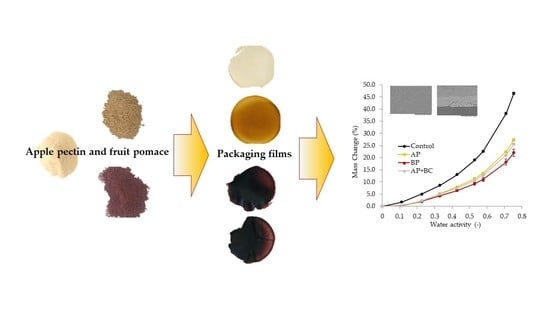
The Potential of Apple and Blackcurrant Pomace Powders as the Components of Pectin Packaging Films
Abstract
:1. Introduction
2. Materials and Methods
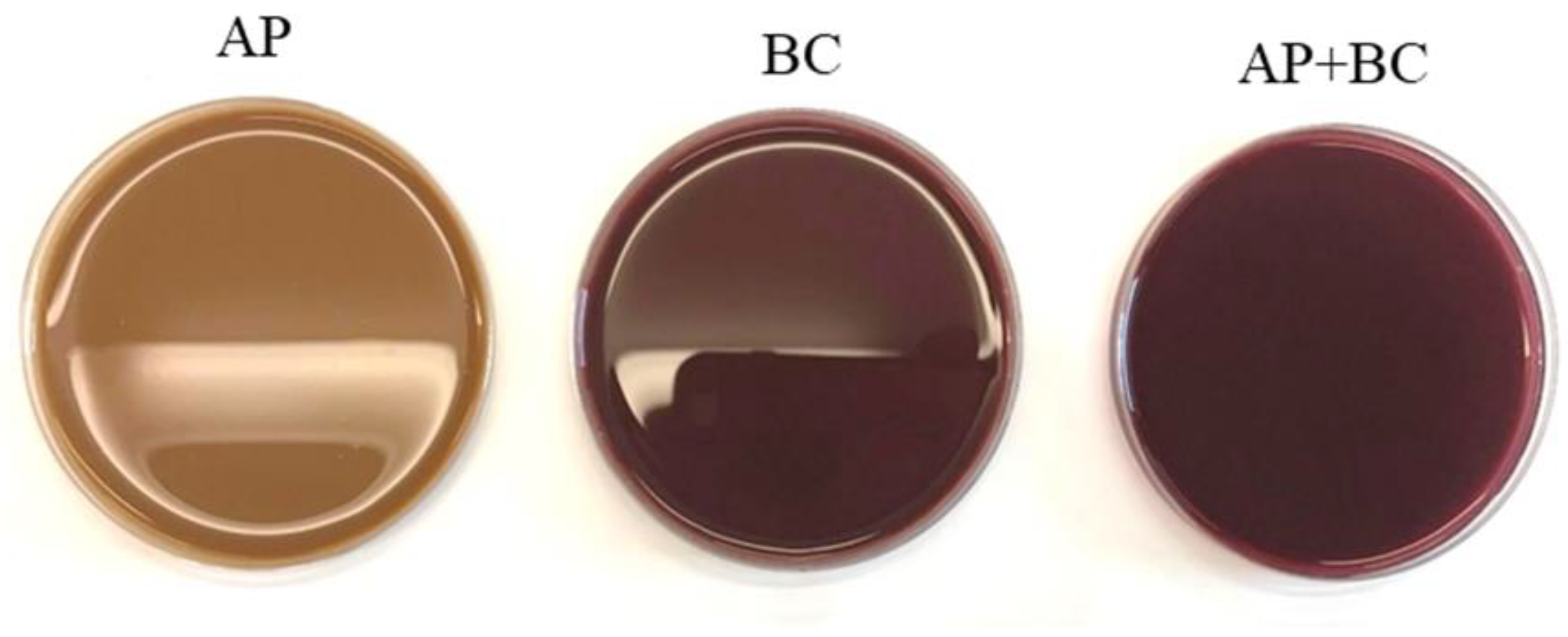
2.1. Film Preparation
2.2. Film Thickness
2.3. Water Content
2.4. Water Solubility
2.5. Swelling Index
2.6. Optical Properties
2.6.1. Color
2.6.2. Film Opacity
2.7. Water Contact Angle
2.8. Water Vapor Sorption Isotherms
2.9. Mechanical Properties
2.9.1. Tensile Strength
2.9.2. Elongation at Break
2.10. Microstructure
2.11. Statistical Analysis
3. Results and Discussion
3.1. Film Characterization
3.2. The Effect of Fruit Pomace Addition on the Thickness of Pectin Films
3.3. The Effect of Fruit Pomace on the Water Content of Pectin Films
3.4. The Effect of Fruit Pomace on the Water Solubility of Pectin Films
3.5. The Effect of Fruit Pomace on the Swelling Index of Pectin Films
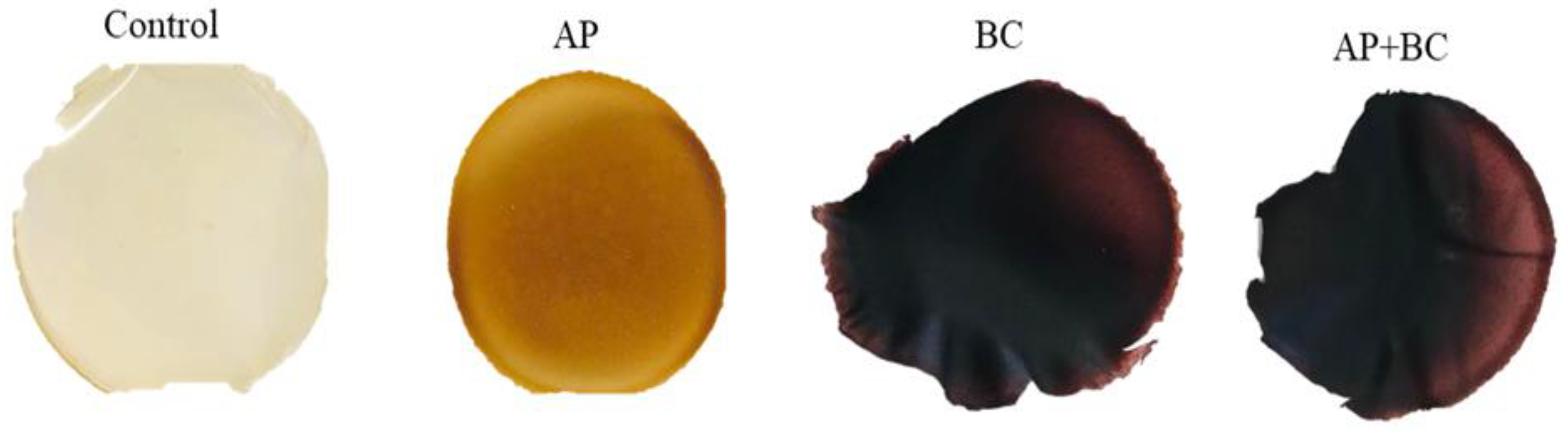
3.6. The Effect of Fruit Pomace on the Optical Properties of Pectin Films
3.7. The Effect of the Fruit Pomace on the Water Contact Angle of Pectin Films
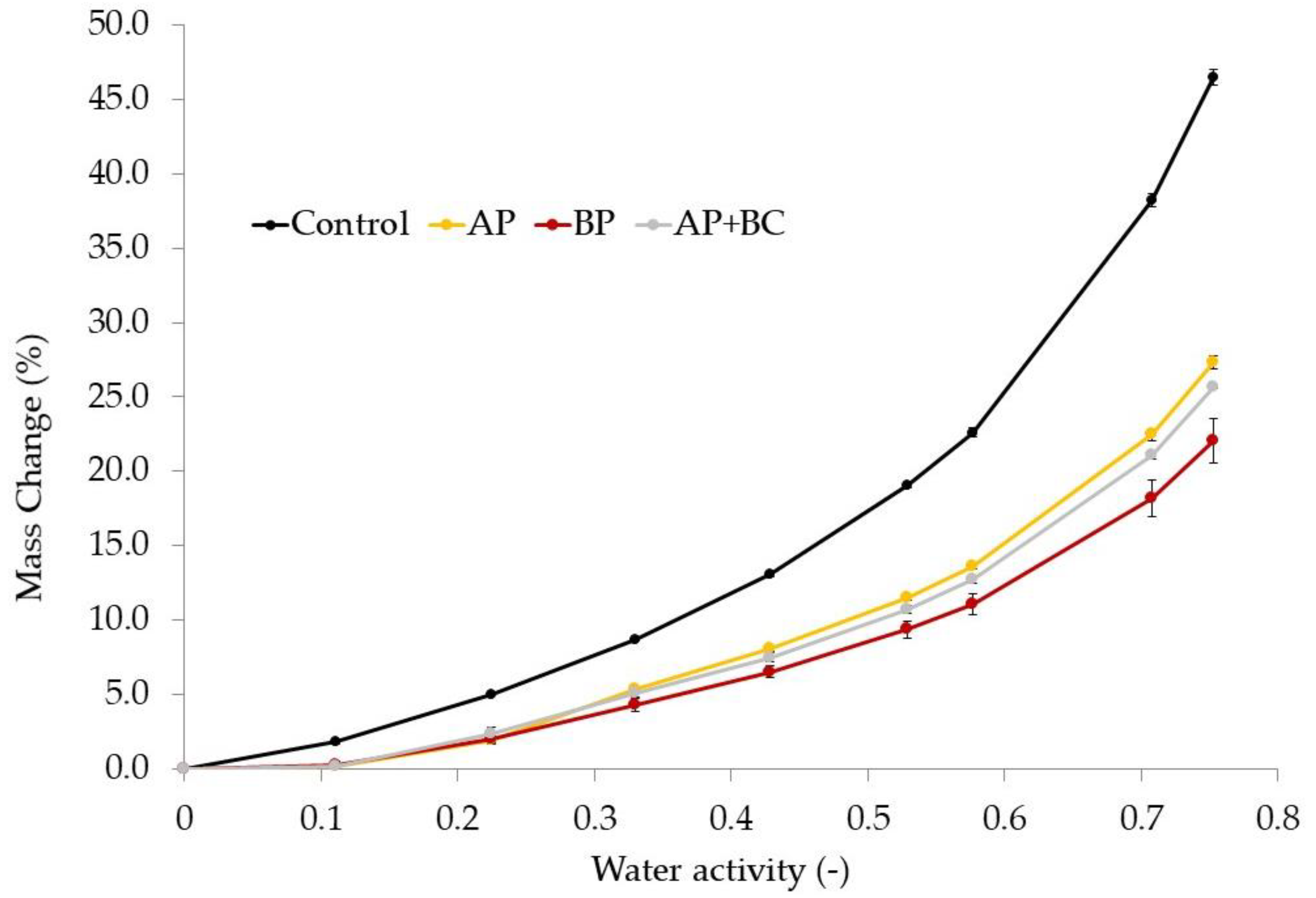
3.8. The Effect of Fruit Pomace Addition on the Sorption Isotherms of Pectin Films
3.9. The Effect of Fruit Pomace on the Mechanical Properties of Pectin Films
3.10. The Effect of the Fruit Pomace on the Microstructure of Pectin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advances in edible coatings and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Verma, M.K.; Shakya, S.; Kumar, P.; Madhavi, J.; Murugaiyan, J.; Rao, M.V.R. Trends in packaging material for food products: Historical background, current scenario, and future prospects. J. Food Sci. Technol. 2021, 58, 4069–4082. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, J.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2. [Google Scholar] [CrossRef]
- Hong, L.; Yuhana, A.Y.; Zawawi, E.Z.E. Review of bioplastic as food packaging materials. AIMS Mater. Sci. 2021, 8, 166–184. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Hu, L. Recent Advances of Proteins, Polysaccharides and Lipids-Based Edible Films/Coatings for Food Packaging Applications: A Review. Food Biophys. 2023, 1–17. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; Abdel-Monem, M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Dwivedi, P.; Mishra, P.K.; Mondal, M.K.; Srivastava, N. Non-biodegradable polymeric waste pyrolysis for energy recovery. Heliyon 2019, 5, e02198. [Google Scholar] [CrossRef] [Green Version]
- Gupta, I.; Cherwoo, L.; Bhatia, R.; Setia, H. Biopolymers: Implications and application in the food industry. Biocatal. Agric. Biotechnol. 2022, 46, 102534. [Google Scholar] [CrossRef]
- Kumar, A.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in edible packaging films and its prospective future in food: A review. Appl. Food Res. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2022, 3, 2. [Google Scholar] [CrossRef]
- Perveen, S.; Anwar, M.J.; Ismail, T.; Hameed, A.; Naqvi, S.S.; Mahomoodally, M.F.; Al Jbawi, E. Utilization of biomaterials to develop the biodegradable food packaging. Int. J. Food Prop. 2023, 26, 1122–1139. [Google Scholar] [CrossRef]
- Dong, M.; Tian, L.; Li, J.; Jia, J.; Dong, Y.; Tu, Y.; Liu, X.; Tan, C.h.; Duan, X. Improving physicochemical properties of edible wheat gluten protein films with proteins, polysaccharides and organic acid. LWT—Food Sci. Technol. 2022, 154, 112868. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and characterization of novel composite films based on soy protein isolate and oilseed flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, N.; Upadhyay, A.; Pratibha Anurag, R.K. Edible packaging from fruit processing waste: A comprehensive review. Food Rev. Int. 2023, 39, 2075–2106. [Google Scholar] [CrossRef]
- Erinle, T.J.; Adewole, D.I. Fruit pomaces—Their nutrient and bioactive components, effects on growth and health of poultry species, and possible optimization techniques. Anim. Nutr. 2022, 9, 357–377. [Google Scholar] [CrossRef]
- Radzymińska, M.; Siemianowska, E.; Platta, A. Fruit Pomace as a Potential Active Food Ingredient to the Production Ecological Innovative Confectionery Products. Zesz. Nauk. Wyższej Szkoły Ekon. Społecznej Ostrołęce 2017, 25, 383–398. [Google Scholar] [CrossRef]
- Susmitha, A.; Sasikumar, K.; Rajan, D.; Nampoothiri, K.M. Development and characterization of corn starch-gelatin based edible films incorporated with mango and pineapple for active packaging. Food Biosci. 2021, 41, 100977. [Google Scholar] [CrossRef]
- Aloui, H.; Baraket, K.; Sendon, R.; Silva, A.S.; Khwaldia, K. Development and characterization of novel composite glycerol-plasticized films based on sodium caseinate and lipid fraction of tomato pomace by-product. Int. J. Biol. Macromol. 2019, 139, 128–138. [Google Scholar] [CrossRef]
- Okoro, V.O.; Amenaghawon, A.; Podstawczyk, D.; Alimoradi, H.; Reza Khalili, M.; Anwar, M.; Brouki Milan, P.; Nie, L.; Shavandi, S. Fruit pomace—Lignin as a sustainable biopolymer for biomedical application. J. Clean. Prod. 2021, 328, 129498. [Google Scholar] [CrossRef]
- Jeya, J.; Jeevahan, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto Joseph, G.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Zhang, H.; Sablani, S. Biodegradable packaging reinforced with plant based food waste and by product. Curr. Opin. Food Sci. 2021, 42, 61–68. [Google Scholar] [CrossRef]
- Method ASTM D882–02; Standard Test Methods for Tensile Properties of Plastics. American Society for Testing and Materials: Philadelphia, PA, USA, 2002.
- Kumar, S.; Reddy, A.R.L.; Basumatary, I.B.; Nayak, A.; Dutta, D.; Konwar, J.; Purkayastha, M.D.; Mukherjee, A. Recent progress in pectin extraction and their applications in developing films and coatings for sustainable food packaging: A review. Int. J. Biol. Macromol. 2023, 239, 124281. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Effect of protein concentration on kinetics of water vapour adsorption by coatings prepared on the basis of whey protein isolate. Zywnosc Nauka Technol. Jakosc 2011, 6, 66–73. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Yun, D.; Liu, J. Recent advances on the development of food packaging films based on citrus processing wastes: A review. J. Agric. Food Res. 2022, 9, 100316. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, I.H.B.T.; Soares, C.T.; Fakhouri, F.M.; de Oliveira, R.A. Development and Characterization of Arrowroot Starch Films Incorporated with Grape Pomace Extract. Polysaccharides 2022, 3, 14. [Google Scholar] [CrossRef]
- Sutharsan, J.; Boyer, C.A.; Zhao, J. Physicochemical properties of chitosan edible films incorporated with different classes of flavonoids. Carbohydr. Polym. Technol. Appl. 2022, 4, 100232. [Google Scholar] [CrossRef]
- Lin, L.; Peng, S.; Shi, C.; Li, C.h.; Hua, Z.; Cui, H. Preparation and characterization of cassava starch/sodium carboxymethyl cellulose edible film incorporating apple polyphenols. Int. J. Biol. Macromol. 2022, 212, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N.; Goh, K.K.T.; Waterland, M.R.; Huffman, L.M.; Weeks, M.; Matia-Merino, L. Complexation of Anthocyanin-Bound Blackcurrant Pectin and Whey Protein: Effect of pH and Heat Treatment. Molecules 2022, 27, 4202. [Google Scholar] [CrossRef] [PubMed]
- Pirsa, S.; Shamusi, T. Intelligent and active packaging of chicken thigh meat by conducting nano structure cellulose-polypyrrole-ZnO film. Mater. Sci. Eng. 2019, 102, 798–809. [Google Scholar] [CrossRef]
- Salem, A.; Jridi, M.; Abdelhedi, O.; Fakhfakh, N.; Nasri, M.; Debeaufort, F.; Zouari, N. Development and characterization of fish gelatin-based biodegradable film enriched with Lepidium sativum extract as active packaging for cheese preservation. Heliyon 2021, 7, 10. [Google Scholar] [CrossRef]
- Tabatabaian, F. Color aspect of monolithic zirconia restorations: A review of the literature. J. Prosthodont. 2019, 28, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Hou, H. Effects of hydrophobic agents on the physicochemical properties of edible agar/maltodextrin films. Food Hydrocoll. 2019, 88, 283–290. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Reißner, A.M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turnerb, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Yang, J.M.; Chang, Y.H. Development of chitosan, graphene oxide, and cerium oxide composite blended films: Structural, physical, and functional properties. Cellulose 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Optical, mechanical, and moisture sorption properties of whey protein edible films. J. Food Process Eng. 2019, 42, 6. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]


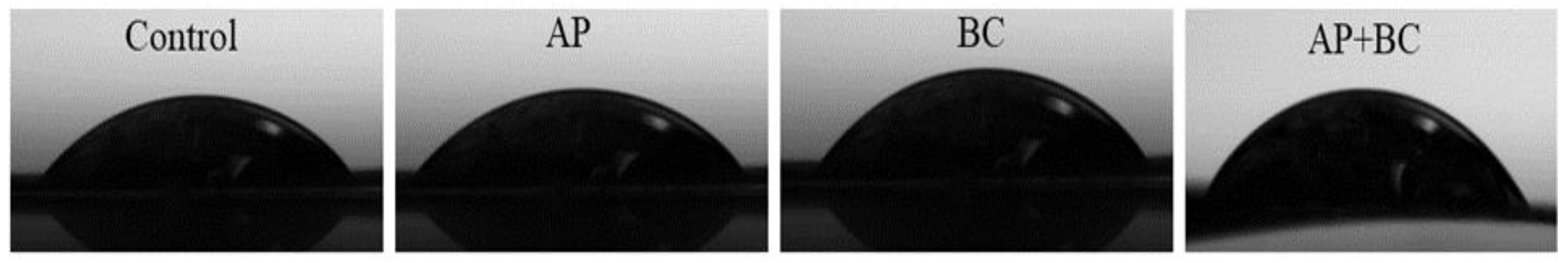

| Film | Thickness (mm) | Water Content (%) | Swelling Index (%) | Water Contact Angle (°) |
|---|---|---|---|---|
| Control | 0.097 ± 0.011 a | 1.425 ± 0.035 b | - | 50.69 ± 2.62 a |
| AP | 0.102 ± 0.005 ab | 1.305 ± 0.078 a | 117.70 ± 0.08 a | 53.32 ± 3.33 a |
| BC | 0.098 ± 0.011 a | 1.330 ± 0.014 a | 181.13 ± 0.10 c | 50.90 ± 9.01 a |
| AP+BC | 0.107 ± 0.002 b | 1.318 ± 0.039 a | 154.17 ± 0.03 b | 70.89 ± 4.45 b |
| Film | L* | a* | b* | ∆E | Film Opacity (A/mm) |
|---|---|---|---|---|---|
| Control | 87.24 ± 0.68 d | (−1.15) ± 0.04 a | 13.75 ± 1.90 c | 14.05 ± 2.01 a | 1.03 ± 0.18 a |
| AP | 62.22 ± 3.89 c | 9.78 ± 2.13 c | 39.08 ± 1.56 d | 49.95 ± 3.92 b | 10.64 ± 0.56 b |
| BC | 28.69 ± 3.69 b | 13.51 ± 3.18 d | 7.12 ± 1.95 b | 65.62 ± 2.69 c | 17.14 ± 0.87 c |
| AP+BC | 21.09 ± 0.70 a | 2.44 ± 0.87 b | (−0.03) ± 0.21 a | 71.34 ± 0.67 d | 14.79 ± 3.62 c |
| Film | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| Control | 2.97 ± 1.19 a | 10.16 ± 2.02 b |
| AP | 5.71 ± 1.71 bc | 5.11 ± 1.22 a |
| BC | 3.11 ± 1.41 a | 6.07 ± 1.45 a |
| AP+BC | 6.72 ± 1.14 c | 5.76 ± 1.35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakulska, A.; Bartosiewicz, E.; Galus, S. The Potential of Apple and Blackcurrant Pomace Powders as the Components of Pectin Packaging Films. Coatings 2023, 13, 1409. https://doi.org/10.3390/coatings13081409
Pakulska A, Bartosiewicz E, Galus S. The Potential of Apple and Blackcurrant Pomace Powders as the Components of Pectin Packaging Films. Coatings. 2023; 13(8):1409. https://doi.org/10.3390/coatings13081409
Chicago/Turabian StylePakulska, Anna, Edyta Bartosiewicz, and Sabina Galus. 2023. "The Potential of Apple and Blackcurrant Pomace Powders as the Components of Pectin Packaging Films" Coatings 13, no. 8: 1409. https://doi.org/10.3390/coatings13081409
APA StylePakulska, A., Bartosiewicz, E., & Galus, S. (2023). The Potential of Apple and Blackcurrant Pomace Powders as the Components of Pectin Packaging Films. Coatings, 13(8), 1409. https://doi.org/10.3390/coatings13081409