Solution-Processable Benzo[b]thieno[2,3-d]thiophene Derivatives as Organic Semiconductors for Organic Thin-Film Transistors
Abstract
:1. Introduction
2. Experimental
2.1. General Substances and Procedures
2.2. Synthesis
2.2.1. Synthesis of Benzo[b]thieno[2,3-d]thiophen-2-yltributylstannane (1)
2.2.2. Preparation of 2-(benzo[b]thieno[2,3-d]thiophen-2-yl)dibenzo[b,d]thiophene (2)
2.2.3. Preparation of 2-(benzo[b]thiophen-6-yl)benzo[b]thieno[2,3-d]thiophene (3)
2.3. Theoretical Calculation
2.4. Device Production
2.5. Device and Film Characterization
3. Results and Discussion
3.1. Synthesis
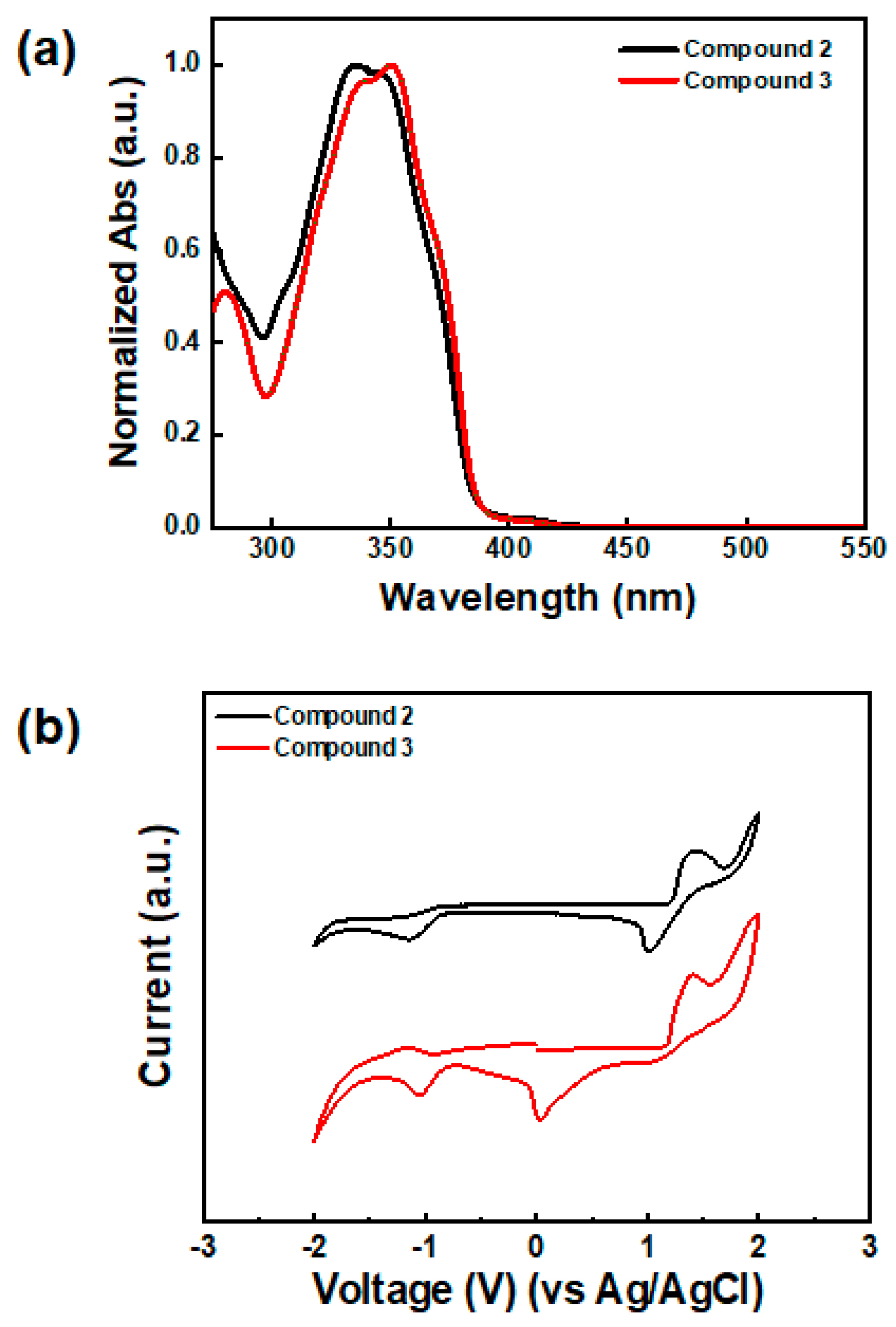
3.2. Thermal, Optical, and Electrochemical Features of Synthesized Compounds
3.3. Theoretical Calculation
3.4. Characterization of the Microstructure and Morphology of the Thin-Films
3.5. Field-Effect Transistor Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arias, A.C.; MacKenzie, J.D.; McCulloh, I.; Rivnay, J.; Salleo, A. Materials and applications for large area electronics: Solution-based approaches. Chem. Rev. 2010, 110, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Berggren, M.; Nilsson, D.; Robinson, N.D. Organic materials for printed electronics. Nat. Mater. 2007, 6, 3–5. [Google Scholar] [CrossRef]
- Brütting, W. Introduction to the Physics of Organic Semiconductors; Physics of Organic Semiconductors: Weinheim, Germany, 2005; pp. 1–14. [Google Scholar]
- Caironi, M.; Noh, Y.-Y. Large Area and Flexible Electronics; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mamada, M.; Shima, H.; Yoneda, Y.; Shimano, T.; Yamada, N.; Kakita, K.; Machida, T.; Tanaka, Y.; Aotsuka, S.; Kumaki, D.; et al. A unique solution-processable n-type semiconductor material design for high-performance organic field-effect transistors. Chem. Mater. 2015, 27, 141–147. [Google Scholar] [CrossRef]
- Ozdemir, M.; Choi, D.; Kwon, G.; Zorlu, Y.; Cosut, B.; Kim, H.; Facchetti, A.; Kim, C.; Usta, H. Solution-processable BODIPY-based small molecules for semiconducting microfibers in organic thin-film transistors. ACS Appl. Mater. Interfaces 2016, 8, 14077–14087. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.; Garcia-Frutos, E.M.; Hennrich, G.; Gómez-Lor, B. Organic semiconductors toward electronic devices: High mobility and easy processability. J. Phys. Chem. Lett. 2012, 3, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Zhu, D. Advances in organic field-effect transistors. J. Mater. Chem. 2005, 15, 53–65. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Facchetti, A. Semiconductors for organic transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Huang, F.; Yip, H.-L.; Cao, Y. Polymer Photovoltaics: Materials, Physics, and Device Engineering; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Lee, S.-H.; Park, H.-L.; Kang, S.; Kim, M.-H.; Lee, S.D. Organic thin-film transistors with liquid crystalline polymer insulator integrated for solution-processed organic light-emitting devices. Semicond. Sci. Technol. 2019, 34, 105012. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F. Organic thin-film transistors for chemical and biological sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef]
- Murphy, A.R.; Frechet, J.M. Organic semiconducting oligomers for use in thin film transistors. Chem. Rev. 2007, 107, 1066–1096. [Google Scholar] [CrossRef] [PubMed]
- Reese, C.; Roberts, M.; Ling, M.; Bao, Z. Organic thin film transistors. Mater. Today 2004, 7, 20–27. [Google Scholar] [CrossRef]
- Tang, M.L.; Okamoto, T.; Bao, Z. High-performance organic semiconductors: Asymmetric linear acenes containing sulphur. J. Am. Chem. Soc. 2006, 128, 16002–16003. [Google Scholar] [CrossRef]
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic field-effect transistor sensors: A tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Duan, L.; Hou, L.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. A comparison study of the organic small molecular thin films prepared by solution process and vacuum deposition: Roughness, hydrophilicity, absorption, photoluminescence, density, mobility, and electroluminescence. J. Phys. Chem. C 2011, 115, 14278–14284. [Google Scholar] [CrossRef]
- Xing, X.; Zhong, L.; Zhang, L.; Chen, Z.; Qu, B.; Chen, E.; Xiao, L.; Gong, Q. Essential differences of organic films at the molecular level via vacuum deposition and solution processes for organic light-emitting diodes. J. Phys. Chem. C 2013, 117, 25405–25408. [Google Scholar] [CrossRef]
- Diao, Y.; Tee, B.C.-K.; Giri, G.; Xu, J.; Kim, D.H.; Becerril, H.A.; Stoltenberg, R.M.; Lee, T.H.; Xue, G.; Mannsfeld, S.C.B.; et al. Solution coating of large-area organic semiconductor thin films with aligned single-crystalline domains. Nat. Mater. 2013, 12, 665–671. [Google Scholar] [CrossRef]
- Shaw, L.; Bao, Z. The Large-Area, Solution-Based Deposition of Single-Crystal Organic Semiconductors. Isr. J. Chem. 2014, 54, 496–512. [Google Scholar] [CrossRef]
- Ling, M.M.; Gomez, M.; Koenemann, M.; Locklin, J.; Bao, Z. Air-stable n-channel organic semiconductors based on perylene diimide derivatives without strong electron withdrawing groups. Adv. Mater. 2007, 19, 1123–1127. [Google Scholar] [CrossRef]
- Park, S.K.; Jackson, T.N.; Anthony, J.E.; Mourey, D.A. High mobility solution processed 6, 13-bis (triisopropyl-silylethynyl) pentacene organic thin film transistors. Appl. Phys. Lett. 2007, 91, 063514. [Google Scholar] [CrossRef]
- Song, D.; Wang, H.; Zhu, F.; Yang, J.; Tian, H.; Geng, Y.; Yan, D. Phthalocyanato Tin (IV) Dichloride: An Air-Stable, High-Performance, n-Type Organic Semiconductor with a High Field-Effect Electron Mobility. Adv. Mater. 2008, 20, 2142–2144. [Google Scholar] [CrossRef]
- Tian, H.K.; Shi, J.W.; Yan, D.H.; Wang, L.X.; Geng, Y.H.; Wang, F.S. Naphthyl End-Capped Quarterthiophene: A Simple Organic Semiconductor with High Mobility and Air Stability. Adv. Mater. 2006, 18, 2149–2152. [Google Scholar] [CrossRef]
- Zschieschang, U.; Ante, F.; Yamamoto, T.; Takimiya, K.; Kuwabara, H.; Ikeda, M.; Sekitani, T.; Someya, T.; Kern, K.; Klauk, H. Flexible low-voltage organic transistors and circuits based on a high-mobility organic semiconductor with good air stability. Adv. Mater. 2010, 22, 982–985. [Google Scholar] [CrossRef]
- Lim, B.; Sun, H.; Noh, Y.-Y. Highly soluble small-molecule organic semiconductor with trihexylsilyloxy side chain for high-performance organic field-effect transistors with mobility of up to 3.10 cm2 V−1 s−1. Dye. Pigment. 2017, 142, 17–23. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, G.; Cai, Z.; Chen, X.; Luo, H.; Li, Y.; Wang, J.; Zhang, D. New organic semiconductors with imide/amide-containing molecular systems. Adv. Mater. 2014, 26, 6965–6977. [Google Scholar] [CrossRef] [PubMed]
- Vegiraju, S.; Hsieh, C.-M.; Huang, D.-Y.; Chen, Y.-C.; Priyanka, P.; Ni, J.-S.; Esya, F.A.; Kim, C.; Yau, S.L.; Chen, C.-P.; et al. Synthesis and characterization of solution-processable diketopyrrolopyrrole (DPP) and tetrathienothiophene (TTA)-based small molecules for organic thin film transistors and organic photovoltaic cells. Dye. Pigment. 2016, 133, 280–291. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-conjugated systems in field-effect transistors: A material odyssey of organic electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Aydin, M.; Arsu, N.; Sekerci, M. A small-molecule organic semiconductor. Semiconductors 2004, 38, 468–471. [Google Scholar] [CrossRef] [Green Version]
- Youn, J.; Vegiraju, S.; Emery, J.D.; Leever, B.J.; Kewalramani, S.; Lou, S.J.; Zhang, S.; Prabakaran, K.; Ezhumalai, Y.; Kim, C.; et al. Diperfluorophenyl fused thiophene semiconductors for n-type organic thin film transistors (OTFTs). Adv. Electron. Mater. 2015, 1, 1500098. [Google Scholar] [CrossRef]
- Ashraf, R.S.; Gilot, J.; Janssen, R.A. Fused ring thiophene-based poly (heteroarylene ethynylene) s for organic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1759–1766. [Google Scholar] [CrossRef]
- Kumaresan, P.; Vegiraju, S.; Ezhumalai, Y.; Yau, S.L.; Kim, C.; Lee, W.-H.; Chen, M.-C. Fused-thiophene based materials for organic photovoltaics and dye-sensitized solar cells. Polymers 2014, 6, 2645–2669. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.-Q.; Zhou, Y.-N.; Yan, X.-Y.; Shi, K.; Zheng, Y.-Q.; Luo, M.; Wang, X.-C.; Pei, J.; Xia, H.; Zoppi, L.; et al. Thiophene-fused bowl-shaped polycyclic aromatics with a dibenzo [a, g] corannulene core for organic field-effect transistors. Chem. Commun. 2015, 51, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, Y.; Zhu, D. π-Conjugated molecules with fused rings for organic field-effect transistors: Design, synthesis and applications. Chem. Soc. Rev. 2010, 39, 1489–1502. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Liao, Q.; Koh, C.W.; Chen, J.; Su, M.; Zhou, X.; Tang, Y.; Wang, Y.; Zhang, Y.; Woo, H.Y.; et al. Improved photovoltaic performance of a nonfullerene acceptor based on a benzo [b] thiophene fused end group with extended π-conjugation. J. Mater. Chem. A 2019, 7, 9822–9830. [Google Scholar] [CrossRef]
- Huang, P.-Y.; Chen, L.-H.; Chen, Y.-Y.; Chang, W.-J.; Wang, J.-J.; Lii, K.-H.; Yan, J.-Y.; Ho, J.-C.; Lee, C.-C.; Kim, C.; et al. Enhanced Performance of Benzothieno [3, 2-b] thiophene (BTT)-Based Bottom-Contact Thin-Film Transistors. Chem.–A Eur. J. 2013, 19, 3721–3728. [Google Scholar] [CrossRef]
- Ra, C.-S.; Yim, S.-G.; Park, G.-S. DFT studies of band gaps of the fused thiophene oligomers. Bull. Korean Chem. Soc. 2008, 29, 891–893. [Google Scholar]
- Takimiya, K.; Ebata, H.; Sakamoto, K.; Izawa, T.; Otsubo, T.; Kunugi, Y. 2, 7-Diphenyl [1] benzothieno [3, 2-b] benzothiophene, a new organic semiconductor for air-stable organic field-effect transistors with mobilities up to 2.0 cm2 V−1 s−1. J. Am. Chem. Soc. 2006, 128, 12604–12605. [Google Scholar] [CrossRef]
- Chen, H.; Cui, Q.; Yu, G.; Guo, Y.; Huang, J.; Zhu, M.; Guo, X.; Liu, Y. Synthesis and characterization of novel semiconductors based on thieno [3, 2-b][1] benzothiophene cores and their applications in the organic thin-film transistors. J. Phys. Chem. C 2011, 115, 23984–23991. [Google Scholar] [CrossRef]
- Hyodo, K.; Hagiwara, H.; Toyama, R.; Mori, H.; Soga, S.; Nishihara, Y. Bis [1] benzothieno [2, 3-d: 2′, 3′-d′] anthra [1, 2-b: 5, 6-b′] dithiophene: Synthesis, characterization, and application to organic field-effect transistors. RSC Adv. 2017, 7, 6089–6092. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Z.; Luo, H.; Xie, X.; Ai, L.; Ge, Z.; Yu, G.; Liu, Y. Benzothieno [2, 3-b] thiophene semiconductors: Synthesis, characterization and applications in organic field-effect transistors. J. Mater. Chem. C 2014, 2, 8804–8810. [Google Scholar] [CrossRef]
- Mathis, T.; Liu, Y.; Ai, L.; Ge, Z.; Lumpi, D.; Horkel, E.; Holzer, B.; Froehlich, J.; Batlogg, B. Stable organic field-effect-transistors with high mobilities unaffected by supporting dielectric based on phenylene-bridged thienobenzothiophene. J. Appl. Phys. 2014, 115, 043707. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Shen, Y.; Song, Y. A theoretical study of the electronic structure and charge transport properties of thieno [2, 3-b] benzothiophene based derivatives. Phys. Chem. Chem. Phys. 2016, 18, 8401–8411. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Huang, P.-Y.; Zhang, S.; Liu, C.-W.; Vegiraju, S.; Prabakaran, K.; Stern, S.; Kim, C.; Chen, M.-C.; Facchetti, A.; et al. Functionalized benzothieno [3, 2 b] thiophenes (BTT s) for high performance organic thin-film transistors (OTFTs). J. Mater. Chem. C 2014, 2, 7599–7607. [Google Scholar] [CrossRef]
- Park, S.; Ryu, S.; Ho, D.; Chae, W.; Earmme, T.; Kim, C.; Seo, S. Novel benzo [b] thieno [2, 3-d] thiophene derivatives with an additional alkyl-thiophene core: Synthesis, characterization, and p-type thin film transistor performance. New J. Chem. 2022, 46, 12196–12205. [Google Scholar] [CrossRef]
- Niemczak, M.; Rzemieniecki, T.; Sobiech, Ł.; Skrzypczak, G.; Praczyk, T.; Pernak, J. Influence of the alkyl chain length on the physicochemical properties and biological activity in a homologous series of dichlorprop-based herbicidal ionic liquids. J. Mol. Liq. 2019, 276, 431–440. [Google Scholar] [CrossRef]
- Othman Zailani, N.H.Z.; Yunus, N.M.; Ab Rahim, A.H.; Bustam, M.A. Thermophysical properties of newly synthesized ammonium-based protic ionic liquids: Effect of temperature, anion and alkyl chain length. Processes 2020, 8, 742. [Google Scholar] [CrossRef]
- Kim, S.O.; An, T.K.; Chen, J.; Kang, I.; Kang, S.H.; Chung, D.S.; Kwon, S. K. H-aggregation strategy in the design of molecular semiconductors for highly reliable organic thin film transistors. Adv. Funct. Mater. 2011, 21, 1616–1623. [Google Scholar] [CrossRef]
- Ha, T.-J.; Sonar, P.; Dodabalapur, A. Charge transport study of high mobility polymer thin-film transistors based on thiophene ssubstituted diketopyrrolopyrrole copolymers. Phys. Chem. Chem. Phys. 2013, 15, 9735–9741. [Google Scholar] [CrossRef]
- Chen, S.-C.; Zhang, Q.; Zheng, Q.; Tang, C.; Lu, C.Z. Angular-shaped naphthalene tetracarboxylic diimides for n-channel organic transistor semiconductors. Chem. Commun. 2012, 48, 1254–1256. [Google Scholar] [CrossRef]
- Kim, H.; Reddy, M.R.; Kwon, G.; Choi, D.; Kim, C.; Seo, S. Synthesis and characterization of 2, 7-diethynyl-benzo [b] benzo [4, 5] thieno [2, 3-d] thiophene derivative as organic semiconductors for organic thin-film transistors. Synth. Met. 2016, 220, 599–605. [Google Scholar] [CrossRef]
- Nowok, A.; Dulski, M.; Grelska, J.; Szeremeta, A.Z.; Jurkiewicz, K.; Grzybowska, K.; Pawlus, S. Phenyl ring: A steric hindrance or a source of different hydrogen bonding patterns in self-organizing systems? J. Phys. Chem. Lett. 2021, 12, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Berliocchi, M.; Manenti, M.; Di Carlo, A.; Lugli, P.; Lmimouni, K.; Dufour, C. Effects of grain boundaries, field-dependent mobility, and interface trap states on the electrical characteristics of pentacene TFT. IEEE Trans. Electron Devices 2004, 51, 1997–2003. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, P.; Ghosh, S. Optimization of surface morphology to reduce the effect of grain boundaries and contact resistance in small molecule based thin film transistors. Appl. Phys. Lett. 2012, 101, 193307. [Google Scholar] [CrossRef]
- Di Carlo, A.; Piacenza, F.; Bolognesi, A.; Stadlober, B.; Maresch, H. Influence of grain sizes on the mobility of organic thin-film transistors. Appl. Phys. Lett. 2005, 86, 263501. [Google Scholar] [CrossRef]
- Locklin, J.; Bao, Z. Effect of morphology on organic thin film transistor sensors. Anal. Bioanal. Chem. 2006, 384, 336–342. [Google Scholar] [CrossRef] [PubMed]




| Td (°C) a | Tm (°C) b | λmax (nm) c | λonset (nm) c | Egap (eV) c | Eoxonset (V) d | EHOMO (eV) d | ELUMO (eV) e | ||
|---|---|---|---|---|---|---|---|---|---|
| Solution | Film | ||||||||
| 2 | 281 | 194 | 335 | 311 | 384 | 3.23 | 1.16 | −5.49 | −2.26 |
| 3 | 248 | 191 | 350 | 328 | 385 | 3.22 | 1.18 | −5.51 | −2.29 |
| Compound | µavg (cm2/Vs) (µmax (cm2/Vs)) | Ion/Ioff | Vth (V) |
|---|---|---|---|
| 2 | 0.0003± 0.0001 (0.0003) | (1.0 ± 0.3) × 105 | −3 ± 1.0 |
| 3 | 0.0045 ± 0.0005 (0.005) | (1.7 ± 0.5) × 106 | −5 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Ryu, S.; Ahn, J.; Kim, D.; Marrocchi, A.; Kim, C.; Seo, S. Solution-Processable Benzo[b]thieno[2,3-d]thiophene Derivatives as Organic Semiconductors for Organic Thin-Film Transistors. Coatings 2023, 13, 1417. https://doi.org/10.3390/coatings13081417
Kim S, Ryu S, Ahn J, Kim D, Marrocchi A, Kim C, Seo S. Solution-Processable Benzo[b]thieno[2,3-d]thiophene Derivatives as Organic Semiconductors for Organic Thin-Film Transistors. Coatings. 2023; 13(8):1417. https://doi.org/10.3390/coatings13081417
Chicago/Turabian StyleKim, Seongyun, Soomin Ryu, Jihae Ahn, Dongkyu Kim, Assunta Marrocchi, Choongik Kim, and SungYong Seo. 2023. "Solution-Processable Benzo[b]thieno[2,3-d]thiophene Derivatives as Organic Semiconductors for Organic Thin-Film Transistors" Coatings 13, no. 8: 1417. https://doi.org/10.3390/coatings13081417
APA StyleKim, S., Ryu, S., Ahn, J., Kim, D., Marrocchi, A., Kim, C., & Seo, S. (2023). Solution-Processable Benzo[b]thieno[2,3-d]thiophene Derivatives as Organic Semiconductors for Organic Thin-Film Transistors. Coatings, 13(8), 1417. https://doi.org/10.3390/coatings13081417








