The Corrosion Behavior of Al/Al2O3 Composite Films with Ultra-Dense Structure Exposed to Lead-Bismuth Eutectic at 450 to 650 °C
Abstract
:1. Introduction
2. Experimental
2.1. Coating Deposition
2.2. Material Characterization
2.3. Mechanical Properties
2.4. Corrosion Properties in LBE
3. Results
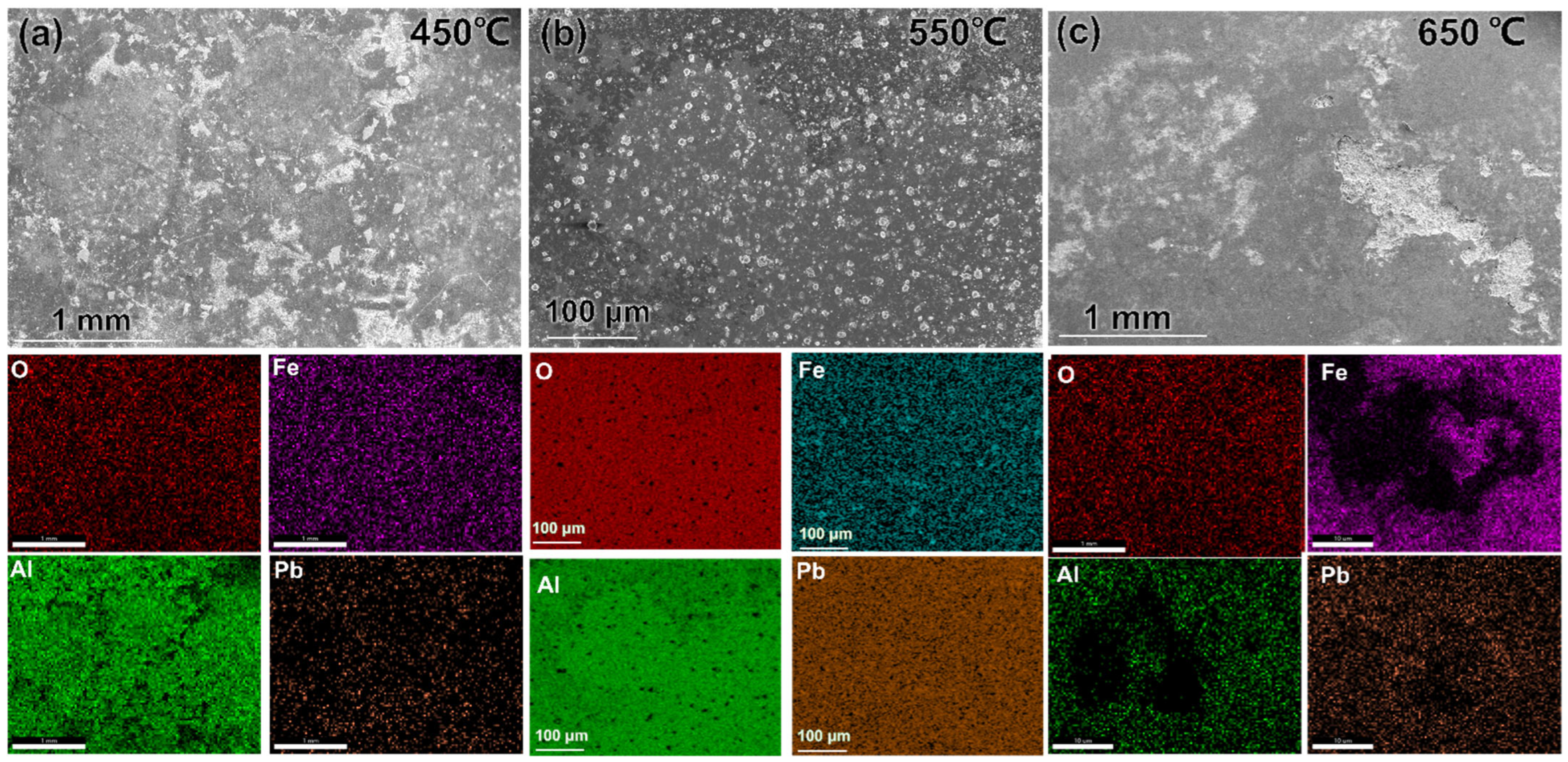
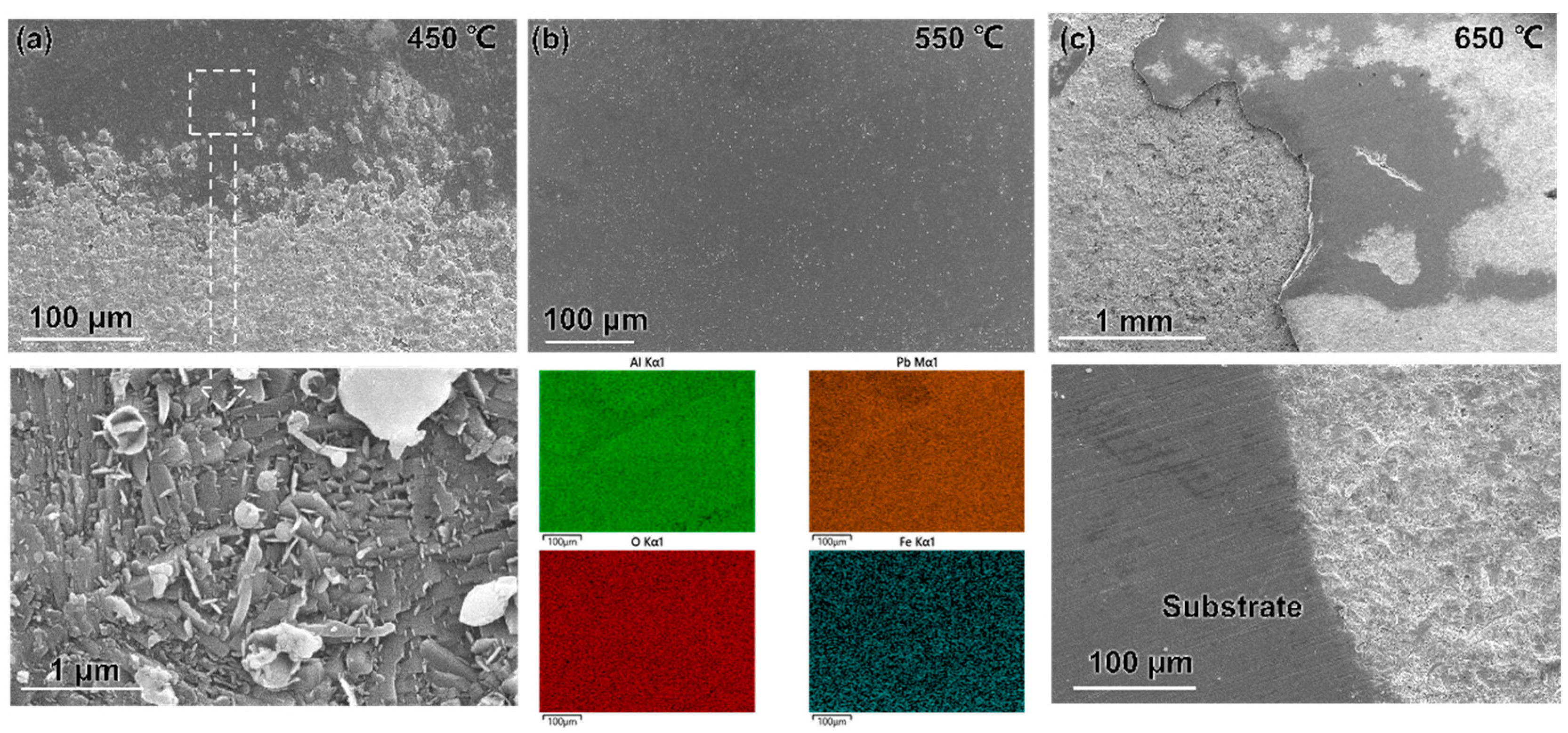
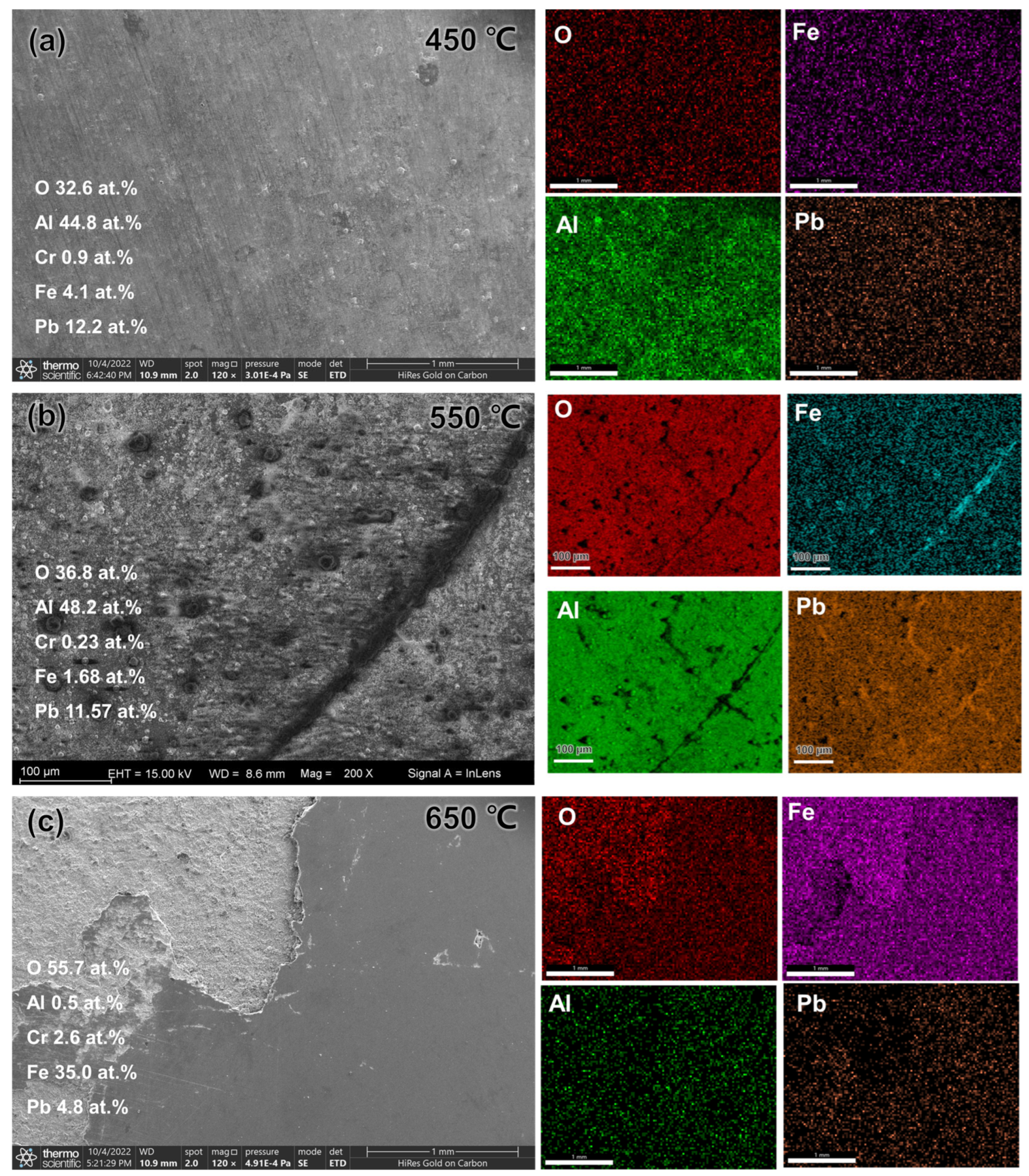
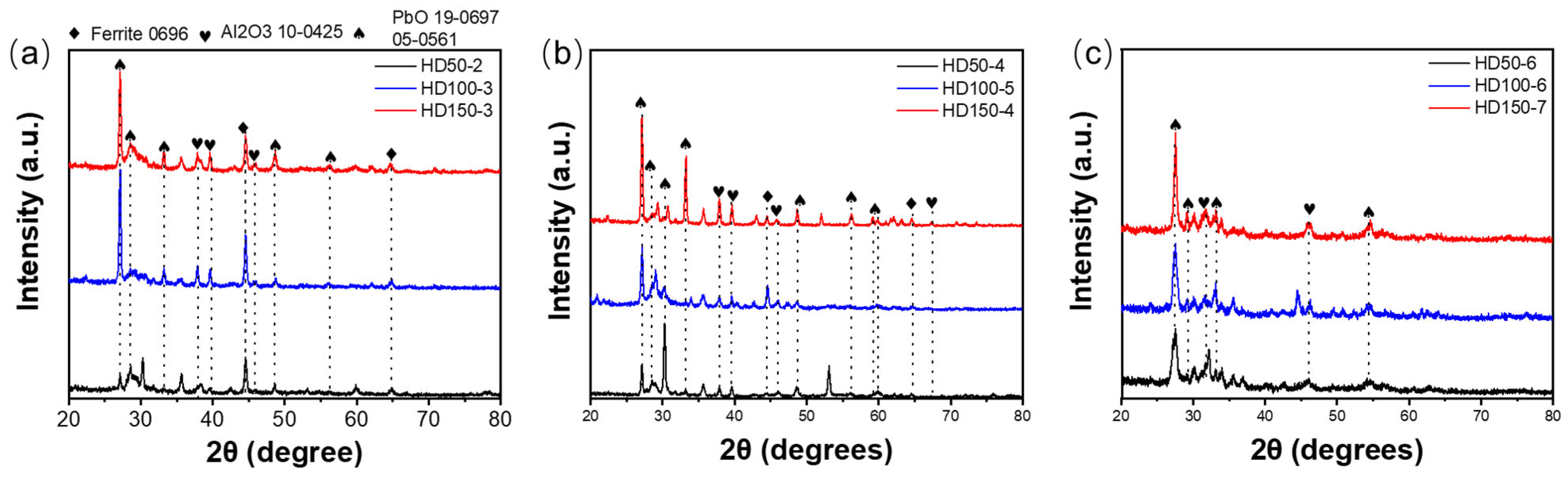
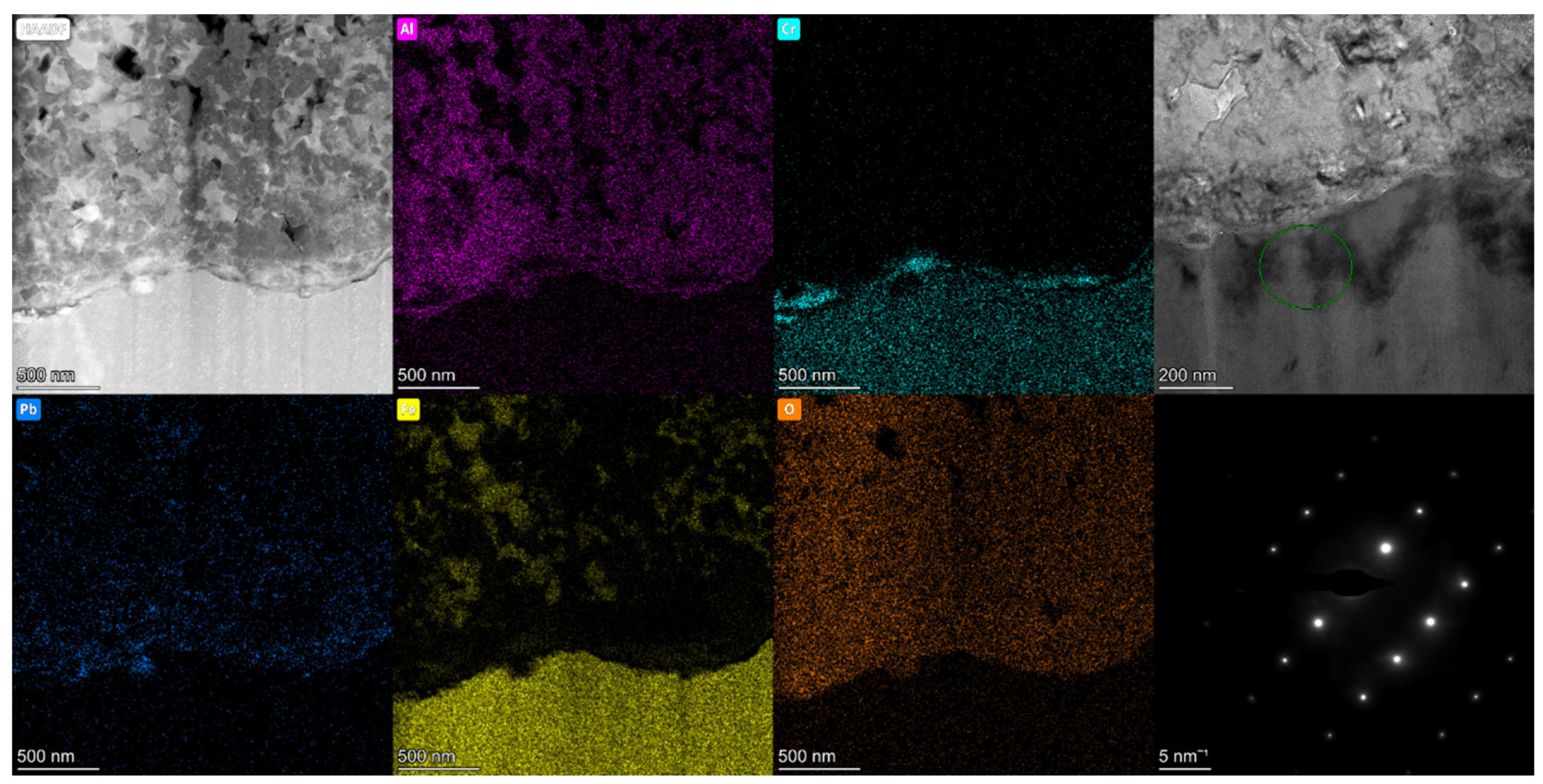
3.1. Microstructure and Composition
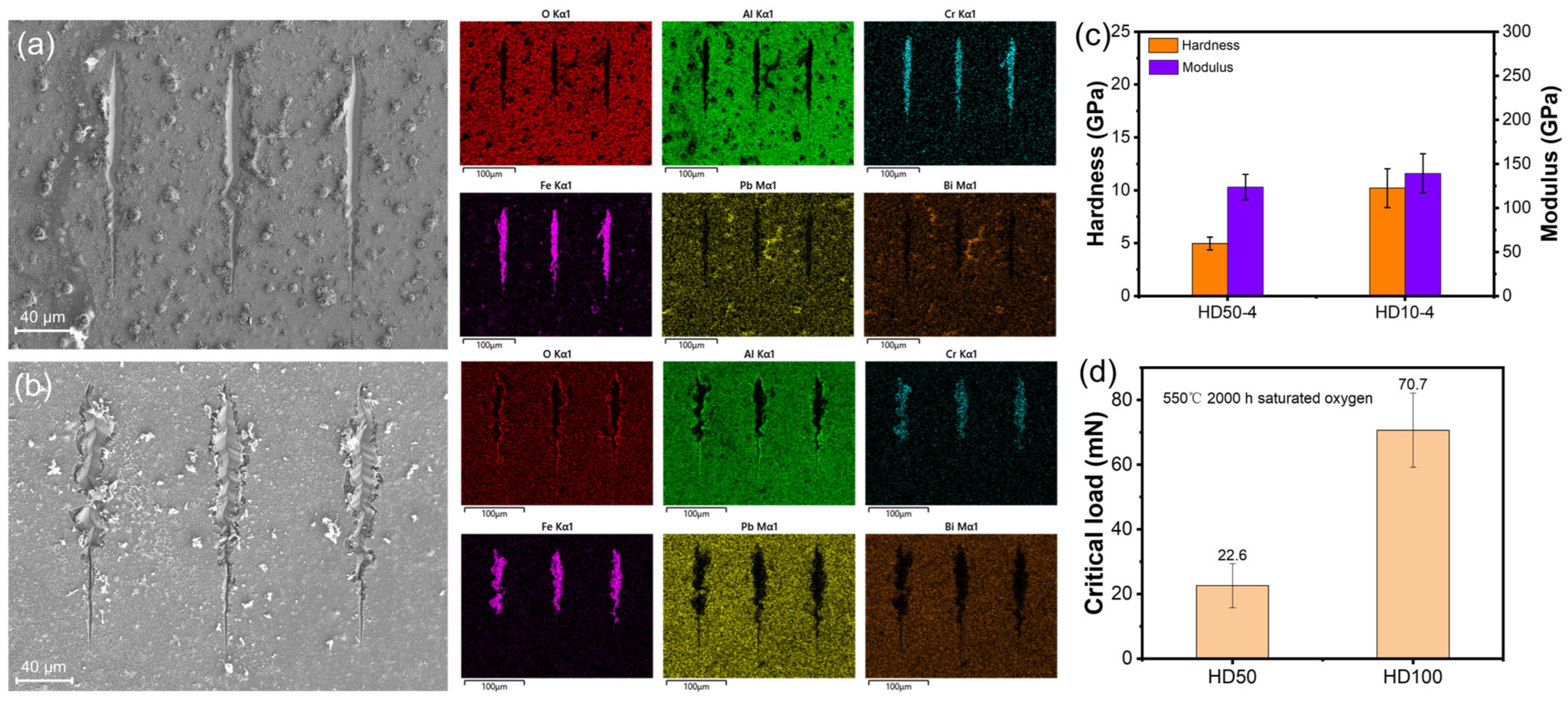
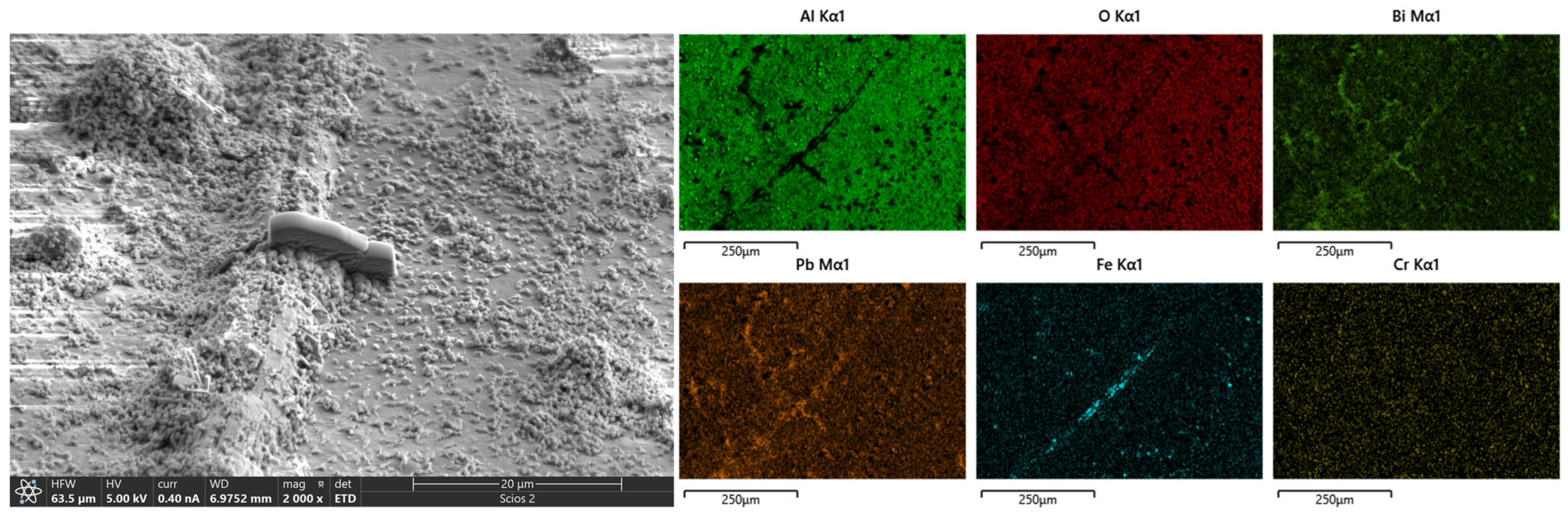
3.2. Morphology, Structure, Mechanical Properties after LBE Corrosion
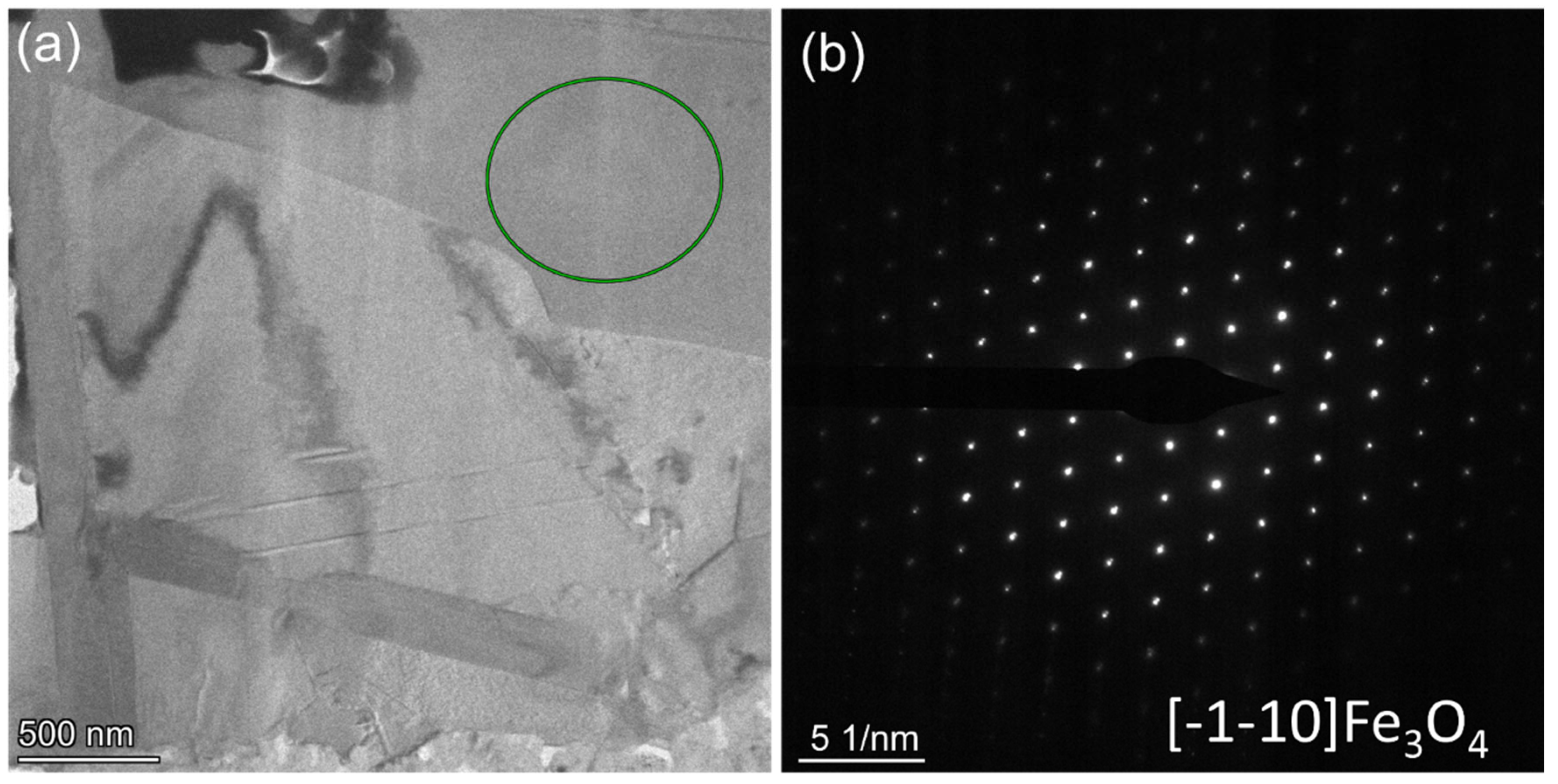
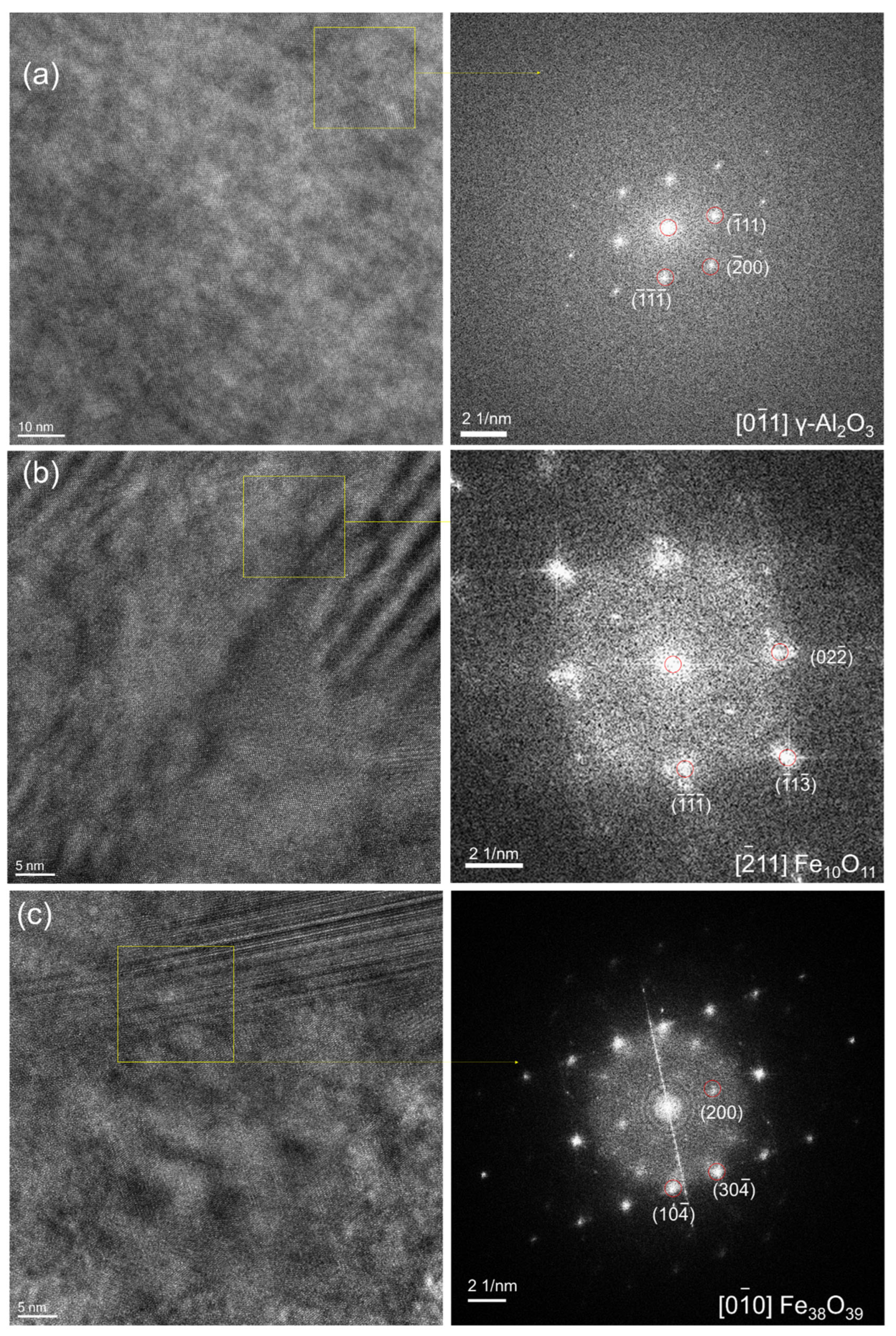
3.3. TEM Analysis of Corrosion Resistance of Al2O3 for 2000 h
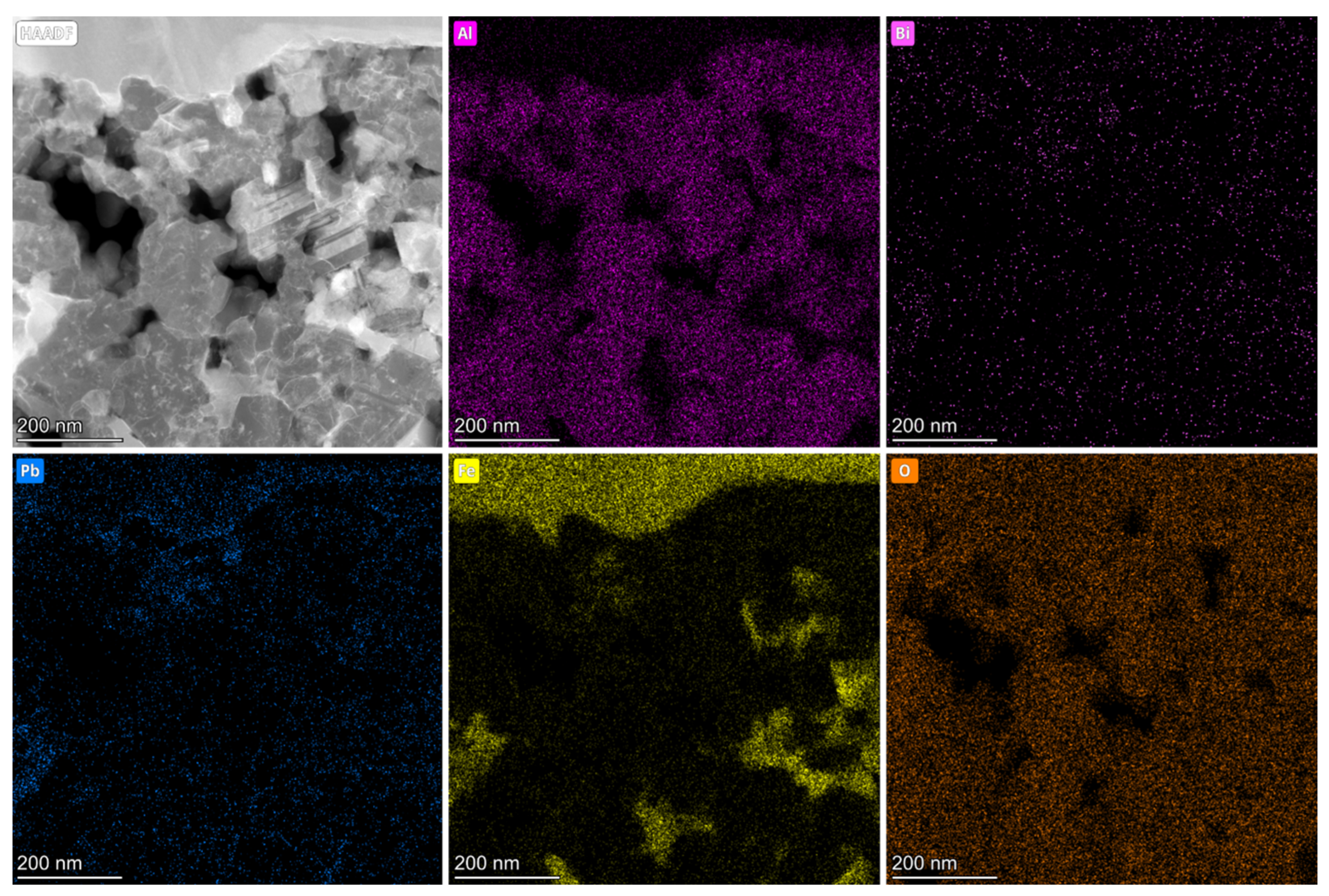
3.4. TEM Analysis of Al2O3 Coatings after LBE Corrosion for 4000 h
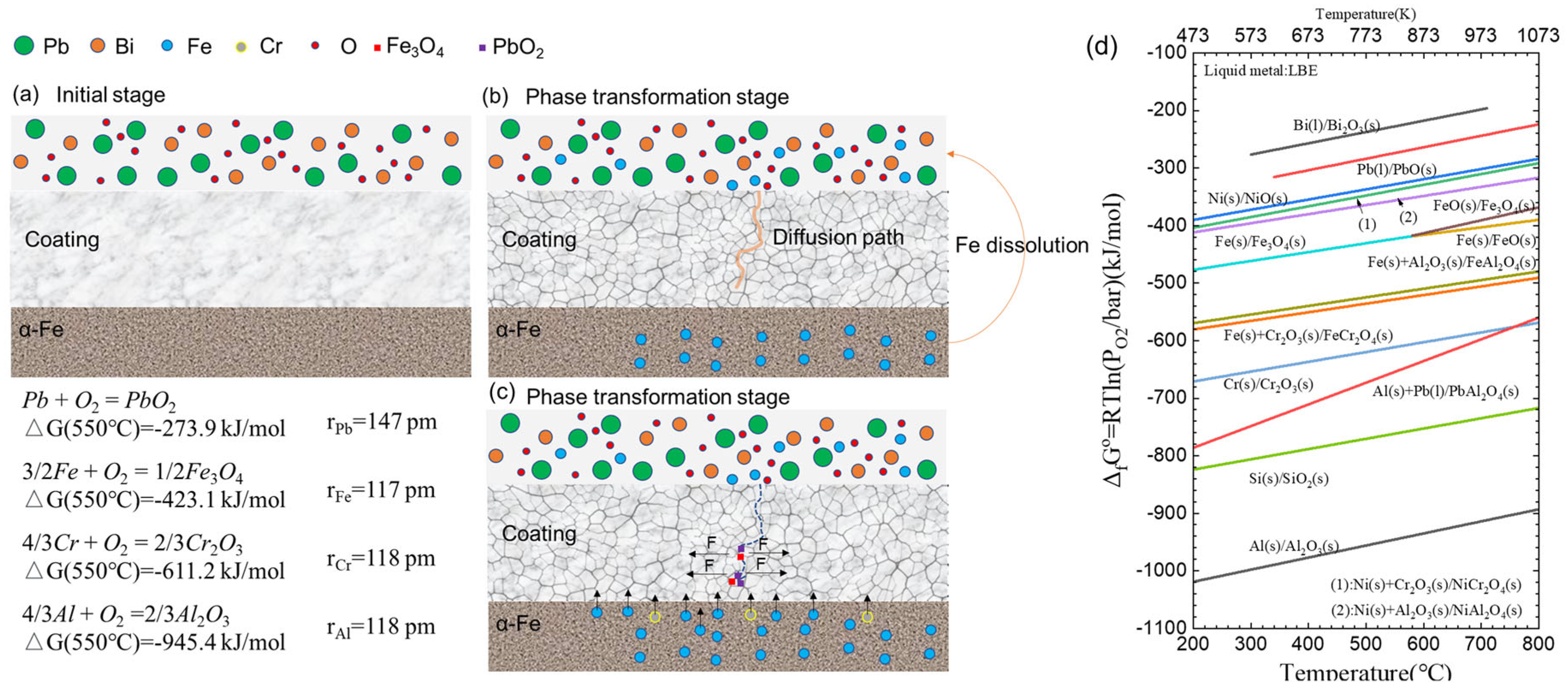
4. Discussion
5. Conclusions
- Amorphous Al2O3 coatings with an ultra-dense structure were successfully deposited at bias voltages of −50 V, −100 V, and 150 V. As the temperature increased from 450 to 650 °C, the coatings experienced severe LBE corrosion, resulting in the formation of nodule-shaped corrosion products during the initial stage of corrosion.
- The samples tested at 550 °C exhibited phase transformation from amorphous to γ- and κ-Al2O3 coatings, leading to a reduction in the volume of small grains and the presence of pores between grains. However, the formation of corrosion products consisting of Fe and Pb caused volume expansion, resulting in stress corrosion cracking.
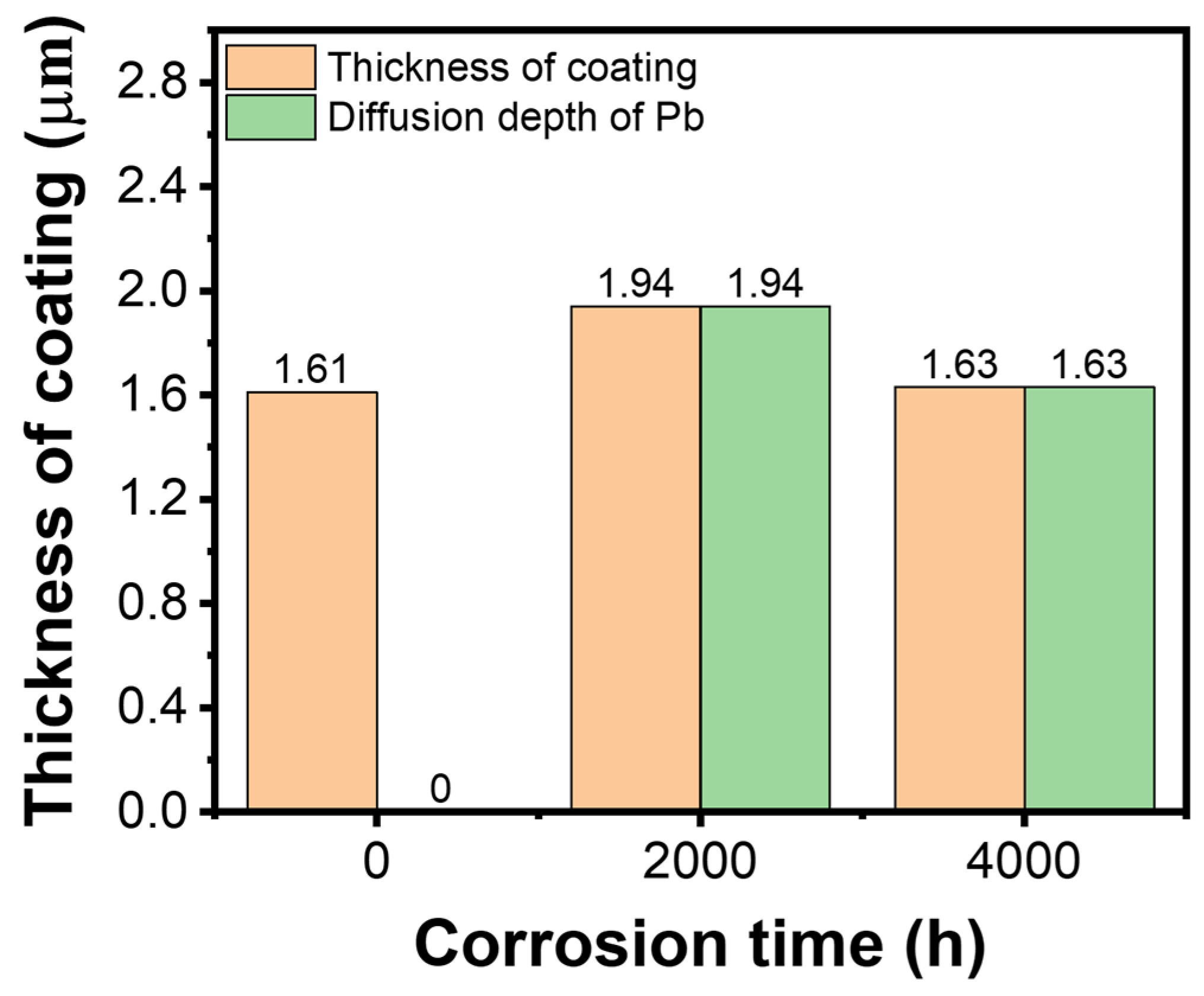
- During the corrosion time of up to 4000 h, the corrosion products of Fe and Pb permeated throughout the entire coating matrix. No phase changes were observed in the α-Fe substrate. Importantly, no corrosion products were detected within the inner regions of γ-Al2O3 small grains, indicating the significant potential of this material for practical applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cinotti, L.; Smith, C.F.; Sekimoto, H.; Mansani, L.; Reale, M.; Sienicki, J.J. Lead-cooled system design and challenges in the frame of Generation IV International Forum. J. Nucl. Mater. 2011, 415, 245–253. [Google Scholar] [CrossRef]
- Kelly, J.E. Generation IV International Forum: A decade of progress through international cooperation. Prog. Nucl. Energy 2014, 77, 240–246. [Google Scholar] [CrossRef]
- Locatelli, G.; Mancini, M.; Todeschini, N. Generation IV nuclear reactors: Current status and future prospects. Energy Policy 2013, 61, 1503–1520. [Google Scholar] [CrossRef]
- Abu-Khader, M.M. Recent advances in nuclear power: A review. Prog. Nucl. Energy 2009, 51, 225–235. [Google Scholar] [CrossRef]
- Lorusso, P.; Bassini, S.; Del Nevo, A.; Di Piazza, I.; Giannetti, F.; Tarantino, M.; Utili, M. GEN-IV LFR development: Status & perspectives. Prog. Nucl. Energy 2018, 105, 318–331. [Google Scholar]
- Alemberti, A.; Smirnov, V.; Smith, C.F.; Takahashi, M. Overview of lead-cooled fast reactor activities. Prog. Nucl. Energy 2014, 77, 300–307. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, J.; Luan, H.; Yang, Z.; Wang, W.; Yan, W.; Shan, Y.; Yang, K. Oxidation behavior of ferritic/martensitic steels in stagnant liquid LBE saturated by oxygen at 600 °C. J. Nucl. Mater. 2015, 457, 135–141. [Google Scholar] [CrossRef]
- Yvon, P.; Carré, F. Structural materials challenges for advanced reactor systems. J. Nucl. Mater. 2009, 385, 217–222. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H.; Fetzer, R.; Heinzel, A.; Weisenburger, A.; Wang, K.; Jianu, A.; Müller, G. Influence of Si addition on the corrosion behavior of 9 wt% Cr ferritic/martensitic steels exposed to oxygen-controlled molten Pb-Bi eutectic at 550 and 600 °C. Corros. Sci. 2021, 193, 109871. [Google Scholar] [CrossRef]
- Chen, Y.; Long, Y.; Luo, H.; Xie, Z.; Lin, W.; Guo, L.; Wang, H.; An, X.; Kong, Q.; Zhang, W. Effects of different silicon content on irradiation defects and hardening in 9Cr ferritic/martensitic steel. J. Nucl. Mater. 2022, 571, 154026. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, J.; Wang, H.; Chen, Y.; Yin, X.; Guo, N. Corrosion behavior and surface treatment of cladding materials used in high-temperature lead-bismuth eutectic alloy: A review. Coatings 2021, 11, 364. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N. Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 2008, 373, 351–377. [Google Scholar] [CrossRef]
- Shi, K.; Yang, J.; Chen, Q.; Sun, Y.; Zhang, W.; He, K.; Tang, R.; Ning, Z.; Liao, J.; Yang, Y. Corrosion behavior of ion-irradiated 12Cr2W2Mn F/M steel in liquid LBE. Nucl. Mater. Energy 2021, 28, 101033. [Google Scholar] [CrossRef]
- Chen, Q.; Bai, F.; Wang, P.; Yang, J.; Zhu, C.; Zhang, W.; Liu, H.; Zhong, Y.; Deng, J.; Liu, N. Microstructure response and lead-bismuth eutectic corrosion behavior of 11Cr1Si ferritic/martensitic steel after Au-ion irradiation. Corros. Sci. 2022, 198, 110101. [Google Scholar] [CrossRef]
- Schroer, C.; Voß, Z.; Wedemeyer, O.; Novotny, J.; Konys, J. Oxidation of steel T91 in flowing lead–bismuth eutectic (LBE) at 550 °C. J. Nucl. Mater. 2006, 356, 189–197. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, X.; Cao, S.; Wang, D.; Zhu, Q.; Lei, Y. Improvement of the mechanical properties and LBE cavitation erosion resistance of laser cladded FeCrAlTiC coating by Y2O3 addition. Mater. Lett. 2022, 326, 132882. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, W.; Chen, Q.; Yang, J.; Zhu, C.; Li, Q.; Yang, J.; Liu, N.; Yang, J. Effect of LBE corrosion on microstructure of amorphous Al2O3 coating by magnetron sputtering. J. Surf. Coat. Technol. 2022, 443, 128598. [Google Scholar] [CrossRef]
- Yang, J.; Shi, K.; Zhang, W.; Chen, Q.; Ning, Z.; Zhu, C.; Liao, J.; Yang, Y.; Liu, N.; Yang, J. A novel AlCrFeMoTi high-entropy alloy coating with a high corrosion-resistance in lead-bismuth eutectic alloy. J. Corros. Sci. 2021, 187, 109524. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, C.; Yang, J.; Zhang, W.; He, L.; Zhang, R.; Yang, H.; Wang, J.; Long, J.; Chang, H. Mechanical and high-temperature corrosion properties of AlTiCrNiTa high entropy alloy coating prepared by magnetron sputtering for accident-tolerant fuel cladding. Surf. Coat. Technol. 2021, 417, 127228. [Google Scholar] [CrossRef]
- Vogt, J.-B.; Serre, I.P. A review of the surface modifications for corrosion mitigation of steels in lead and LBE. Coatings 2021, 11, 53. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Kim, J.-K.; Kim, B.-S.; Shim, S.-H.; Lee, B.-T. Characterization of SiO2 and TiO2 films prepared using rf magnetron sputtering and their application to anti-reflection coating. Vacuum 2004, 76, 507–515. [Google Scholar] [CrossRef]
- Lin, J.; Wu, Z.; Zhang, X.; Mishra, B.; Moore, J.; Sproul, W. A comparative study of CrNx coatings Synthesized by dc and pulsed dc magnetron sputtering. Thin Solid Films 2009, 517, 1887–1894. [Google Scholar] [CrossRef]
- Hong, R.; Jiang, X.; Szyszka, B.; Sittinger, V.; Pflug, A. Studies on ZnO: Al thin films deposited by in-line reactive mid-frequency magnetron sputtering. Appl. Surf. Sci. 2003, 207, 341–350. [Google Scholar] [CrossRef]
- Zhang, T.; Liao, H.; Huang, W.; Ruan, H.; Su, Y.; Yang, X.; Xu, Z.; Yin, S.; Wang, J. High temperature steam oxidation behavior of textured Cr coatings with different grain structures. J. Surf. Coat. Technol. 2023, 459, 129358. [Google Scholar] [CrossRef]
- Riedl, H.; Kagerer, S.; Zauner, L.; Wojcik, T.; Kolozsvári, S.; Capek, J.; Kozák, T.; Zeman, P.; Mayrhofer, P.H. Influence of transition metal dopants on the reactive HiPIMS deposition of γ-Al2O3 thin films. In Proceedings of the PLATHINIUM (Plasma Thin film International Union Meeting), Antibes, France, 13–17 September 2019. [Google Scholar]
- Ghailane, A.; Makha, M.; Larhlimi, H.J.; Alami, J. Design of hard coatings deposited by HiPIMS and dcMS. Mater. Lett. 2020, 280, 128540. [Google Scholar] [CrossRef]
- Cui, S.; Liu, L.; Jin, Z.; Zhou, L.; Ruan, Q.; Wu, Z.; Fu, R.K.; Tian, X.; Chu, P.K.; Wu, Z. Characteristics of continuous high power magnetron sputtering (C-HPMS) in reactive O2/Ar atmospheres. J. Appl. Phys. 2021, 129, 243301. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Bhushan, B.; Li, X. Nanomechanical characterisation of solid surfaces and thin films. Int. Mater. Rev. 2003, 48, 125–164. [Google Scholar] [CrossRef]
- Ferré, F.G.; Bertarelli, E.; Chiodoni, A.; Carnelli, D.; Gastaldi, D.; Vena, P.; Beghi, M.G.; Di Fonzo, F. The mechanical properties of a nanocrystalline Al2O3/a-Al2O3 composite coating measured by nanoindentation and Brillouin spectroscopy. Acta Mater. 2013, 61, 2662–2670. [Google Scholar] [CrossRef]
- Ma, H.; Gong, X.; Zhu, J.; Chen, H.; Li, J.; Liu, Y.; Ren, Q.; Zhang, F.; Pang, Z. Liquid metal embrittlement susceptibility of an oxide-dispersion strengthened FeCrAl alloy in contact with liquid lead-bismuth eutectic at 350 °C. Corros. Sci. 2022, 207, 110603. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Jancelewicz, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS characterization of ALD Al2O3 coated porous silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Brajpuriya, R.; Shripathi, T. Investigation of Fe/Al interface as a function of annealing temperature using XPS. Appl. Surf. Sci. 2009, 255, 6149–6154. [Google Scholar] [CrossRef]
- Wan, X.; Zhao, S.; Yang, Y.; Gong, J.; Sun, C. Effects of nitrogen pressure and pulse bias voltage on the properties of Cr–N coatings deposited by arc ion plating. Surf. Coat. Technol. 2010, 204, 1800–1810. [Google Scholar] [CrossRef]
- Nordahl, C.S.; Messing, G.L. Thermal analysis of phase transformation kinetics in α-Al2O3 seeded boehmite and γ-Al2O3. Thermochim. Acta 1998, 318, 187–199. [Google Scholar] [CrossRef]
- Landälv, L.; Carlström, C.-F.; Lu, J.; Primetzhofer, D.; Jõesaar, M.; Ahlgren, M.; Göthelid, E.; Alling, B.; Hultman, L.; Eklund, P. Phase composition and transformations in magnetron-sputtered (Al,V)2O3 coatings. Thin Solid Films 2019, 688, 137369. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Y.; Wang, H.; Zhao, K.; Sun, Y.; Xiao, J.; Zhao, Y.; Gong, F.; Chen, Y. Corrosion behavior and failure mechanism of amorphous Al2O3 coating at high-temperature LBE. Vacuum 2023, 215, 112251. [Google Scholar] [CrossRef]
- Sahu, B.P.; Dutta, A.; Mitra, R. Influence of substrate bias voltage on structure and properties of DC magnetron sputtered Ni–Zr alloy thin films. J. Mater. Res. 2020, 35, 1543–1555. [Google Scholar] [CrossRef]
- Murdzek, J.A.; Rajashekhar, A.; Makala, R.S.; George, S.M. Thermal atomic layer etching of amorphous and crystalline Al2O3 films. J. Vac. Sci. Technol. A 2021, 39, 042602. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkov, S.S.; Kulkova, S.E. Adhesion properties of clean and doped Ti3Al/Al2O3 interface. Appl. Surf. Sci. 2021, 536, 147639. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Li, X.; Wang, H.; Zhao, K.; Wang, J.; Chen, L.; Wu, Z.; Chen, Y. The Corrosion Behavior of Al/Al2O3 Composite Films with Ultra-Dense Structure Exposed to Lead-Bismuth Eutectic at 450 to 650 °C. Coatings 2023, 13, 1274. https://doi.org/10.3390/coatings13071274
Yin X, Li X, Wang H, Zhao K, Wang J, Chen L, Wu Z, Chen Y. The Corrosion Behavior of Al/Al2O3 Composite Films with Ultra-Dense Structure Exposed to Lead-Bismuth Eutectic at 450 to 650 °C. Coatings. 2023; 13(7):1274. https://doi.org/10.3390/coatings13071274
Chicago/Turabian StyleYin, Xing, Xiteng Li, Hao Wang, Ke Zhao, Jun Wang, Le Chen, Zhongzhen Wu, and Yong Chen. 2023. "The Corrosion Behavior of Al/Al2O3 Composite Films with Ultra-Dense Structure Exposed to Lead-Bismuth Eutectic at 450 to 650 °C" Coatings 13, no. 7: 1274. https://doi.org/10.3390/coatings13071274
APA StyleYin, X., Li, X., Wang, H., Zhao, K., Wang, J., Chen, L., Wu, Z., & Chen, Y. (2023). The Corrosion Behavior of Al/Al2O3 Composite Films with Ultra-Dense Structure Exposed to Lead-Bismuth Eutectic at 450 to 650 °C. Coatings, 13(7), 1274. https://doi.org/10.3390/coatings13071274




