Whey Protein Isolate and Garlic Essential Oil as an Antimicrobial Coating to Preserve the Internal Quality of Quail Eggs
Abstract
:1. Introduction
2. Materials and Methods
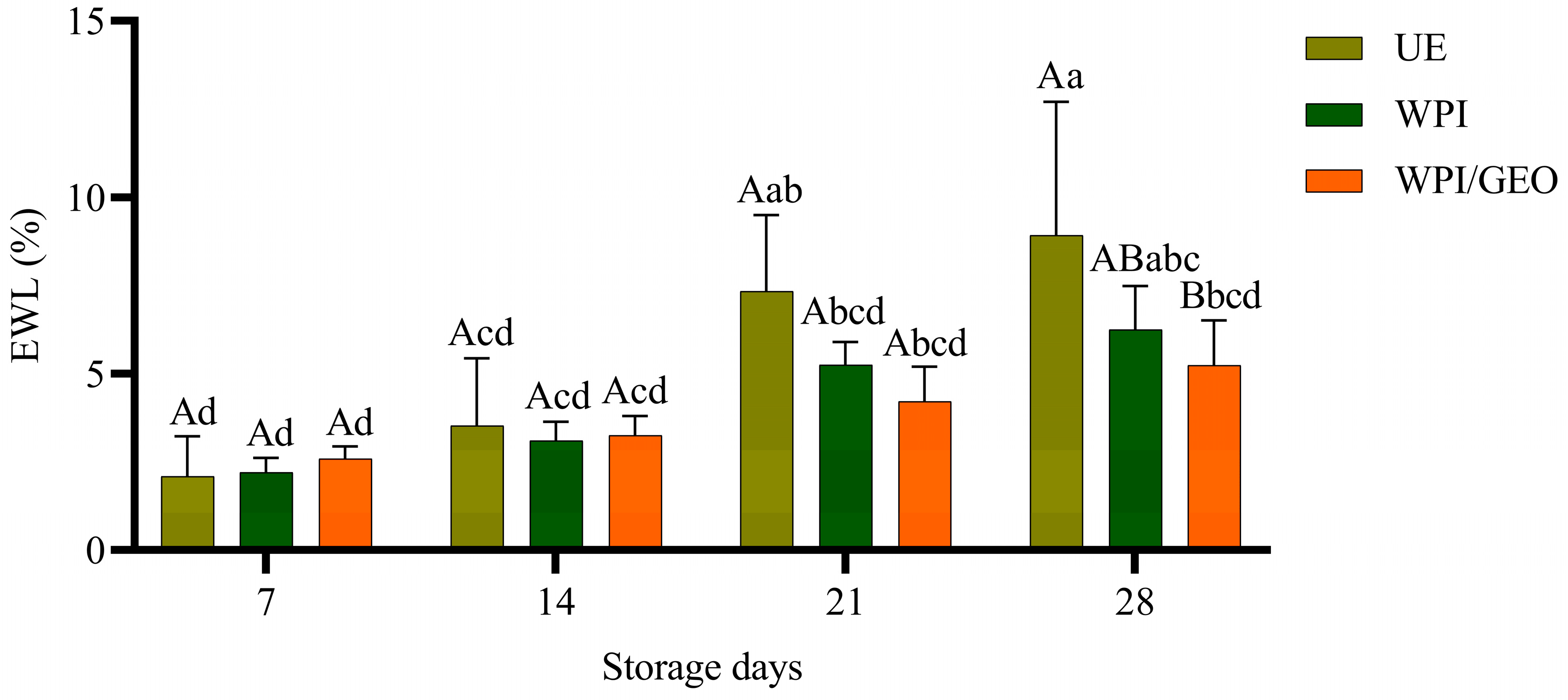
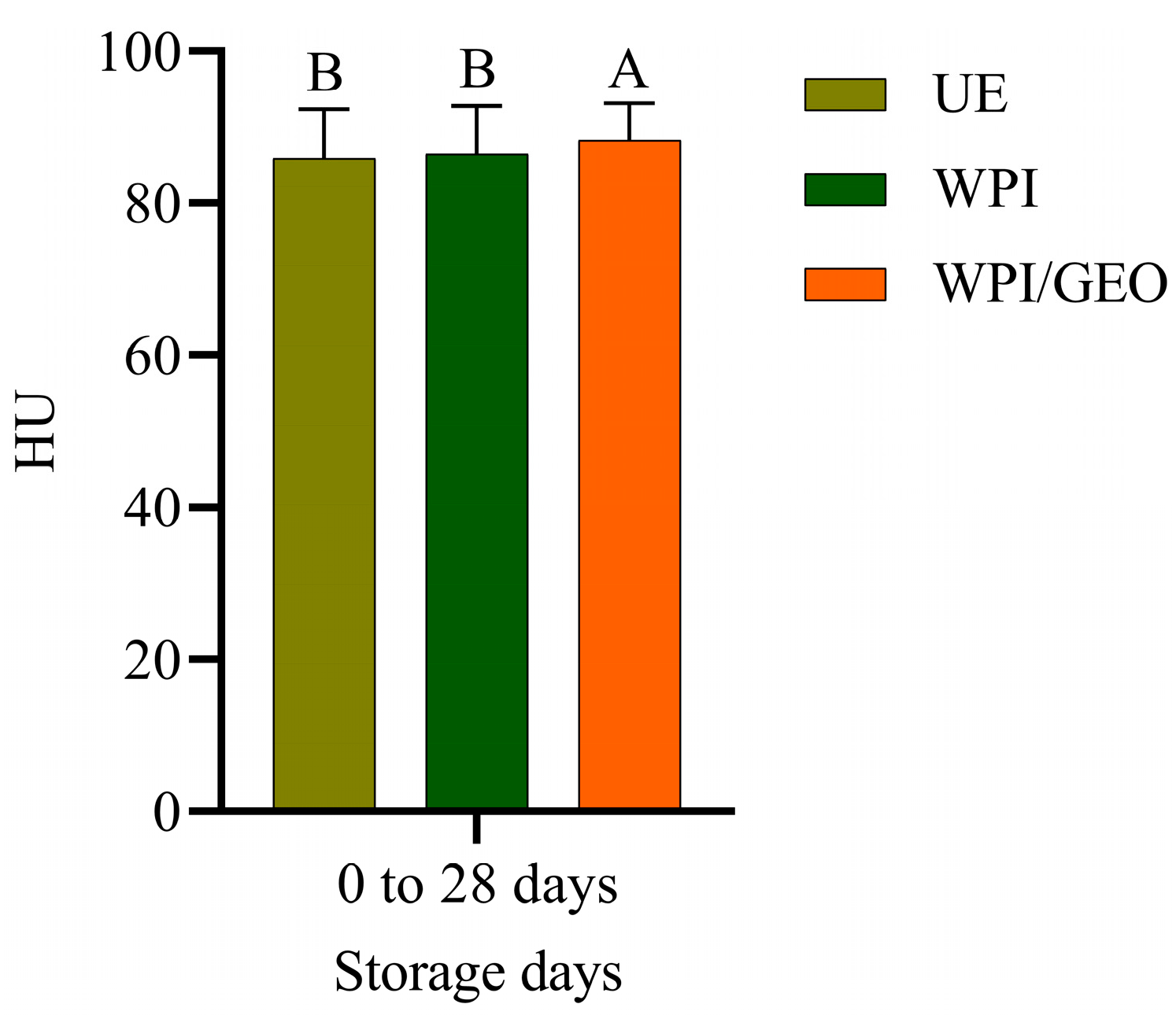
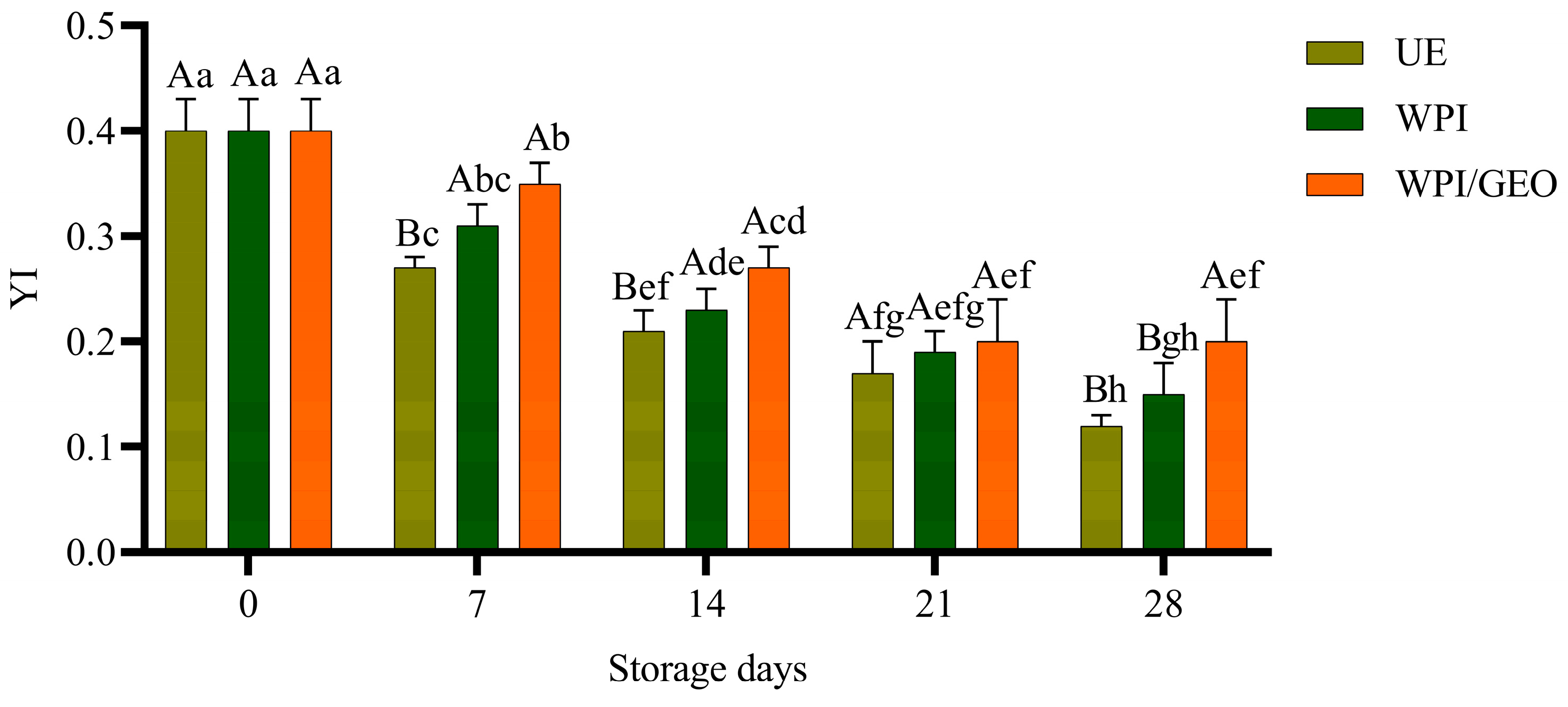
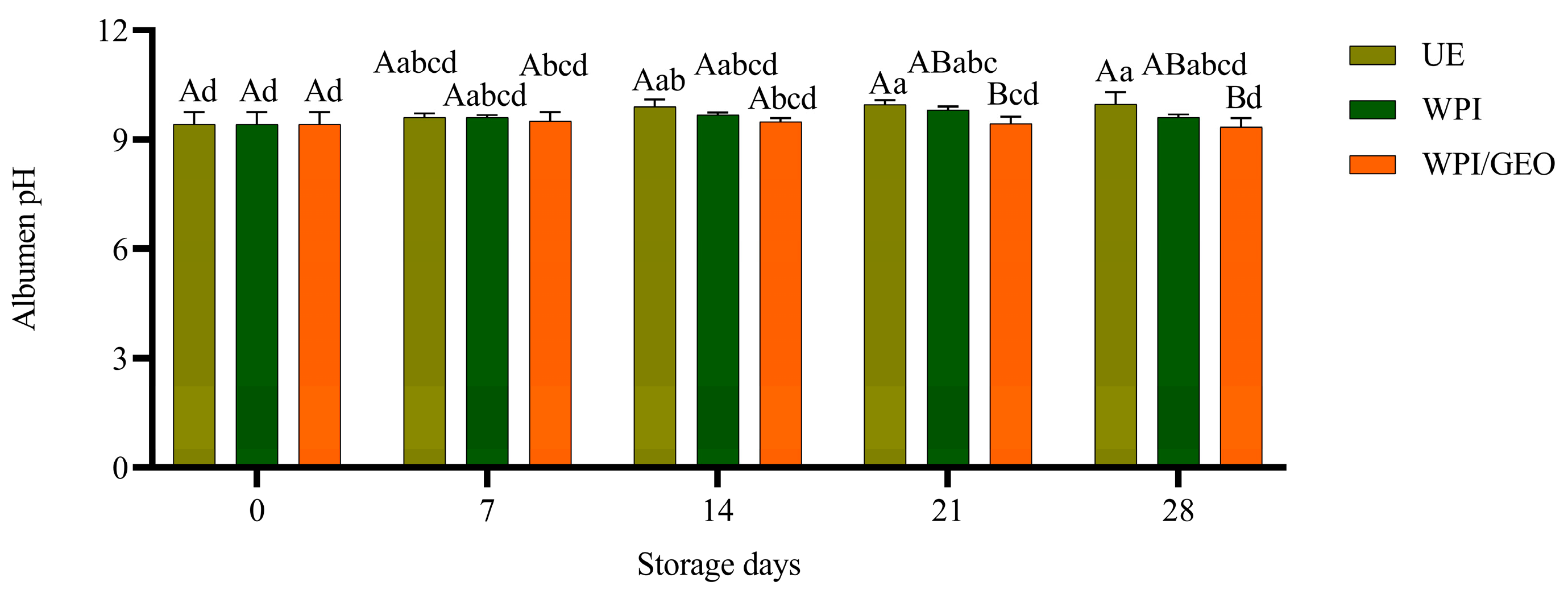
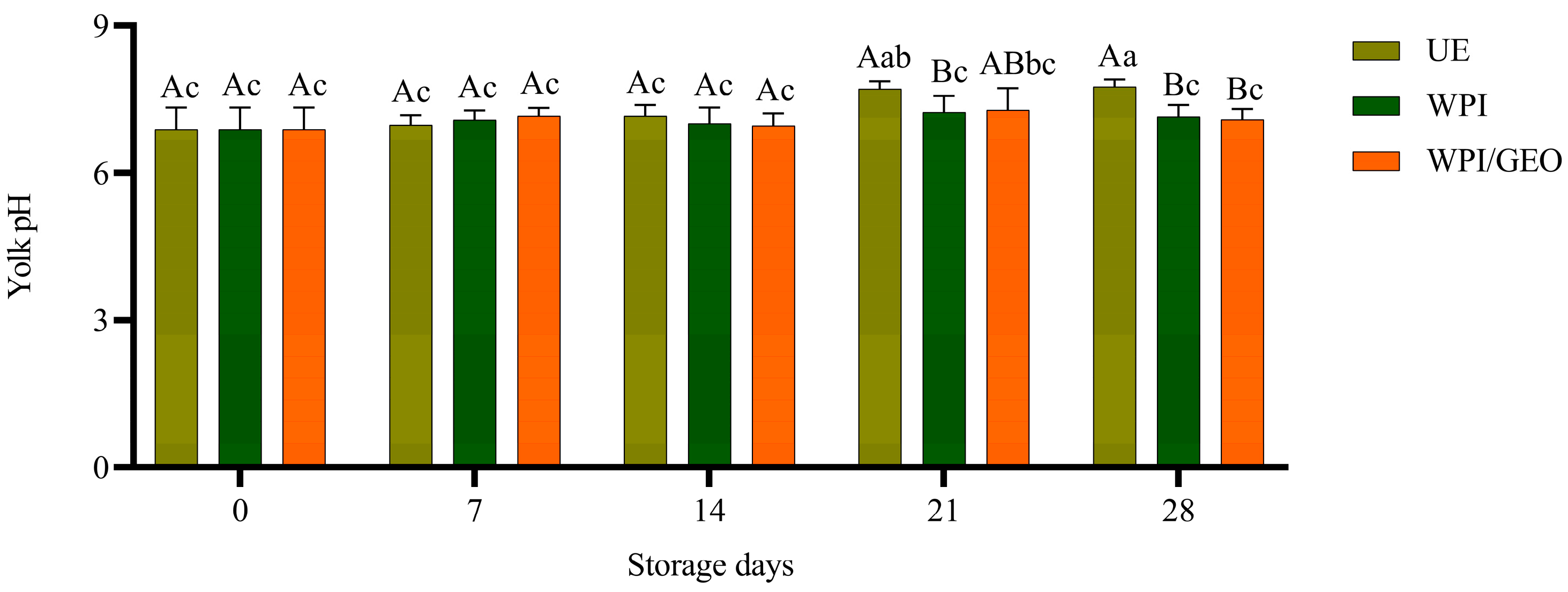
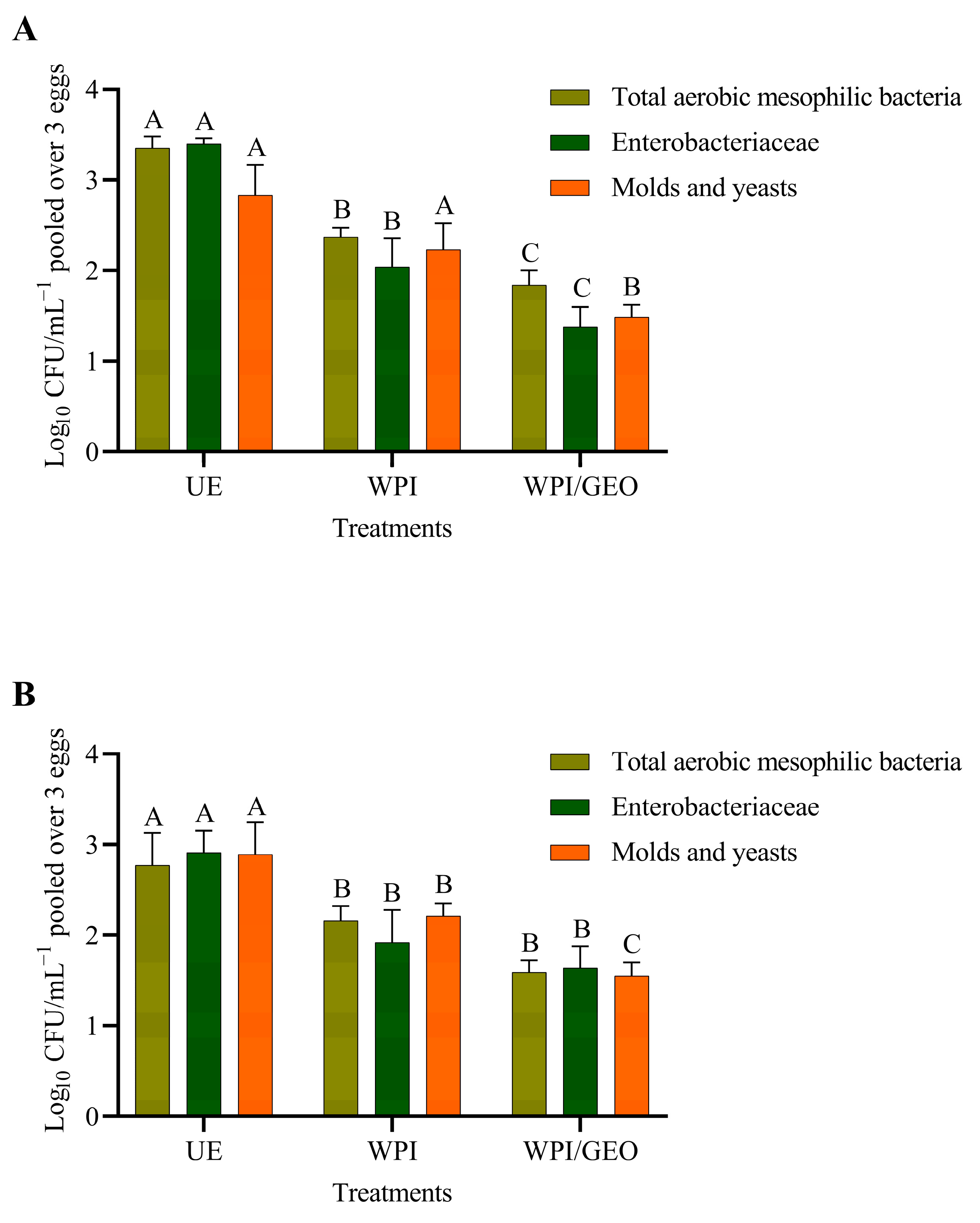
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, D.R.; Ward, G.E.; Regmi, P.; Karcher, D.M. Impact of egg handling and conditions during extended storage on egg quality. Poult. Sci. 2018, 97, 716–723. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; dos Santos, V.M.; Rodrigues, J.C.; Santana, Â.P. Conservation of the Internal Quality of Eggs Using a Biode-gradable Coating. Poult. Sci. 2020, 99, 7207–7213. [Google Scholar] [CrossRef]
- Wardy, W.; Torrico, D.D.; No, H.K.; Prinyawiwatkul, W.; Saalia, F.K. Edible coating affects physico-functional properties and shelf life of chicken eggs during refrigerated and room temperature storage. Int. J. Food Sci. Technol. 2010, 45, 2659–2668. [Google Scholar] [CrossRef]
- Pires, P.G.d.S.; Bavaresco, C.; Oliveira, G.d.S.; McManus, C.; dos Santos, V.M.; Andretta, I. Rice, soy, and whey protein coatings as carriers to extend egg shelf life. Acta Aliment. 2022, 51, 605–612. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; dos Santos, V.M. Essential oils and propolis as additives in egg coatings. World’s Poult. Sci. J. 2022, 78, 1053–1066. [Google Scholar] [CrossRef]
- Xie, L.; Hettiarachchy, N.S.; Ju, Z.Y.; Meullenet, J.; Wang, H.; Slavik, M.F.; Janes, M.E. Edible Film Coating to Minimize Eggshell Breakage and Reduce Post-Wash Bacterial Contamination Measured by Dye Penetration in Eggs. J. Food Sci. 2002, 67, 280–284. [Google Scholar] [CrossRef]
- Biladeau, A.M.; Keener, K.M. The effects of edible coatings on chicken egg quality under refrigerated storage. Poult. Sci. 2009, 88, 1266–1274. [Google Scholar] [CrossRef]
- Caner, C.; Yüceer, M. Efficacy of various protein-based coating on enhancing the shelf life of fresh eggs during storage. Poult. Sci. 2015, 94, 1665–1677. [Google Scholar] [CrossRef]
- Tokur, B.K.; Sert, F.; Aksun, E.T.; Özoğul, F. The Effect of Whey Protein Isolate Coating Enriched with Thyme Essential Oils on Trout Quality at Refrigerated Storage (4 ± 2 °C). J. Aquat. Food Prod. Technol. 2016, 25, 585–596. [Google Scholar] [CrossRef]
- Verma, T.; Aggarwal, A.; Dey, P.; Chauhan, A.K.; Rashid, S.; Chen, K.-T.; Sharma, R. Medicinal and therapeutic properties of garlic, garlic essential oil, and garlic-based snack food: An updated review. Front. Nutr. 2023, 10, 1120377. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; dos Santos, V.M. Garlic as active principle of sanitiser for hatching eggs. World’s Poult. Sci. J. 2022, 78, 1037–1052. [Google Scholar] [CrossRef]
- Esmaeili, H.; Cheraghi, N.; Khanjari, A.; Rezaeigolestani, M.; Basti, A.A.; Kamkar, A.; Aghaee, E.M. Incorporation of Nano-encapsulated Garlic Essential Oil into Edible Films: A Novel Approach for Extending Shelf Life of Vacuum-Packed Sausages. Meat Sci. 2020, 166, 108135. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Caner, C. Whey protein isolate coating and concentration effects on egg shelf life. J. Sci. Food Agric. 2005, 85, 2143–2148. [Google Scholar] [CrossRef]
- Haugh, R.R. A New Method for Determining the Quality of an Egg. US Egg Poult. 1937, 39, 27–49. [Google Scholar]
- Funk, E.M. The relation of the Yolk Index Determined in Natural Position to the Yolk Index as Determined after Separating the Yolk from the Albumen. Poult. Sci. 1948, 27, 367. [Google Scholar] [CrossRef]
- Nwamo, A.C.; Oshibanjo, D.O.; Sati, N.M.; Emennaa, P.E.; Mbuka, J.J.; Njam, R.L.; Bature, E.; Ejidare, D.A.; Gyang, B.D.; Adeniyi, A.K.; et al. Egg quality and sensory evaluation as affected by temperature and storage days of fertile and non-fertile eggs. Niger. J. Anim. Prod. 2021, 48, 23–32. [Google Scholar] [CrossRef]
- Wells, J.B.; Coufal, C.D.; Parker, H.M.; McDaniel, C.D. Disinfection of eggshells using ultraviolet light and hydrogen peroxide independently and in combination. Poult. Sci. 2010, 89, 2499–2505. [Google Scholar] [CrossRef]
- Figueiredo, T.C.; Assis, D.C.S.; Menezes, L.D.M.; Oliveira, D.D.; Lima, A.L.; Souza, M.R.; Heneine, L.G.D.; Cançado, S.V. Effects of packaging, mineral oil coating, and storage time on biogenic amine levels and internal quality of eggs. Poult. Sci. 2014, 93, 3171–3178. [Google Scholar] [CrossRef]
- Zeweil, H.S.; Rizk, R.E.; Bekhet, G.M.; Ahmed, M.R. Comparing the effectiveness of egg disinfectants against bacteria and mitotic indices of developing chick embryos. J. Basic Appl. Zool. 2015, 70, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Eddin, A.S.; Tahergorabi, R. Efficacy of Sweet Potato Starch-Based Coating to Improve Quality and Safety of Hen Eggs during Storage. Coatings 2019, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- Pires, P.G.S.; Leuven, A.F.R.; Franceschi, C.H.; Machado, G.S.; Pires, P.D.S.; Moraes, P.O.; Kindlein, L.; Andretta, I. Effects of rice protein coating enriched with essential oils on internal quality and shelf life of eggs during room temperature storage. Poult. Sci. 2020, 99, 604–611. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; dos Santos, V.M.; Nascimento, S.T. Essential oils as sanitisers for hatching eggs. World’s Poult. Sci. J. 2021, 77, 605–617. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; Nascimento, S.T.; dos Santos, V.M.; Silva, M.G. Clove essential oil in the sanitation of fertile eggs. Poult. Sci. 2020, 99, 5509–5516. [Google Scholar] [CrossRef]
- Sun, R.; Song, G.; Zhang, H.; Zhang, H.; Chi, Y.; Ma, Y.; Li, H.; Bai, S.; Zhang, X. Effect of Basil Essential Oil and Beeswax Incorporation on The Physical, Structural, and Antibacterial Properties of Chitosan Emulsion Based Coating for Eggs Preservation. LWT 2021, 150, 112020. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; Pires, P.G.d.S.; dos Santos, V.M. Rice flour coating supplemented with rosemary essential oil to preserve the internal, microbiological, and sensory quality of quail eggs. Acta Aliment. 2023, 52, 294–304. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; Pires, P.G.D.S.; dos Santos, V.M. Combination of cassava starch biopolymer and essential oils for coating table eggs. Front. Sustain. Food Syst. 2022, 6, 957229. [Google Scholar] [CrossRef]
- Song, G.; Sun, R.; Li, H.; Zhang, H.; Xia, N.; Guo, P.; Jiang, L.W.; Zhang, X.; Rayan, A.M. Effects of Pine Needle Essential Oil Combined with Chitosan Shellac on Physical and Antibacterial Properties of Emulsions for Egg Preservation. Food Biophys. 2022, 17, 260–272. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, D.; Ma, M. Egg Freshness Indexes Correlations with Ovomucin Concentration during Storage. J. Food Qual. 2022, 2022, 9562886. [Google Scholar] [CrossRef]
- Farnejad, S.; Nouri, M.; Dolatabad, S.S. Obtaining of Chickpea Protein Isolate and its Application as Coating Enriched with Essential Oils from Satureja hortensis and Satureja mutica in Egg at Room Temperature. Int. J. Food Sci. Technol. 2022, 57, 400–407. [Google Scholar] [CrossRef]
- Yuceer, M.; Caner, C. Antimicrobial lysozyme-chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J. Sci. Food Agric. 2014, 94, 153–162. [Google Scholar] [CrossRef]
- Qi, L.; Zhao, M.-C.; Li, Z.; Shen, D.-H.; Lu, J. Non-destructive testing technology for raw eggs freshness: A review. SN Appl. Sci. 2020, 2, 1113. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Oleforuh-Okoleh, V.U.; Wang, J.; Zhang, H.-J.; Qi, G.-H.; Qiu, K.; Wu, S.-G. Potential Implications of Natural Antioxidants of Plant Origin on Oxidative Stability of Chicken Albumen during Storage: A Review. Antioxidants 2022, 11, 630. [Google Scholar] [CrossRef]
- Kim, S.H.; Youn, D.K.; No, H.K.; Choi, S.W.; Prinyawiwatkul, W. Effects of chitosan coating and storage position on quality and shelf life of eggs. Int. J. Food Sci. Technol. 2009, 44, 1351–1359. [Google Scholar] [CrossRef]
- Obanu, Z.A.; Mpieri, A.A. Efficiency of dietary vegetable oils in preserving the quality of shell eggs under ambient tropical conditions. J. Sci. Food Agric. 1984, 35, 1311–1317. [Google Scholar] [CrossRef]
- Valero, M.; Giner, M.J. Effects of antimicrobial components of essential oils on growth of Bacillus cereus INRA L2104 in and the sensory qualities of carrot broth. Int. J. Food Microbiol. 2006, 106, 90–94. [Google Scholar] [CrossRef]
- Benkeblia, N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT 2004, 37, 263–268. [Google Scholar] [CrossRef]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical composition, extraction sources and action mechanisms of essential oils: Natural preservative and limitations of use in meat products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef]
- Upadhyaya, I.; Yin, H.-B.; Surendran Nair, M.; Chen, C.-H.; Lang, R.; Darre, M.J.; Venkitanarayanan, K. Inactivation of Salmonella Enteritidis on Shell Eggs by Coating with Phytochemicals. Poult. Sci. Assoc. 2016, 95, 2106–2111. [Google Scholar] [CrossRef]
- Araújo, M.V.; Oliveira, G.d.S.; McManus, C.; Vale, I.R.R.; Salgado, C.B.; Pires, P.G.d.S.; de Campos, T.A.; Gonçalves, L.F.; Ameida, A.P.C.; Martins, G.d.S.; et al. Preserving the Internal Quality of Quail Eggs Using a Corn Starch-Based Coating Combined with Basil Essential Oil. Processes 2023, 11, 1612. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; Pires, P.G.d.S.; de Figueiredo Sousa, H.A.; da Silva, E.R.; dos Santos, V.M. Antimicrobial Coating Based on Tahiti Lemon Essential Oil and Green Banana Flour to Preserve the Internal Quality of Quail Eggs. Animals 2023, 13, 2123. [Google Scholar] [CrossRef] [PubMed]

| Retention Time | Concentration (Peak Area %) | Kovats Index | Kovats Index Literature | NIST 14 Library Similarity (%) | Compound |
|---|---|---|---|---|---|
| 3.167 | 4.87 | - | 835 | 97 | Diallyl sulfide |
| 3.550 | 0.10 | - | 889 | 94 | (E)-Allyl(prop-1-en-1-yl)sulfane |
| 3.600 | 0.12 | - | 889 | 94 | (E)-Allyl(prop-1-en-1-yl)sulfane |
| 3.835 | 0.03 | 910 | 905 | 90 | 3,4-dimethyl-thiophene |
| 4.027 | 3.13 | 922 | 923 | 93 | methyl-2-propenyl-disulfide |
| 4.283 | 0.22 | 936 | 925 | 95 | (Z)-Methyl propenyl disulfide |
| 4.443 | 0.34 | 945 | 964 | 97 | (E)-Methyl propenyl disulfide |
| 4.841 | 0.67 | 964 | 997 | 97 | 3H-1,2-Dithiole |
| 5.094 | 0.87 | 975 | 982 | 93 | Dimethyl trisulfide |
| 6.424 | 0.07 | 1028 | 1026 | 93 | p-cymene |
| 6.532 | 0.08 | 1032 | 1036 | 92 | D-Limonene |
| 8.260 | 18.95 | 1087 | 1085 | 91 | Diallyl disulphide |
| 8.588 | 1.68 | 1095 | 1099 | 94 | (Z)-1-Allyl-2-(prop-1-en-1-yl)disulfane |
| 8.803 | 1.99 | 1101 | 1099 | 94 | (E)-1-Allyl-2-(prop-1-en-1-yl)disulfane |
| 10.245 | 11.84 | 1145 | 1116 | 95 | allyl methyl trisulfide |
| 10.535 | 0.04 | 1152 | 1130 | 93 | Methyl-propyl-trisulfide |
| 10.599 | 0.49 | 1154 | 1142 | 92 | 4-Methyl-1,2,3-trithiolane |
| 11.905 | 0.32 | 1186 | 1156 | 96 | 3-Vinyl-1,2-dithiacyclohex-4-ene |
| 12.941 | 1.06 | 1212 | 1148 | 97 | 2-Vinyl-4H-1,3-dithiine |
| 13.033 | 0.21 | 1215 | 1232 | 89 | Dimethyl-tetrasulfide |
| 16.922 | 30.72 | 1307 | 1292 | 95 | di-2-propenyl-trisulfide |
| 17.122 | 0.45 | 1313 | - | 97 | 1-allyl-3-propyl-trisulfide |
| 17.471 | 0.16 | 1322 | 1306 | 95 | (E)-1-Allyl-3-(prop-1-en-1-yl)trisulfane |
| 17.691 | 0.19 | 1327 | - | 96 | (E)-1-Allyl-3-(prop-1-en-1-yl)trisulfane |
| 18.937 | 0.48 | 1358 | 1364 | 95 | 5-Methyl-1,2,3,4-tetrathiane |
| 22.420 | 0.24 | 1441 | - | 88 | Methyl 1-(methylthio)propyl disulfide |
| 26.511 | 9.65 | 1542 | 1531 | 91 | diallyl Tetrasulfide |
| 26.718 | 0.35 | 1547 | 1573 | 86 | 6-Ethyl-4,5,7-trithia-2,8-decadiene |
| 27.985 | 0.12 | 1579 | 1581 | - | 7-Methyl-4,5,8-trithia-1,10-undecadiene |
| 28.361 | 0.28 | 1588 | 1591 | 94 | 6-Methyl-4,5,8-trithia-1,10-undecadiene |
| 36.463 | 1.38 | 1808 | - | 96 | 1-Allyl-3-(2-(allylthio)propyl)trisulfane |
| 44.374 | 0.23 | 2049 | - | 94 | 1-Allyl-3-(2-(allyldisulfanyl)propyl)trisulfane |
| 61.610 | 2.80 | - | - | 91 | 3-(Octanoyloxy)propane-1,2-diyl bis(decanoate) |
| - | 7.34 | - | - | - | Unidentified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vale, I.R.R.; Oliveira, G.d.S.; McManus, C.; de Araújo, M.V.; Salgado, C.B.; Pires, P.G.d.S.; de Campos, T.A.; Gonçalves, L.F.; Almeida, A.P.C.; Martins, G.d.S.; et al. Whey Protein Isolate and Garlic Essential Oil as an Antimicrobial Coating to Preserve the Internal Quality of Quail Eggs. Coatings 2023, 13, 1369. https://doi.org/10.3390/coatings13081369
Vale IRR, Oliveira GdS, McManus C, de Araújo MV, Salgado CB, Pires PGdS, de Campos TA, Gonçalves LF, Almeida APC, Martins GdS, et al. Whey Protein Isolate and Garlic Essential Oil as an Antimicrobial Coating to Preserve the Internal Quality of Quail Eggs. Coatings. 2023; 13(8):1369. https://doi.org/10.3390/coatings13081369
Chicago/Turabian StyleVale, Igor Rafael Ribeiro, Gabriel da Silva Oliveira, Concepta McManus, Maria Viviane de Araújo, Cristiane Batista Salgado, Paula Gabriela da Silva Pires, Tatiana Amabile de Campos, Laura Fernandes Gonçalves, Ana Paula Cardoso Almeida, Gustavo dos Santos Martins, and et al. 2023. "Whey Protein Isolate and Garlic Essential Oil as an Antimicrobial Coating to Preserve the Internal Quality of Quail Eggs" Coatings 13, no. 8: 1369. https://doi.org/10.3390/coatings13081369






