New Azo Dyes-Based Mg Complex Pigments for Optimizing the Anti-Corrosion Efficiency of Zinc-Pigmented Epoxy Ester Organic Coatings
Abstract
:1. Introduction
2. Materials
3. Experimental Part
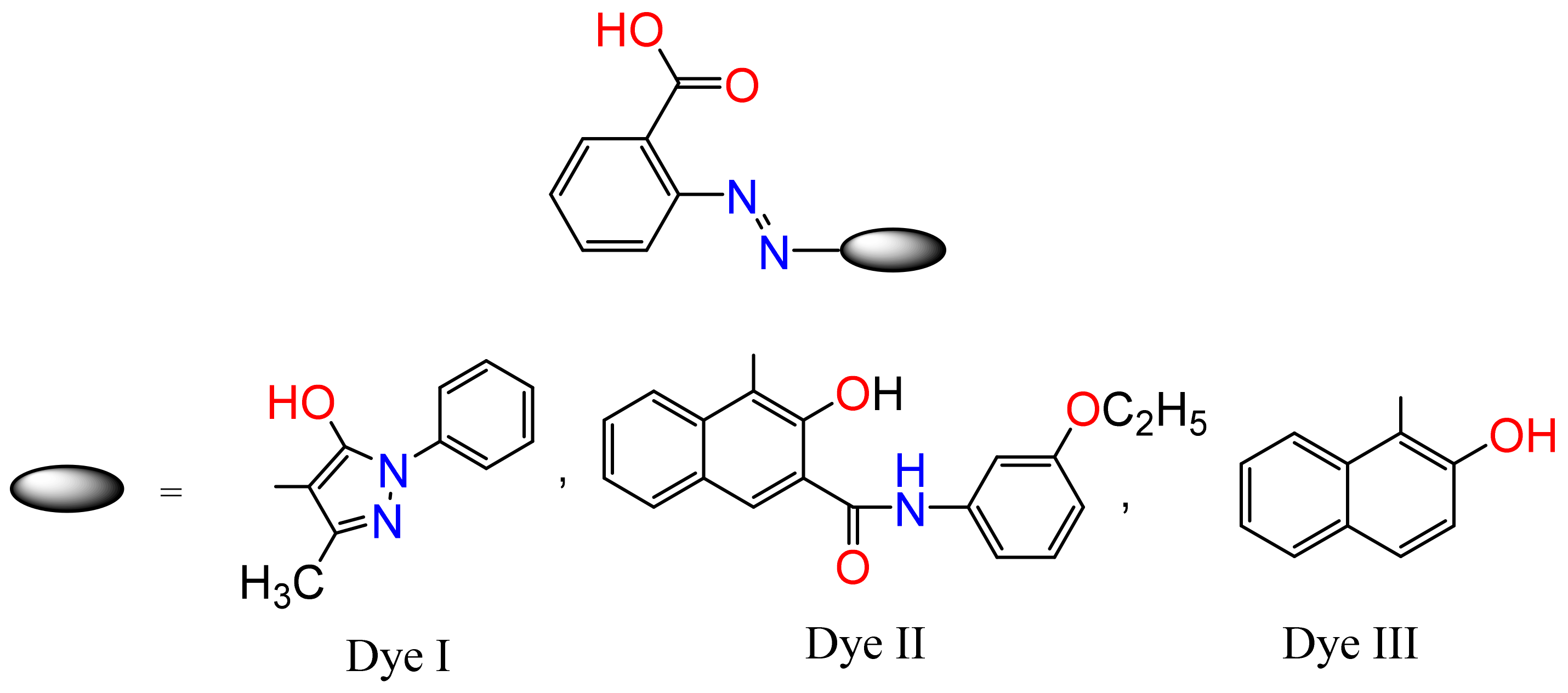
3.1. The Synthesis of Azo Dyes and Magnesium Complexes
3.1.1. Synthesis of Magnesium Complex Mg-Dye-I (C34H26MgN8O6)
Diazotization of Anthranilic Acid
Coupling to 5-Methyl-2-phenyl-3-pyrazolone, Dye I (C17H14N4O3)
Preparation of Magnesium Complex Mg-Dye-I (C34H26MgN8O6)
3.1.2. Synthesis of Magnesium Complex Mg-Dye-II (C26H19MgN3O5)
Coupling Reaction to Naphthol AS-PH, Dye II (C26H21N3O5)
Preparation of Magnesium Complex Mg-Dye-II (C26H19MgN3O5)
3.1.3. Synthesis of Magnesium Complex Mg-Dye-III (C17H10MgN2O3)
Coupling Reaction to 2-Naphthol, Dye III (C17H12N2O3)
Preparation of Magnesium Complex Mg-Dye-III (C17H10MgN2O3)
3.1.4. Synthesis of Magnesium Complex Mg-Dye-IV (C25H18MgN4O6)
Diazotization of 2-Amino-5-nitrophenol
Coupling to Naphthol AS-PH, Dye-IV (C25H20N4O6)
Preparation of Magnesium Complex Mg-Dye-IV (C25H18MgN4O6)
3.2. Characterization of the Synthesized Organic Dyes and Pigments (Complexes) by Analytical Methods
3.2.1. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
3.2.2. Elemental Analysis
3.2.3. Energy Dispersive X-ray Spectroscopy (EDX)
3.2.4. Mass Spectrometry—MALDI
3.2.5. X-ray Diffraction
3.2.6. Infrared Spectroscopy (IR)
3.3. Characterization of the Studied Pigments and the Used Binder by Methods Used in the Field of Paint Materials
3.3.1. Pigment Parameter Determination
3.3.2. SEM and EDX Measurements of Pigments
3.3.3. Specification of the Binder for Coatings
3.4. Formulation and Preparation of the Organic Coatings
3.5. Mechanical Properties of the Paints
3.6. Corrosion Test Procedures and Evaluation of Results after Corrosion Tests
3.6.1. Accelerated Cyclic Corrosion Testing in an Atmosphere of NaCl with Water Steam Condensation (Derived from ISO 9227)
3.6.2. Accelerated Cyclic Corrosion Testing in an Atmosphere of SO2 with Water Condensation (ISO 6988)
3.6.3. Accelerated Cyclic Corrosion/Weather Resistance Testing with Exposure to a Salt Electrolyte and UV Radiation
3.6.4. Evaluation of Samples after Corrosion Tests
3.7. Electron Microanalysis Studied Organic Coatings
3.8. Electrochemical Methods
3.9. Determination of pH and Specific Electrical Conductivity and Corrosion Loss from Aqueous Extracts of Pigments and of Loose Paint Films
4. Results and Discussion
4.1. Synthesis and Characterization of New Azo Organic Pigments by Analytical Methods
4.2. Maldi Characterization
- HR-MALDI-MS (DHB): calcd for C17H14N4O3 m/z 323.1139 ([M+H]), found 323.1144.
- HR-MALDI-MS (DHB): calcd for C17H14N4O3 m/z 345.0958 ([M+Na]), found 345.0963.
- HR-MALDI-MS (DHB): calcd for C17H14N4O3 m/z 367.0778 ([M+2Na]), found 367.0783.
- HR-MALDI-MS (DHB): calcd for C26H21N3O5 m/z 456.1554 ([M+H]), found 456.1562.
- HR-MALDI-MS (DHB): calcd for C26H21N3O5 m/z 478.1373 ([M+Na), found 478.1381.
- HR-MALDI-MS (DHB): calcd for C17H12N2O3 m/z 293.0921 ([M+H]), found 293.0924.
- HR-MALDI-MS (DHB): calcd for C17H12N2O3 m/z 315.0740 ([M+Na]), found 315.0744.
- HR-MALDI-MS (DHB): calcd for C25H20N4O6 m/z 473.1456 ([M+H]), found 473.1464.
- HR-MALDI-MS (DHB): calcd for C25H20N4O6 m/z 495.1275 ([M+Na), found 495.1282.
- HR-MALDI-MS (DHB): calcd for C34H26MgN8O6 m/z 667.1899 ([M+H]), found 667.1915.
- HR-MALDI-MS (DHB): calcd for C34H26MgN8O6 m/z 689.1718 ([M+Na]), found 689.1735.
- HR-MALDI-MS (DHB): calcd for C26H19MgN3O5 m/z 478.1248 ([M+H]), found 478.1263.
- HR-MALDI-MS (DHB): calcd for C26H22N3O5 m/z 456.1554 ([M+H]), found 456.1566.
- HR-MALDI-MS (DHB): calcd for C26H19N3O5 m/z 478.1373 ([M+Na]), found 478.1386.
- HR-MALDI-MS (DHB): calcd for C17H12N2O3 m/z 315.0740 ([M+Na]), found 315.0744.
- HR-MALDI-MS (DHB): calcd for C25H20N4O6 m/z 495.1275 ([M+Na]), found 495.1287.
4.3. Energy Dispersive X-ray Spectroscopy (EDX)
4.4. X-ray Powder Diffraction Patterns
4.5. Infrared Spectroscopy (IR)
4.6. Characterization of the Studied Pigments
Pigment Specification
4.7. Results of Mechanical Properties of the Protective Coatings
4.8. Results of Corrosion Tests of the Protective Coatings
4.8.1. Accelerated Corrosion Tests in a Salt Mist Atmosphere
4.8.2. Accelerated Corrosion Tests in Atmosphere Containing SO2
4.8.3. Cyclic Corrosion/Weather Resistance Test with Exposure to a Salt Electrolyte (NaCl + (NH4)2SO4) and UV Radiation
4.9. Compositional Characterization of the Organic Coating after Exposure to the Salt Atmosphere
4.10. Potentiodynamic Polarization Studies and Electrochemical Impedance Spectroscopy
4.11. Determination of pH and Specific Electrical Conductivity and Corrosion Loss from Aqueous Extracts of Pigments and of Loose Paint Films
4.12. Assumed Corrosion Protection Mechanism of Studied Organic Pigment in the Organic Coatings Containing Zinc Pigment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, H.; Liu, J.; Lu, X.; Wang, D.; Geng, T.; Feng, L.; Liang, D.; Ma, X.; Hu, Z. Wettability and anticorrosion behavior of organic-inorganic hybrid superhydrophobic epoxy coatings containing triazine corrosion inhibitor loaded in a mesoporous molecular sieve. J. Taiwan Inst. Chem. Eng. 2022, 141, 104604. [Google Scholar] [CrossRef]
- Fortes, J.C.; Castilla-Gutierrrez, J.; Sarmiento, A.; Grande, J.A. Corrosion of Carbon Steel in Extreme Environments by Acid Mine Water: Experimental Study of the Process Using a Factorial Analysis Tool. Minerals 2022, 12, 1030. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhi, H.; Guo, L.; Fu, A.; Xiang, T.; Jin, Y. Experimental and molecular modeling studies of multi-active tetrazole derivative bearing sulfur linker for protecting steel from corrosion. J. Mol. Liq. 2022, 351, 118638. [Google Scholar] [CrossRef]
- Armelin, E.; Pla, R.; Liesa, F.; Ramis, X.; Iribarren, J.I.; Alemán, C. Corrosion protection with polyaniline and polypyrrole as anticorrosive additives for epoxy paint. Corros. Sci. 2008, 50, 721–728. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Emira, H.S.; Selim, M.M. Anticorrosive performance of ion-exchange zeolites in alkyd-based paints. Pigment Resin Technol. 2011, 40, 91–99. [Google Scholar] [CrossRef]
- Zubielewicz, M.; Kaminska-Tarnawska, E.; Kozlowska, A. Protective properties of organic phosphate pigmented coatings on phosphate steel substrates. Prog. Org. Coat. 2005, 53, 276–285. [Google Scholar] [CrossRef]
- Shen, L.; He, S.; Xie, W.; Miao, L.; Liu, J.; Zhou, H.; Li, B.; Qiang, Y. Corrosion protection of PPy-TiC-modifed epoxy zinc-rich coatings in dilute NaCl solution. Prog. Org. Coat. 2022, 172, 107148. [Google Scholar] [CrossRef]
- Zunita, M.; Kevin, Y.J. Ionic liquids as corrosion inhibitor: From research and development to commercialization. Result Eng. 2022, 15, 100562. [Google Scholar] [CrossRef]
- Fakir, S.H.; Kumar, K.C.; Ali, H.; Rawat, J.; Farooqi, I.H. Synthesis and evaluation of green corrosion inhibitor from rice husk to mitigate corrosion of carbon steel in NaCl environment. Mater. Today Proc. 2022, 71, 29–37. [Google Scholar] [CrossRef]
- Sanaei, Z.; Ramezanzadeh, B.; Shahrabi, T. Anti-corrosion performance of an epoxy ester coating filled with a new generation of hybrid green organic/inorganic inhibitive pigment; electrochemical and surface characterizations. Appl. Surf. Sci. 2018, 454, 1–15. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.; Li, H.; Fan, X.; Zhu, M. Ti3C2 MXene nanosheets toward high-performance corrosion inhibitor for epoxy coating. Prog. Org. Coat. 2019, 135, 156–167. [Google Scholar] [CrossRef]
- Zhu, Y.; Qu, S.; Shen, Y.; Liu, X.; Lai, N.; Dai, Z.; Liu, J. Investigation on the synergistic effects and mechanism of oleic imidazoline and mercaptoethanol corrosion inhibitors by experiment and molecular dynamic simulation. J. Mol. Struct. 2023, 1274, 134512. [Google Scholar] [CrossRef]
- Verma, C.; Haque, J.; Quraishi, M.A.; Ebenso, E.E. Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: A review. J. Mol. Liq. 2019, 275, 18–40. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and nonferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Quraishi, M.A.; Tripathy, D.B.; Abai, E.J. Effect of akyl chain length, flow, and temperature on the corrosion inhibition of carbon steel in a simulated acidizing environment by an imidazoline-based inhibitor. J. Pet. Sci. Eng. 2020, 187, 106801. [Google Scholar] [CrossRef]
- Li, Y.Z.; Xu, N.; Guo, X.P.; Zhang, G.A. Inhibition effect of imidazoline inhibitor on the crevice corrosion of N80 carbon steel in the CO2-saturated NaCl solution containing acetic acid. Corros. Sci. 2017, 126, 127–141. [Google Scholar] [CrossRef]
- Mazumder, M.A.J.; Nazal, M.K.; Faiz, M.; Ali, S.A. Imidazolines containing single-, twin- and triple-tailed hydrophobes and hydrophilic pendants (CH2CH2NH)n H as inhibitors of mild steel corrosion in CO2-0.5 M NaCl. RSC Adv. 2016, 6, 12348–12362. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hou, B.S.; Xu, N.; Liu, H.F.; Zhang, G.A. Two novel thiadiazole derivatives as highly efficient inhibitors for the corrosion of mild steel in the CO2-saturated oilfield produced water. J. Taiwan Inst. Chem. Eng. 2019, 96, 588–598. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hou, B.S.; Zhang, G.A. Inhibitive and adsorption behavior of thiadiazole derivatives on carbon steel corrosion in CO2-saturated oilfield produced water: Effect of substituent group on efficiency. J. Colloid Interface Sci. 2020, 572, 91–106. [Google Scholar] [CrossRef]
- Chiter, F.; Costa, D.; Maurice, V.; Marcus, P. DFT investigation of 2-mercaptobenzothia zole adsorption on model oxidized copper surfaces and relationship with corrosion inhibition. Appl. Surf. Sci. 2021, 537, 147802. [Google Scholar] [CrossRef]
- Hamadi, L.; Mansouri, S.; Oulmi, K.; Kareche, A. The use of amino acids as corrosion inhibitors for metals: A review. Egypt. J. Pet. 2018, 27, 1157–1165. [Google Scholar] [CrossRef]
- Maddah, M.; Maddah, M.; Peyvandi, K. Molecular dynamics simulation of methane hydrate formation in presence and absence of amino acid inhibitors. J. Mol. Liq. 2018, 269, 721–732. [Google Scholar] [CrossRef]
- Ibrahimi, B.E.; Jmiai, A.; Bazzi, L.; Issami, S.E. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Han, P.; Li, W.H.; Tian, H.W.; Gao, X.; Ding, R.; Xiong, C.S.; Song, L.Y.; Zhang, X.Y.; Wang, W.; Chen, C.F. Comparison of inhibition performance of pyridine derivatives containing hydroxyl and sulfhydryl groups: Experimental and theoretical calculations. Mater. Chem. Phys. 2018, 214, 345–354. [Google Scholar] [CrossRef]
- Huang, J.; Yang, M.; Zhu, W.; Tang, K.; Zhang, H.; Chen, J.; Noel, J.N.; Barker, I.; Zhang, H.; Zhu, J. Extrusion-free fabrication of zinc-rich powder coatings: Press bonding. Chem. Eng. J. 2022, 442, 135925. [Google Scholar] [CrossRef]
- Li, Z.; Bi, H.; Weinell, C.E.; Ravenni, G.; Benedini, L.; Dam-Johansen, K. Investigation of zinc epoxy coatings modified with pyrolyzed and gasified biochar nanoparticles for corrosion protection. Prog. Org. Coat. 2023, 178, 107477. [Google Scholar] [CrossRef]
- Meroufel, A.; Touzain, S. EIS characterisation of new zinc-rich powder coatings. Prog. Org. Coat. 2007, 59, 197–205. [Google Scholar] [CrossRef]
- Li, Y.; Ravenni, G.; Bi, H.; Weinell, C.E.; Ulusoy, B.; Zhang, Z.; Dam-Johansen, K. Effects of biochar nanoparticles on anticorrosive performance of zinc-rich epoxy coatings. Prog. Org. Coat. 2021, 158, 106351. [Google Scholar] [CrossRef]
- Abreu, C.M.; Izquierdo, M.; Keddam, M.; Nóvoa, X.R.; Takenouti, H. Electrochemical behaviour of zinc-rich epoxy paints in 3% NaCl solution. Electrochim. Acta 1996, 41, 2405–2415. [Google Scholar] [CrossRef]
- Feliu, S.; Barajas, R.; Bastidas, J.D.; Morcillo, M. Mechanism of cathodic protection of zinc-rich points by electrochemical impedance spectroscopy. I: Galvanic stage. J. Coat. Technol. 1989, 61, 775. [Google Scholar]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra Ch, S. Research progress in organic zinc rich primer coatings for cathodic protection of metals—A comprehensive review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Jalili, M.; Rostami, M.; Ramezanzadeh, B. An investigation of the electrochemical action of the epoxy zinc-rich coatings containing surface modified aluminum nanoparticle. Appl. Surf. Sci. 2015, 328, 95–108. [Google Scholar] [CrossRef]
- Lan, Y.-X.; Cho, Y.-C.; Liu, W.-R.; Wong, W.-T.; Sun, C.-F.; Yeh, J.-M. Small-load rGO as partial replacement for the large amount of zinc dust in epoxy zinc-rich composites applied in heavy-duty anticorrosion coatings. Prog. Org. Coat. 2023, 175, 107332. [Google Scholar] [CrossRef]
- Lamprakou, Z.; Bi, H.; Weinell, C.E.; Dam-Johansen, K. Tannin-based inhibitive pigment for sustainable epoxy coatings formulation. Prog. Org. Coat. 2022, 167, 106841. [Google Scholar] [CrossRef]
- Hrdina, R.; Burgert, L.; Kalendová, A.; Alafid, A.; Panák, F.; Držková, O.; Kohl, M. Use of Perylenic Acid Salts as Anticorrosive Substances. Czech Republic No. 308991, 29 September 2021. [Google Scholar]
- Miao, M.; Yuan, X.Y.; Wang, X.G.; Lu, Y.; Liu, J.K. One step self-heating synthesis and their excellent anticorrosion performance of zinc phosphate/benzotriazole composite pigments. Dye. Pigment. 2017, 141, 74–82. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Prochon, M. New Organic/Inorganic Pigments Based on Azo Dye and Aluminum-Magnesium Hydroxycarbonates with Various Mg/Al Ratios. Materials 2019, 12, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manku, G.S.; Chadha, R.C.; Nayar, N.K.; Sethi, M.S. The formation constants of lanthanon (III) complexes with some 1-(substituted) phenylazo-2-naphthols. J. Less Common Met. 1971, 25, 55–59. [Google Scholar] [CrossRef]
- Zollinger, H. Colour Chemistry: Syntheses, Properties, and Applications of Organic Dyes; Wiley-VCH: Weinheim, Germany, 2003; p. 637. ISBN 3-906390-23-3. [Google Scholar]
- Roy, M.; Devi, S.S.; Roy, S.; Singh, C.B.; Singh, K.S. Inorg. Synthesis, characterization, crystal structures and in vitro antimicrobial activities of triorganotin(IV) complexes of azo-dicarboxylates. Inorganica Chim. Acta 2015, 426, 89–98. [Google Scholar] [CrossRef]
- Lyčka, A.; Luštinec, D.; Holeček, J.; Nadvornik, M.; Holčapek, M. 27Al, 15N, 13C and 1H NMR spectra and negative-ion electrospray mass spectra of the 2:1 aluminium(III) complexes of azo dyes derived from anthranilic acid. Dye. Pigment. 2001, 50, 203–209. [Google Scholar] [CrossRef]
- Smitha, P.; Asha, S.K.; Pillai, C.K.S. Synthesis, characterization, and hyperpolarizability measurements of main-chain azobenzene molecules. J. Polym. Sci.—Polym. Chem. 2005, 43, 4455–4468. [Google Scholar] [CrossRef]
- Fierz-David, H.E.; Blangey, L. Grundlegende Operationen der Farbenchemie, 5th ed.; Julius Springer: Vienna, Austria, 1938. [Google Scholar]
- Kohl, M.; Alafid, F.; Bouška, M.; Krejčová, A.; Raycha, Y.; Kalendová, A.; Hrdina, R.; Burgert, L. New Corrosion Inhibitors Based on Perylene Units in Epoxy Ester Resin Coatings. Coatings 2022, 12, 923. [Google Scholar] [CrossRef]
- Kohl, M.; Kalendová, A.; Deshpande, P.P.; Schmidová, E. Effects of conductive polymers (type and concentration) in coatings with zinc particles of different shapes. J. Coat. Technol. Res. 2019, 16, 949–962. [Google Scholar] [CrossRef]
- Kohl, M.; Kalendová, A. Assessment of the impact of polyaniline saltson corrosion properties of organic coatings. Corros. Mater. Prot. 2014, 58, 113–119. [Google Scholar]
- Kalendová, A.; Veselý, D.; Sapurina, I.; Stejskal, J. Anticorrosion efficiency of organic coatings depending on the pigment volume concentration of polyaniline phosphate. Prog. Org. Coat. 2008, 63, 228–237. [Google Scholar] [CrossRef]
- Kalendová, A.; Veselý, D.; Stejskal, J.; Trchová, M. Anticorrosion properties of inorganic pigments surface-modified with a polyaniline phosphate layer. Prog. Org. Coat. 2008, 63, 209–221. [Google Scholar] [CrossRef]
- Kalendová, A. Methods for testing and evaluating the flash corrosion. Prog. Org. Coat. 2002, 44, 201–209. [Google Scholar] [CrossRef]
- Kukačková, H.; Vraštilová, A.; Kalendová, A. Non-toxic Anticorrosive Pigments Intended for Applications in High-solids and Waterborne Paints. Phys. Procedia 2013, 44, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Kalendová, A. Alkalising and neutralising effects of anticorrosive pigments containing Zn, Mg, Ca, and Sr cations. Prog. Org. Coat. 2000, 38, 199–206. [Google Scholar] [CrossRef]
- TenHuisen, S.K.; Brown, W.P. Formation of calcium-deficient hydroxyapatite from α-tricalcium phosphate. Biomaterials 1998, 19, 2209–2217. [Google Scholar] [CrossRef]
- Eddy, N.O.; Odoemelam, S.A.; Ogoko, E.C.; Ita, B.I. Inhibition of the Corrosion of Zinc in 0.01–0.04 M H2SO4 by Erythromycin. Port. Electrochim. Acta 2010, 28, 15–26. [Google Scholar] [CrossRef]
- Kalendová, A.; Ryšánek, P.; Nechvílová, K. Investigation of the anticorrosion efficiency of ferrites MgZnFeO with different particle morphology and chemical composition in epoxy-ester resin-based coatings. Prog. Org. Coat. 2015, 86, 147–163. [Google Scholar] [CrossRef]
- Deshpande, P.; Kolekar, A.; Bhopale, A.; Kalendova, A.; Kohl, M. Impressed current cathodic protection (ICCP) of mild steel in association with zinc based paint coating. Mater. Today Proc. 2022, 50, 552–559. [Google Scholar] [CrossRef]
- Kohl, M.; Kalendová, A.; Schmidová, E. Enhancing corrosion resistance of zinc-filled protective coatings using conductive polymers. Chem. Pap. 2017, 71, 409–421. [Google Scholar] [CrossRef]
- Kalendová, A.; Veselý, D.; Kohl, M.; Stejskal, J. Anticorrosion efficiency of zinc-filled epoxy coatings containing conducting polymers and pigments. Prog. Org. Coat. 2015, 78, 1–20. [Google Scholar] [CrossRef]




























| Compound | Chemical Formula | Molecular Weight [g·mol−1] | Elemental Analysis (Found/Calculated) [%] | Yield [%] |
|---|---|---|---|---|
| Dye-I | C17H14N4O3 | 322.32 | C—58.21/63.35 H—3.84/4.38 N—15.69/17.38 | 97.4 |
| Dye-II | C26H21N3O5 | 455.47 | C—62.53/68.56 H—4.11/4.65 N—8.20/9.23 | 86.7 |
| Dye-III | C17H12N2O3 | 292.29 | C—70.48/69.86 H—3.99/4.14 N—8.14/9.58 | 89.9 |
| Dye-IV | C25H20N4O6 | 472.46 | C—60.92/63.56 H—4.39/4.27 N—11.08/11.86 | 68.3 |
| Compound | Chemical Formula | Molecular Weight [g·mol−1] | Elemental Analysis (Found/Calculated) [%] | Yield [%] | Content of Mg (Prepared/Theoretical) [mg·kg−1] |
|---|---|---|---|---|---|
| Mg-Dye-I | C34H26MgN8O6 | 666.90 | C—56.70/58.09 H—4.36/4.30 N—15.28/15.94 Mg—3.46/4.14 | 68.3 | 34,600/41,400 |
| Mg-Dye-II | C26H19MgN3O5 | 477.76 | C—62.61/65.36 H—4.63/4.01 N—8.17/8.80 Mg—5.59/5.09 | 85.6 | 55,900/50,900 |
| Mg-Dye-III | C17H10MgN2O3 | 314.58 | C—54.06/58.24 H—3.77/4.02 N—7.20/7.99 Mg—7.15/6.93 | 97.5 | 71,500/69,300 |
| Mg-Dye-IV | C25H18MgN4O6 | 494.75 | C—53.67/56.57 H—4.12/4.18 N—9.67/10.56 Mg—4.32/4.58 | 99.2 | 43,200/45,800 |
| Pigment | Density [g·cm−3] | Oil Absorption [g/100g] | CPVC [-] |
|---|---|---|---|
| Mg-Dye-I C34H26MgN8O6 | 1.38 ± 0.02 | 45.9 | 59 |
| Mg-Dye-II C26H19MgN3O5 | 1.37 ± 0.02 | 56.8 | 54 |
| Mg-Dye-III C17H10MgN2O3 | 1.55 ± 0.02 | 43.2 | 58 |
| Mg-Dye-IV C25H18MgN4O6 | 1.41 ± 0.02 | 75.8 | 46 |
| MgO | 3.01 ± 0.02 | 48.1 | 36 |
| Ca-Mg-HPO4 | 2.72 ± 0.02 | 37.5 | 48 |
| Zn | 7.14 ± 0.02 | 6.4 | 67 |
| Complex | Element [Atomic %] | ||||||
|---|---|---|---|---|---|---|---|
| C | N | O | Na | Mg | P | Ca | |
| Mg-Dye-I C34H26MgN8O6 | 65.3 ± 0.9 | 15.1 ± 0.5 | 17.8 ± 0.7 | - | 1.8 ± 0.2 | - | - |
| Mg-Dye-II C26H19MgN3O5 | 68.9 ± 0.5 | 8.0 ± 0.4 | 20.2 ± 0.3 | 0.5 ± 0.1 | 2.4 ± 0.2 | - | - |
| Mg-Dye-III C17H10MgN2O3 | 68.2 ± 0.2 | 8.1 ± 0.3 | 20.4 ± 0.2 | 0.9 ± 0.2 | 2.4 ± 0.2 | - | - |
| Mg-Dye-IV C25H18MgN4O6 | 67.0 ± 1.0 | 8.6 ± 0.7 | 22.2 ± 0.5 | - | 2.2 ± 0.3 | - | - |
| MgO | - | - | 52.5 ± 0.7 | - | 47.5 ± 0.3 | - | - |
| Ca-Mg-HPO4 | - | - | 68.0 ± 0.5 | - | 1.5 ± 0.3 | 12.8 ± 0.5 | 17.7 ± 0.5 |
| Pigment | PVC [%] | Blistering | Corrosion | ||
|---|---|---|---|---|---|
| On the Film Area [dg] | In the Cut [dg] | In the Cut [mm] | Metal Base [%] | ||
| Mg-Dye-I C34H26MgN8O6 | 1 | 6F | 4M | 4–4.5 | 1 |
| 3 | - | 6M | 4–4.5 | 0.1 | |
| 5 | - | 8M | 4–4.5 | 0.03 | |
| 10 | - | 8M | 3.5–4 | 0.03 | |
| Mg-Dye-II C26H19MgN3O5 | 1 | 8F | 2MD | 5.5–6 | 1 |
| 3 | 8F | 2MD | 5.5–6 | 3 | |
| 5 | 8F | 2D | 6–6.5 | 3 | |
| 10 | 8F | 4M | 5–5.5 | 1 | |
| Mg-Dye-III C17H10MgN2O3 | 1 | 8MD | 2MD | 6–6.5 | 100 |
| 3 | 8D | 2D | 9–9.5 | 100 | |
| 5 | 8MD | 2M | 6–6.5 | 100 | |
| 10 | 8MD | 2M | 6.5–7 | 50 | |
| Mg-Dye-IV C25H18MgN4O6 | 1 | 8M | 4M | 6–6.5 | 3 |
| 3 | 8F | 2D | 6–6.5 | 10 | |
| 5 | 8M | 2D | 6–6.5 | 16 | |
| 10 | 8M | 4M | 6–6.5 | 16 | |
| MgO | 1 | - | 4M | 6–6.5 | - |
| 3 | 8F | 6M | 6–6.5 | 0.01 | |
| 5 | - | 6M | 5–5.5 | - | |
| 10 | - | 6M | 5–5.5 | - | |
| CaMgHPO4 | 1 | - | 4MD | 6.5–7 | 3 |
| 3 | 8MD | 4MD | 6.5–7 | 50 | |
| 5 | 8MD | 4MD | 6.5–7 | 50 | |
| 10 | - | 2MD | 6–6.5 | 0.1 | |
| Zn | - | - | 2D | 8–8.5 | 0.3 |
| Pigment | PVC [%] | Blistering | Corrosion | ||
|---|---|---|---|---|---|
| On the Film Area [dg] | In the Cut [dg] | In the Cut [mm] | Metal Base [%] | ||
| Mg-Dye-I C34H26MgN8O6 | 1 | 6M | 6F | 0.5–1 | 0.3 |
| 3 | 8M | 6F | 0.5–1 | 0.3 | |
| 5 | 8F | 8F | 0.5–1 | 0.1 | |
| 10 | 8F | 6F | 0–0.5 | 0.1 | |
| Mg-Dye-II C26H19MgN3O5 | 1 | 8MD | 8F | 0.5–1 | 0.3 |
| 3 | 6F | 6F | 0.5–1 | 0.3 | |
| 5 | 8MD | 8MD | 0–0.5 | 0.3 | |
| 10 | 6M | 8F | 0–0.5 | 0.3 | |
| Mg-Dye-III C17H10MgN2O3 | 1 | 8MD | 4MD | 3–3.5 | 1 |
| 3 | 6MD | 8F | 3–3.5 | 50 | |
| 5 | 8M | 4F | 3–3.5 | 3 | |
| 10 | 6M | 6M | 2–2.5 | 10 | |
| Mg-Dye-IV C25H18MgN4O6 | 1 | 6F | 8F | 0.5–1 | 3 |
| 3 | 6M | - | 1–1.5 | 3 | |
| 5 | 6M | - | 0–0.5 | 3 | |
| 10 | 8D | 8D | 0–0.5 | 1 | |
| MgO | 1 | 8F | - | 0–0.5 | - |
| 3 | 8F | 8F | 0.5–1 | - | |
| 5 | 8D | 8MD | 0.5–1 | 0.1 | |
| 10 | 8D | 8D | 0.5–1 | 0.1 | |
| Ca-Mg-HPO4 | 1 | 6MD | 8F | 0–0.5 | 1 |
| 3 | 6M | 6F | 1–1.5 | 0.3 | |
| 5 | 6M | 6F | 0.5–1 | 0.3 | |
| 10 | 4F | 6MD | 0.5–1 | 0.3 | |
| Zn | - | 4F | 8F | 1–1.5 | 3 |
| Pigment | PVC [%] | Blistering | Corrosion | ||
|---|---|---|---|---|---|
| On the Film Area [dg] | In the Cut [dg] | In the Cut [mm] | Metal Base [%] | ||
| Mg-Dye-I C34H26MgN8O6 | 1 | 8F | 6M | 2–2.5 | 0.01 |
| 3 | 8F | 8F | 2–2.5 | - | |
| 5 | 8F | 8M | 1.5–2 | - | |
| 10 | - | 8M | 1.5–2 | - | |
| Mg-Dye-II C26H19MgN3O5 | 1 | 8M | 6M | 2.5–3 | 0.1 |
| 3 | 6MD | 6M | 2.5–3 | 0.1 | |
| 5 | 6M | 6M | 2.5–3 | 0.1 | |
| 10 | 8F | 6MD | 2.5–3 | 0.1 | |
| Mg-Dye-III C17H10MgN2O3 | 1 | 8MD | 6M | 2.5–3 | 0.1 |
| 3 | 8D | 6M | 2.5–3 | 0.1 | |
| 5 | 8F | 6M | 2.5–3 | 0.1 | |
| 10 | 8F | 6M | 2.5–3 | 0.1 | |
| Mg-Dye-IV C25H18MgN4O6 | 1 | 6F | 6F | 2.5–3 | 1 |
| 3 | 8F | 6M | 2.5–3 | 0.01 | |
| 5 | 8M | 6M | 2.5–3 | 0.03 | |
| 10 | 8M | 6M | 2–2.5 | 0.1 | |
| MgO | 1 | 8F | 6M | 2–2.5 | - |
| 3 | 8F | 6M | 2–2.5 | - | |
| 5 | 8F | 8M | 2–2.5 | - | |
| 10 | 8F | 8M | 2–2.5 | - | |
| Ca-Mg-HPO4 | 1 | 8M | 8M | 2–2.5 | 0.1 |
| 3 | 8F | 6M | 2.5–3 | 0.1 | |
| 5 | - | 6M | 2–2.5 | 0.1 | |
| 10 | 8F | 6M | 2–2.5 | 0.1 | |
| Zn | - | 8M | 4M | 3–3.5 | 0.1 |
| Pigment | PVC [%] | Ecorr [mV] | Icorr [µA·cm−2] | Corrosion Rate [mpy] | Zmod [Ω·cm−2] | Zreal [Ω·cm−2] |
|---|---|---|---|---|---|---|
| Mg-Dye-I C34H26MgN8O6 | 1 | −748 | 19.6 | 1.86 | 134.4 | 113.7 |
| 3 | −755 | 10.2 | 0.97 | 151.1 | 133.7 | |
| 5 | −726 | 18.1 | 1.71 | 113.7 | 98.01 | |
| 10 | −703 | 9.69 | 0.92 | 197.4 | 183.5 | |
| Mg-Dye-II C26H19MgN3O5 | 1 | −722 | 43.1 | 4.10 | 57.26 | 31.77 |
| 3 | −716 | 51.1 | 4.90 | 31.70 | 17.82 | |
| 5 | −721 | 42.7 | 4.04 | 74.00 | 61.40 | |
| 10 | −672 | 44.2 | 4.19 | 45.82 | 23.44 | |
| Mg-Dye-III C17H10MgN2O3 | 1 | −694 | 72.3 | 6.84 | 25.09 | 11.91 |
| 3 | −714 | 79.4 | 7.52 | 29.33 | 15.68 | |
| 5 | −700 | 83.9 | 7.94 | 22.19 | 13.37 | |
| 10 | −710 | 91.6 | 8.37 | 25.94 | 14.16 | |
| Mg-Dye-IV C25H18MgN4O6 | 1 | −719 | 41.5 | 3.92 | 64.02 | 42.86 |
| 3 | −703 | 35.1 | 3.33 | 74.00 | 49.25 | |
| 5 | −726 | 32.6 | 3.10 | 90.45 | 59.08 | |
| 10 | −687 | 48.0 | 4.54 | 69.21 | 46.20 | |
| MgO | 1 | −776 | 10.3 | 0.97 | 151.1 | 133.7 |
| 3 | −718 | 13.3 | 1.26 | 111.8 | 98.01 | |
| 5 | −732 | 14.7 | 1.39 | 54.76 | 34.87 | |
| 10 | −720 | 15.3 | 1.45 | 66.94 | 46.37 | |
| Ca-Mg-HPO4 | 1 | −719 | 49.3 | 4.67 | 59.21 | 28.38 |
| 3 | −687 | 51.7 | 4.89 | 43.82 | 24.31 | |
| 5 | −700 | 49.2 | 4.66 | 54.76 | 28.47 | |
| 10 | −716 | 51.1 | 4.86 | 40.08 | 23.37 | |
| Zn | - | −737 | 23.8 | 2.25 | 22.44 | 11.38 |
| Pigment | a | a | b [μS·cm−1] | b [μS·cm−1] | Kmp [g·m−2] | XHp [%] | URp [mm] | VKp [g·m−2·d−1] |
|---|---|---|---|---|---|---|---|---|
| Mg-Dye-I C34H26MgN8O6 | 10.28 | 10.48 | 449 | 468 | 0.471 | 34.6 | 6.0 × 10−5 | 6.7 × 10−2 |
| Mg-Dye-II C26H19MgN3O5 | 10.45 | 10.54 | 301 | 523 | 0.819 | 60.2 | 1.0 × 10−4 | 1.2 × 10−1 |
| Mg-Dye-III C17H10MgN2O3 | 10.55 | 10.98 | 890 | 911 | 1.265 | 92.9 | 1.6 × 10−4 | 1.8 × 10−1 |
| Mg-Dye-IV C25H18MgN4O6 | 10.33 | 10.53 | 428 | 521 | 1.262 | 92.7 | 1.6 × 10−4 | 1.8 × 10−1 |
| MgO | 10.23 | 10.32 | 640 | 854 | 0.597 | 43.9 | 7.6 × 10−5 | 8.5 × 10−2 |
| Ca-Mg-HPO4 | 6.41 | 6.51 | 980 | 998 | 1.139 | 83.7 | 1.5 × 10−4 | 1.6 × 10−1 |
| H2O (distilled) | 7.19 | 7.10 | 50 | 59 | 1.361 | 100 | 1.7 × 10−4 | 1.9 × 10−1 |
| Pigment | PVCpigm [%] | a | a | b [μS·cm−1] | b [μS·cm−1] | KMf [g·m−2] | XHf [%] | URf [mm] | VKf [g·m−2·d−1] |
|---|---|---|---|---|---|---|---|---|---|
| Mg-Dye-I C34H26MgN8O6 | 1 | 6.41 | 6.80 | 26 | 55 | 0.695 | 51.0 | 8.9 × 10−5 | 9.9 × 10−2 |
| 10 | 6.74 | 6.95 | 64 | 72 | 0.587 | 43.1 | 7.5 × 10−5 | 8.4 × 10−2 | |
| Mg-Dye-II C26H19MgN3O5 | 1 | 6.22 | 6.58 | 44 | 58 | 1.111 | 74.2 | 1.4 × 10−4 | 1.6 × 10−1 |
| 10 | 6.33 | 6.86 | 46 | 69 | 0.945 | 69.3 | 1.2 × 10−4 | 1.4 × 10−1 | |
| Mg-Dye-III C17H10MgN2O3 | 1 | 6.48 | 6.62 | 40 | 61 | 1.354 | 99.3 | 1.7 × 10−4 | 1.9 × 10−1 |
| 10 | 6.58 | 6.80 | 47 | 77 | 1.289 | 96.4 | 1.6 × 10−4 | 1.8 × 10−1 | |
| Mg-Dye-IV C25H18MgN4O6 | 1 | 6.12 | 6.67 | 43 | 92 | 1.359 | 99.7 | 1.7 × 10−4 | 1.9 × 10−1 |
| 10 | 6.39 | 6.92 | 56 | 95 | 1.291 | 96.7 | 1.6 × 10−4 | 1.8 × 10−1 | |
| MgO | 1 | 6.81 | 6.92 | 60 | 96 | 0.696 | 50.7 | 8.9 × 10−5 | 9.9 × 10−2 |
| 10 | 7.27 | 7.42 | 96 | 101 | 0.678 | 49.7 | 8.6 × 10−5 | 9.7 × 10−2 | |
| Ca-Mg-HPO4 | 1 | 6.86 | 6.66 | 55 | 102 | 1.347 | 98.8 | 1.7 × 10−4 | 1.9 × 10−1 |
| 10 | 6.64 | 6.55 | 89 | 150 | 1.245 | 91.3 | 1.6 × 10−4 | 1.8 × 10−1 | |
| Zn | - | 6.83 | 6.56 | 40 | 83 | 1.215 | 89.1 | 1.5 × 10−4 | 1.7 × 10−1 |
| non-pigmented film | - | 5.75 | 5.55 | 48 | 53 | 1.236 | 90.7 | 1.6 × 10−4 | 1.8 × 10−1 |
| H2O (distilled) | - | 7.18 | 7.09 | 49 | 60 | 1.363 | 100 | 1.7 × 10−4 | 1.9 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohl, M.; Alafid, F.; Boštíková, K.; Bouška, M.; Krejčová, A.; Svoboda, J.; Slang, S.; Michalíčková, L.; Kalendová, A.; Hrdina, R.; et al. New Azo Dyes-Based Mg Complex Pigments for Optimizing the Anti-Corrosion Efficiency of Zinc-Pigmented Epoxy Ester Organic Coatings. Coatings 2023, 13, 1276. https://doi.org/10.3390/coatings13071276
Kohl M, Alafid F, Boštíková K, Bouška M, Krejčová A, Svoboda J, Slang S, Michalíčková L, Kalendová A, Hrdina R, et al. New Azo Dyes-Based Mg Complex Pigments for Optimizing the Anti-Corrosion Efficiency of Zinc-Pigmented Epoxy Ester Organic Coatings. Coatings. 2023; 13(7):1276. https://doi.org/10.3390/coatings13071276
Chicago/Turabian StyleKohl, Miroslav, Fouzy Alafid, Karolína Boštíková, Marek Bouška, Anna Krejčová, Jan Svoboda, Stanislav Slang, Ludmila Michalíčková, Andréa Kalendová, Radim Hrdina, and et al. 2023. "New Azo Dyes-Based Mg Complex Pigments for Optimizing the Anti-Corrosion Efficiency of Zinc-Pigmented Epoxy Ester Organic Coatings" Coatings 13, no. 7: 1276. https://doi.org/10.3390/coatings13071276
APA StyleKohl, M., Alafid, F., Boštíková, K., Bouška, M., Krejčová, A., Svoboda, J., Slang, S., Michalíčková, L., Kalendová, A., Hrdina, R., Burgert, L., Schmidová, E., Deshpande, P. P., & Bhopale, A. A. (2023). New Azo Dyes-Based Mg Complex Pigments for Optimizing the Anti-Corrosion Efficiency of Zinc-Pigmented Epoxy Ester Organic Coatings. Coatings, 13(7), 1276. https://doi.org/10.3390/coatings13071276









