Preparation and Study of Ca/Tb Co-Doped HfO2 Infrared Coatings with Different Atomic Ratios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Coatings
2.2. Characterization
3. Results and Discussion
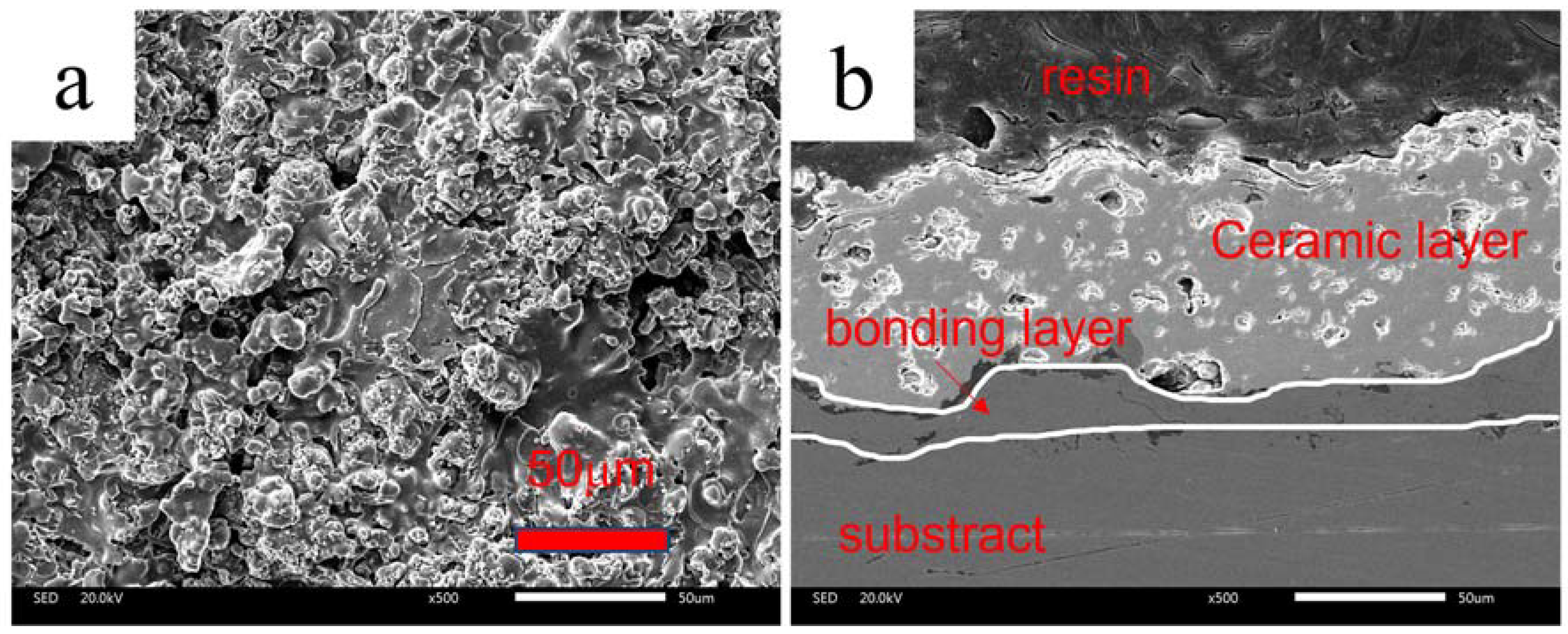
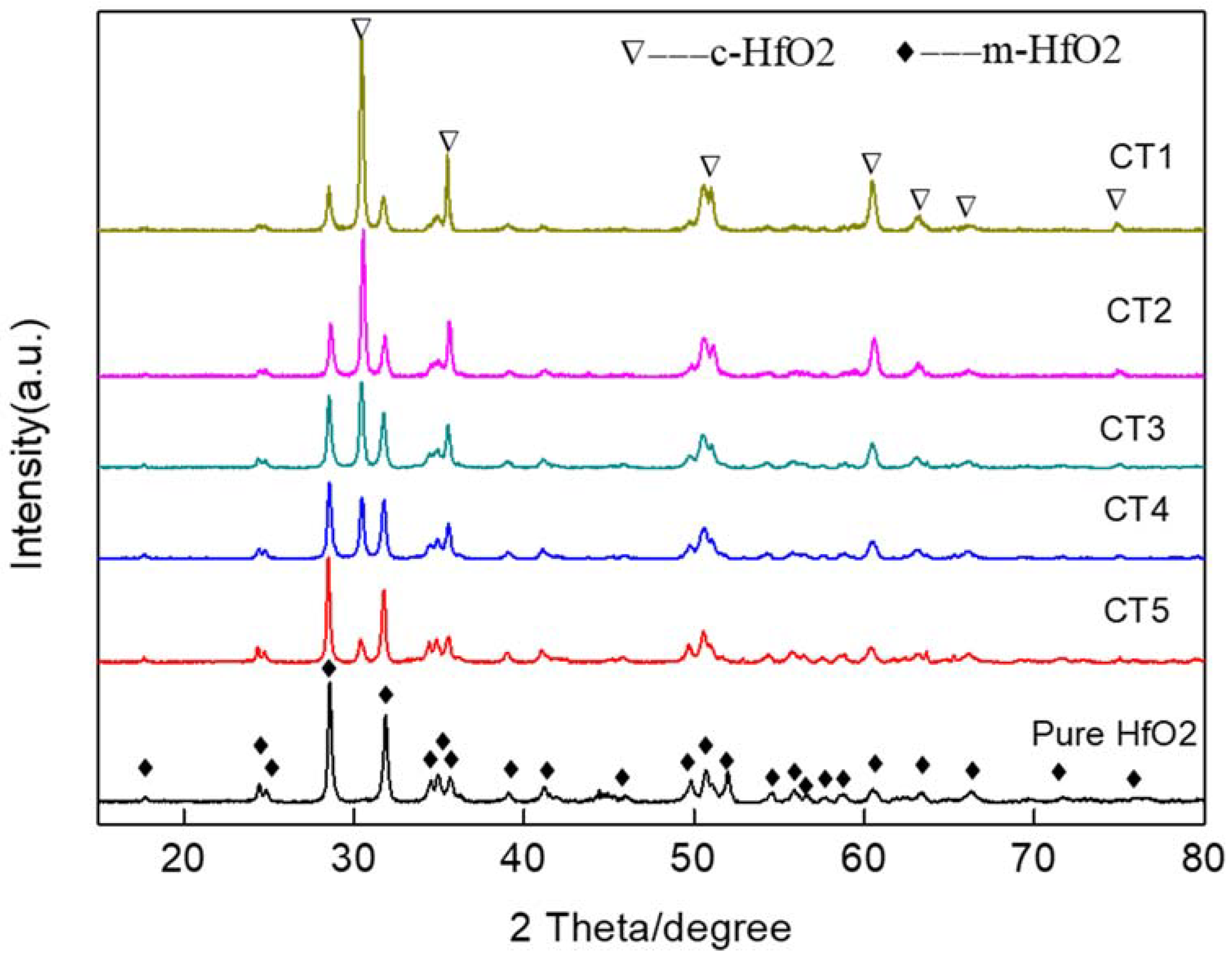
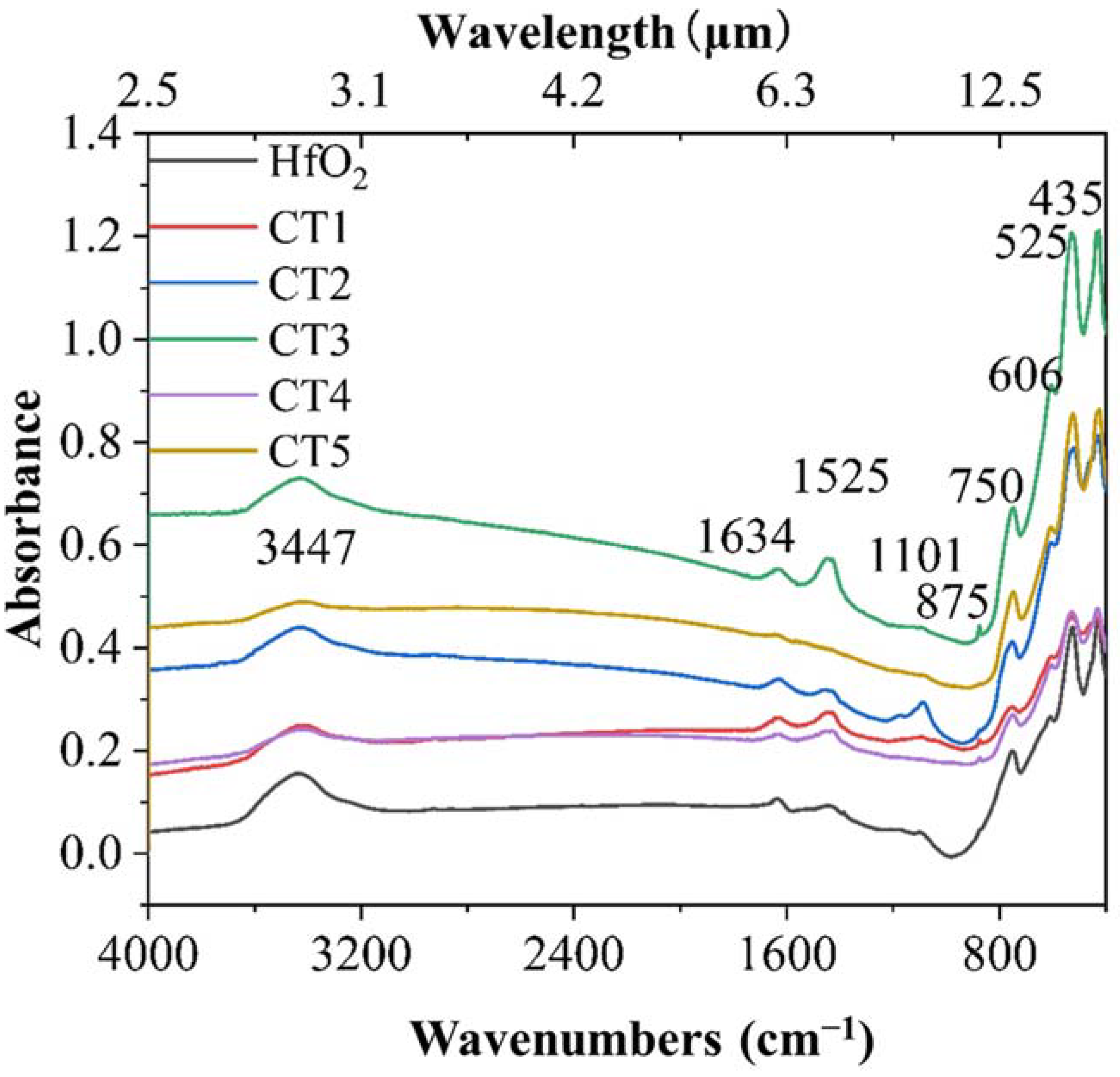
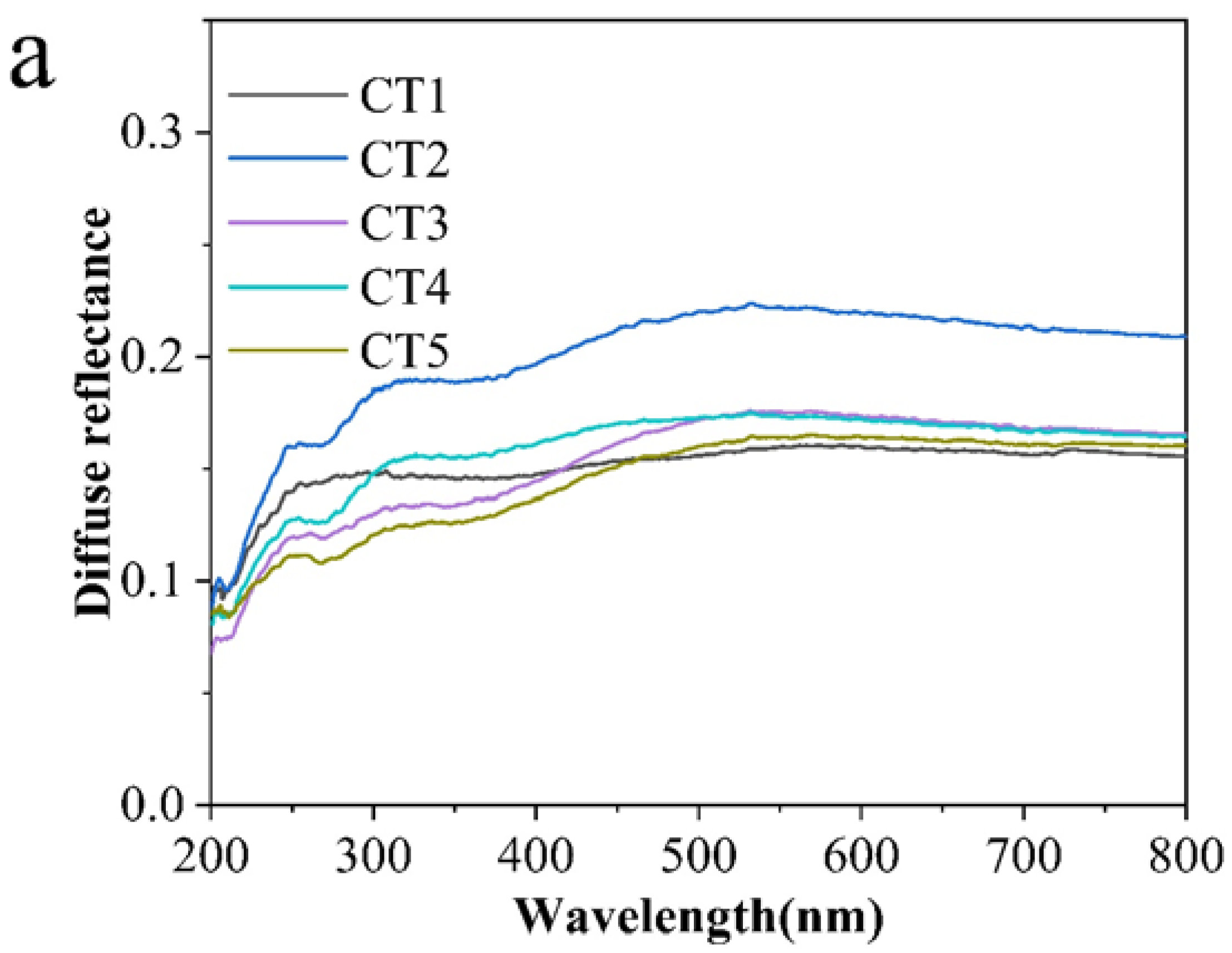
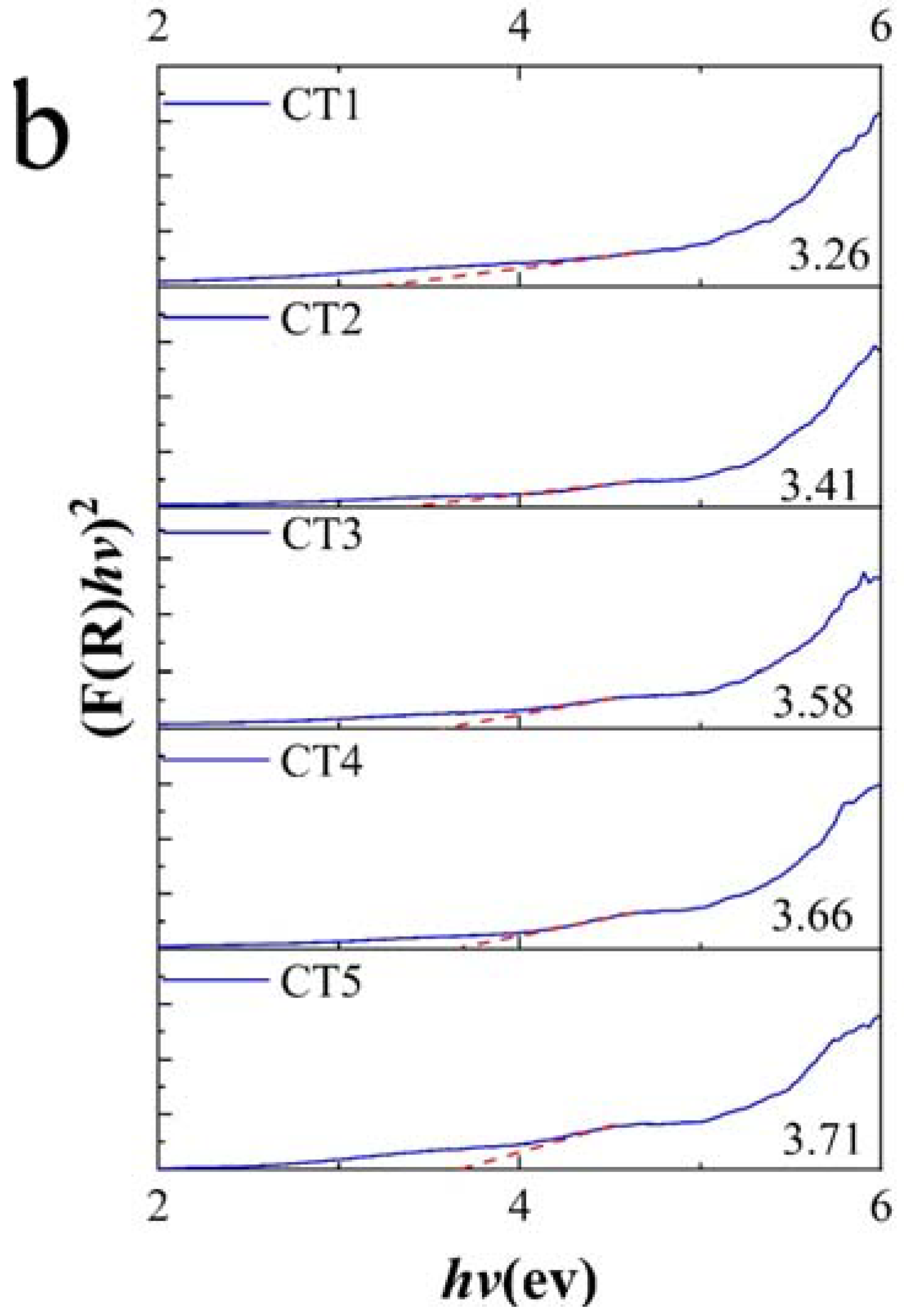
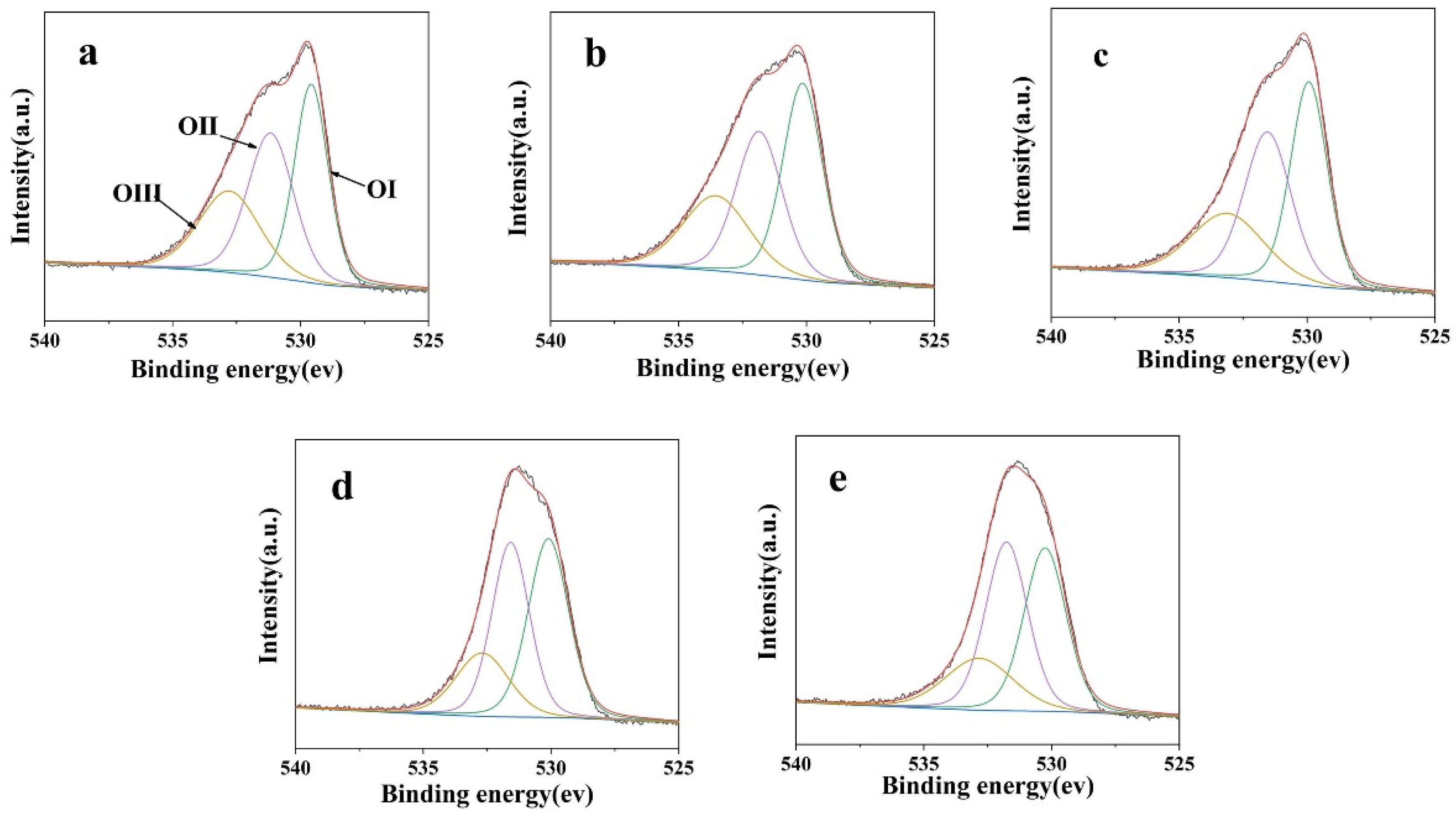
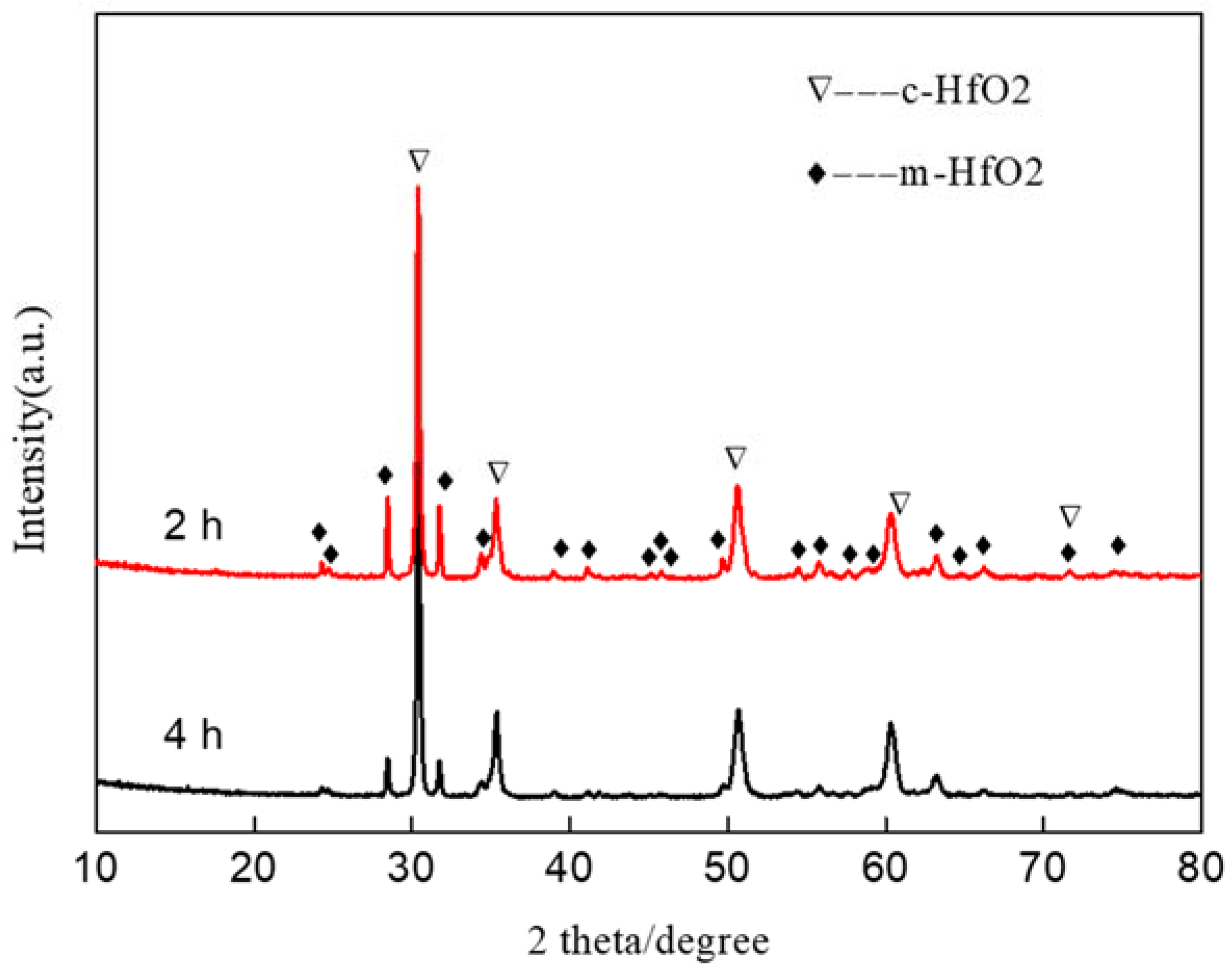
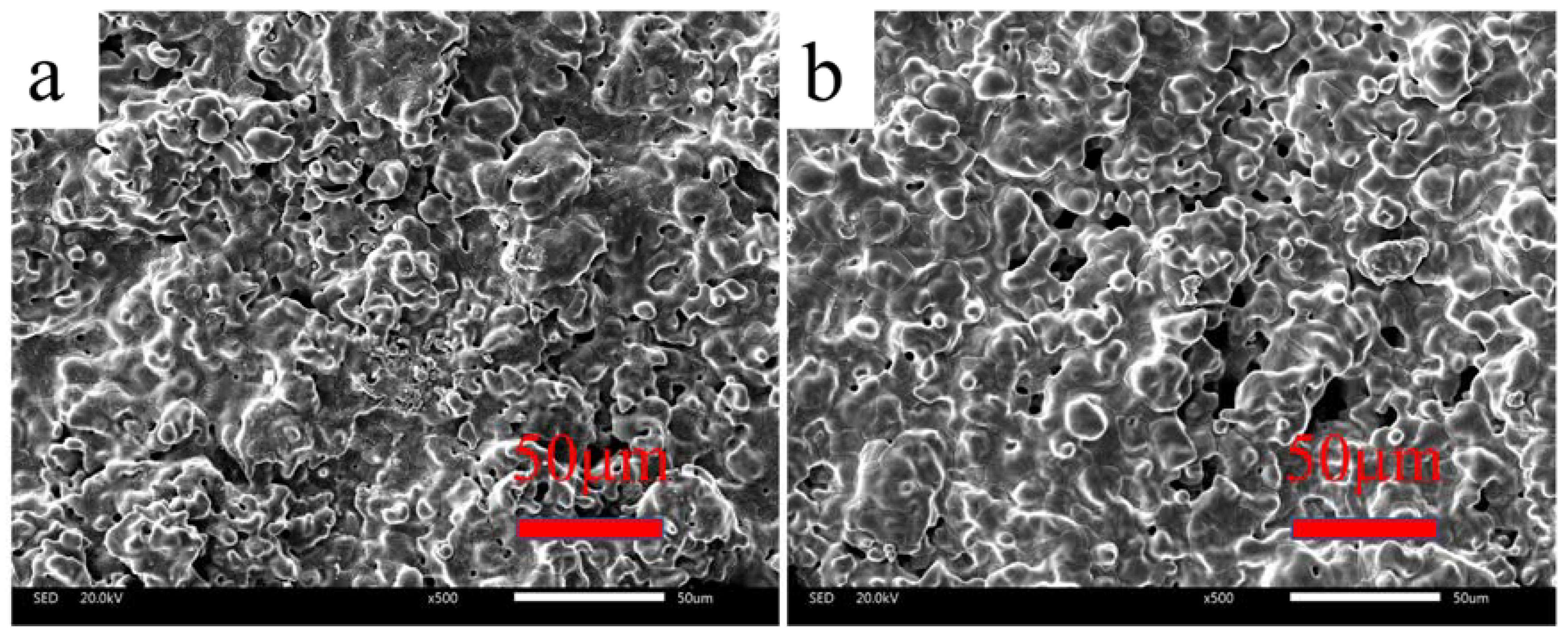
3.1. Microstructure and Chemical Composition
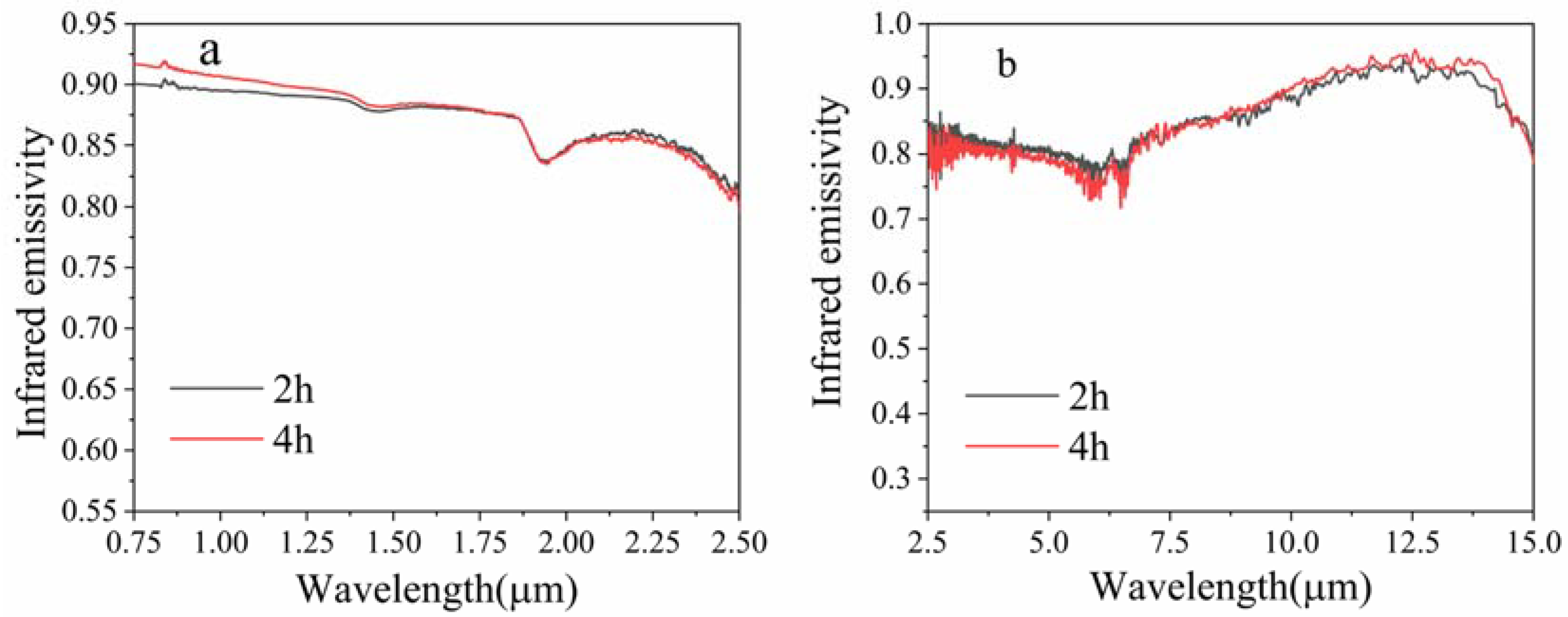
3.2. Room Temperature Infrared Radiation Property
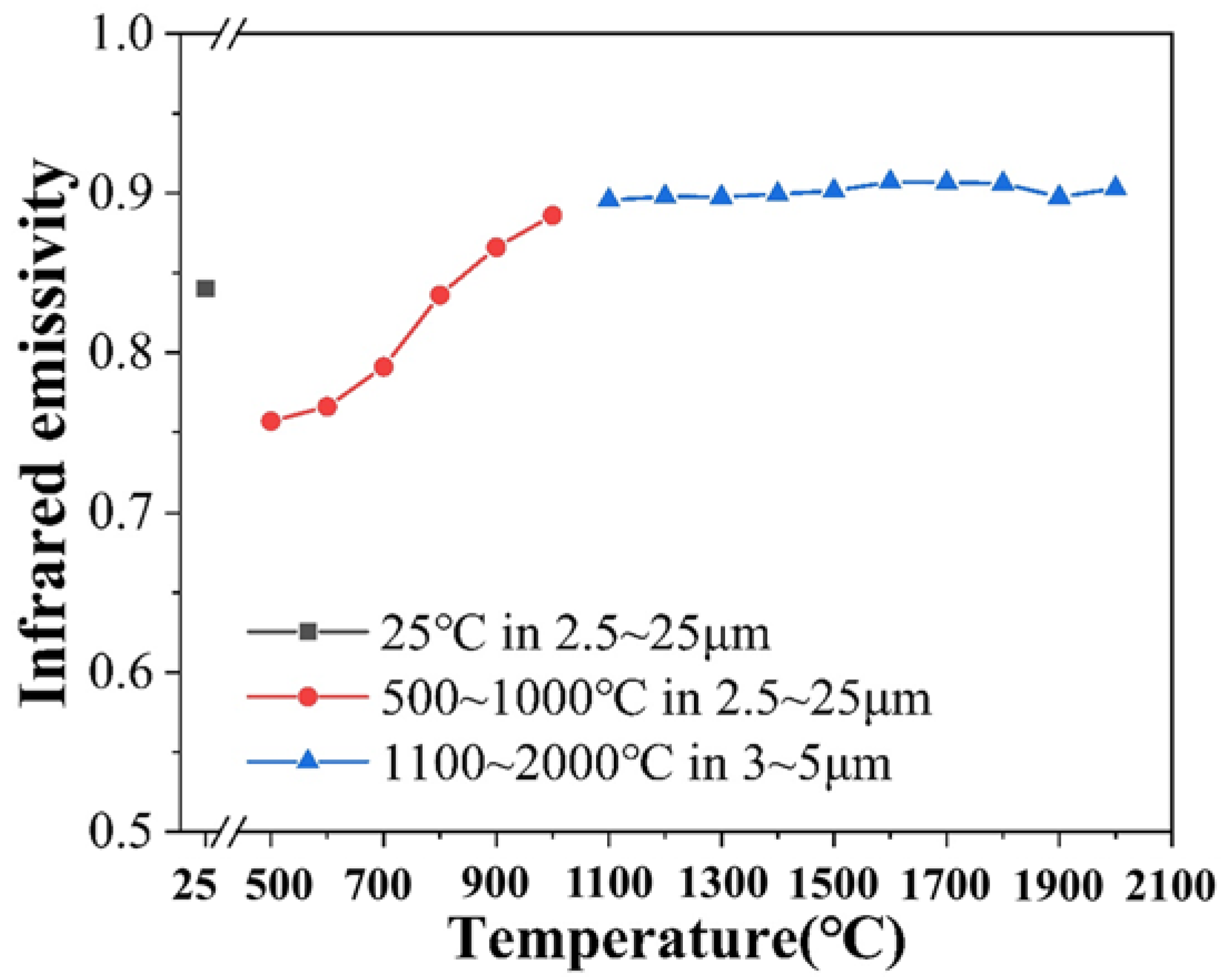
3.3. High-Temperature Infrared Radiation Performance
3.4. High-Temperature Thermal Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Chen, Z.; Pan, Z.; Tang, H. Development and Properties of Infrared High Radiation Energy-Saving Coatings for Industrial Furnace. IOP Conf. Ser. Mater. Sci. Eng. 2019, 252, 022058. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Wei, G.; Lv, Y. A robust Janus fibrous membrane with switchable infrared radiation properties for potential building thermal management applications. J. Mater. Chem. A 2019, 7, 8344–8352. [Google Scholar] [CrossRef]
- Tang, H.; Tao, W.; Wang, H.; Song, Y.; Jian, X.; Yin, L.; Wang, X.; Scarpa, F. High-performance infrared emissivity of micro-arc oxidation coatings formed on titanium alloy for aerospace applications. Int. J. Appl. Ceram. Technol. 2018, 15, 579–591. [Google Scholar] [CrossRef] [Green Version]
- Paul, A.; Jayaseelan, D.; Venugopal, S.D. UHTC composites for hypersonic applications. Am. Ceram. Soc. Bull. 2012, 91, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, Y.; Fu, Q. Ablation resistance of carbides-coated C/C composites. Adv. Surf. Eng. Mater. 2017, 33, 803–809. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, W.; Tian, T.; Xiong, X.; Zhang, H. Effect of sintering additives on the sintering and ablation resistance of novel HfO2-ThO2 high-temperature multiphase oxides. Ceram. Int. 2023, 49, 18977–18987. [Google Scholar] [CrossRef]
- Ren, L.; Yang, L.; Zhang, S.; Li, H.; Zhou, Y.; Ai, D.; Xie, Z.; Zhao, X.; Peng, Z.; Liao, R.; et al. Largely enhanced dielectric properties of polymer composites with HfO2 nanoparticles for high-temperature film capacitors. Compos. Sci. Technol. 2021, 201, 108528. [Google Scholar] [CrossRef]
- Ding, Q.; Tan, X.; Jiang, L.; Fan, X.; He, B.; Wang, C.; Zhuo, X.; Zhou, K.; Zhang, X. High-temperature performances of Si-HfO2-based environmental barrier coatings via atmospheric plasma spraying. Ceram. Int. 2022, 48, 23127–23136. [Google Scholar] [CrossRef]
- Zhang, Z.; Park, Y.; Kim, D.H.; Xue, Z.; Ji, X.; Park, H.; Zhang, S.; Byon, E.; Koo, B.H. High-temperature oxidation performance of novel environmental barrier coating 50HfO2-50SiO2/YxYb(2−x)Si2O7 at 1475 °C. J. Eur. Ceram. Soc. 2023, 43, 1127–1140. [Google Scholar] [CrossRef]
- Khoshman, J.M.; Kordesch, M.E. Optical properties of a-HfO2 thin films. Surf. Coat. Technol. 2006, 201, 3530–3535. [Google Scholar] [CrossRef]
- Dubinov, A.; Kitaev, I. Generalized Wien’s displacement law and Stefan–Boltzmann law for thermal radiation with a nonzero chemical potential. J. Opt. Technol. 2018, 6, 314–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Duan, Y.; Liu, B.; Liang, J. Preparation and performance of Ce-doped far-infrared radiation ceramics by single iron ore tailings. Ceram. Int. 2022, 48, 11709–11717. [Google Scholar] [CrossRef]
- Zhang, K.; Deng, Y.; Yang, Y.; Liao, Y.; Wang, B.; Gong, B.; Yang, W. Effect of lanthanum doping on the far-infrared emission property of vanadium–titanium slag ceramic. RSC Adv. 2017, 7, 13509–13516. [Google Scholar] [CrossRef]
- Liu, J.; Meng, J.; Liang, J.; Duan, X.; Huo, X.; Tang, Q. Effect of rare earth Ce on the far infrared radiation property of iron ore tailings ceramics. Mater. Res. Bull. 2015, 66, 26–31. [Google Scholar] [CrossRef]
- Umezawa, M.; Kurahashi, H.; Nigoghosian, K.; Okubo, K.; Soga, K. Designing Er3+/Ho3+-Doped Near-Infrared (NIR-II) Fluorescent Ceramic Particles for Avoiding Optical Absorption by Water. J. Photopolym. Sci. Technol. 2022, 35, 9–16. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Ma, Z.; Zhu, S. Effects of Ca2+-Sr2+ doping on the infrared emissivity of LaCrO3. Ceram. Int. 2020, 46, 19738–19742. [Google Scholar] [CrossRef]
- Liu, F.; Cheng, X.; Mao, J.; Li, Q.; Zeng, X. Effects of rare-earth oxide doping on the thermal radiation performance of HfO2 coating. Ceram. Int. 2019, 45, 13004–13010. [Google Scholar] [CrossRef]
- De Souza, E.F.; Appel, L.G. Oxygen vacancy formation and their role in the CO2 activation on Ca doped ZrO2 surface: An ab-initio DFT study. Appl. Surf. Sci. 2021, 553, 149589. [Google Scholar] [CrossRef]
- Wang, H.; Sun, T.; Chang, L.; Liu, F.; Liu, B.; Zhao, C.; Xue, X.; Xiong, X. Preparation of Ca doping ZrO2 coating on NiTi shape memory alloy by cathodic plasma electrolytic deposition and its structure, in-vitro bioactivity and biocompatibility analysis. Surf. Coat. Technol. 2017, 325, 136–144. [Google Scholar] [CrossRef]
- Zeng, X.; Tong, X.; Liu, Z.; Xiong, Y.; Cao, Q.; Cheng, X. Fabrication and investigation of Ca/Tb co-doped HfO2 infrared coatings. J. Eur. Ceram. Soc. 2022, 42, 3542–3549. [Google Scholar] [CrossRef]
- Ng, H.W.; Gan, Z. A finite element analysis technique for predicting as-sprayed residual stresses generated by the plasma spray coating process. Finite Elem. Anal. Des. 2005, 41, 1235–1254. [Google Scholar] [CrossRef]
- Andrievskaya, E.R. Phase equilibria in the refractory oxide systems of zirconia, hafnia and yttria with rare-earth oxides. J. Eur. Ceram. Soc. 2008, 28, 2363–2388. [Google Scholar] [CrossRef]
- Rahm, M.; Hoffmann, R.; Ashcroft, N.W. Atomic and Ionic Radii of Elements 1–96. Chem. Eur. J. 2016, 22, 14625–14632. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, C.; Zhou, X.; Chen, J. DFT study on the interaction of H2O and O2 with α-Fe2O3 (001) surface. Vacuum 2021, 188, 110164. [Google Scholar] [CrossRef]
- Auerhammer, J.M.; Eliel, E.R. Frequency doubling of mid-infrared radiation in gallium selenide. Opt. Lett. 1996, 21, 773–775. [Google Scholar] [CrossRef]
- Sturm, J.C.; Reaves, C.M. Silicon temperature measurement by infrared absorption. Fundamental processes and doping effects. IEEE Trans. Electron Devices 1992, 39, 81–88. [Google Scholar] [CrossRef]
- Martins, G.M.; Siqueira, K.; Fantini, C. New insight on the use of diffuse reflectance spectroscopy for the optical characterization of Ln2Ge2O7 (Ln = lanthanides) pyrogermanates. J. Lumin. 2021, 238, 118312. [Google Scholar] [CrossRef]
- Foster, A.S.; Lopez Gejo, F.; Shluger, A.L.; Nieminen, R.M. Vacancy and interstitial defects in hafnia. Phys. Rev. B 2002, 65, 174117. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, Y.; Fang, X.; Cheng, Y. Regulating oxygen species for promoted deep oxidation of toluene: A comparative study of adsorbed oxygen and lattice oxygen. Fuel 2022, 321, 124116. [Google Scholar] [CrossRef]
- Chen, D.; Zhong, L.; Liu, F.; Liu, J.; Yu, J.; Wan, G.; He, S.; Luo, Y. Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization. Appl. Catal. B 2017, 218, 249–259. [Google Scholar] [CrossRef]
- Das, R. Wavelength- and Frequency-Dependent Formulations of Wien’s Displacement Law. J. Chem. Educ. 2015, 92, 1130–1134. [Google Scholar] [CrossRef]
- Han, R.; Tariq, N.; Liu, H. Development of high infrared emissivity porous ceramic coating using pre-synthesized flower-like CeO2 powder for high temperature applications. Ceram. Int. 2022, 48, 1340–1348. [Google Scholar] [CrossRef]
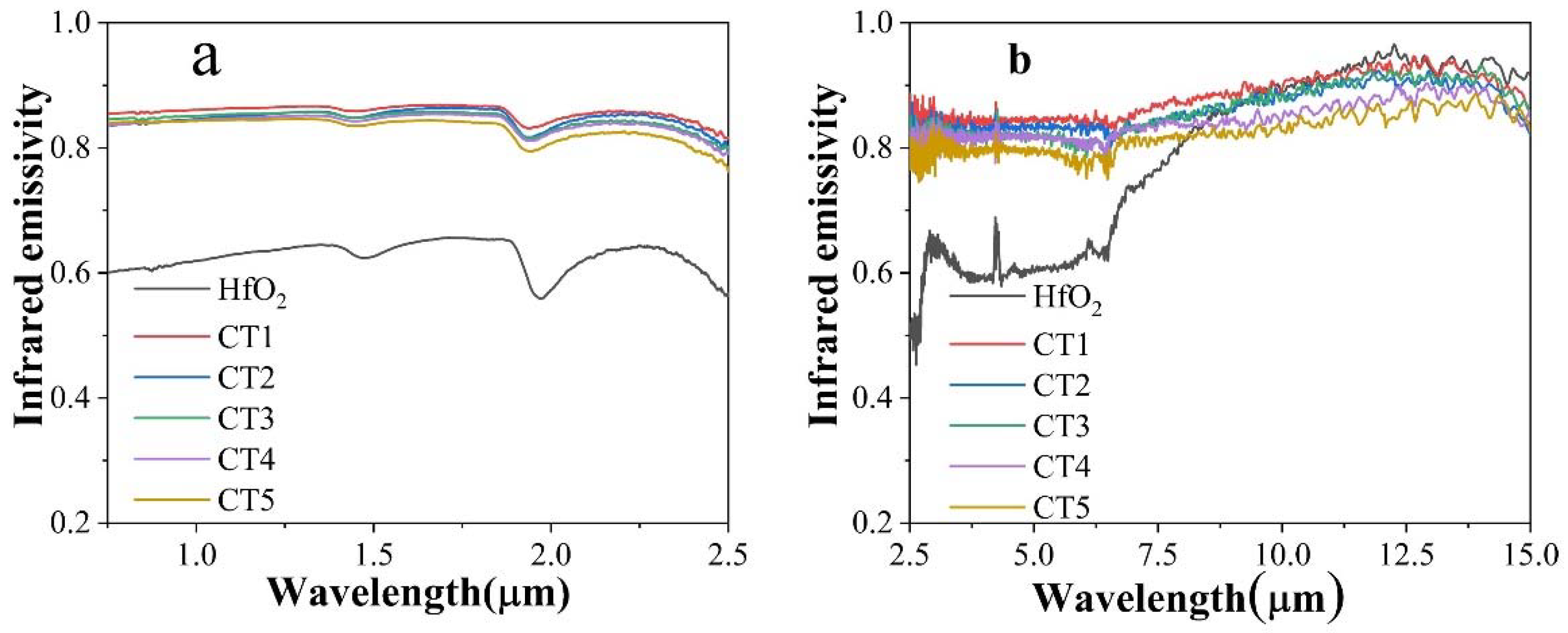
| Number | 0.75~6.5 μm | 6.5~15 μm |
|---|---|---|
| HfO2 | 0.618 | 0.870 |
| CT1 | 0.844 | 0.900 |
| CT2 | 0.832 | 0.880 |
| CT3 | 0.820 | 0.884 |
| CT4 | 0.811 | 0.872 |
| CT5 | 0.785 | 0.865 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Cheng, X.; Hu, C.; Tong, X.; Zeng, X.; Chen, Y. Preparation and Study of Ca/Tb Co-Doped HfO2 Infrared Coatings with Different Atomic Ratios. Coatings 2023, 13, 1386. https://doi.org/10.3390/coatings13081386
Zou J, Cheng X, Hu C, Tong X, Zeng X, Chen Y. Preparation and Study of Ca/Tb Co-Doped HfO2 Infrared Coatings with Different Atomic Ratios. Coatings. 2023; 13(8):1386. https://doi.org/10.3390/coatings13081386
Chicago/Turabian StyleZou, Jun, Xudong Cheng, Chengwei Hu, Xu Tong, Xian Zeng, and Yaping Chen. 2023. "Preparation and Study of Ca/Tb Co-Doped HfO2 Infrared Coatings with Different Atomic Ratios" Coatings 13, no. 8: 1386. https://doi.org/10.3390/coatings13081386
APA StyleZou, J., Cheng, X., Hu, C., Tong, X., Zeng, X., & Chen, Y. (2023). Preparation and Study of Ca/Tb Co-Doped HfO2 Infrared Coatings with Different Atomic Ratios. Coatings, 13(8), 1386. https://doi.org/10.3390/coatings13081386







