Battery-Type Behavior of Al-Doped CuO Nanoflakes to Fabricate a High-Performance Hybrid Supercapacitor Device for Superior Energy Storage Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Al-Doped CuO Synthesis Procedure
2.3. Characterizations
2.4. Electrochemical Characterizations (Three-Electrode System)
2.5. Hybrid Supercapacitor (HSC) Device Fabrication (Two-Electrode System)
3. Results
3.1. Physico-Chemical Properties
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandal, S.; Hu, J.; Shi, S.Q. A comprehensive review of hybrid supercapacitor from transition metal and industrial crop based activated carbon for energy storage applications. Mater. Today Commun. 2023, 34, 105207. [Google Scholar] [CrossRef]
- Navathe, G.J.; Prasad, S.R.; Mane, A.M.; Barge, S.H.; Dongale, T.D.; Shaikh, V.; Karanjkar, M.M.; Teli, S.B.; Patil, P.S.; Prasad, N.R. A Critical Review on Design and Development of New Generation Energy Storage Devices. ES Energy Environ. 2022, 17, 11–32. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication, and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Babu, B.; Simon, P.; Balducci, A. Fast Charging Materials for High Power Applications. Adv. Energy Mater. 2020, 10, 2001128. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Wang, S.; Liu, H.-K.; Li, L. Transition metal-based battery-type electrodes in hybrid supercapacitors: A review. Energy Storage Mater. 2020, 28, 122–145. [Google Scholar] [CrossRef]
- Hou, C.; Yang, W.; Kimura, H.; Xie, X.; Zhang, X.; Sun, X.; Yu, Z.; Yang, X.; Zhang, Y.; Wang; et al. Boosted lithium storage performance by local build-in electric field derived by oxygen vacancies in 3D holey N-doped carbon structure decorated with molybdenum dioxide. J. Mater. Sci. Technol. 2023, 142, 185–195. [Google Scholar] [CrossRef]
- Bhise, S.C.; Awale, D.V.; Vadiyar, M.M.; Patil, S.K.; Kokare, B.N.; Kolekar, S.S. Facile synthesis of CuO nanosheets as electrode for supercapacitor with long cyclic stability in novel methyl imidazole-based ionic liquid electrolyte. J Solid State Electrochem. 2017, 21, 2585–2591. [Google Scholar] [CrossRef]
- Kasap, S.; Kaya, I.I.; Repp, S.; Erdem, E. Superbat: Battery-like supercapacitor utilized by graphene foam and zinc oxide (ZnO) electrodes induced by structural defects. Nanoscale Adv. 2019, 1, 2586–2597. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Gund, G.S.; Lokhande, C.D.; Holze, R. CuO cauliflowers for supercapacitor application: Novel potentiodynamic deposition. Mater. Res. Bull. 2013, 48, 923–928. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Zhan, J.; Sun, X.; Kang, L.; Jiang, F.; Zhang, X.; Shao, Q.; Dong, M.; Liu, H.; et al. Biological cell template synthesis of nitrogen-doped porous hollow carbon spheres/MnO2 composites for high-performance asymmetric supercapacitors. Electrochim. Acta. 2019, 296, 907–915. [Google Scholar] [CrossRef]
- Moosavifard, S.E.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Designing 3D Highly Ordered Nanoporous CuO Electrodes for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.M.; Kim, J.; Inamdar, A.I.; Woo, H.; Jo, Y.; Pawar, B.S.; Cho, S.; Kim, H.; Im, H. Multi-functional reactively-sputtered copper oxide electrodes for supercapacitor and electro-catalyst in direct methanol fuel cell applications. Sci. Rep. 2016, 6, 21310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senthilkumar, V.; Kim, Y.S.; Chandrasekaran, S.; Rajagopalan, B.; Kim, E.J.; Chung, J.S. Comparative supercapacitance performance of CuO nanostructures for energy storage device application. RSC Adv. 2015, 5, 20545–20553. [Google Scholar] [CrossRef]
- Nandy, S.; Banerjee, A.; Fortunato, E.; Martins, R. A Review on Cu2O and CuI-Based p-Type Semiconducting Transparent Oxide Materials: Promising Candidates for New Generation Oxide Based Electronics. Rev. Adv. Sci. Eng. 2013, 2, 273–304. [Google Scholar] [CrossRef]
- Mahendra, G.; Malathi, R.; Kedhareswara, S.P.; Lakshmi-Narayana, A.; Dhananjaya, M.; Guruprakash, N.; Hussain, O.M.; Mauger, A.; Julien, C.M. RF Sputter-Deposited Nanostructured CuO Films for Micro-Supercapacitors. Appl. Nano 2021, 2, 46–66. [Google Scholar] [CrossRef]
- Kumar, P.S.M.; Kyaw, H.H.; Myint, M.T.Z.; Al-Haj, L.; Al-Muhtaseb, A.H.; Al-Abri, M.; Thanigaivel, V.; Ponnusamy, V.K. Green route synthesis of nanoporous copper oxide for efficient supercapacitor and capacitive deionization performances. Int. J. Energy Res. 2020, 44, 10682–10694. [Google Scholar] [CrossRef]
- Mao, Y.; Qian, Y.; Li, L.; Li, Y.; Xie, J.; Hu, W. Novel Ag configurations decorated CuO hybrid electrode for high-performance asymmetric supercapacitor. J. Mater. Sci. 2020, 55, 6963–6975. [Google Scholar] [CrossRef]
- Arfan, M.; Siddiqui, D.N.; Shahid, T.; Iqbal, Z.; Majeed, Y.; Akram, I.; Noreen; Bagheri, R.; Song, Z.; Zeb, A. Tailoring of nanostructures: Al doped CuO synthesized by composite-hydroxide-mediated approach. Results Phys. 2019, 13, 102187. [Google Scholar] [CrossRef]
- Shinde, S.K.; Dubal, D.P.; Ghodake, G.S.; Gomez-Romero, P.; Kim, S.; Fulari, V.J. Influence of Mn incorporation on the supercapacitive properties of hybrid CuO/Cu(OH)2 electrodes. RSC Adv. 2015, 5, 30478–30484. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, M.; Singh, M.; Kumar, A.; Prachi; Gautam, Y.K.; Malik, A.K.; Kumar, Y.; Singh, B.P. Experimental investigation of Co and Fe-Doped CuO nanostructured electrode material for remarkable electrochemical performance. Ceram. Int. 2021, 47, 2094–2106. [Google Scholar] [CrossRef]
- Khlifi, N.; Mnif, S.; Nasr, F.B.; Fourati, N.; Zerrouki, C.; Chehimi, M.M.; Guermazi, H.; Aifa, S.; Guermazi, S. Non-doped and transition metal-doped CuO nano-powders: Structure-physical properties and anti-adhesion activity relationship. RSC Adv. 2022, 12, 23527–23543. [Google Scholar] [CrossRef]
- Rodney, J.D.; Deepapriya, S.; Cyril, R.M.; Jerome, D.S. Impact of synthesis technique on dysprosium doped CuO for supercapacitor applications. AIP Conf. Proc. 2020, 2265, 030635. [Google Scholar] [CrossRef]
- Du, Y.; Gao, X.; Meng, X. Single-phase indium-doped cupric oxide films prepared through DC reactive magnetron sputtering: Influence of substrate temperature on microstructure, optical and electrical behavior. J. Alloys Compd. 2020, 815, 152462. [Google Scholar] [CrossRef]
- John, D.R.; Deepapriya, S.; Cyril, R.M.; Annie, V.P.; Jose, M.; Jerome, D.S. Co-precipitation synthesized lanthanum doped copper oxide nanoparticles for supercapacitor applications. AIP Conf. Proc. 2020, 2244, 070018. [Google Scholar] [CrossRef]
- Lv, W.; Li, L.; Meng, Q.; Zhang, X. Molybdenum-doped CuO nanosheets on Ni foams with extraordinary specific capacitance for advanced hybrid supercapacitors. J. Mater. Sci. 2020, 55, 2492–2502. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, T.-T.; Xu, W.; Zhu, H.-L.; Zheng, Y.-Q. Ultrathin nanosheets-assembled CuO flowers for highly efficient electrocatalytic water oxidation. J. Mater. Sci. 2018, 53, 8141–8150. [Google Scholar] [CrossRef]
- Ponnar, M.; Thangamani, C.; Monisha, P.; Gomathi, S.S.; Pushpanathan, K. Influence of Ce doping on CuO nanoparticles synthesized by microwave irradiation method. Appl. Surf. Sci. 2018, 449, 132–143. [Google Scholar] [CrossRef]
- Nishiwaki-Akine, Y.; Kanazawa, S.; Uneyama, T.; Nitta, K.; Yamamoto-Ikemoto, R.; Watanabe, T. Transparent Woody Film Made by Dissolution of Finely Divided Japanese Beech in Formic Acid at Room Temperature. ACS Sustain. Chem. Eng. 2017, 5, 11536–11542. [Google Scholar] [CrossRef]
- Chuai, M.; Chen, X.; Zhang, K.; Zhang, J.; Zhang, M. CuO–SnO2 reverse cubic heterojunctions as high-performance supercapacitor electrodes. J. Mater. Chem. A. 2019, 7, 1160–1167. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Anand, S.; Fatima, A.; Yang, D.-J.; Choudhury, A. Facile synthesis of copper oxide nanoparticles-decorated polyaniline nanofibers with enhanced electrochemical performance as supercapacitor electrode. Polym. Adv. Technol. 2021, 32, 4070–4081. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Li, F.; Wang, Z.; Alamusi; Hu, N.; Wen, Z.; Liu, Q. Merging of Kirkendall Growth and Ostwald Ripening: CuO@MnO2 Core-shell Architectures for Asymmetric Supercapacitors. Sci. Rep. 2014, 4, 4518. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Wu, H.; Wang, Y.; Li, N.; Chen, H.; Song, W.; Wang, Z.; Sun, W.; Sun, K. Metal–organic framework derived hollow porous CuO–CuCo2O4 dodecahedrons as a cathode catalyst for Li–O2 batteries. RSC Adv. 2019, 9, 16288–16295. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Tian, W.; Zhou, J.; Qing, Y.; Huang, Q.; Li, X.; Wei, S.; Zhang, L.; Liu, X.; Wu, Y. Green anisotropic carbon-stabilized polylaminate copper oxide as a novel cathode for high-performance hybrid supercapacitors. Mater. Des. 2021, 198, 109309. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Sun, H.; Guo, X.; Xu, J.; Zhang, H.; Zhang, X. In situ Growth of Cu2O/CuO Nanosheets on Cu Coating Carbon Cloths as a Binder-Free Electrode for Asymmetric Supercapacitors. Front. Chem. 2019, 7, 420. Available online: https://www.frontiersin.org/articles/10.3389/fchem.2019.00420 (accessed on 29 June 2023). [CrossRef] [Green Version]
- Islam, M.R.; Obaid, J.E.; Saiduzzaman, M.; Nishat, S.S.; Debnath, T.; Kabir, A. Effect of Al doping on the structural and optical properties of CuO nanoparticles prepared by solution combustion method: Experiment and DFT investigation. J. Phys. Chem. Solids 2020, 147, 109646. [Google Scholar] [CrossRef]
- Gnanasekar, T.; Valanarasu, S.; Das, H.T.; Chidhambaram, N.; Isaac, R.S.R.; Al-Enizi, A.M.; Ubaidullah, M.; Reddy, V.R.M. Enhanced opto-electronic properties of X-doped (X = Al, Ga, and In) CuO thin films for photodetector applications. J. Mater. Sci. Mater. Electron. 2022, 33, 18786–18797. [Google Scholar] [CrossRef]
- Baghdadi, N.; Saeed, A.; Ansari, A.R.; Hammad, A.H.; Afify, A.; Salah, N. Controlled nanostructuring via aluminum doping in CuO nanosheets for enhanced thermoelectric performance. J. Alloys Compd. 2021, 869, 159370. [Google Scholar] [CrossRef]
- Mbu, E.E.; Dodoo-Arhin, D.; Ntwampe, S.K.; Malenga, E.; Fosso-Kankeu, E. Photocatalytic Degradation of AZO Dye and Rhodamine Dyes Using Copper (II) Oxide Nanoparticles. Proceedings of 10th Int’l Conference on Advances in Science, Engineering, Technology & Healthcare (ASETH-18), Cape Town, South Africa, 19–20 November 2018. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Pub. Co. Inc.: Boston, MA, USA, 1978; Available online: https://www.academia.edu/28079760/Elements_of_X_RAY_DIFFRACTION_SECOND_EDITION (accessed on 30 June 2023).
- Qadri, S.B.; Skelton, E.F.; Hsu, D.; Dinsmore, A.D.; Yang, J.; Gray, H.F.; Ratna, B.R. Size-induced transition-temperature reduction in nanoparticles of ZnS. Phys. Rev. B 1999, 60, 9191–9193. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, C.; Qi, K.; Cui, X. Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst. Sci. Rep. 2017, 7, 39695. [Google Scholar] [CrossRef] [PubMed]
- Krstulović, N.; Salamon, K.; Budimlija, O.; Kovač, J.; Dasović, J.; Umek, P.; Capan, I. Parameters optimization for synthesis of Al-doped ZnO nanoparticles by laser ablation in water. Appl. Surf. Sci. 2018, 440, 916–925. [Google Scholar] [CrossRef]
- Lu, W.; Iwasa, Y.; Ou, Y.; Jinno, D.; Kamiyama, S.; Petersen, P.M.; Ou, H. Effective optimization of surface passivation on porous silicon carbide using atomic layer deposited Al2O3. RSC Adv. 2017, 7, 8090–8097. [Google Scholar] [CrossRef] [Green Version]
- Vidhyadharan, B.; Misnon, I.I.; Aziz, R.A.; Padmasree, K.P.; Yusoff, M.M.; Jose, R. Superior supercapacitive performance in electrospun copper oxide nanowire electrodes. J. Mater. Chem. A 2014, 2, 6578–6588. [Google Scholar] [CrossRef]
- Goswami, S.; Dillip, G.R.; Nandy, S.; Banerjee, A.N.; Pimentel, A.; Joo, S.W.; Martins, R.; Fortunato, E. Biowaste-derived carbon black applied to polyaniline-based high-performance supercapacitor microelectrodes: Sustainable materials for renewable energy applications. Electrochim. Acta 2019, 316, 202–218. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Banerjee, A.N.; Nallapureddy, R.R.; Joo, S.W. Urea-assisted hydrothermal synthesis of MnMoO4/MnCO3 hybrid electrochemical electrode and fabrication of high-performance asymmetric supercapacitor. J. Mater. Sci. Technol. 2022, 96, 332–344. [Google Scholar] [CrossRef]
- Reddy, N.R.; Banerjee, A.N.; Reddy, P.M.; Joo, S.W. Functionalization of 0-D and 2-D carbon nitride nanostructures on bio-derived carbon spheres for sustainable electrochemical supercapacitors. J. Electroanal. Chem. 2021, 902, 115808. [Google Scholar] [CrossRef]
- Ryoken, H.; Sakaguchi, I.; Ohashi, N.; Sekiguchi, T.; Hishita, S.; Haneda, H. Non-equilibrium defects in aluminum-doped zinc oxide thin films grown with a pulsed laser deposition method. J. Mater. Res. 2005, 20, 2866–2872. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Nallapureddy, R.R.; Goli, H.R.; Banerjee, A.N.; Reddy, G.R.; Joo, S.W. Bioinspired tailoring of nanoarchitectured nickel sulfide@nickel permeated carbon composite as highly durable and redox chemistry enabled battery-type electrode for hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 25208–25219. [Google Scholar] [CrossRef]
- Merum, D.; Nallapureddy, R.R.; Pallavolu, M.R.; Mandal, T.K.; Gutturu, R.R.; Parvin, N.; Banerjee, A.N.; Joo, S.W. Pseudocapacitive Performance of Freestanding Ni3V2O8 Nanosheets for High Energy and Power Density Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 5561–5578. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Xiao, L.; Yan, M.; Bai, H.; Zhu, F.; Lei, Y.; Shi, W. Self-templated transformation of MOFs into layered double hydroxide nanoarrays with selectively formed Co9S8 for high-performance asymmetric supercapacitors. Chem. Eng. J. 2018, 354, 716–726. [Google Scholar] [CrossRef]
- Merum, D.; Ambadi, L.N.; Mahammad, H.O.; Pallavolu, M.R.; Goddati, M.; Lee, J.; Al-Asbahi, B.A.; Pitcheri, R.; Banerjee, A.N.; Joo, S.W. Direct growth of cobalt-doped nickel vanadate shelf-like architectures on Ni foam electrodes for solid-state alkaline battery. J. Alloys Compd. 2023, 950, 169771. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Prabhu, S.; Nallapureddy, R.R.; Kumar, A.S.; Banerjee, A.N.; Joo, S.W. Bio-derived graphitic carbon quantum dot encapsulated S- and N-doped graphene sheets with unusual battery-type behavior for high-performance supercapacitor. Carbon 2023, 202, 93–102. [Google Scholar] [CrossRef]
- Suresh, R.; Tamilarasan, K.; Vadivu, D.S. Pseudocapacitive Features of CuO Nanoworms and Mn Doped CuO Nanoflakes and Their Prospects as Electrode Materials for Supercapacitors. Dig. J. Nanomater. Biostructures 2016, 11, 795–803. Available online: https://chalcogen.ro/795_SureshR.pdf (accessed on 2 July 2023).
- Zhang, J.; Zhang, G.; Luo, W.; Sun, Y.; Jin, C.; Zheng, W. Graphitic Carbon Coated CuO Hollow Nanospheres with Penetrated Mesochannels for High-Performance Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 105–111. [Google Scholar] [CrossRef]
- Pendashteh, A.; Mousavi, M.F.; Rahmanifar, M.S. Fabrication of anchored copper oxide nanoparticles on graphene oxide nanosheets via an electrostatic coprecipitation and its application as supercapacitor. Electrochim. Acta 2013, 88, 347–357. [Google Scholar] [CrossRef]
- Tian, L.; Liu, B.; Zheng, X.; Xing, Z. MnO2 Films Deposited on CuO Nanomaterials as Electrode Materials for Supercapacitors. J. Alloys Comp. 2022, 911, 165003. [Google Scholar] [CrossRef]
- Ghazal, N.; Madkour, M.; Nazeer, A.A.; Obayya, S.S.A.; Mohamed, S.A. Electrochemical capacitive performance of thermally evaporated Al-doped CuI thin films. RSC Adv. 2021, 11, 39262–39269. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Tamilarasan, K.; Vadivu, D.S. Electrochemical Features of Symmetric and Asymmetric Supercapacitors Based on Nanostructured Mn-CuO Electrodes. Orient. J. Chem. 2018, 34, 3058–3063. [Google Scholar] [CrossRef]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gómez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Xie, J.; Liu, X.; Lu, X. Recent progress and challenges of carbon materials for Zn-ion hybrid supercapacitors. Carbon Energy 2020, 2, 521–539. [Google Scholar] [CrossRef]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.-S.; Madhavi, S. Insertion-Type Electrodes for Nonaqueous Li-Ion Capacitors. Chem. Rev. 2014, 114, 11619–11635. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]


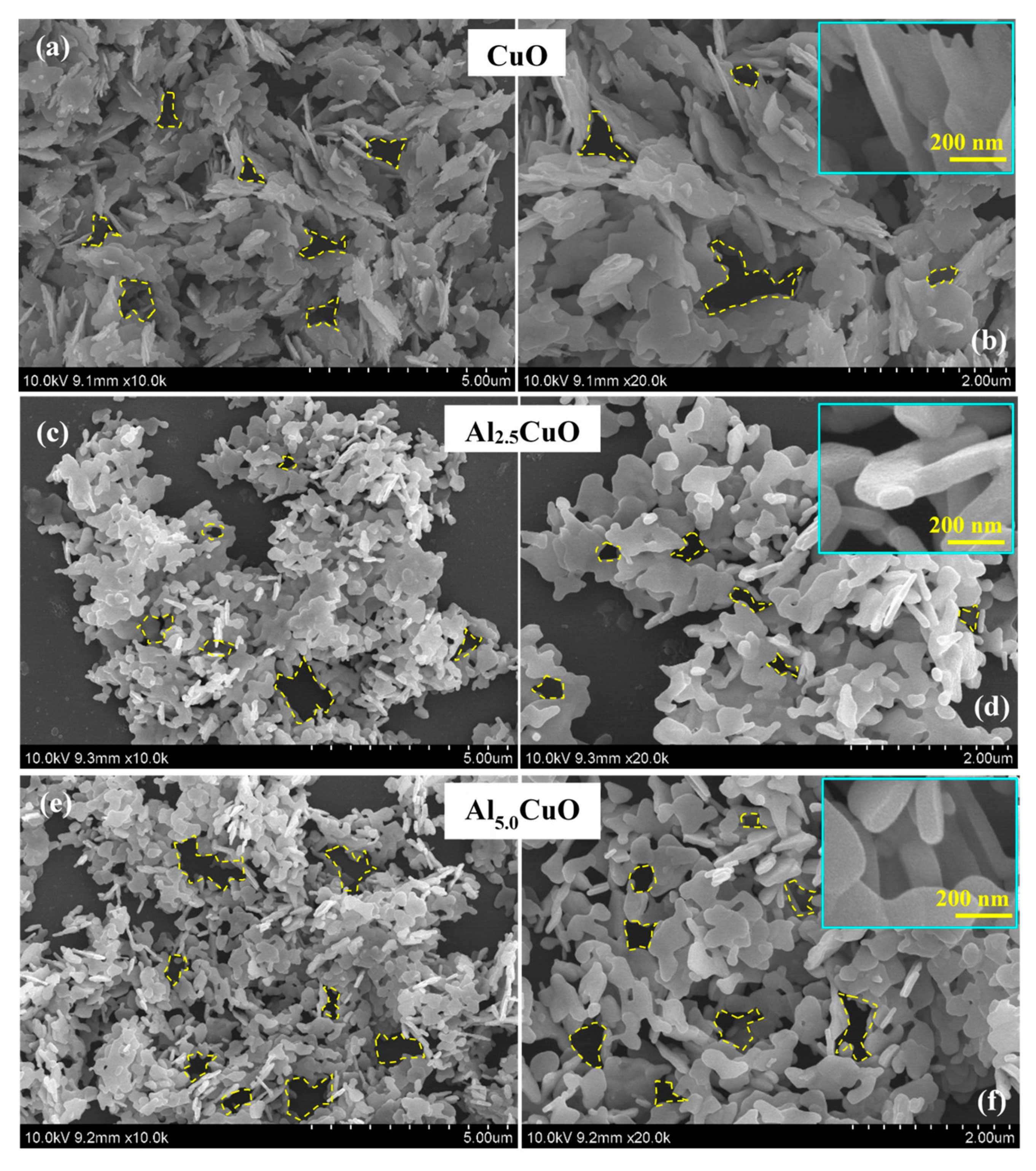
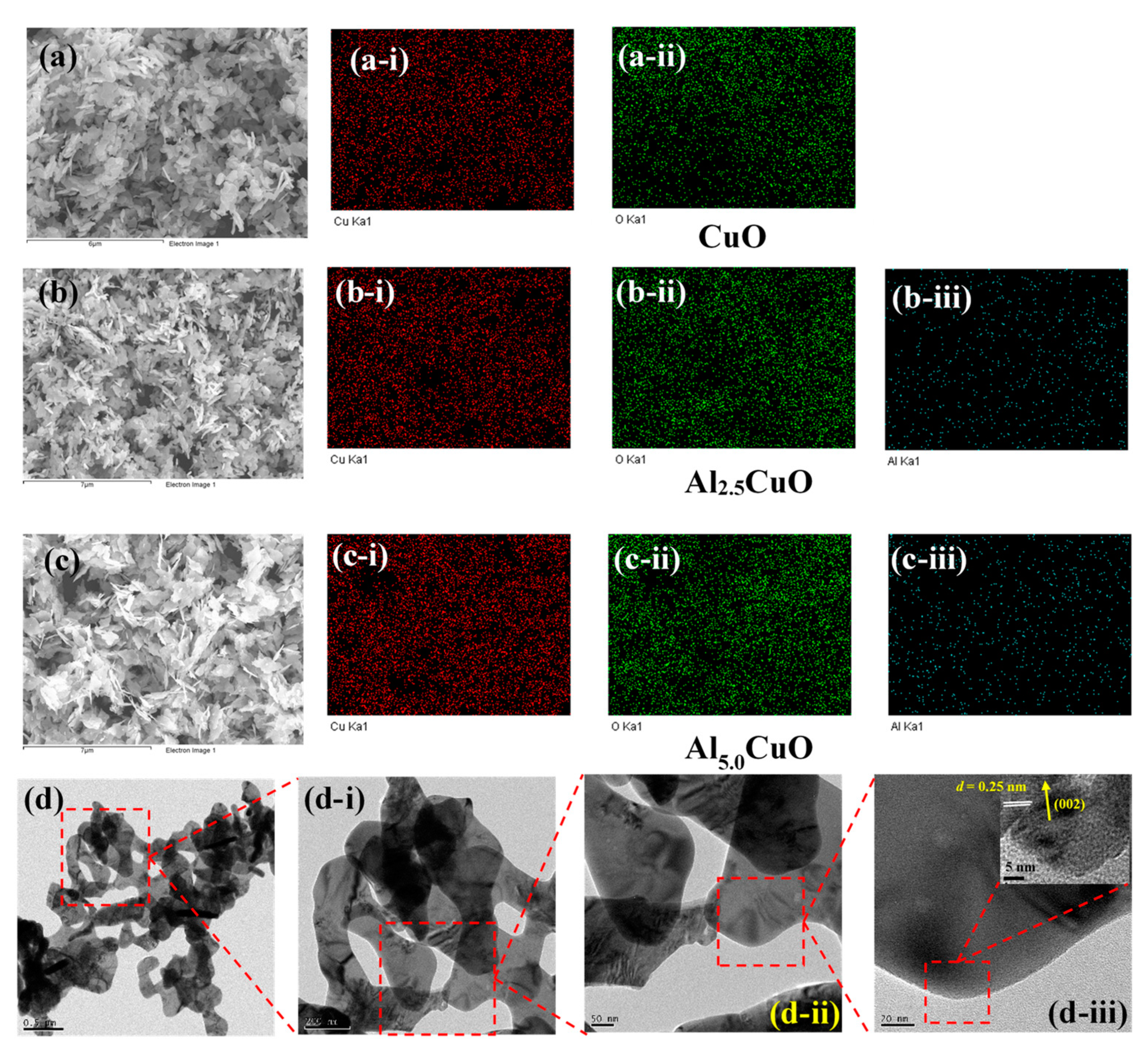
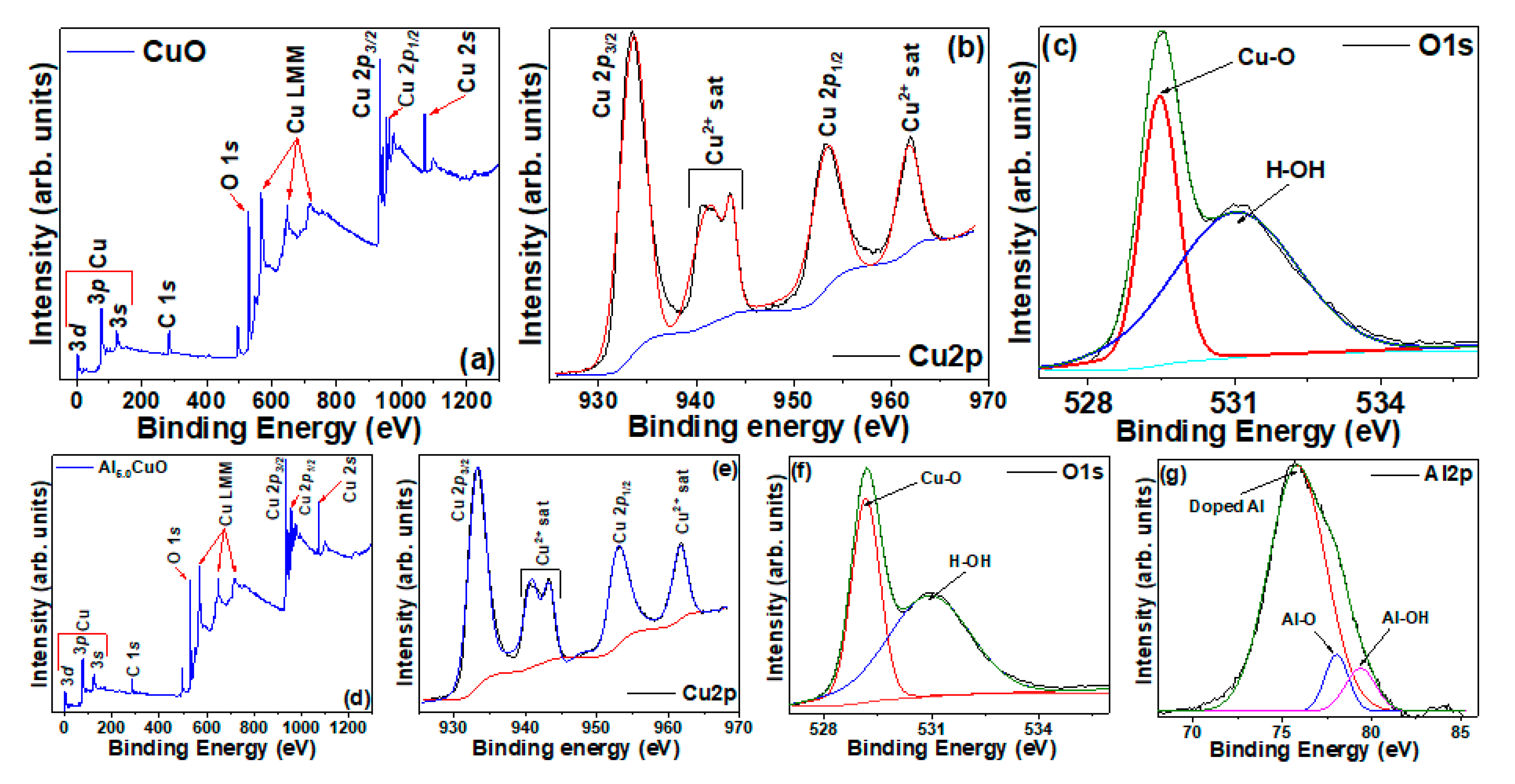
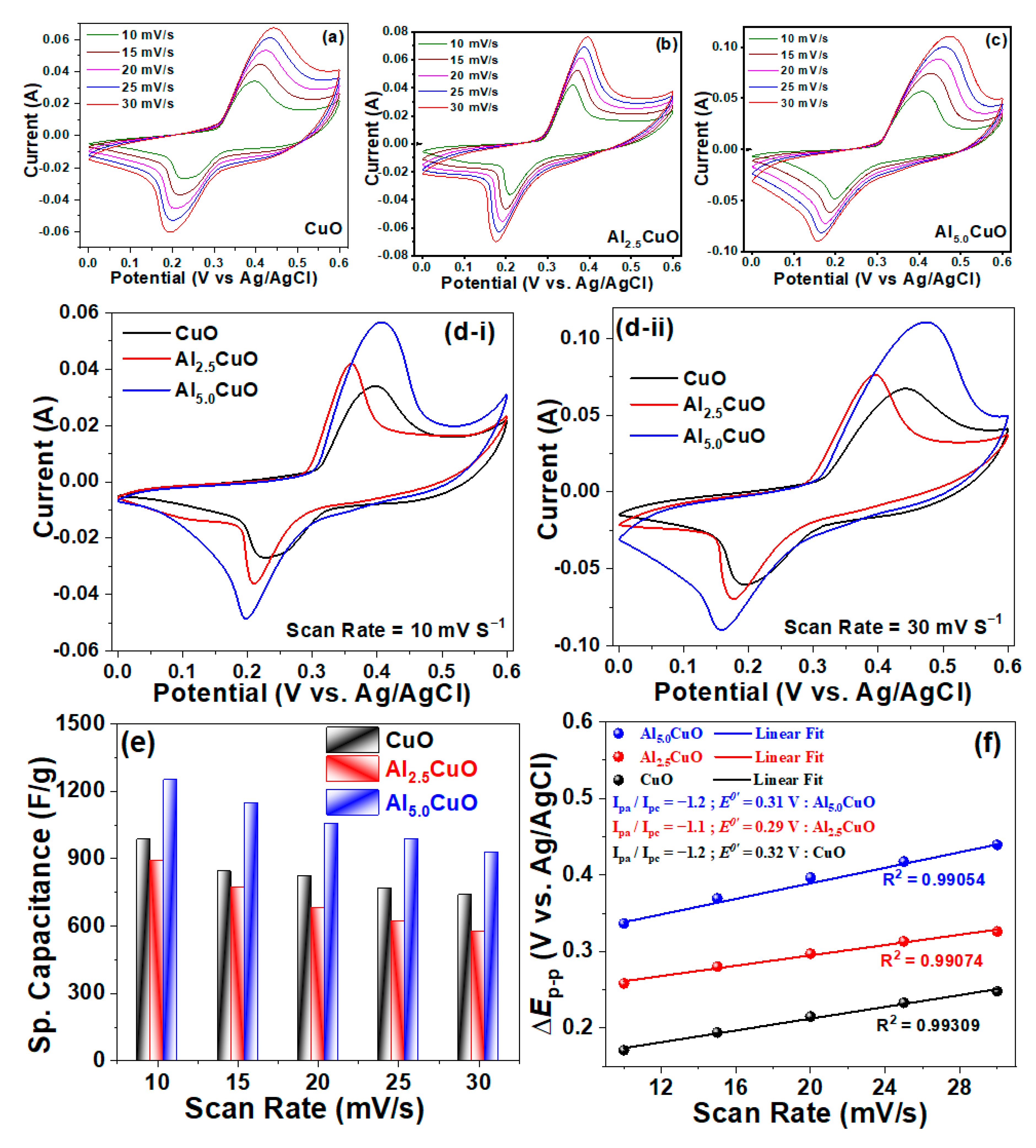
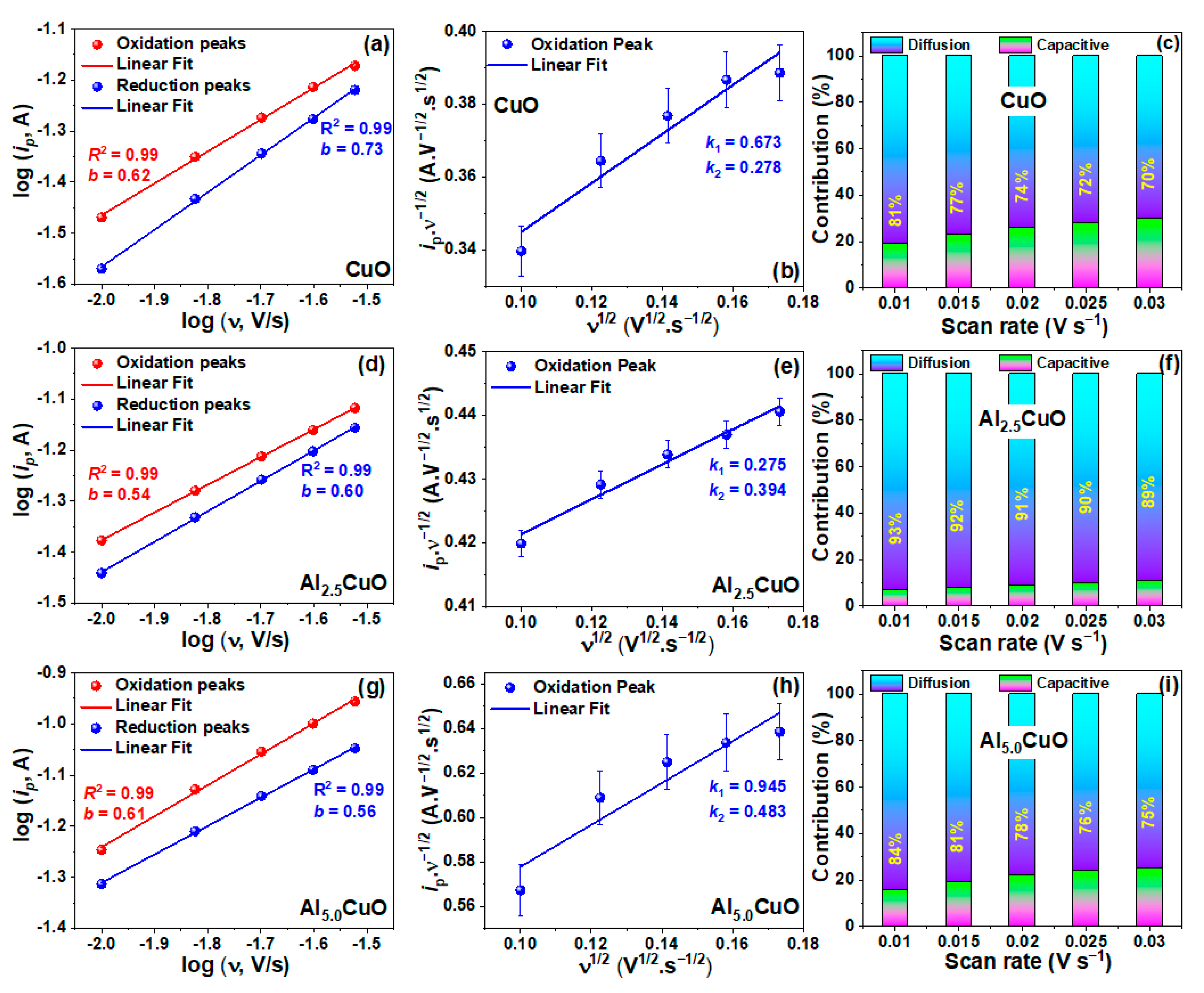
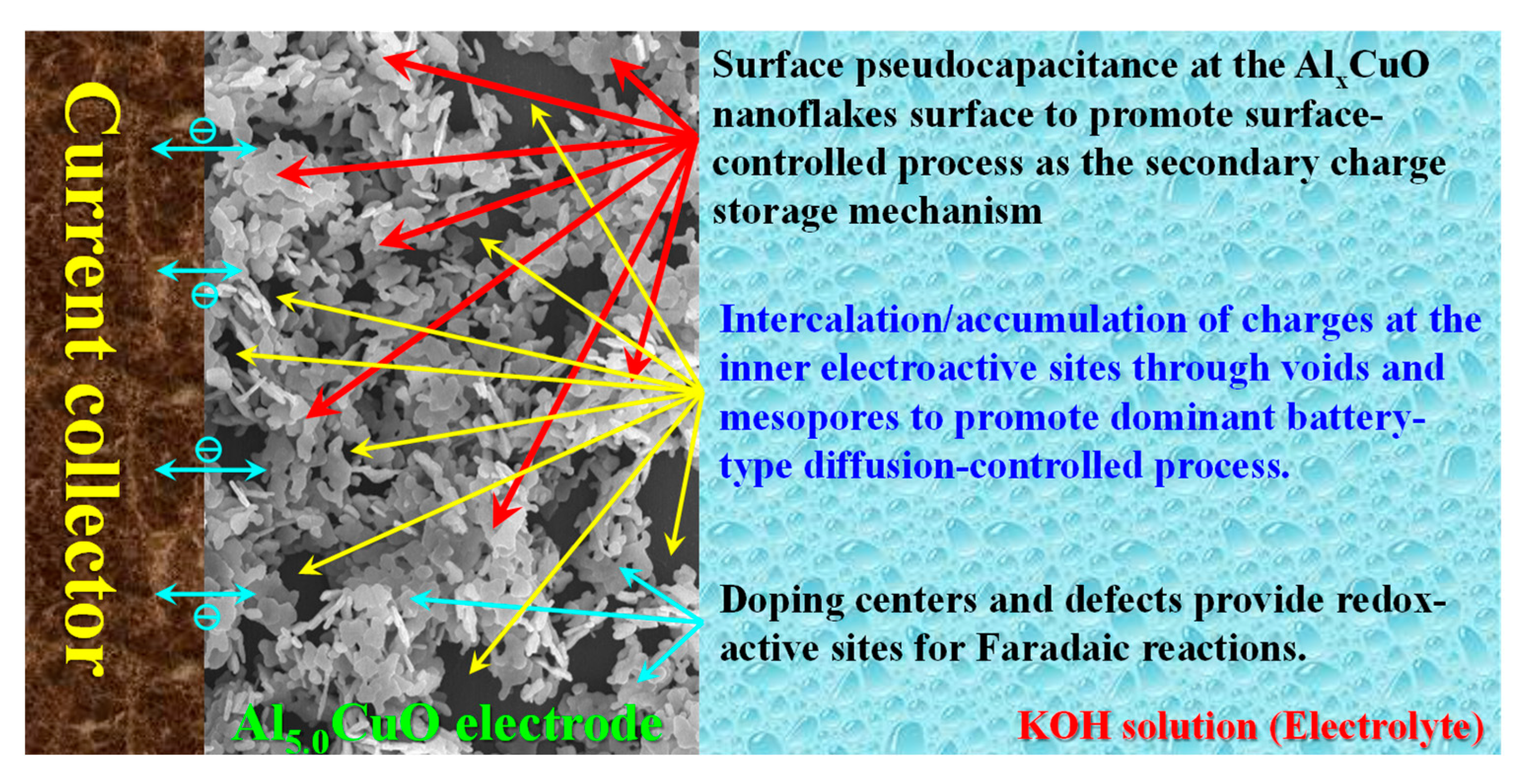
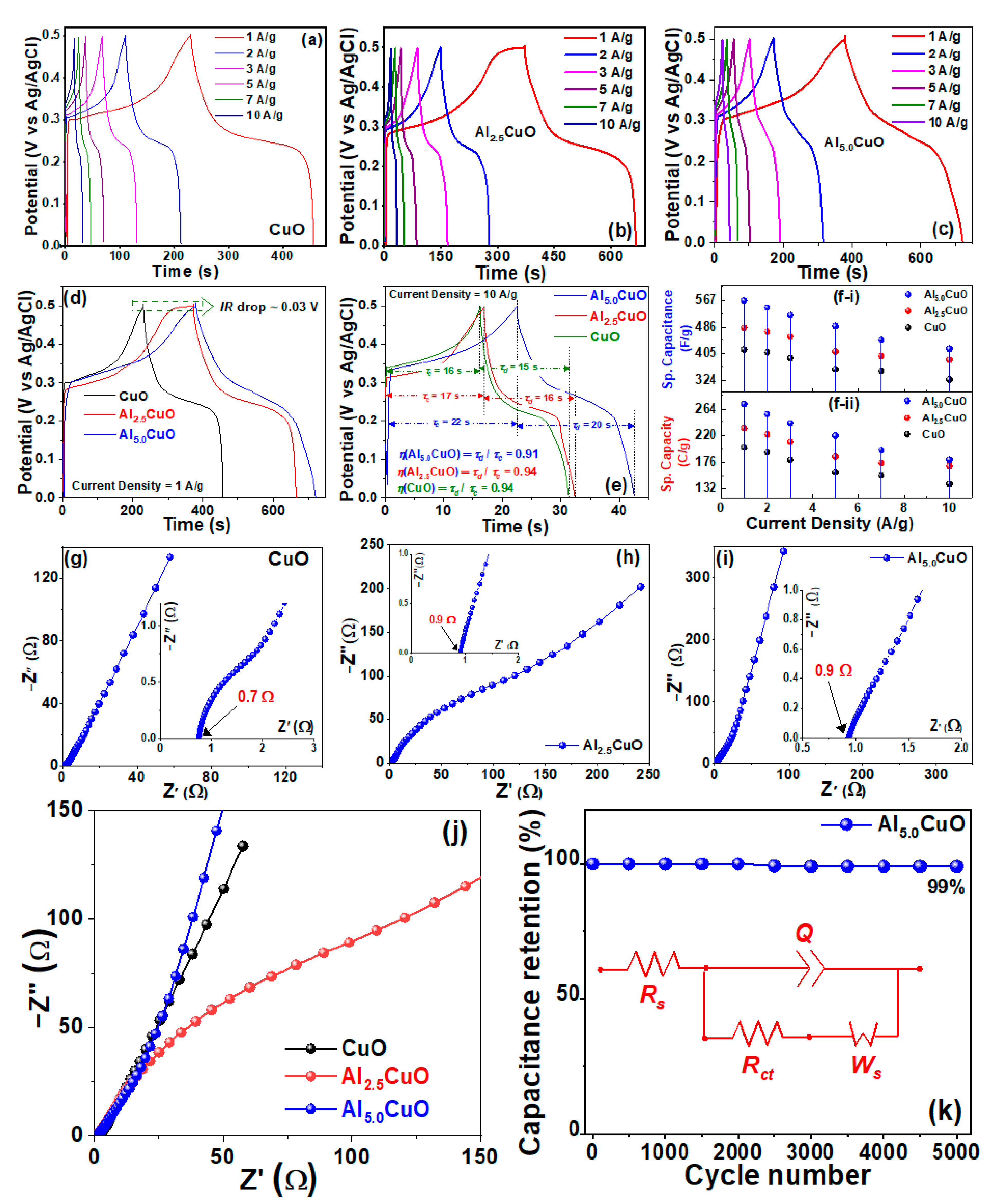
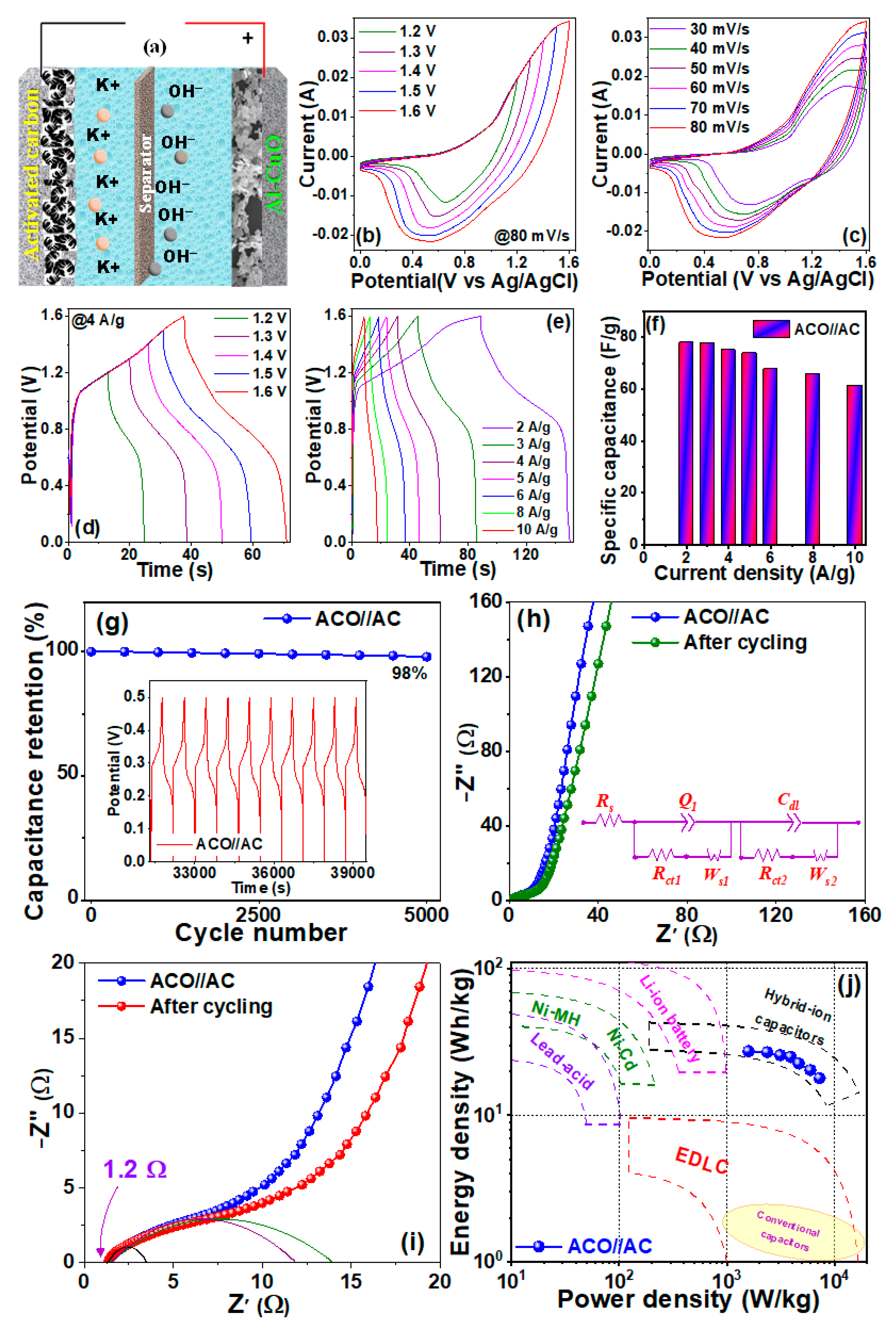

| Sample | Peak | Strain (ε) (× 10−3) | Crystallite Size (L) (nm) | Atomic Percentage (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| (002) | (111) | ||||||||
| 2θ (Deg.) | β (Rad.) | 2θ (Deg.) | β (Rad.) | Cu | O | Al | |||
| CuO | 35.49 | 5.2 × 10−3 | 38.71 | 6.5 × 10−3 | 5.9 | 47 | 51.2 | 48.8 | -- |
| Al2.5CuO | 35.59 | 6.3 × 10−3 | 38.81 | 7.5 × 10−3 | 6.3 | 36 | 52.1 | 46.1 | 1.8 |
| Al5.0CuO | 35.59 | 6.6 × 10−3 | 38.80 | 7.9 × 10−3 | 6.5 | 35 | 48.7 | 48.0 | 3.3 |
| Electrode Material | Synthesis Method | Electrolyte | Specific Capacitance (F/g)/Capacity (C/g) | Scan Rate (mV/s)/Current Density (A/g) | Cyclic Stability | Refs. | |
|---|---|---|---|---|---|---|---|
| Capacity Retention | Number of Cycles @ Current Density | ||||||
| Nanoporous CuO | Nitrate combustion | 3 M KOH | 431 F/g | 1 A/g | 93% | 3000 @ 2 A/g | [12] |
| Nanoporous CuO | Microwave-assisted green synthesis | 3 M KOH | 238 F/g | 5 mV/s | 75% | 3000 @ 5 A/g | [17] |
| 218 F/g | 1 A/g | ||||||
| CuO nanostructure | Chemical synthesis | 6 M KOH | 535 F/g | 2 A/g | 94% | 2000 @ 4 A/g | [14] |
| CuO nanowire | Electro-spinning | 6 M KOH | 710 F/g | 2 mV/s | 90% | 2000 @ 5 A/g | [45] |
| 620 F/g | 2 A/g | ||||||
| CuO cauliflowers | Potentiodynamic deposition | 1 M Na2SO4 | 179 F/g | 5 mV/s | 81% | 2000 @100 mV/s | [10] |
| 162 F/g | 2 mA/cm2 | ||||||
| CuO nanosheet | Reflux deposition | 0.1 M aqueous [MOPMIM] [Cl] | 180 F/g | 10 mV/s | 87% | 5000 @ 100 mV/s | [8] |
| CuO nano-worms | Chemical synthesis | 2 M KOH | 375 F/g | 2 mV/s | - | - | [55] |
| 206 F/g | 2 A/g | ||||||
| Nanostructured CuO thin film | RF sputter deposition | Aqueous phosphate-buffered saline | 387 F/g | 1 mA/cm2 | 95% | 1000 @ 1 mA/cm2 | [16] |
| Granular CuO Thin Film | RF Sputter deposition | 6 M KOH | 272 F/g | 5 mV/s | 85% | 3000 @ 100 mV/s | [13] |
| ~260 F/g | 1.47 A/g | ||||||
| Graphitic caron-CuO hollow nanosphere | Solvothermal-calcination | 3 M KOH | 677 F/g | 1 A/g | 86.7% | 8000 @ 5 A/g | [56] |
| Ag-decorated CuO nanorod | Chemical synthesis | 6 M KOH | 812 F/g | 2 A/g | 110% a | 5000 @ 16.67 A/g | [18] |
| Carbon stabilized CuO laminate | Hydrothermal-calcination | 3 M KOH | 695 F/g | 0.5 A/g | 87.3% | 5000 @ 10 A/g | [34] |
| Co-doped CuO | Co-precipitation | 1 M Na2SO4 | 168 F/g | 5 mV/s | 83% | 5000 @ 2.5 A/g | [21] |
| 136 F/g | 0.5 A/g | ||||||
| Fe-doped CuO | Co-precipitation | 1 M Na2SO4 | 186 F/g | 5 mV/s | 90.5% | 5000 @ 2.5 A/g | |
| 170 F/g | 0.5 A/g | ||||||
| Cu2O-CuO nanosheet | Electroless Cu plating | 6 M KOH | 835 F/g | 3.5 A/g | 85.6% | 5000 @ 5 mA/cm2 | [35] |
| CuO nanoparticle-graphene oxide nanosheet | Sonochemical- assisted precipitation | 1 M Na2SO4 | 245 F/g | 0.1 A/g | 79% | 1000 @ 0.25 A/g | [57] |
| CuO@MnO2 core-shell | Chemical synthesis | 1 M Na2SO4 | 276 F/g | 0.6 A/g | 92.1% | 1000 @ 3 A/g | [32] |
| CuO-MnO2 nanocomposite | Electrodeposition | 1 M Na2SO4 | (152.7) mF/cm2 | 1 mA/cm2 | 82.3% | 1000 @ 3 mA/cm2 | [58] |
| CuO@PANI nanocomposite | In situ chemical deposition | 0.5 M H2SO4 | 486.9 F/g | 0.5 mA/cm2 | 80% | 2000 @ 1 mA/cm2 | [31] |
| Dy-doped CuO | Combustion | 1 M Na2SO4 | 25.31 F/g | 5 mV/s | - | - | [23] |
| La-doped CuO nanoparticles | Co-precipitation | 1 M Na2SO4 | 47 F/g | 5 mV/s | - | - | [25] |
| Mn-doped CuO/Cu(OH)2 hybrid | Ionic layer deposition | 1 M Na2SO4 | 600 F/g b | 5 mV/s | ~90% | 1000 @ 5 mA/cm2 | [20] |
| Al-doped CuI thin film | Co-precipitation and thermal evaporation | 0.1 M Na2SO4 | 142.8 F/g c | 2 mV/s | 89.1% | 2000 @ 1 A/g | [59] |
| 103.4 F/g | 2 mA/cm2 | ||||||
| Al-doped CuO nanoflake d | Co-precipitation | 2 M KOH | 1250 F/g | 10 mV/s | 99% | 5000 @ 1 A/g | Current study |
| 567 F/g | 1 A/g | ||||||
| 272 C/g | 1 A/g | ||||||
| Device (Positrode//Negatrode) | Potential Window (V) | Specific Capacitance @ Current Density | Max. Energy Density (Wh/kg) | Max. Power Density (kW/kg) | Cyclic Stability | Refs. | |
|---|---|---|---|---|---|---|---|
| Capacity Retention (%) | Number of Cycles @ Current Density | ||||||
| CuO//AC | 1.4 | 72.4 F/g @ 1.0 A/g | 19.7 | ~7.0 | 96.0 | 3000 @ 2.00 A/g | [12] |
| Ag-CuO//AC | 1.5 | 134.98 F/g @ 1.5 A/g | 40.0 | ~7.5 | 96.7 | 5000 @ 10.0 A/g | [18] |
| Mo-CuO//AC | 1.6 | ~100 F/g @ 1.0 A/g | 36.0 | 10.3 | 81.0 | 5000 @ NA | [26] |
| C//C-CuO | 1.5 | 50.1 F/g @ 0.5 A/g | 13.6 | 7.0 | 91.2 | 5000 @ 10.0 A/g | [34] |
| Cu2O-CuO//AC | 1.6 | 180 F/g @ 0.5 A/g | 60.26 | 7.0 | 90.3 | 5000 @ 2.00 A/g | [35] |
| CuO@MnO2//Graphite oxide | 1.8 | 49.2 F/g @ 0.25 A/g | 22.1 | ~5.0 | 101.5 a | 10,000 @ 3.00 A/g | [32] |
| CuO//AC b | 1.0 | ~70 F/g @ 2.0 A/g | 29.4 | 3.01 | - | - | [14] |
| CuO//CuO b | 1.0 | ~82 F/g @ 1.0 A/g | ~12.0 | 3.93 | |||
| CuO@graphitic carbon//AC | 1.6 | 108.7 F/g @ 0.5 A/g | 38.6 | 14.0 | 90.2 | 10,000 @ 5.00 A/g | [29] |
| Mn-CuO//AC | 0.6 | 72.0 F/g @ 0.5 A/g | 7.4 | - | 71.0 | 300 @ 10.00 A/g | [60] |
| Mn-CuO//Mn-CuO | 57.0 F/g @ 0.5 A/g | 5.8 | 51.0 | ||||
| Al-CuO//AC c | 1.6 | 80.0 F/g @ 2.0 A/g | 30.0 | 7.2 | 98.0 | 5000 @ 10.00 A/g | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallavolu, M.-R.; Banerjee, A.-N.; Joo, S.-W. Battery-Type Behavior of Al-Doped CuO Nanoflakes to Fabricate a High-Performance Hybrid Supercapacitor Device for Superior Energy Storage Applications. Coatings 2023, 13, 1337. https://doi.org/10.3390/coatings13081337
Pallavolu M-R, Banerjee A-N, Joo S-W. Battery-Type Behavior of Al-Doped CuO Nanoflakes to Fabricate a High-Performance Hybrid Supercapacitor Device for Superior Energy Storage Applications. Coatings. 2023; 13(8):1337. https://doi.org/10.3390/coatings13081337
Chicago/Turabian StylePallavolu, Mohan-Reddy, Arghya-Narayan Banerjee, and Sang-Woo Joo. 2023. "Battery-Type Behavior of Al-Doped CuO Nanoflakes to Fabricate a High-Performance Hybrid Supercapacitor Device for Superior Energy Storage Applications" Coatings 13, no. 8: 1337. https://doi.org/10.3390/coatings13081337
APA StylePallavolu, M.-R., Banerjee, A.-N., & Joo, S.-W. (2023). Battery-Type Behavior of Al-Doped CuO Nanoflakes to Fabricate a High-Performance Hybrid Supercapacitor Device for Superior Energy Storage Applications. Coatings, 13(8), 1337. https://doi.org/10.3390/coatings13081337







