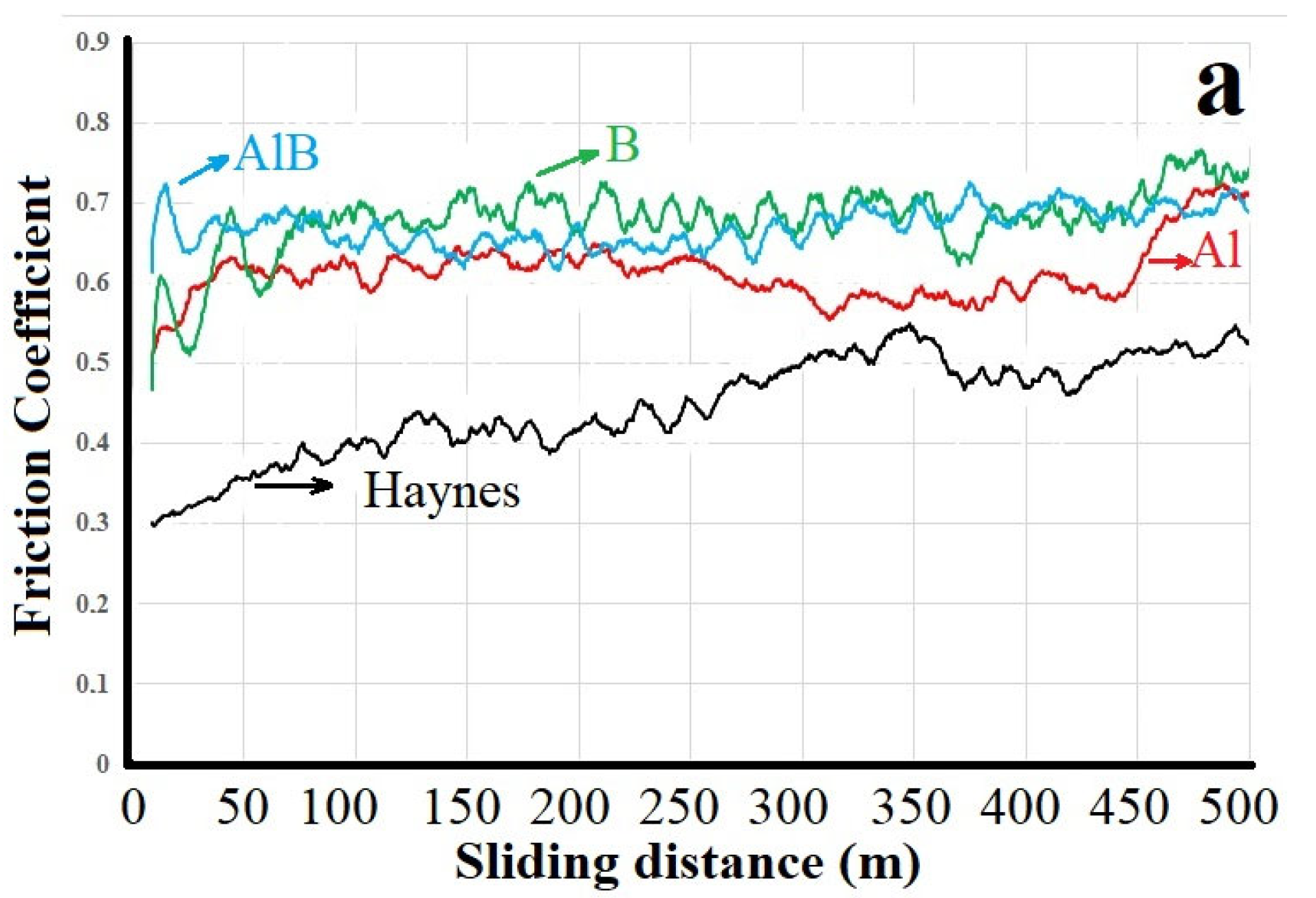
3.2. Friction and Wear Behavior
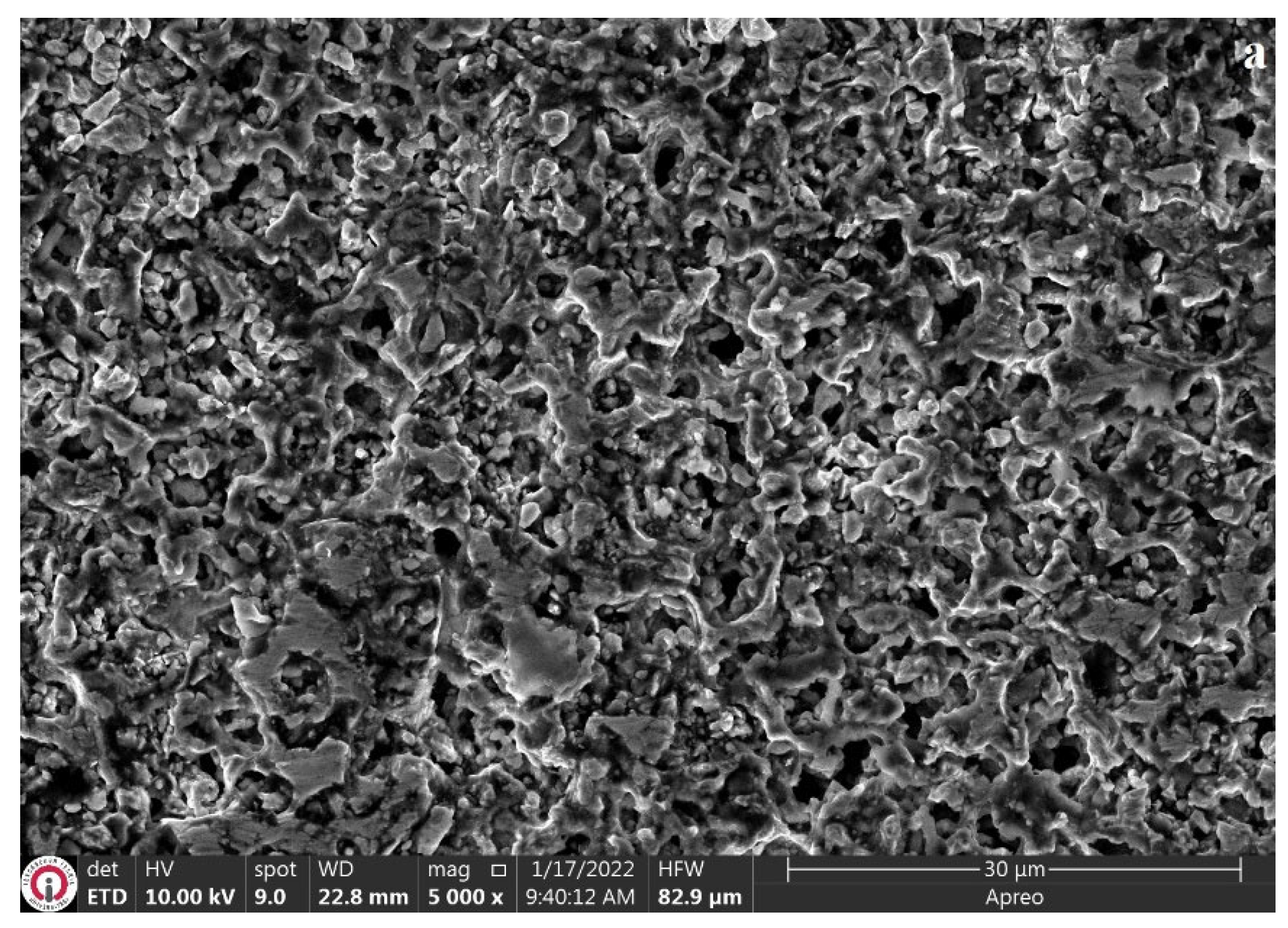
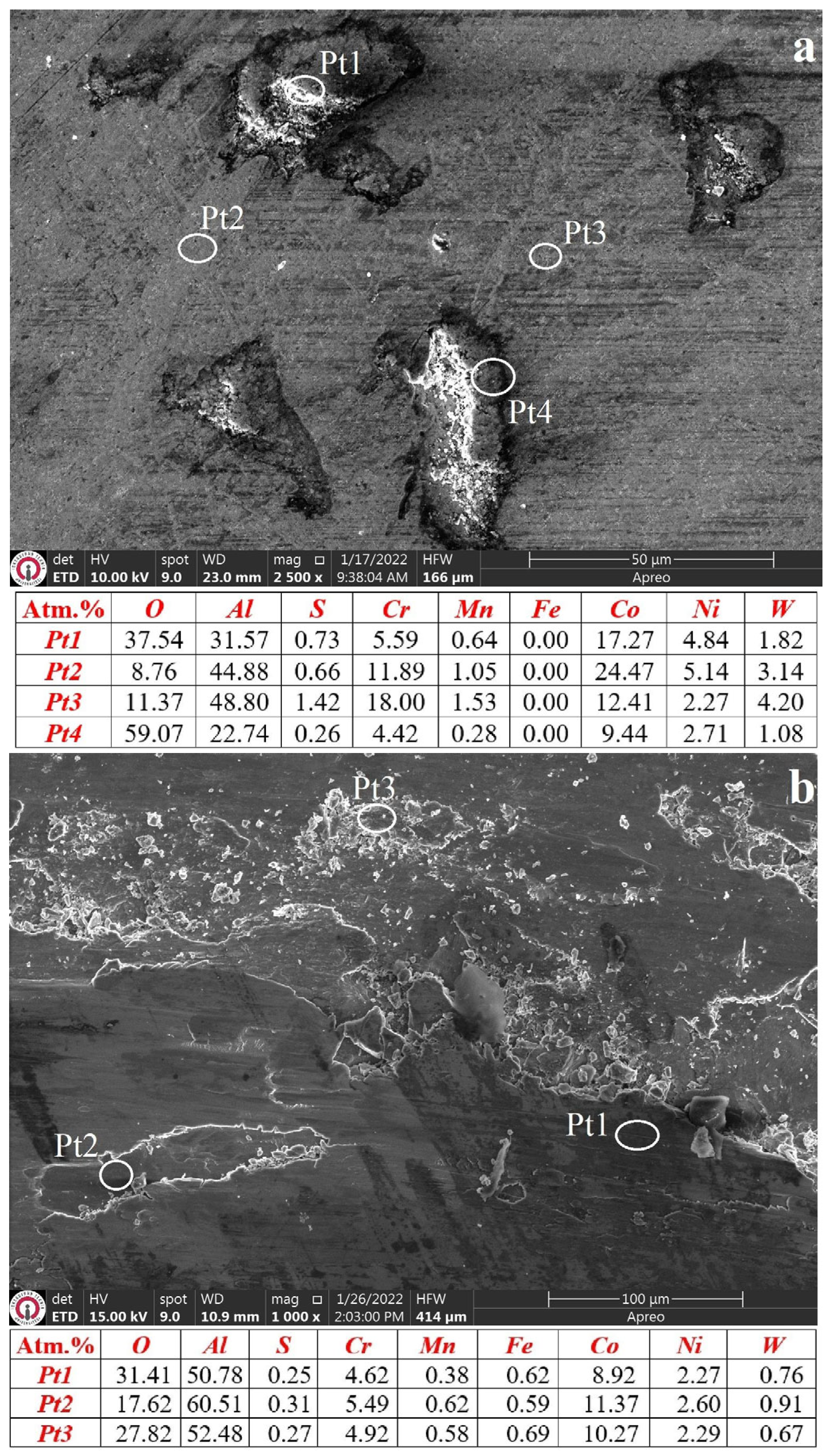
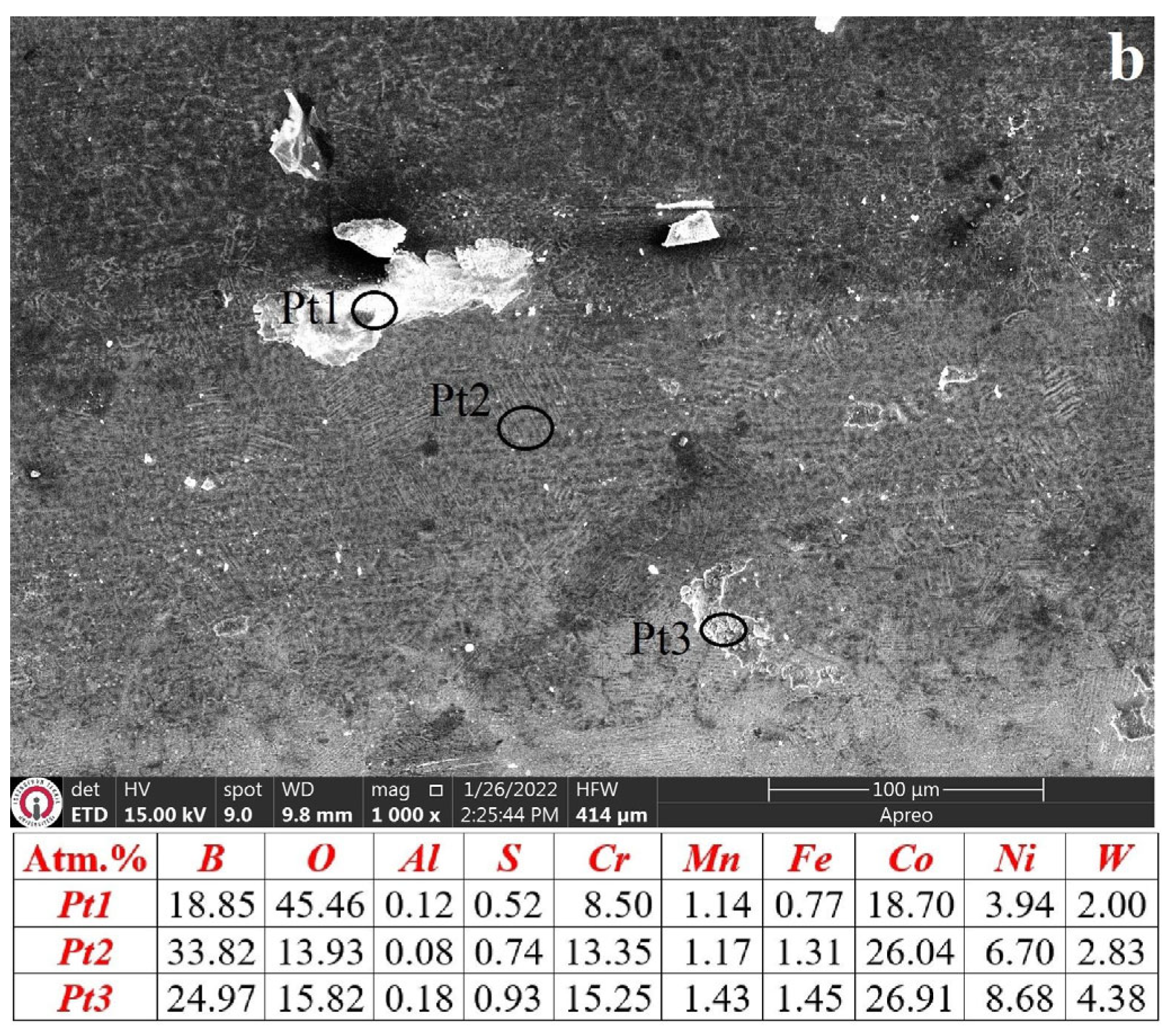
3.3. Characterization of Wear Mechanisms
4. Conclusions
- Chemically bonded, crack-free, and porosity-free aluminide, boride, and boro-aluminide coatings were obtained on the surface of the Haynes 25 superalloy using cost-effective package cementation techniques.
- The obtained coatings had thicknesses ranging from 14.73 to 140 µm and hardness values ranging from 1270 to 2833 HV0.1, depending on the coating method.
- The dominant phases obtained through the coatings were Co2Al5 in aluminide coating, CoB and Co2B in boride coating, and CoAl and Co4B in boro-aluminide coating.
- Thermochemical coatings improved the wear resistance of the Haynes 25 alloy both at room temperature and at 500 °C.
- The highest wear resistance was achieved in the boronized sample with the highest hardness at room temperature, while at 500 °C, the best wear resistance was obtained in the boron-aluminized sample with the highest H3/E2′ and optimal fracture toughness.
- The wear mechanism is dependent on the wear temperature, surface characteristics of the coatings, and mechanical properties of the coatings. A significant increase in oxidative and adhesive wear mechanisms was observed at high temperatures.
- This study has shown that the wear resistance of the widely used Haynes-25 alloy in high-temperature applications can be improved 10-fold with boro-aluminide coating based on the relationship between hardness, elastic modulus, and fracture toughness.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geddes, B.; Leon, H.; Huang, X. Overview of Superalloys. In Superalloys: Alloying and Performance; ASM International: Almere, The Netherlands, 2010; pp. 9–16. [Google Scholar]
- Gialanella, S.; Malandruccolo, A.; Gialanella, S.; Malandruccolo, A. Superalloys. In Aerospace Alloys; Springer: Berlin/Heidelberg, Germany, 2020; pp. 267–386. [Google Scholar]
- De Faria Cunha, F.A.; de Andrade Reis, R.; Gonçalves, S.P.; Fernandes, F.A.P.; Baldan, R.; de Sousa Malafaia, A.M. Cyclic Oxidation Behavior of Conventional and Niobium-Modified MAR-M246 Superalloy at 900 and 1000 °C. Coatings 2023, 13, 519. [Google Scholar] [CrossRef]
- Youdelis, W.V.; Kwon, O. Carbide phases in cobalt base superalloy: Role of nucleation entropy in refinement. Met. Sci. 1983, 17, 379–384. [Google Scholar] [CrossRef]
- Jokisaari, A.M.; Naghavi, S.S.; Wolverton, C.; Voorhees, P.W.; Heinonen, O.G. Predicting the morphologies of γ precipitates in cobalt-based superalloys. Acta Mater. 2017, 141, 273–284. [Google Scholar] [CrossRef]
- Coutsouradis, D.; Davin, A.; Lamberigts, M. Cobalt-based superalloys for applications in gas turbines. Mater. Sci. Eng. 1987, 88, 11–19. [Google Scholar] [CrossRef]
- Gui, W.; Zhang, H.; Yang, M.; Jin, T.; Sun, X.; Zheng, Q. The investigation of carbides evolution in a cobalt-base superalloy at elevated temperature. J. Alloys Compd. 2017, 695, 1271–1278. [Google Scholar] [CrossRef]
- Akande, I.G.; Oluwole, O.O.; Fayomi, O.S.I.; Odunlami, O.A. Overview of mechanical, microstructural, oxidation properties and high-temperature applications of superalloys. Mater. Today Proc. 2021, 43, 2222–2231. [Google Scholar] [CrossRef]
- Vorontsov, V.A.; McAuliffe, T.P.; Hardy, M.C.; Dye, D.; Bantounas, I. Precipitate dissolution during deformation induced twin thickening in a CoNi-base superalloy subject to creep. Acta Mater. 2022, 232, 117936. [Google Scholar] [CrossRef]
- Nourpoor, P.; Javadian, S.; Sabour Rouh Aghdam, A.; Ghadami, F. Microstructure Investigation and Cyclic Oxidation Resistance of Ce-Si-Modified Aluminide Coating Deposited by Pack Cementation on Inconel 738LC. Coatings 2022, 12, 1491. [Google Scholar] [CrossRef]
- Cuao-Moreu, C.A.; Campos-Silva, I.; Delgado-Brito, A.M.; Garcia-Sanchez, E.O.; Juarez-Hernandez, A.; Diabb-Zavala, J.M.; Hernandez-Rodriguez, M.A.L. Effect of laser surface texturing and boriding on the tribocorrosion resistance of an ASTM F-1537 cobalt alloy. Wear 2023, 523, 204799. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, Y.; Yuan, C.; Kong, W.; Wang, Y.; Wen, X.; Liu, X. Effect of internal nitridation on microstructure and mechanical properties of cobalt-based superalloy under high-temperature nitrogen atmosphere. Mater. Charact. 2023, 200, 112851. [Google Scholar] [CrossRef]
- Xiong, J.C.; Li, J.R.; Liu, S.Z.; Zhao, J.Q.; Han, M. Effects of carburization on recrystallization behavior of a single crystal superalloy. Mater. Charact. 2010, 61, 749–755. [Google Scholar] [CrossRef]
- Günen, A.; Gürol, U.; Koçak, M.; Çam, G. Investigation into the influence of boronizing on the wear behavior of additively manufactured Inconel 625 alloy at elevated temperature. Prog. Addit. Manuf. 2023, 1–21. [Google Scholar] [CrossRef]
- Winter, K.M.; Kalucki, J.; Koshel, D. Process technologies for thermochemical surface engineering. In Thermochemical Surface Engineering of Steels; Woodhead Publishing: Cambridge, UK, 2015; pp. 141–206. [Google Scholar]
- Günen, A.; Kanca, Y.; Karahan, İ.H.; Karakaş, M.S.; Gök, M.S.; Kanca, E.; Çürük, A. A comparative study on the effects of different thermochemical coating techniques on corrosion resistance of STKM-13A steel. Metall. Mater. Trans. A 2018, 49, 5833–5847. [Google Scholar] [CrossRef]
- Günen, A.; Lindner, T.; Karakaş, M.S.; Kanca, E.; Töberling, G.; Vogt, S.; Gök, M.S.; Lampke, T. Effect of the boriding environment on the wear response of laser-clad AlCoCrFeNi high entropy alloy coatings. Surf. Coat. Technol. 2022, 447, 128830. [Google Scholar] [CrossRef]
- Yener, T.; Doleker, K.M.; Erdogan, A.; Oge, M.; Er, Y.; Karaoglanli, A.C.; Zeytin, S. Wear and oxidation performances of low temperature aluminized IN600. Surf. Coat. Technol. 2022, 436, 128295. [Google Scholar] [CrossRef]
- Mu, D.; Shen, B.L. Oxidation resistance of boronized CoCrMo alloy. Int. J. Refract. Met. Hard Mater. 2010, 28, 424–428. [Google Scholar] [CrossRef]
- Doñu-Ruiz, M.; López-Perrusquia, N.; Renteria-Salcedo, A.; Flores-Martinez, M.; Anda, E.R.-D.; Muhl, S.; Hernández-Navarro, C.; García, E. Tribocorrosion behavior of boride coating on CoCrMo alloy produced by thermochemical process in 0.35% NaCl solution. Surf. Coat. Technol. 2021, 425, 127698. [Google Scholar] [CrossRef]
- Öğe, M.; Küçük, Y.; Öğe, T.Ö.; Günen, A.; Kanca, Y.; Gök, M.S. Effect of boriding on high temperature tribological behavior of CoCrMo alloy. Tribol. Int. 2023, 187, 108697. [Google Scholar] [CrossRef]
- Donachie, M.J.; Donachie, S.J. Superalloys: A Technical Guide; ASM International: Almere, The Netherlands, 2002. [Google Scholar]
- Kramer, D.P.; McDougal, J.R.; Ruhkamp, J.D.; McNeil, D.C.; Koehler, F.A.; Booher, R.A.; Howell, E.I. Application of the cobalt based superalloy Haynes Alloy 25 (L605) in the fabrication of future radioisotope power systems. In AIP Conference Proceedings; American Institute of Physics: College Park, MI, USA, 1998; Volume 420, pp. 1167–1172. [Google Scholar]
- Lee, J.W.; Kuo, Y.C. Cyclic oxidation behavior of a cobalt aluminide coating on Co-base superalloy AMS 5608. Surf. Coat. Technol. 2005, 200, 1225–1230. [Google Scholar] [CrossRef]
- Delgado-Brito, A.M.; López-Suero, D.; Ruiz-Ríos, A.; García-León, R.A.; Martínez-Trinidad, J.; Oseguera-Peña, J.; Campos-Silva, I. Effect of the diffusion annealing process in the indentation properties of cobalt boride layer. Ceram. Int. 2019, 45, 7767–7777. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, T.W.; Son, K.S.; Yoon, J.H.; Kim, H.S.; Leisk, G.G.; Mitton, D.B.; Latanision, R.M. Aluminizing and boroaluminizing treatments of Mar-M247 and their effect on hot corrosion resistance in Na2SO4–NaCl molten salt. Met. Mater. Int. 2003, 9, 303–310. [Google Scholar] [CrossRef]
- Paoletti, L.; Bruni, B.M.; Gianfagna, A.; Mazziotti-Tagliani, S.; Pacella, A. Quantitative energy dispersive X-ray analysis of submicrometric particles using a scanning electron microscope. Microsc. Microanal. 2011, 17, 710–717. [Google Scholar] [CrossRef]
- Günen, A.; Döleker, K.M.; Korkmaz, M.E.; Gök, M.S.; Erdogan, A. Characteristics, high temperature wear and oxidation behavior of boride layer grown on nimonic 80A Ni-based superalloy. Surf. Coat. Technol. 2021, 409, 126906. [Google Scholar] [CrossRef]
- Gök, M.S.; Küçük, Y.; Erdoğan, A.; Öge, M.; Kanca, E.; Günen, A. Dry sliding wear behavior of borided hot-work tool steel at elevated temperatures. Surf. Coat. Technol. 2017, 328, 54–62. [Google Scholar] [CrossRef]
- Hardell, J.; Kassfeldt, E.; Prakash, B. Friction and wear behaviour of high strength boron steel at elevated temperatures of up to 800 °C. Wear 2008, 264, 788–799. [Google Scholar] [CrossRef]
- Chen, C.; Lv, B.; Ma, H.; Sun, D.; Zhang, F. Wear behavior and the corresponding work hardening characteristics of Hadfield steel. Tribol. Int. 2018, 121, 389–399. [Google Scholar] [CrossRef]
- Kato, K. Wear in relation to friction—A review. Wear 2000, 241, 151–157. [Google Scholar] [CrossRef]
- Beake, B.D. The influence of the H/E ratio on wear resistance of coating systems—Insights from small-scale testing. Surf. Coat. Technol. 2022, 442, 128272. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Günen, A.; Bölükbaşı, Ö.S.; Özgürlük, Y.; Özkan, D.; Odabaş, O.; Somunkıran, İ. Effect of Cr addition on properties and tribological behavior at elevated temperature of boride layers grown on borosintered powder metallurgy alloys. Met. Mater. Int. 2023, 29, 748–766. [Google Scholar] [CrossRef]
- Wu, J.M.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chen, H.C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Dhakne, A.; Jaju, S.; Shukla, S. Review on analysis of enhancing wear properties through thermo-mechanical treatment and grain size. Mater. Today Proc. 2022, 60, 2270–2272. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Chen, K.M.; Wang, L.; Cui, X.H.; Wang, S.Q. Characteristics of oxidative wear and oxidative mildwear. Tribol. Int. 2013, 61, 214–223. [Google Scholar] [CrossRef]
- Erdogan, A.; Yener, T.; Doleker, K.M.; Korkmaz, M.E.; Gök, M.S. Low-temperature aluminizing influence on degradation of nimonic 80A surface: Microstructure, wear and high temperature oxidation behaviors. Surf. Interfaces 2021, 25, 101240. [Google Scholar] [CrossRef]
- Polcar, T.; Cavaleiro, A. High-temperature tribological properties of CrAlN, CrAlSiN and AlCrSiN coatings. Surf. Coat. Technol. 2011, 206, 1244–1251. [Google Scholar] [CrossRef]
- Erdemir, A. Boron-Based Solid Nanolubricants and Lubrication Additives; Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 203–223. [Google Scholar]
- Wang, L.; Nie, X. Effect of annealing temperature on tribological properties and material transfer phenomena of CrN and CrAlN coatings. J. Mater. Eng. Perform. 2014, 23, 560–571. [Google Scholar] [CrossRef]







| Sample | Ra (µm) | Microhardness | Nanohardness | |||
|---|---|---|---|---|---|---|
| Sample | Ra (µm) | (HV) | Layer 1 | Layer 2 | Layer 3 | Layer 4 |
| Al | 1.25 | Max. 1270 | 12.23 ± 0.9 | 15.14 ± 1.0 | 7.66 ± 0.3 | --------- |
| B | 0.26 | Max. 2833 | 26.34 ± 2.33 | 23.54 ± 1.8 | 11.0 ± 4.6 | 7.9 ± 1.73 |
| Al-B | 0.21 | Max. 2386 | 23.46 ± 1.29 | 18.25 ± 0.61 | 12.92 ± 0.32 | 7.43 ± 0.14 |
| Properties | Elasticity modulus (GPa) | |||||
| Sample | Fracture toughness | Coating thickness (µm) | Layer 1 | Layer 2 | Layer 3 | Layer 4 |
| Al | 1.88 ± 0.08 | 140 ± 1.5 | 152.56 ± 16.26 | 196.96 ± 5.3 | 177.97 ± 6.9 | --- |
| B | 0.94 ± 0.05 | 37.58 ± 2.85 | 206.36 ± 3.68 | 200.89 ± 7.40 | 151.66 ± 28.22 | 135.08 ± 22.26 |
| Al-B | 1.06 ± 0.06 | 14.73 ± 1.71 | 177.56 ± 8.44 | 203.03 ± 13.30 | 117.16 ± 8.6 | 126.14 ± 5.43 |
| Wear Test Temperature | Samples | Wear Trace Width (µm) | Wear Trace Depth (µm) | Wear Rate mm3/Nm |
|---|---|---|---|---|
| 24 °C | Untreated | 1515.67 ± 37.89 | 48.33 ± 1.93 | 23.89 ± 0.7 |
| Al | 797.23 ± 15.94 | 70.00 ± 2.80 | 18.20 ± 0.7 | |
| B | 775.17 ± 12.92 | 8.00 ± 0.32 | 2.02 ± 0.09 | |
| AlB | 815.87 ± 20.40 | 8.67 ± 0.35 | 2.31 ± 0.1 | |
| 500 °C | Untreated | 1934.33 ± 55.27 | 55.33 ± 2.77 | 34.90 ± 1.2 |
| Al | 1279.00 ± 30.45 | 54.00 ± 1.80 | 22.52 ± 0.7 | |
| B | 941.83 ± 19.62 | 16.33 ± 0.63 | 5.02 ± 0.2 | |
| AlB | 982.53 ± 32.75 | 10.00 ± 0.36 | 3.20 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günen, A.; Ergin, Ö. A Comparative Study on Characterization and High-Temperature Wear Behaviors of Thermochemical Coatings Applied to Cobalt-Based Haynes 25 Superalloys. Coatings 2023, 13, 1272. https://doi.org/10.3390/coatings13071272
Günen A, Ergin Ö. A Comparative Study on Characterization and High-Temperature Wear Behaviors of Thermochemical Coatings Applied to Cobalt-Based Haynes 25 Superalloys. Coatings. 2023; 13(7):1272. https://doi.org/10.3390/coatings13071272
Chicago/Turabian StyleGünen, Ali, and Ömer Ergin. 2023. "A Comparative Study on Characterization and High-Temperature Wear Behaviors of Thermochemical Coatings Applied to Cobalt-Based Haynes 25 Superalloys" Coatings 13, no. 7: 1272. https://doi.org/10.3390/coatings13071272
APA StyleGünen, A., & Ergin, Ö. (2023). A Comparative Study on Characterization and High-Temperature Wear Behaviors of Thermochemical Coatings Applied to Cobalt-Based Haynes 25 Superalloys. Coatings, 13(7), 1272. https://doi.org/10.3390/coatings13071272






