Effect of Different Emulsifiers on the Preparation Process of Aloe-Emodin Microcapsules and Waterborne Coating Properties
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Preparation Method of the Aloe-Emodin Microcapsules
- (1)
- Preparation of wall material: Formaldehyde solution and urea were combined thoroughly, triethanolamine was added to bring the pH level down to about 8, and then 0.1 g of polyvinyl alcohol was added. The mixture was then placed in a magnetic stirrer and heated to 80 °C while being stirred at a rate of 600 rpm for one hour.
- (2)
- Preparation of core material: In order to fully emulsify the core material, for 45 min different types of emulsifiers were mixed with water and thoroughly stirred before the core material, aloe-emodin, was added and placed into the magnetic stirrer. The reaction temperature was then set to 60 °C and the stirring rate to 1000 rpm.
- (3)
- Microencapsulation: The solution of urea-formaldehyde resin wall material was slowly added into the core material with a dropper at 600 rpm. Citric acid monohydrate crystals were added in small amounts to adjust the pH of the solution to 2.5–3.0. The reaction was then continued for another two hours while being stirred with the addition of the proper amounts of NaCl and SiO2 powder. The final product was aged for 24 h at room temperature. After aging, the product was filtered several times with ethanol and water using a filtering machine and put into an oven and dried at 40 °C for 24 h. After drying, the product was ground and the resulting powder was microencapsulated.
2.3. Preparation Method of the Paint Films
2.4. Testing and Characterization
2.4.1. Yield and Coverage Rate Test of the Microcapsules
2.4.2. Micromorphology and Chemical Composition Test
2.4.3. Antibacterial Test of the Paint Films
2.4.4. Optical Properties Test of the Paint Films
2.4.5. Roughness Test of the Paint Films
3. Results and Discussion
3.1. Analysis of Microcapsule Preparation Results
3.1.1. Analysis of Microcapsule Yield and Coverage Rate
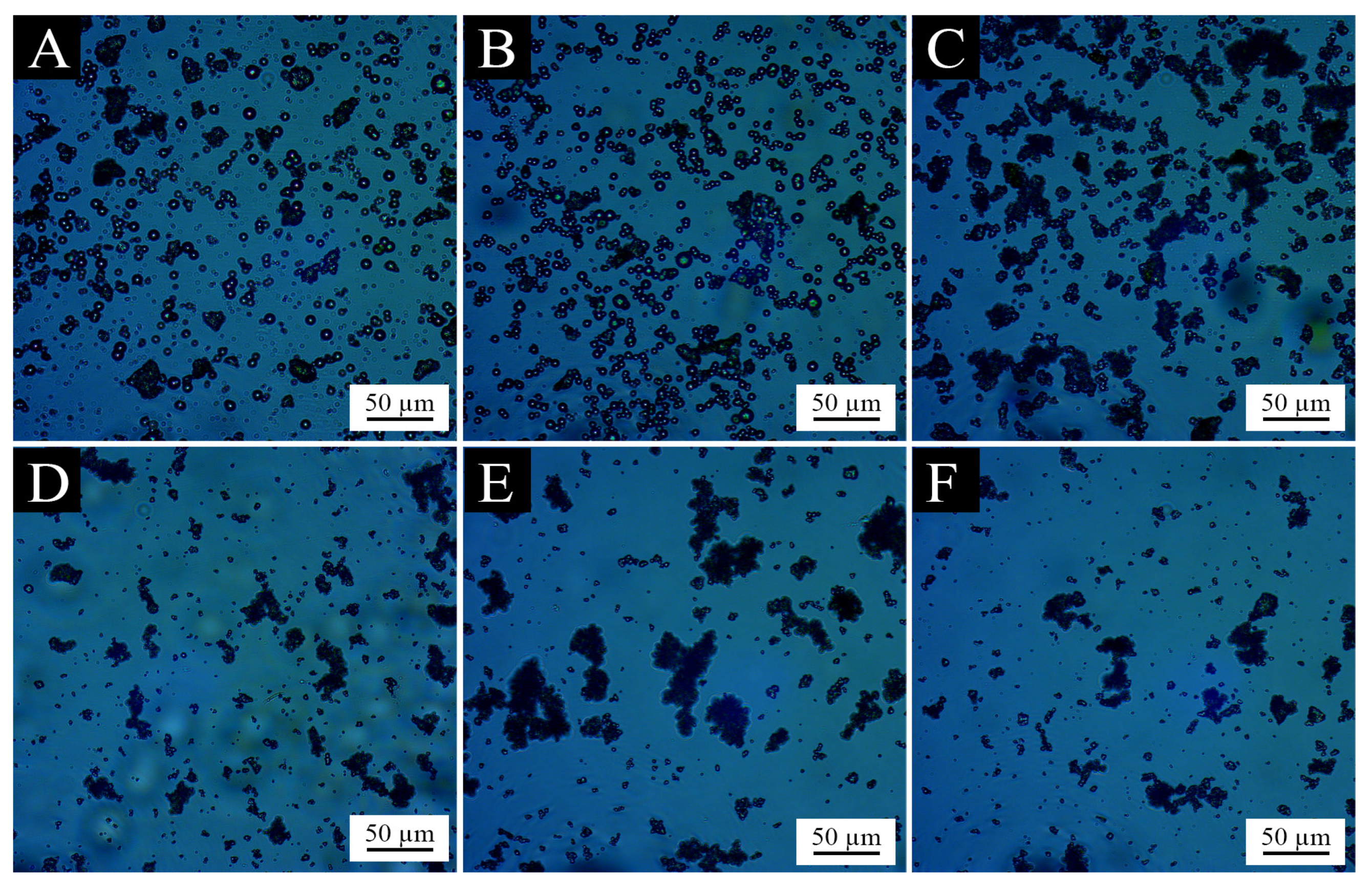
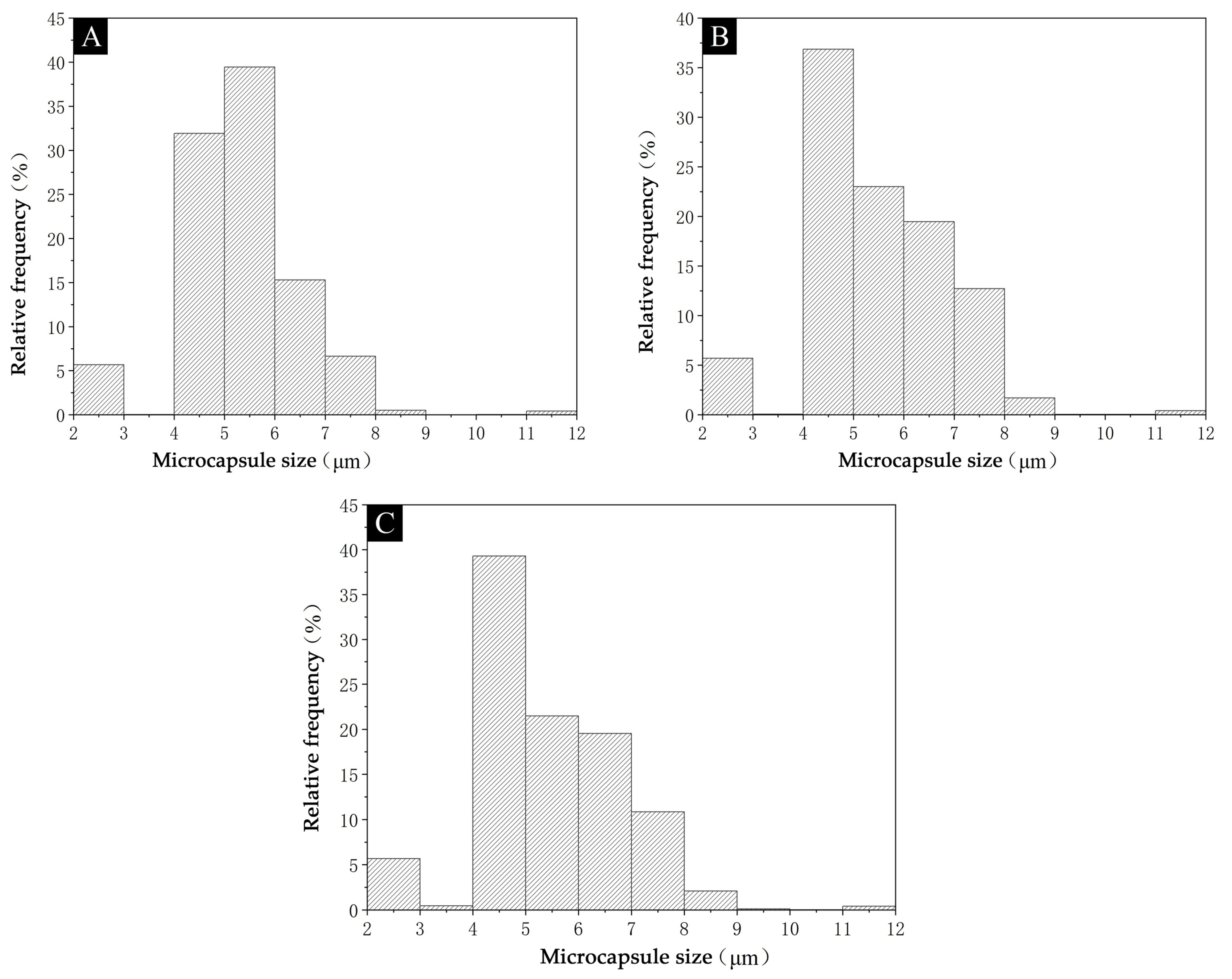
3.1.2. Analysis of Microcapsule Morphology
3.1.3. Analysis of Chemical Composition of Microcapsules
3.2. Analysis of Paint Film Properties
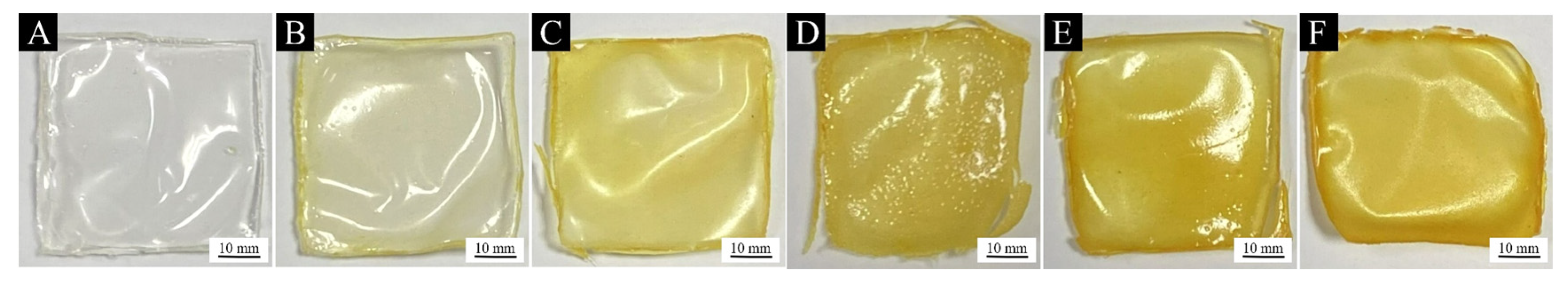
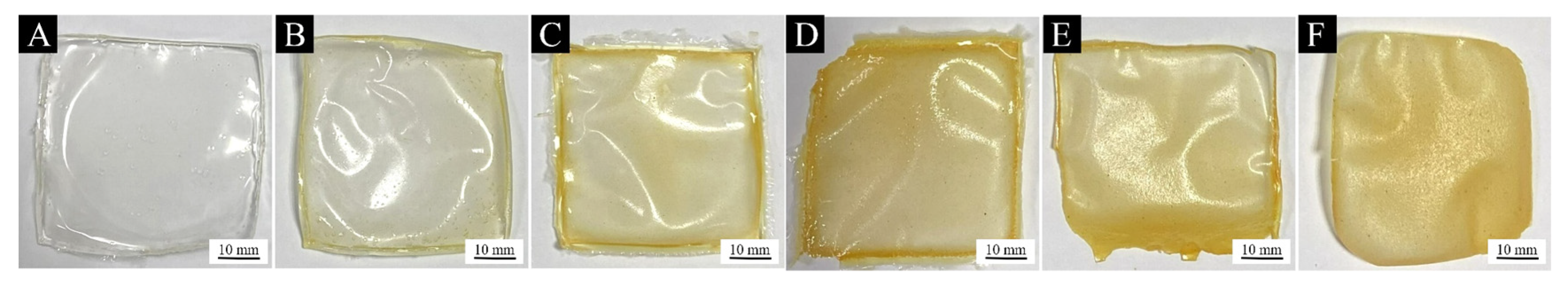
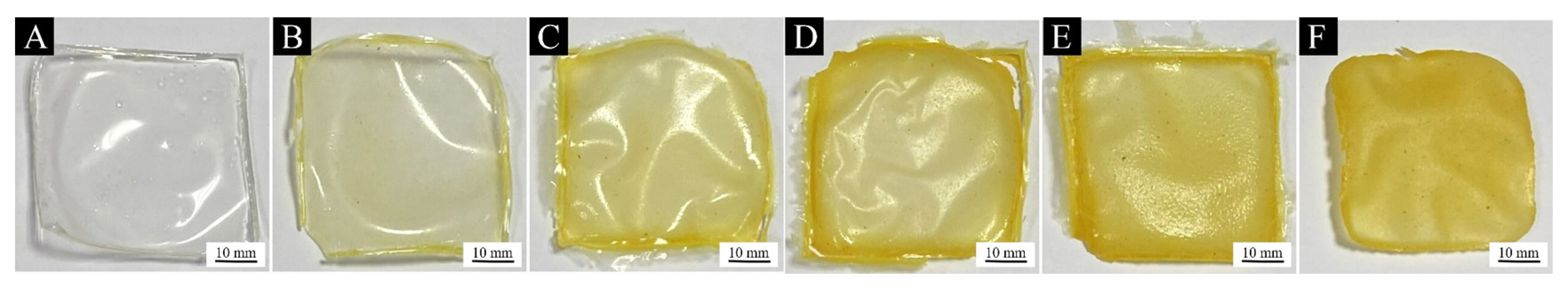
3.2.1. Analysis of Paint Film Morphology
3.2.2. Analysis of Chemical Composition of Paint Film
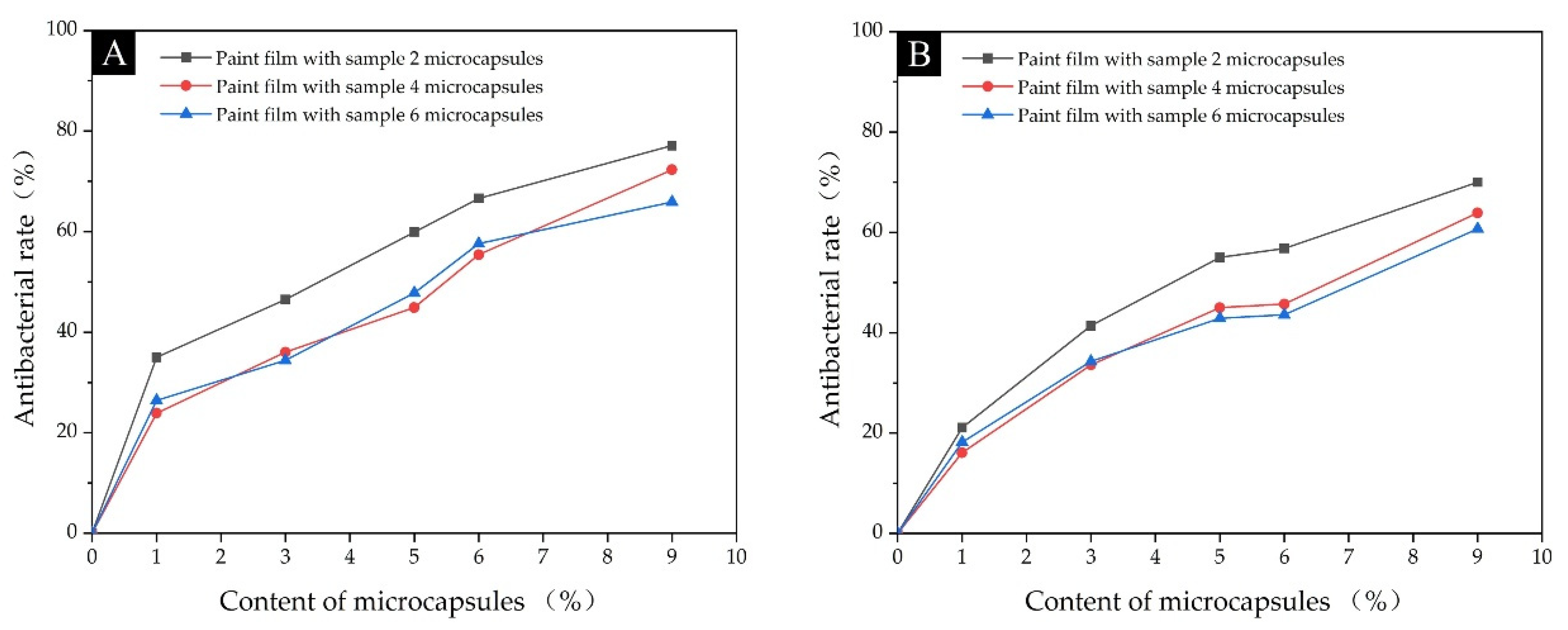
3.2.3. Analysis of Antimicrobial Properties of Paint Film
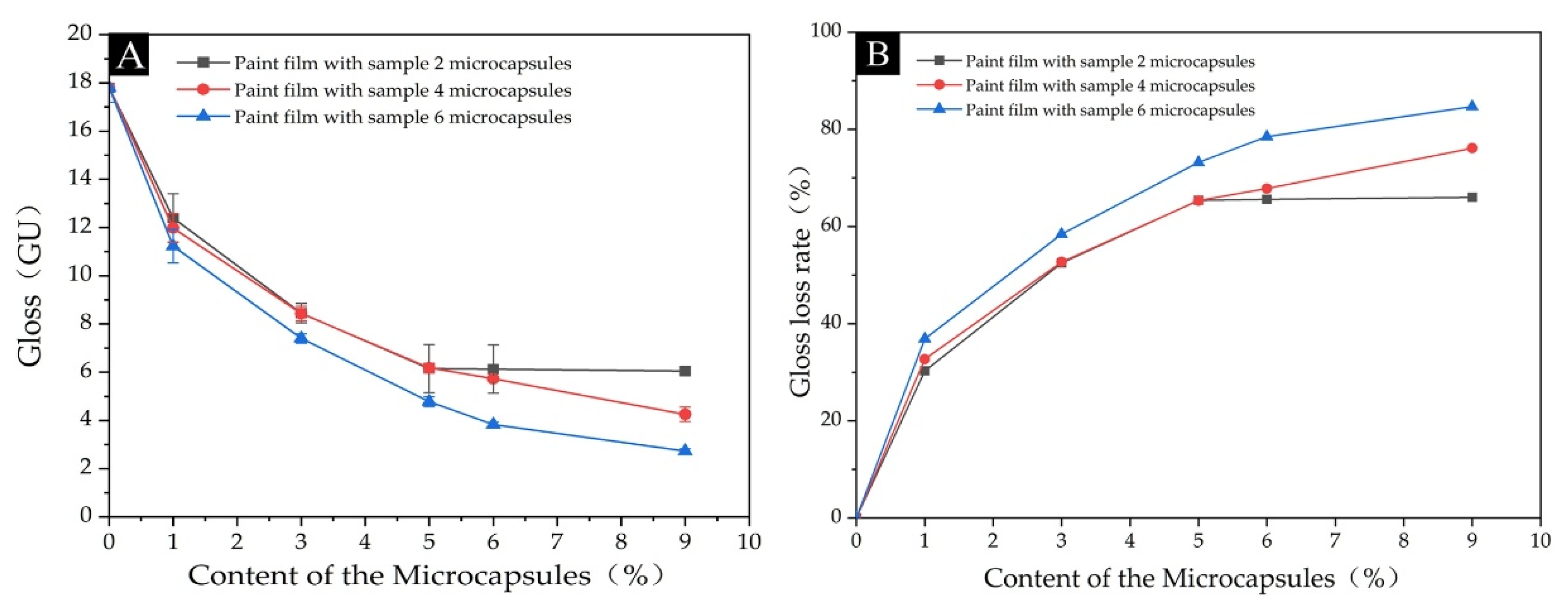
3.2.4. Analysis of Optical Properties of Paint Film
3.2.5. Analysis of Paint Film Roughness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.J.; Xie, C.Y. City garden environment research: Optimal design can improve the therapeutic benefit of therapy garden. Fresenius Environ. Bull. 2020, 29, 11615–11623. [Google Scholar]
- Hu, W.G.; Yu, R.Z. Mechanical and acoustic characteristics of four wood species subjected to bending load. Maderas-Cienc. Tecnol. 2023, 25, 39. [Google Scholar]
- Hu, W.G.; Luo, M.Y.; Hao, M.M.; Tang, B.; Wan, C. Study on the Effects of Selected Factors on the Diagonal Tensile Strength of Oblique Corner Furniture Joints Constructed by Wood Dowel. Forests 2023, 14, 1149. [Google Scholar] [CrossRef]
- Hu, W.G.; Yu, R.Z.; Luo, M.Y.; Konukcu, A.C. Study on tensile strength of single dovetail joint: Experimental, numerical, and analytical analysis. Wood Mater. Sci. Eng. 2022, 17, 2155875. [Google Scholar] [CrossRef]
- Han, M.J.N.; Kim, M.J. Green environments and happiness level in housing areas toward a sustainable life. Sustainability 2019, 11, 4768. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, R.; Zhang, Z.M.; Song, D.D.; Li, X.G. Effect of membrane structure of waterborne coatings on the transport process of corrosive medium. Prog. Org. Coat. 2018, 124, 8–15. [Google Scholar] [CrossRef]
- Ge, H.W.; Zhang, J.F.; Yuan, Y.; Liu, J.C.; Liu, R.; Liu, X.Y. Preparation of organicinorganic hybrid silica nanoparticles with contact antibacterial properties and their application in UV-curable coatings. Prog. Org. Coat. 2017, 106, 20–26. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.D.; Mustata, F.; Rosu, D.; Rosca, I.; Rusu, T. Epoxy coatings based on modified vegetable oils for wood surface protection against fungal degradation. ACS Appl. Mater. Interfaces 2020, 12, 14443–14458. [Google Scholar] [CrossRef]
- Huang, N.; Yan, X. Preparation of Aloe-Emodin Microcapsules and Its Effect on Antibacterial and Optical Properties of Water-Based Coating. Polymers 2023, 15, 1728. [Google Scholar] [CrossRef]
- Luo, Y.R.; Xu, W. Optimization of Panel Furniture Plates Rework Based on Intelligent Manufacturing. Bioresources 2023, 18, 5198–5208. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Xu, W.; Wu, S.S. Performances of Green Velvet Material (PLON) Used in Upholstered Furniture. Bioresources 2023, 18, 5108–5119. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xiao, Y.F.; Gan, L.; Wu, Y.; Wu, F.; Gu, Z.W. The effect of antibacterial ingredients and coating microstructure on the antibacterial properties of plasma sprayed hydroxyapatite coatings. Surf. Coat. Technol. 2012, 206, 2986–2990. [Google Scholar]
- Lu, Z.; Hu, J.B.; Zhang, M.H.; Chang, S.S.; Liu, Y.; Zheng, L.; Li, X.J. Research on wettability of wood surface with waterborne-resin priming paint. J. For. Eng. 2021, 6, 178–183. [Google Scholar]
- Han, S.J.; Chen, Y.P.; Lyu, S.Y.; Chen, Z.L.; Wang, S.Q.; Fu, F. Effects of processing conditions on the properties of paraffin/melamine-urea-formaldehyde microcapsules prepared by in situ polymerization. Colloids Surf. B 2019, 585, 124046. [Google Scholar] [CrossRef]
- You, K.X.; Gao, B.; Wang, M.Y.; Wang, X.Y.; Okoro, K.C.; Rakhimbekzoda, A.; Feng, Y.K. Versatile polymer-based strategies for antibacterial drug delivery systems and antibacterial coatings. J. Mater. Chem. B 2022, 10, 1005–1018. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, J.; Zheng, X.; Pan, B.; Leng, W. Effects of three kinds of fungi on color, chemical composition and route of infection of Picea sitchensis. J. For. Eng. 2021, 6, 88–93. [Google Scholar]
- Das, S.; Das, A.; Bhavya, T.; Nivashini, S.R. Molecular characterisation and antibacterial activity of Aloe barbadensis miller on textiles. J. Text. Inst. 2020, 111, 1116–1122. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Li, X.B.; Zhu, Z.F.; Zhang, E.D.; Shi, J.; Lu, M.G. Antibacterial and alkali-responsive cationic waterborne polyurethane based on modification of aloe emodin. Chem. Res. Chin. Univ. 2023, 39, 266–275. [Google Scholar] [CrossRef]
- Xiang, H.; Cao, F.J.; Ming, D.; Zheng, Y.Y.; Dong, X.Y.; Zhong, X.B.; Mu, D.; Li, B.B.; Zhong, L.; Cao, J.J. Aloe-emodin inhibits Staphylococcus aureus biofilms and extracellular protein production at the initial adhesion stage of biofilm development. Appl. Microbiol. Biotechnol. 2017, 101, 6671–6681. [Google Scholar] [CrossRef]
- Hu, X.K.; Ma, Y.; Liu, Z.D.; Zhao, M.X.; Dong, S.M.; Yang, H.; Dai, C.M. Microcalorimetric evaluation of the effects of three anthraquinone derivatives from Chinese Rhubarb and the synergistic effect of the mixture on Staphylococcus aureus. J. Therm. Anal. Calorim. 2020, 141, 739–749. [Google Scholar] [CrossRef]
- Ding, L.J.; Wang, H.; Liu, D.; Zheng, Z.N. Surface attachment of natural antimicrobial coatings onto conventional polypropylene nonwoven fabric and its antimicrobial performance assessment. J. Food Protect. 2018, 81, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.X.; Yi, T.; Shen, Z.Y.; Teng, Z.H.; Wang, J.F. Aloe-emodin attenuates staphylococcus aureus pathogenicity by interfering with the oligomerization of alpha-toxin. Front. Cell. Infect. Microbiol. 2019, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Donkor, A.M.; Donkor, M.N.; Kuubabongnaa, N. Evaluation of anti-infective potencies of formulated aloin A ointment and aloin A isolated from Aloe barbadensis Miller. BMC Chem. 2020, 14, 8. [Google Scholar] [CrossRef]
- Coopoosamy, R.M.; Magwa, M.L. Antibacterial activity of aloe emodin and aloin A isolated from Aloe excelsa. Afr. J. Biotechnol. 2006, 5, 1092–1094. [Google Scholar]
- Alvaro, G.; Schlangen, E. Preparation of capsules containing rejuvenators for their use in asphalt concrete. J. Hazard. Mater. 2010, 184, 603–611. [Google Scholar]
- Zotiadis, C.; Patrikalos, I.; Loukaidou, V.; Korres, D.M.; Karantonis, A.; Vouyiouka, S. Self-healing coatings based on poly(urea-formaldehyde) microcapsules: In situ polymerization, capsule properties and application. Prog. Org. Coat. 2021, 161, 106475. [Google Scholar] [CrossRef]
- An, S.; Lee, M.W.; Yarin, A.L.; Yoon, S.S. A review on corrosion-protective extrinsic self-healing: Comparison of microcapsulebased systems and those based on core-shell vascular networks. Chem. Eng. J. 2018, 344, 206–220. [Google Scholar] [CrossRef]
- Scheiner, M.; Dickens, T.J.; Okoli, O. Progress towards self-healing polymers for composite structural applications. Polymer 2016, 83, 260–282. [Google Scholar] [CrossRef]
- Farooq, A.; Lee, H.W.; Jae, J.; Kwon, E.E.; Park, Y.K. Emulsification characteristics of ether extracted pyrolysis-oil in diesel using various combinations of emulsifiers (Span 80, Atlox 4916 and Zephrym PD3315) in double reactor system. Environ. Res. 2020, 184, 109267. [Google Scholar] [CrossRef]
- Liu, R.D.; Yan, J.L.; Li, Z.Y.; Zhang, Y.C.; Tian, C.Y.; Fu, P.; Li, X.B.; Zhou, L.; Xu, X.W. Research on the Upgrading of Bio-Oil Based on Low-Carbon Alcohol Solvents and Ultrasonic-Emulsification. BioResources 2022, 17, 4241–4261. [Google Scholar] [CrossRef]
- Yan, X.X.; Peng, W.W.; Qian, X.Y. Effect of water-based acrylic acid microcapsules on the properties of paint film for furniture surface. Appl. Sci. 2021, 11, 7586. [Google Scholar] [CrossRef]
- Yamazaki, N.; Du, Y.Z.; Nagai, M.; Omi, S. Preparation of polyepoxide microcapsule via interfacial polyaddition reaction in W/O and O/W emulsion systems. Colloids Surf. B Biointerfaces 2003, 29, 159–169. [Google Scholar] [CrossRef]
- Tao, Y.; Yan, X.X. Influence of HLB Value of Emulsifier on the Properties of Microcapsules and Self-Healing Properties of Waterborne Coatings. Polymers 2022, 14, 1304. [Google Scholar] [CrossRef]
- GB/T 21866-2008; Test Method and Effect for Antibacterial Capability of Paints Film. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T 4789.2-2016; National Food Safety Standard Food Microbiological Examination: Aerobic Plate Count. Standardization Administration of the People’s Republic of China: Beijing, China, 2016.
- GB/T 11186.3-1989; Methods for Measuring the Colour of Paint Films. Part III: Calculation of Colour Differences. Standardization Administration of the People’s Republic of China: Beijing, China, 1990.
- GB/T 4893.4-2013; Test of Surface Coatings of Furniture. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 6062-1985; Stylus Instruments for the Measurement of Surface Roughness by the Profile Method Profile Recording Instruments and Profile Meters by the System M. Standardization Administration of the People’s Republic of China: Beijing, China, 1985.
- Chen, S.W.; Lu, X.C.; Wang, T.Z.; Zhang, Z.M. Preparation and characterization of urea-formaldehyde resin/reactive kaolinite composites. Particuology 2016, 24, 203–209. [Google Scholar] [CrossRef]
- Poljansek, I.; Sebenik, U.; Krajnc, M. Characterization of phenol-urea-formaldehyde resin by infine FTIR spectroscopy. J. Appl. Polym. Sci. 2006, 99, 2016–2028. [Google Scholar] [CrossRef]
- Xie, Y.F.; Huan, N.; Cao, Y.Y.; Wang, H.Y.; Zhong, Y.; Yao, W.R.; Qian, H. Structural characterization and spectroscopic analysis of the aloin. Spectrosc. Spect. Anal. 2014, 34, 385–388. [Google Scholar]







| Material | Molecular Formula | Mw (g/mol) | CAS No. | Concentration (%) |
|---|---|---|---|---|
| urea | CH4N2O | 60.06 | 57-13-6 | 99.0 |
| formaldehyde solution | - | - | - | 37.0 |
| triethanolamine | C6H15NO3 | 149.19 | 102-71-6 | 99.9 |
| polyvinyl alcohol | [C2H4O]n | - | 9002-89-5 | |
| aloe-emodin | C15H10O5 | 270.2369 | 481-72-1 | 98.0 |
| citric acid monohydrate | C6H10O8 | 210.14 | 5949-29-1 | 99.9 |
| anhydrous ethanol | C2H6O | 46.07 | 64-17-5 | 99.9 |
| waterborne acrylic resin | - | - | 9003-01-4 | - |
| Escherichia coli | - | - | - | - |
| Staphylococcus aureus | - | - | - | - |
| nutrient agar medium | - | - | - | - |
| nutritional broth | - | - | - | - |
| sodium chloride | NaCl | 58.4428 | 7647-14-5 | 99.5 |
| silicon dioxide | SiO2 | 60.084 | 14808-60-7 | 99.5 |
| polyethylene film | - | - | - | - |
| Petri dish | - | - | - | - |
| sodium dodecylbenzene sulfonate (SDBS) | C18H29NaO3S | 348.48 | 25155-30-0 | 99.0 |
| octylphenol polyoxyethylene ether -10 (OP-10) | (C2H4O)N·C14H22O | 602.797 | 9002-93-1 | 99.0 |
| polyoxyethylene dehydrated sorbitan monooleate (Tween-80) | C24H44O6 | 428.60 | 9005-65-6 | 99.0 |
| Name of Emulsifier | HLB Value | Type of Emulsifier |
|---|---|---|
| SDBS | 10.6 | Anionic type |
| OP-10 | 13.4 | Non-ionic |
| Tween-80 | 15.0 | Non-ionic |
| Sample | Types of Emulsifiers | Emulsifier Concentration (%) | n (Urea):n (Formaldehyde) | m (Core Material):m (Wall Material) | Temperature (°C) | Stirring Speed (rpm) |
|---|---|---|---|---|---|---|
| 1 | SDBS | 1 | 1:1.2 | 1:15 | 50 | 600 |
| 2 | SDBS | 3 | 1:1.2 | 1:15 | 50 | 600 |
| 3 | OP-10 | 1 | 1:1.2 | 1:15 | 50 | 600 |
| 4 | OP-10 | 3 | 1:1.2 | 1:15 | 50 | 600 |
| 4 | Tween-80 | 1 | 1:1.2 | 1:15 | 50 | 600 |
| 6 | Tween-80 | 3 | 1:1.2 | 1:15 | 50 | 600 |
| Sample | Urea (g) | Formaldehyde Solution (g) | Wall Material (g) | Polyvinyl Alcohol (g) | Aloe-Emodin (g) | Deionized Water (g) | Emulsifier (g) | NaCl (g) | SiO2 (g) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.00 | 16.22 | 16.00 | 0.10 | 1.07 | 232.65 | 2.35 | 1.28 | 1.28 |
| 2 | 10.00 | 16.22 | 16.00 | 0.10 | 1.07 | 227.95 | 7.05 | 1.28 | 1.28 |
| 3 | 10.00 | 16.22 | 16.00 | 0.10 | 1.07 | 232.65 | 2.35 | 1.28 | 1.28 |
| 4 | 10.00 | 16.22 | 16.00 | 0.10 | 1.07 | 227.95 | 7.05 | 1.28 | 1.28 |
| 5 | 10.00 | 16.22 | 16.00 | 0.10 | 1.07 | 232.65 | 2.35 | 1.28 | 1.28 |
| 6 | 10.00 | 16.22 | 16.00 | 0.10 | 1.07 | 227.95 | 7.05 | 1.28 | 1.28 |
| Content of the Microcapsules (%) | Microcapsule Weight (g) | Coating Weight (g) |
|---|---|---|
| 0 | 0 | 1.00 |
| 1.0 | 0.01 | 0.99 |
| 3.0 | 0.03 | 0.97 |
| 6.0 | 0.06 | 0.94 |
| 7.0 | 0.07 | 0.93 |
| 9.0 | 0.09 | 0.91 |
| Sample | Types of Emulsifiers | Emulsifier Concentration (%) | Yield (g) | Yield Rate (%) | Coverage Rate (%) |
|---|---|---|---|---|---|
| 1 | SDBS | 1.0 | 10.16 | 3.8 | 9.3 |
| 2 | SDBS | 3.0 | 11.85 | 4.5 | 9.4 |
| 3 | OP-10 | 1.0 | 13.27 | 5.1 | 7.7 |
| 4 | OP-10 | 3.0 | 13.58 | 5.1 | 7.5 |
| 5 | Tween-80 | 1.0 | 14.15 | 5.3 | 8.0 |
| 6 | Tween-80 | 3.0 | 14.25 | 5.4 | 8.0 |
| Sample | Content of the Microcapsules (%) | Average Number of Recovered Escherichia coli (CFU·Piece−1) | Antibacterial Rate against Escherichia coli (%) | Average Number of Recovered Staphylococcus aureus (CFU·Piece−1) | Antibacterial Rate against Staphylococcus aureus (%) |
|---|---|---|---|---|---|
| Paint film with Sample 2 microcapsules | 0 | 314 | - | 280 | - |
| 1 | 204 | 35.0 | 221 | 21.1 | |
| 3 | 168 | 46.5 | 164 | 41.4 | |
| 5 | 126 | 59.9 | 126 | 55.0 | |
| 6 | 105 | 66.6 | 121 | 56.8 | |
| 9 | 72 | 77.1 | 78 | 72.1 | |
| Paint film with Sample 4 microcapsules | 0 | 314 | - | 280 | - |
| 1 | 239 | 23.9 | 235 | 16.1 | |
| 3 | 201 | 36.0 | 186 | 33.6 | |
| 5 | 173 | 44.9 | 154 | 45.0 | |
| 6 | 140 | 55.4 | 152 | 45.7 | |
| 9 | 87 | 72.3 | 101 | 63.9 | |
| Paint film with Sample 6 microcapsules | 0 | 314 | - | 280 | - |
| 1 | 231 | 26.4 | 229 | 18.2 | |
| 3 | 206 | 34.4 | 184 | 34.3 | |
| 5 | 164 | 47.8 | 160 | 42.9 | |
| 6 | 133 | 57.6 | 158 | 43.6 | |
| 9 | 92 | 70.7 | 110 | 60.7 |
| Sample | Content of the Microcapsules (%) | L | a | b | ΔE |
|---|---|---|---|---|---|
| Paint film with Sample 2 microcapsules | 0 | 93.6 ± 0.7 | −0.8± 0.1 | 3.8 ± 0.5 | - |
| 1.0 | 92.0 ± 1.6 | −3.0 ± 0.1 | 18.1 ± 1.2 | 14.5 | |
| 3.0 | 86.2 ± 1.5 | −3.3 ± 0.2 | 42.7 ± 2.3 | 39.7 | |
| 5.0 | 84.2 ± 1.6 | 1.0 ± 0.1 | 55.6 ± 1.8 | 52.7 | |
| 6.0 | 84.5 ± 1.6 | 1.9 ± 0.3 | 61.3 ± 1.2 | 58.3 | |
| 9.0 | 81.7 ± 0.9 | 5.1 ± 0.6 | 63.1 ± 1.4 | 60.8 | |
| Paint film with Sample 4 microcapsules | 0 | 93.6 ± 0.7 | −0.8 ± 0.1 | 3.8 ± 0.5 | - |
| 1.0 | 90.6 ± 2.3 | −1.0 ± 0.1 | 16.2 ± 1.2 | 12.8 | |
| 3.0 | 89.4 ± 0.8 | 0.8 ± 0.1 | 25.4 ± 0.5 | 22.0 | |
| 5.0 | 85.4 ± 3.3 | 2.9 ± 0.6 | 37.4 ± 1.4 | 34.8 | |
| 6.0 | 85.6 ± 2.5 | 1.4 ± 0.3 | 38.8 ± 1.5 | 36.1 | |
| 9.0 | 83.5 ± 2.3 | 6.1 ± 0.5 | 48.1 ± 0.7 | 46.0 | |
| Paint film with Sample 6 microcapsules | 0 | 93.6 ± 0.7 | −0.8 ± 0.1 | 3.8 ± 0.5 | - |
| 1.0 | 91.5 ± 2.0 | −2.9 ± 0.2 | 21.4 ± 1.1 | 17.9 | |
| 3.0 | 86.4 ± 2.0 | −2.0 ± 0.1 | 37.4 ± 0.8 | 34.4 | |
| 5.0 | 85.4 ± 1.0 | −0.5 | 53.9 ± 1.2 | 50.8 | |
| 6.0 | 87.7 ± 1.4 | −0.6 ± 0.1 | 54.5 ± 1.3 | 51.1 | |
| 9.0 | 82.2 ± 1.6 | 6.1 ± 0.2 | 61.9 ± 0.6 | 59.6 |
| Sample | Content of the Microcapsules (%) | 20° (GU) | 60° (GU) | 85° (GU) | Gloss Loss Rate (%) |
|---|---|---|---|---|---|
| Paint film with Sample 2 microcapsules | 0 | 5.9 ± 0.3 | 17.8 ± 0.6 | 7.0 ± 1.0 | - |
| 1.0 | 6.2 ± 0.2 | 12.4 ± 1.0 | 2.8 | 30.3 | |
| 3.0 | 3.8 ± 0.1 | 8.5 ± 0.4 | 3.9 ± 0.2 | 52.5 | |
| 5.0 | 2.0 ± 0.1 | 6.2 ± 0.1 | 3.5 ± 0.1 | 65.4 | |
| 6.0 | 1.8 ± 0.1 | 6.1 ± 0.1 | 3.2 ± 0.1 | 65.6 | |
| 9.0 | 1.7 ± 0.1 | 6.4 ± 0.2 | 1.9 ± 0.1 | 66.0 | |
| Paint film with Sample 4 microcapsules | 0 | 5.9 ± 0.3 | 17.8 ± 0.6 | 7.0 ± 1.0 | - |
| 1.0 | 2.8 ± 0.3 | 12.0 ± 0.6 | 9.8 ± 0.2 | 32.7 | |
| 3.0 | 2.3 ± 0.2 | 8.4 ± 0.3 | 4.7 ± 0.1 | 52.7 | |
| 5.0 | 1.6 ± 0.1 | 6.9 ± 0.2 | 2.8 ± 0.1 | 65.3 | |
| 6.0 | 1.8 ± 0.1 | 5.7 ± 0.1 | 2.1 ± 0.1 | 67.8 | |
| 9.0 | 1.5 ± 0.2 | 4.3 ± 0.3 | 0.5 | 76.1 | |
| Paint film with Sample 6 microcapsules | 0 | 6.0 ± 0.3 | 17.8 ± 0.6 | 7.0 ± 1.0 | - |
| 1.0 | 3.5 ± 0.1 | 11.2 ± 0.7 | 10.9 ± 0.8 | 36.9 | |
| 3.0 | 1.5 ± 0.1 | 7.4 ± 0.2 | 2.0 ± 0.1 | 58.4 | |
| 5.0 | 1.2 ± 0.1 | 4.8 ± 0.2 | 1.0 ± 0.1 | 73.2 | |
| 6.0 | 1.6 ± 0.2 | 3.8 ± 0.1 | 0.5 | 78.5 | |
| 9.0 | 1.2 ± 0.1 | 2.7 ± 0.1 | 0.3 | 84.7 |
| Sample | Content of the Microcapsules (%) | Roughness (μm) |
|---|---|---|
| Paint film with Sample 2 microcapsules | 0 | 0.1 ± 0.1 |
| 1.0 | 0.4± 0.1 | |
| 3.0 | 0.7 ± 0.1 | |
| 5.0 | 1.5 | |
| 6.0 | 1.5 ± 0.2 | |
| 9.0 | 2.1 ± 0.1 | |
| Paint film with Sample 4 microcapsules | 0 | 0.1 ± 0.1 |
| 1.0 | 0.8 ± 0.2 | |
| 3.0 | 1.2 | |
| 5.0 | 2.0 ± 0.1 | |
| 6.0 | 2.6 ±0.1 | |
| 9.0 | 4.2 | |
| Paint film with Sample 6 microcapsules | 0 | 0.1 ± 0.1 |
| 1.0 | 0.7 ± 0.1 | |
| 3.0 | 1.6 | |
| 5.0 | 3.5 ± 0.1 | |
| 6.0 | 4.1 ± 0.3 | |
| 9.0 | 5.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, T.; Huang, N.; Yan, X. Effect of Different Emulsifiers on the Preparation Process of Aloe-Emodin Microcapsules and Waterborne Coating Properties. Coatings 2023, 13, 1355. https://doi.org/10.3390/coatings13081355
Ding T, Huang N, Yan X. Effect of Different Emulsifiers on the Preparation Process of Aloe-Emodin Microcapsules and Waterborne Coating Properties. Coatings. 2023; 13(8):1355. https://doi.org/10.3390/coatings13081355
Chicago/Turabian StyleDing, Tingting, Nan Huang, and Xiaoxing Yan. 2023. "Effect of Different Emulsifiers on the Preparation Process of Aloe-Emodin Microcapsules and Waterborne Coating Properties" Coatings 13, no. 8: 1355. https://doi.org/10.3390/coatings13081355




