The Effects of a Multifunctional Rust Inhibitor on the Rust Resistance Mechanism of Carbon Steel and the Properties of Concrete
Abstract
1. Introduction
2. Experiments
2.1. Materials Preparation
2.2. Preparation of the Multifunctional Rust Inhibitor
2.3. Experimental Method
2.3.1. Weight Loss Method
2.3.2. Compressive Strength Test of Concrete
2.3.3. Chloride Ion Electromigration Experiment
2.3.4. Carbonation Experiment
2.3.5. Electrochemical Test Method
2.3.6. Morphological Characterization
3. Results and Discussion
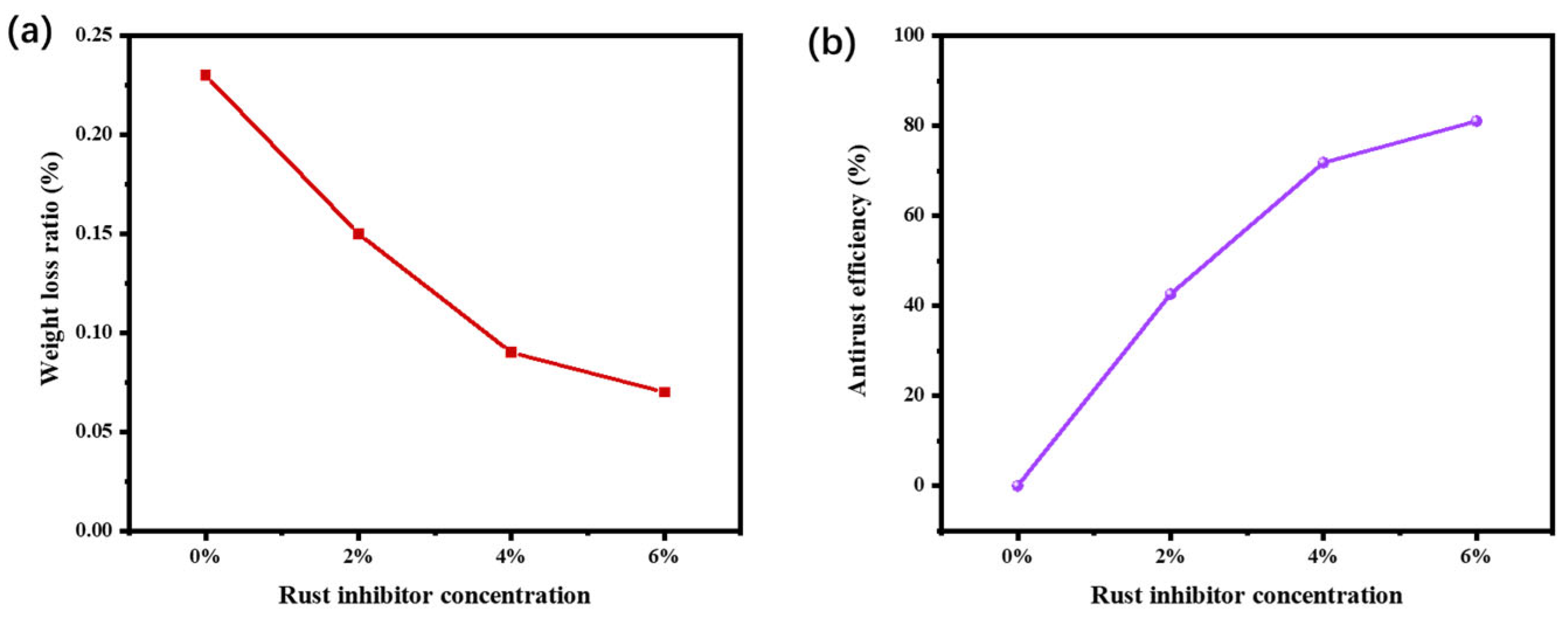
3.1. Weight Loss Analysis
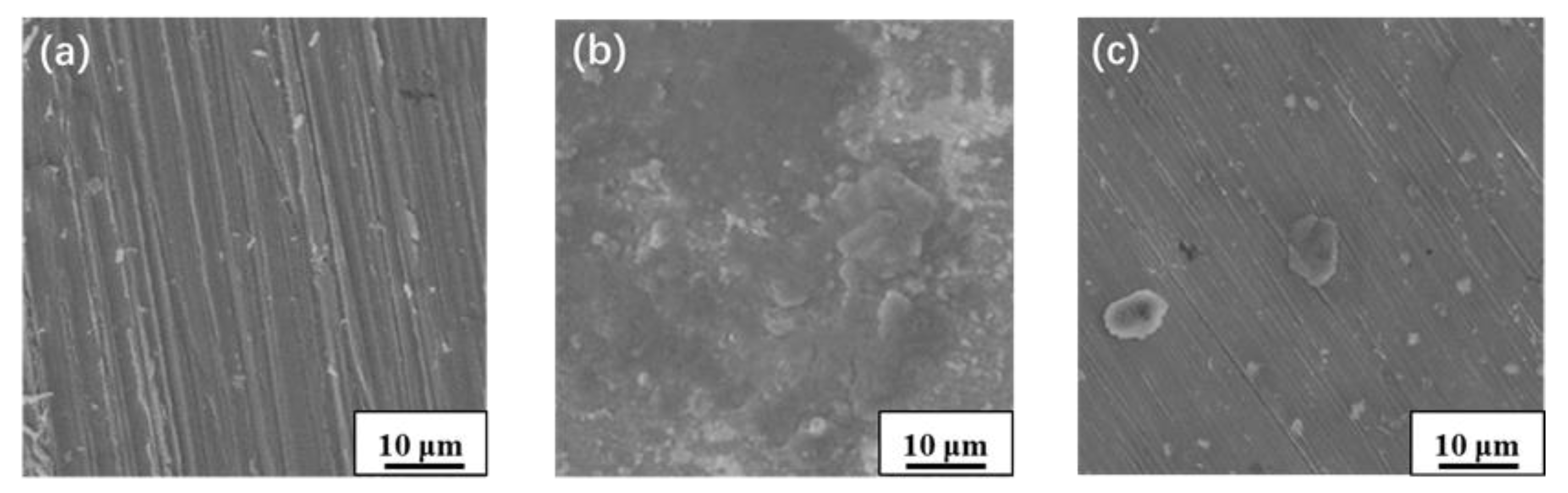
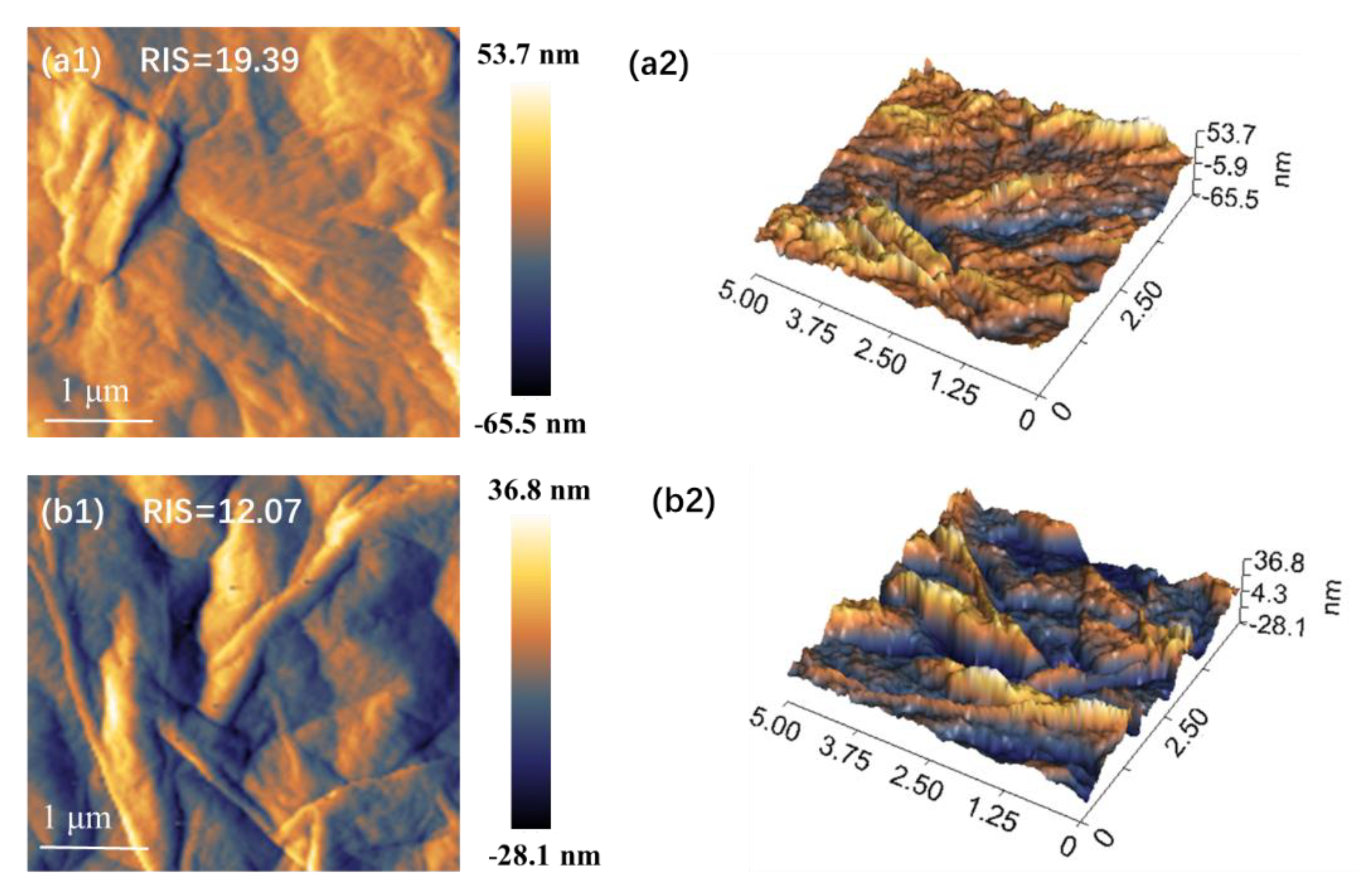
3.2. Micromorphology Analysis
3.3. Effect of Rust Inhibitor Mixed with Concrete Rods in Concrete
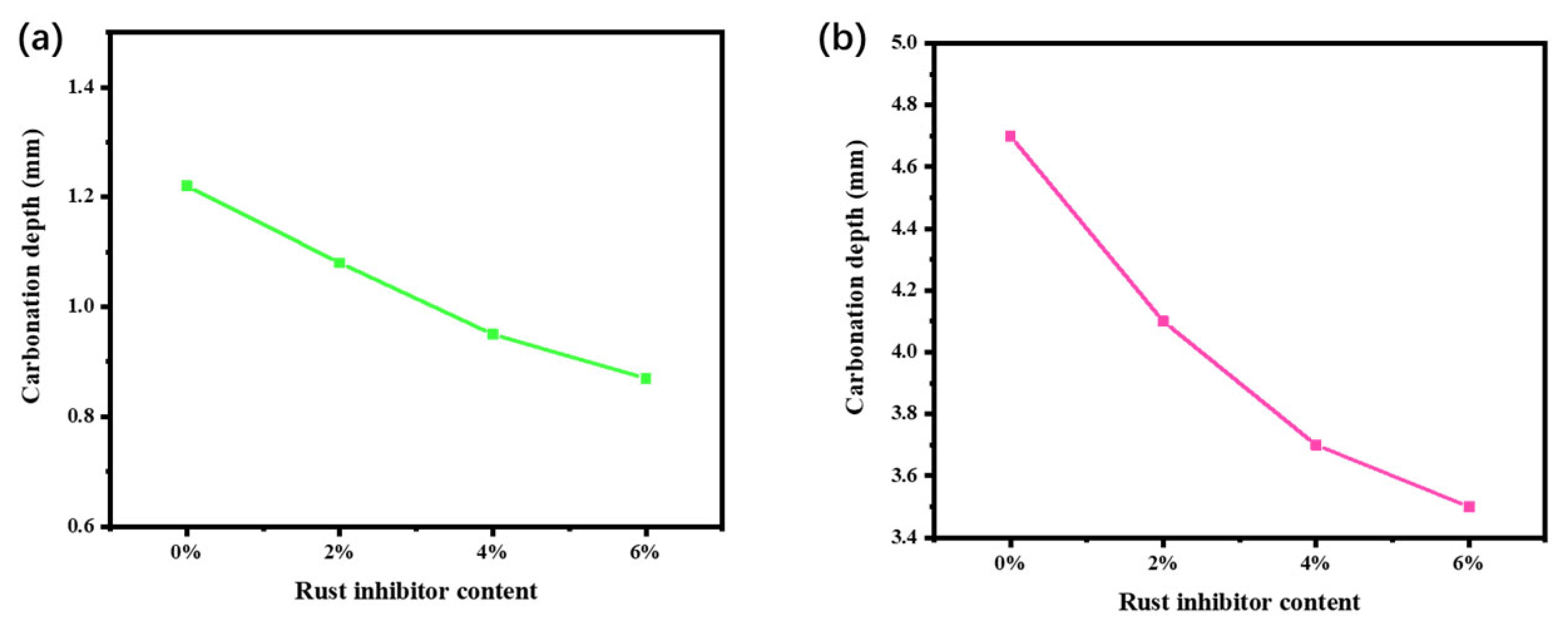
3.4. Effect of Carbonation Resistance of Concrete
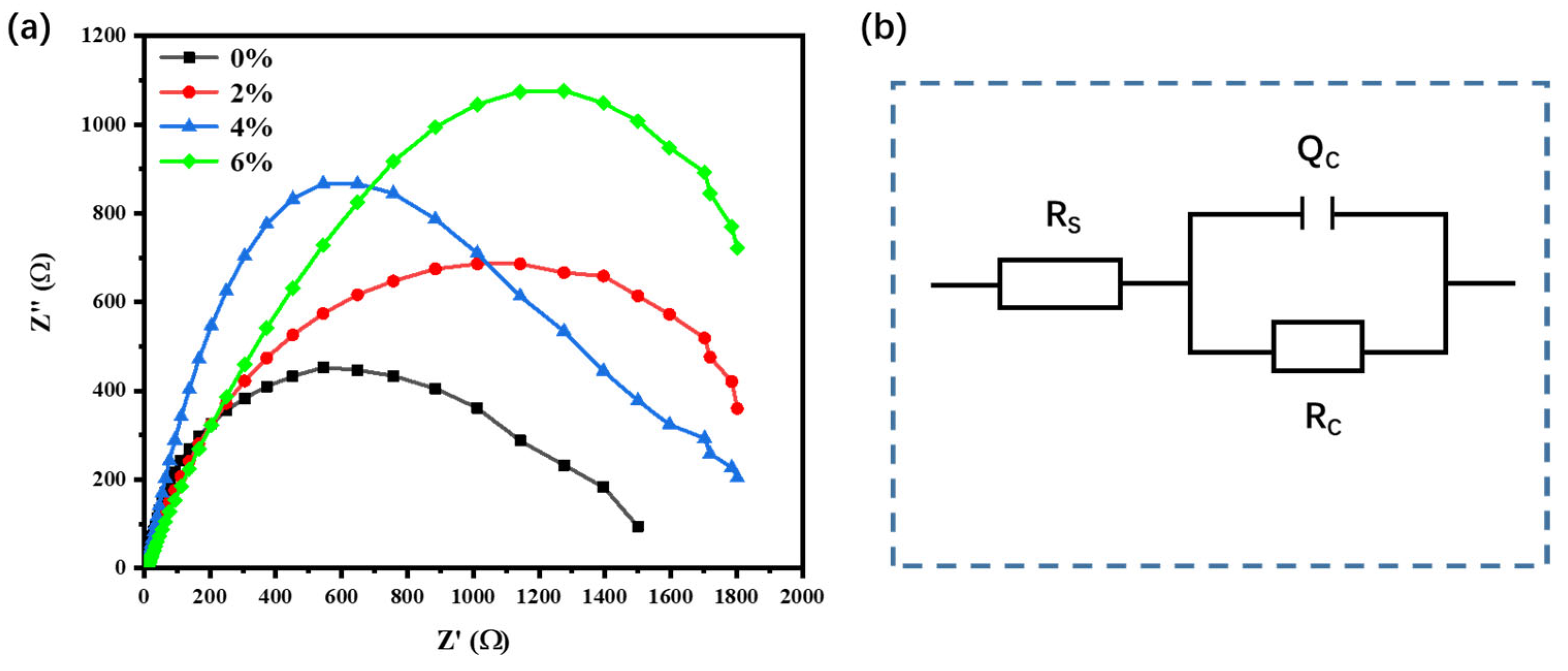
3.5. Electrochemical Test Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ping, L.; Ang, G.Z. Research on Longitudinal Reinforcement Ratio of Reinforced Concrete Rectangular Section Single-reinforced Beam. In Progress in Industrial and Civil Engineering II; PTS 1–4; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2013; pp. 985–988. [Google Scholar] [CrossRef]
- He, Y.; Xu, Z.; Chen, H.; Tang, Y.; Chen, D. The research progress of metal material corrosion protection technology. Appl. Chem. Ind. 2013, 42, 2065–2067. [Google Scholar]
- Li, H.; Zhang, Y.; Li, C.; Zhou, Z.; Nie, X.; Chen, Y.; Cao, H.; Liu, B.; Zhang, N.; Said, Z.; et al. Cutting fluid corrosion inhibitors from inorganic to organic: Progress and applications. Korean J. Chem. Eng. 2022, 39, 1107–1134. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Kuznetsov, Y.I. Organic Inhibitors of Metal Corrosion in Acid Solutions. I. Mechanism of Protective Action. Russ. J. Phys. Chem. A 2023, 97, 413–427. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Q.; Yuan, Y.; Taerwe, L. Comparison of the structural behavior of reinforced concrete tunnel segments with steel fiber and synthetic fiber addition. Tunn. Undergr. Space Technol. 2020, 103, 103506. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Kuznetsov, Y.I. Iron oxide and oxyhydroxide phases formed on steel surfaces and their dissolution in acidic media. Review. Int. J. Corros. Scale Inhib. 2023, 12, 366–409. [Google Scholar] [CrossRef]
- Faske, T.R.; Emerson, M. Multiyear Evaluation of Fungicide Efficacy and Application Timing for Control of Southern Rust in Hybrid Corn in Arkansas. Plant Dis. 2021, 105, 1108–1114. [Google Scholar] [CrossRef]
- Bao, J.; Zhuang, Z.; Zhang, P.; Wei, J.; Gao, S.; Zhao, T. Research Progress of Chloride Corrosion Resistance of Concrete Exposed to Marine Tidal Environment Based on Similarity Theory. Mater. Rev. 2021, 35, 7087–7095. [Google Scholar]
- Jia, M.; Hu, P.; Zhang, X.; Hu, G. Rust Conversion of Proanthocyanidins to Archaeological Steel: A Case Study of Lingzhao Xuan in the Forbidden City. Molecules 2022, 27, 7711. [Google Scholar] [CrossRef]
- Rajendran, L.M.; Vincent, J.; Natarajan, B.; Govindan, V. The Effects of Nano-Based Bio-Carbonates in Superhydrophobic Concrete—A Review. Buildings 2023, 13, 1354. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, L.; Zhi, F.; Jin, W.; Li, G. Effect of cation type on corrosion of steel treated by DNA corrosion inhibitor in simulated concrete pore solution. Mag. Concr. Res. 2023, 75, 271–282. [Google Scholar] [CrossRef]
- Berke, N.S.; Ade, K.M.; DeNicola, P.K.; Rungta, A. Organofunctional Silane Corrosion Inhibitor Surface Treatment Protects Concrete. Mater. Perform. 2017, 56, 52–56. [Google Scholar]
- Chen, Y.; Li, S.; Liu, Z.; Wang, Z. Anticorrosion Property of Alcohol Amine Modified Phosphoric and Tannic Acid Based Rust Converter and Its Waterborne Polymer-Based Paint for Carbon Steel. Coatings 2021, 11, 1091. [Google Scholar] [CrossRef]
- Haldhar, R.; Vanaraj, R.; Dagdag, O.; Berisha, A.; Kim, S.-C. Convolvulus microphyllus Extract as a Green, Effective, and Affordable Corrosion Inhibitor: Theoretical Calculations and Experimental Studies. Coatings 2023, 13, 860. [Google Scholar] [CrossRef]
- Hang, M.; Zhou, X.; Wang, J.; Sun, M. Corrosion behavior and mechanism analysis of triethanolamine and imidazoline in the simulated pore solution of concrete. Case Stud. Constr. Mater. 2023, 18, e01907. [Google Scholar] [CrossRef]
- Mei, K.; He, Z.; Yi, B.; Lin, X.; Wang, J.; Wang, H.; Liu, J. Study on electrochemical characteristics of reinforced concrete corrosion under the action of carbonation and chloride. Case Stud. Constr. Mater. 2022, 17, e01351. [Google Scholar] [CrossRef]
- Raja, P.B.; Ghoreishiamiri, S.; Ismail, M. Natural corrosion inhibitors for steel reinforcement in concrete—A review. Surf. Rev. Lett. 2015, 22, 1550040. [Google Scholar] [CrossRef]
- Ramasubramanian, M.; Haran, B.S.; Popova, S.; Popov, B.N.; Petrou, M.F.; White, R.E. Inhibiting action of calcium nitrite on carbon steel rebars. J. Mater. Civ. Eng. 2001, 13, 10–17. [Google Scholar] [CrossRef]
- Yi, B.; Wang, J.; Feng, L.; Song, Y.; Liu, J.; Shu, H. Research on Inhibiting Performance of Compound Corrosion Inhibitors Based on Nitrite. Adv. Civ. Eng. 2021, 2021, 6655080. [Google Scholar] [CrossRef]
- Tang, Y.B.; Wang, S.N.; Xu, Y.P.; Ni, J.X. Influence of calcium nitrite on the passive films of rebar in simulated concrete pore solution. Anti-Corros. Methods Mater. 2017, 64, 265–272. [Google Scholar] [CrossRef]
- Guo, Q.; Li, X.; Lin, N.; Liu, J. Influence of Rust Inhibitors on the Microstructure of a Steel Passive Film in Chloride Concrete. Coatings 2022, 12, 692. [Google Scholar] [CrossRef]
- Guo, Q.; Li, X.; Liu, J. Effect of rust inhibitor on passive film of rebar in cement paste under carbonation. Rev. Romana Mater.-Rom. J. Mater. 2022, 52, 278–283. [Google Scholar]
- Jia, M.; Hu, P.; Gong, Z.; Sun, J.; Cui, Y.; Hu, D.; Hu, G. Corrosion Inhibition and Rust Conversion of Catechin on Archaeological Iron of Nanhai I. Metals 2022, 12, 714. [Google Scholar] [CrossRef]
- Yu, M.S.; Lee, S.-H.; Choi, J.-W.; Shim, H.-S.; Yang, S.-B. Prediction of Corrosion Inhibition Effect on Steel Coupons and Tap-water Pipe with Zinc Ionization Device. J. Korean Soc. Environ. Technol. 2021, 22, 203–212. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, L.; Lei, B.; Meng, G.; Feng, Z.; Guo, H.; Liao, B.; Zhang, P. A New Imidazole Derivative for Corrosion Inhibition of Q235 Carbon Steel in an Acid Environment. Polymers 2023, 15, 2420. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.L.; Skilbred, A.W.B.; Loken, A.; Henriques, R.R.; Soares, B.G. Effect of accelerated ageing procedures and flash rust inhibitors on the anti-corrosive performance of epoxy coatings: EIS and dynamic-mechanical analysis. Prog. Org. Coat. 2021, 159, 106387. [Google Scholar] [CrossRef]
- Simović, A.R.; Grgur, B.N.; Novaković, J.; Janaćković, P.; Bajat, J. Black Pine (Pinus nigra) Essential Oil as a Green Corrosion Inhibitor for Carbon Steel. Metals 2023, 13, 508. [Google Scholar] [CrossRef]
- Xu, J.; Hang, M.; Cui, L.; Dong, L. Study on the impedance spectroscopy and microscopic morphology of reinforcing bar influenced by corrosion inhibitor under chloride attack. J. Chin. Electron. Microsc. Soc. 2021, 40, 132–137. [Google Scholar]
- Raja, T.; Abuthahir, S.S.S.; Begum, A.S.; Lavanya, M.; Rajendran, S. Corrosion inhibition performance of carbon steel in 1 N hydrochloric acid by an aqueous extract of Syzygium cumini Linn (SCL) plant leaves. Int. J. Corros. Scale Inhib. 2022, 11, 1819–1838. [Google Scholar] [CrossRef]
- Yan, Z.; Kang, M.; Tan, Z.; Li, Q.; Tian, B.; Li, S. Prevention of Q235 Steel Corrosion using Waterborne Rust Inhibitor. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2023, 38, 206–211. [Google Scholar] [CrossRef]
- Adam, L.; Judele, L.; Motrescu, I.; Rusu, I.; Lepadatu, D.; Bucur, R.D. Advanced Design for Experimental Optimisation of Physico-Mechanical Characteristics of Sustainable Local Hemp Concrete. Sustainability 2023, 15, 8484. [Google Scholar] [CrossRef]
- Han, J.; Yan, H.; Huang, Y.; Zhou, L.; Yang, S. Structural Features of Oxide Scales on Weathering Steel and Their Influence on Atmospheric Corrosion. Acta Metall. Sin. 2017, 53, 163–174. [Google Scholar] [CrossRef]

| Density (kg/m3) | Specific Surface Area (kg/m2) | Normal Consistency (%) | Time of Coagulation (min) | Compressive Strength (3d, MPa) |
|---|---|---|---|---|
| Initial Set Final Set | ||||
| 3000 | 360 | 28 | 180 240 | 27.5 |
| SiO2 | CaO | Al2O3 | MgO | SO3 | Fe2O3 | Na2O | Other |
|---|---|---|---|---|---|---|---|
| 21.13 | 62.35 | 4.58 | 1.78 | 3.25 | 4.25 | 0.22 | 2.44 |
| C | Si | Mn | P | Al | Cr | Ni | Fe |
|---|---|---|---|---|---|---|---|
| 4.00 | 3.25 | 20.70 | 0.95 | 1.00 | 1.50 | 1.50 | 67.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Z.; Lu, X.; Luo, Y. The Effects of a Multifunctional Rust Inhibitor on the Rust Resistance Mechanism of Carbon Steel and the Properties of Concrete. Coatings 2023, 13, 1375. https://doi.org/10.3390/coatings13081375
Niu Z, Lu X, Luo Y. The Effects of a Multifunctional Rust Inhibitor on the Rust Resistance Mechanism of Carbon Steel and the Properties of Concrete. Coatings. 2023; 13(8):1375. https://doi.org/10.3390/coatings13081375
Chicago/Turabian StyleNiu, Zhiqiang, Xiaoming Lu, and Yanan Luo. 2023. "The Effects of a Multifunctional Rust Inhibitor on the Rust Resistance Mechanism of Carbon Steel and the Properties of Concrete" Coatings 13, no. 8: 1375. https://doi.org/10.3390/coatings13081375
APA StyleNiu, Z., Lu, X., & Luo, Y. (2023). The Effects of a Multifunctional Rust Inhibitor on the Rust Resistance Mechanism of Carbon Steel and the Properties of Concrete. Coatings, 13(8), 1375. https://doi.org/10.3390/coatings13081375






