A Cost-Effective, Nanoporous, High-Entropy Oxide Electrode for Electrocatalytic Water Splitting
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of High-Entropy Oxides and Fabrication of Electrodes
2.2. Characterization of Electrode Materials
3. Result and Discussion
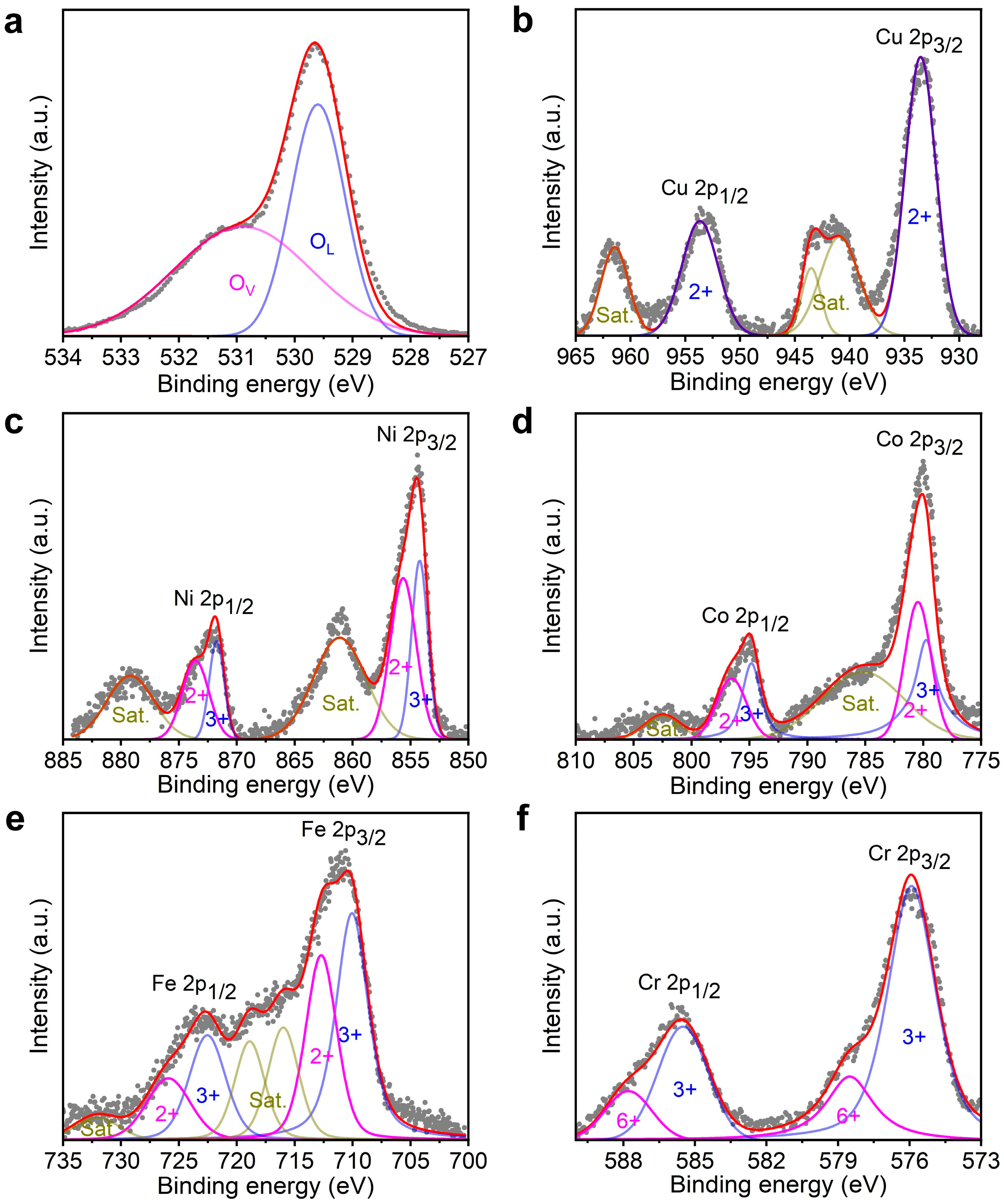
3.1. Characterization of High-Entropy Oxides
3.2. Electrochemical Impedance Studies of High-Entropy Metal Oxide Electrodes
3.3. Electrochemical Measurements of High-Entropy Metal Oxide Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Shahri, O.A.; Ismail, F.B.; Hannan, M.A.; Lipu, M.S.H.; Al-Shetwi, A.Q.; Begum, R.A.; Al-Muhsen, N.F.O.; Soujeri, E. Solar photovoltaic energy optimization methods, challenges and issues: A comprehensive review. J. Clean. Prod. 2021, 284, 125465. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, Y.; Chau, K.T.; Zhao, W.; Lee, C.H.T. Modern electric machines and drives for wind power generation: A review of opportunities and challenges. IET Renew. Power Gener. 2021, 15, 1864–1887. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, X.; Yao, L. Research status and future of hydro-related sustainable complementary multi-energy power generation. Sustain. Futures 2021, 3, 100042. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef] [PubMed]
- Abdin, Z.; Mérida, W. Hybrid energy systems for off-grid power supply and hydrogen production based on renewable energy: A techno-economic analysis. Energy Convers. Manag. 2019, 196, 1068–1079. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of renewable energy power fluctuations on water electrolysis for green hydrogen production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Panchenko, V.A.; Daus, Y.V.; Kovalev, A.A.; Yudaev, I.V.; Litti, Y.V. Prospects for the production of green hydrogen: Review of countries with high potential. Int. J. Hydrogen Energy 2023, 48, 4551–4571. [Google Scholar] [CrossRef]
- Khademi, M.H.; Alipour-Dehkordi, A.; Nalchifard, F. Sustainable hydrogen and syngas production from waste valorization of biodiesel synthesis by-product: Green chemistry approach. Renew. Sust. Energ. Rev. 2023, 175, 113191. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, D.; Deng, T.; He, G.; Chen, A.; Sun, X.; Yang, Y.; Miao, P. Research progress of oxygen evolution reaction catalysts for electrochemical water splitting. ChemSusChem 2021, 14, 5359–5383. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lai, C.-W.; Jiang, W.-C.; Li, Y.-S.; Choi, C.; Yu, H.-C.; Chen, S.-J.; Choi, Y. Fabrication and characterization of P-type semiconducting copper oxide-based thin-film photoelectrodes for solar water splitting. Coatings 2022, 12, 1206. [Google Scholar] [CrossRef]
- Yin, T.-H.; Liu, B.-J.; Lin, Y.-W.; Li, Y.-S.; Lai, C.-W.; Lan, Y.-P.; Choi, C.; Chang, H.-C.; Choi, Y. Electrodeposition of Copper Oxides as Cost-Effective Heterojunction Photoelectrode Materials for Solar Water Splitting. Coatings 2022, 12, 1839. [Google Scholar] [CrossRef]
- Wan, C.; Duan, X.; Huang, Y. Molecular design of single-atom catalysts for oxygen reduction reaction. Adv. Energy Mater. 2020, 10, 1903815. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yang, X.; Guo, R.; Cai, R.; Ouyang, Y.; Yang, P.; Xiao, J. Engineering high-entropy materials for electrocatalytic water splitting. Int. J. Hydrogen Energy 2022, 47, 13561–13578. [Google Scholar] [CrossRef]
- Hussain, I.; Lamiel, C.; Ahmad, M.; Chen, Y.; Shuang, S.; Javed, M.S.; Yang, Y.; Zhang, K. High entropy alloys as electrode material for supercapacitors: A review. J. Energy Storage 2021, 44, 103405. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Lewin, E. Multi-component and high-entropy nitride coatings-A promising field in need of a novel approach. J. Appl. Phys. 2020, 127, 160901. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, H.; Lu, S.; Duan, F.; Du, M. High entropy alloy nitrides with integrated nanowire/nanosheet architecture for efficient alkaline hydrogen evolution reactions. New J. Chem. 2021, 45, 22255–22260. [Google Scholar] [CrossRef]
- Jin, T.; Sang, X.; Unocic, R.R.; Kinch, R.T.; Liu, X.; Hu, J.; Liu, H.; Dai, S. Mechanochemical-Assisted Synthesis of High-Entropy Metal Nitride via a Soft Urea Strategy. Adv. Mater. 2018, 30, 1707512. [Google Scholar] [CrossRef]
- Sarker, P.; Harrington, T.; Toher, C.; Oses, C.; Samiee, M.; Maria, J.-P.; Brenner, D.W.; Vecchio, K.S.; Curtarolo, S. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun. 2018, 9, 4980. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Z.; Du, S.; Zhang, Y.; Li, H.; Xiao, Z.; Chen, W.; Chen, R.; Wang, Y.; Zou, Y. Low-temperature synthesis of small-sized high-entropy oxides for water oxidation. J. Mater. Chem. 2019, 7, 24211–24216. [Google Scholar] [CrossRef]
- Bérardan, D.; Franger, S.; Dragoe, D.; Meena, A.K.; Dragoe, N. Colossal dielectric constant in high entropy oxides. Phys Status Solidi Rapid Res Lett. 2016, 10, 328–333. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Y.; Zhang, J.; Shi, P. Facile Preparation, Microstructure and Dielectric Properties of La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 Perovskite High-Entropy Ceramics. Crystals 2022, 12, 1756. [Google Scholar] [CrossRef]
- Katzbaer, R.R.; dos Santos Vieira, F.M.; Dabo, I.; Mao, Z.; Schaak, R.E. Band Gap Narrowing in a High-Entropy Spinel Oxide Semiconductor for Enhanced Oxygen Evolution Catalysis. J. Am. Chem. Soc. 2023, 145, 6753–6761. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Chen, F.; Shen, Q.; Han, Y.-H.; Fahrenholtz, W.G.; Zhang, L. Microstructural evolution and mechanical properties of (Mg,Co,Ni,Cu,Zn)O high-entropy ceramics. J. Am. Ceram. 2019, 102, 2228–2237. [Google Scholar] [CrossRef]
- Tang, L.; Li, Z.; Chen, K.; Li, C.; Zhang, X.; An, L. High-entropy oxides based on valence combinations: Design and practice. J. Am. Ceram. 2021, 104, 1953–1958. [Google Scholar] [CrossRef]
- Liang, B.; Ai, Y.; Wang, Y.; Liu, C.; Ouyang, S.; Liu, M. Spinel-type (FeCoCrMnZn)3O4 high-entropy oxide: Facile preparation and supercapacitor performance. Materials 2020, 13, 5798. [Google Scholar] [CrossRef]
- Petrovičovà, B.; Xu, W.; Musolino, M.G.; Pantò, F.; Patanè, S.; Pinna, N.; Santangelo, S.; Triolo, C. High-Entropy Spinel Oxides Produced via Sol-Gel and Electrospinning and Their Evaluation as Anodes in Li-Ion Batteries. Appl. Sci. 2022, 12, 5965. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X.; Bi, L. A high-entropy spinel ceramic oxide as the cathode for proton-conducting solid oxide fuel cells. J. Adv. Ceram. 2022, 11, 794–804. [Google Scholar] [CrossRef]
- Abdel-Aal, S.K.; Beskrovnyi, A.I.; Ionov, A.M.; Mozhchil, R.N.; Abdel-Rahman, A.S. Structure investigation by neutron diffraction and X-ray diffraction of graphene nanocomposite CuO-rGO prepared by low-cost method. Phys. Status Solidi A 2021, 218, 2100138. [Google Scholar] [CrossRef]
- Xing, H.; Lei, E.; Guo, Z.; Zhao, D.; Liu, Z. Enhancement in the charge transport and photocorrosion stability of CuO photocathode: The synergistic effect of spatially separated dual-cocatalysts and p-n heterojunction. Chem. Eng. J. 2020, 394, 124907. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Chiang, W.-C.; Wu, J.-K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Cai, Y.; Manladan, S.M.; Luo, Z. Tribological behaviour of the double FeCoNiCrCux middle-entropy alloy coatings. Surf. Eng. 2019, 35, 14–21. [Google Scholar] [CrossRef]
- Wang, W.L.; Hu, L.; Luo, S.B.; Meng, L.J.; Geng, D.L.; Wei, B. Liquid phase separation and rapid dendritic growth of high-entropy CoCrCuFeNi alloy. Intermetallics 2016, 77, 41–45. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, Z.; Shi, J.C.; Wang, Z.Y.; Zhang, J.Y. The effect of Al content on microstructures and comprehensive properties in AlxCoCrCuFeNi high entropy alloys. Vacuum 2019, 161, 143–149. [Google Scholar] [CrossRef]
- Patra, S.; Munichandraiah, N. Supercapacitor studies of electrochemically deposited PEDOT on stainless steel substrate. J. Appl. Polym. Sci. 2007, 106, 1160–1171. [Google Scholar] [CrossRef]
- Eugénio, S.; Silva, T.M.; Carmezim, M.J.; Duarte, R.G.; Montemor, M.F. Electrodeposition and characterization of nickel–copper metallic foams for application as electrodes for supercapacitors. J. Appl. Electrochem. 2014, 44, 455–465. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Zheng, D.; Li, Z.; Zeng, Y.; Lu, X. Carbon cloth as an advanced electrode material for supercapacitors: Progress and challenges. J. Mater. Chem. 2020, 8, 17938–17950. [Google Scholar] [CrossRef]
- Zhang, C.J.; Nicolosi, V. Graphene and MXene-based transparent conductive electrodes and supercapacitors. Energy Stor. Mater. 2019, 16, 102–125. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Aroh, K.; Franson, N.; Satsangi, V.R.; Dass, S.; Ehrman, S. Copper oxide nanoparticle made by flame spray pyrolysis for photoelectrochemical water splitting-Part II. Photoelectrochemical study. Int. J. Hydrogen Energy 2011, 36, 15519–15526. [Google Scholar] [CrossRef]
- Jian, J.; Kumar, R.; Sun, J. Cu2O/ZnO p-n Junction Decorated with NiOx as a Protective Layer and Cocatalyst for Enhanced Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2020, 3, 10408–10414. [Google Scholar] [CrossRef]
- Hossain, R.; Nekouei, R.K.; Al Mahmood, A.; Sahajwalla, V. Value-added fabrication of NiO-doped CuO nanoflakes from waste flexible printed circuit board for advanced photocatalytic application. Sci. Rep. 2022, 12, 12171. [Google Scholar] [CrossRef]
- Liu, F.; Yang, X.; Qiao, Z.; Zhang, L.; Cao, B.; Duan, G. Highly transparent 3D NiO-Ni/Ag-nanowires/FTO micro-supercapacitor electrodes for fully transparent electronic device purpose. Electrochim. Acta 2018, 260, 281–289. [Google Scholar] [CrossRef]
- Shaheen Shah, S.; Aziz, M.A.; Al-Betar, A.-R.; Mahfoz, W. Electrodeposition of polyaniline on high electroactive indium tin oxide nanoparticles-modified fluorine doped tin oxide electrode for fabrication of high-performance hybrid supercapacitor. Arab. J. Chem. 2022, 15, 104058. [Google Scholar] [CrossRef]
- Ferreira, T.A.S.; Waerenborgh, J.C.; Mendonça, M.; Nunes, M.R.; Costa, F.M. Structural and morphological characterization of FeCo2O4 and CoFe2O4 spinels prepared by a coprecipitation method. Solid State Sci. 2003, 5, 383–392. [Google Scholar] [CrossRef]
- Tunell, G.; Posnjak, Ε.; Ksanda, C. Geometrical and optical properties, and crystal structure of tenorite. Z. Krist.—Cryst. Mater. 1935, 90, 120–142. [Google Scholar] [CrossRef]
- Bin Mobarak, M.; Hossain, M.S.; Chowdhury, F.; Ahmed, S. Synthesis and characterization of CuO nanoparticles utilizing waste fish scale and exploitation of XRD peak profile analysis for approximating the structural parameters. Arab. J. Chem. 2022, 15, 104117. [Google Scholar] [CrossRef]
- Liu, X.; Chang, C.-F.; Rata, A.D.; Komarek, A.C.; Tjeng, L.H. Fe3O4 thin films: Controlling and manipulating an elusive quantum material. Npj Quantum Mater. 2016, 1, 16027. [Google Scholar] [CrossRef]
- Bhargava, R.; Khan, S.; Ahmad, N.; Ansari, M.M.N. Investigation of structural, optical and electrical properties of Co3O4 nanoparticles. AIP Conf. Proc. 2018, 1953, 030034. [Google Scholar]
- Zorkipli, N.N.M.; Kaus, N.H.M.; Mohamad, A.A. Synthesis of NiO Nanoparticles through Sol-gel Method. Procedia Chem. 2016, 19, 626–631. [Google Scholar] [CrossRef]
- Wang, Y.K.; Guo, G.Y.; Jeng, H.-T. An ab initio study of the magnetocrystalline anisotropy and magnetoelastic coupling of half-metallic CrO2. J. Magn. Magn. Mater. 2004, 282, 139–142. [Google Scholar] [CrossRef]
- Akrami, S.; Murakami, Y.; Watanabe, M.; Ishihara, T.; Arita, M.; Fuji, M.; Edalati, K. Defective high-entropy oxide photocatalyst with high activity for CO2 conversion. Appl. Catal. B 2022, 303, 120896. [Google Scholar] [CrossRef]
- Yan, Z.; Li, D.; Zhang, X.; Men, Q.; Fan, B.; Guan, L.; Guo, X.; Zhang, R.; Zhao, B. Dual-phase high-entropy (FeCoNiZn)xV2Oy oxides with promising microwave absorption properties. Ceram. Int. 2022, 48, 36871–36879. [Google Scholar] [CrossRef]
- Edalati, P.; Wang, Q.; Razavi-Khosroshahi, H.; Fuji, M.; Ishihara, T.; Edalati, K. Photocatalytic hydrogen evolution on a high-entropy oxide. J. Mater. Chem. 2020, 8, 3814–3821. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. FullProf. CEA/Saclay Fr. 2001, 1045, 132–146. [Google Scholar]
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Jain, S.; Shah, J.; Negi, N.S.; Sharma, C.; Kotnala, R.K. Significance of interface barrier at electrode of hematite hydroelectric cell for generating ecopower by water splitting. Int. J. Energy Res. 2019, 43, 4743–4755. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zeng, Y.; Tong, Y.; Lu, X. Oxygen Defects in Promoting the Electrochemical Performance of Metal Oxides for Supercapacitors: Recent Advances and Challenges. Small Methods 2020, 4, 1900823. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, N.; Wu, B.; Yang, Z.; Sun, S.; Wang, Y. A new spinel high-entropy oxide (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 with fast reaction kinetics and excellent stability as an anode material for lithium ion batteries. RSC Adv. 2020, 10, 9736–9744. [Google Scholar] [CrossRef]
- Bui, N.T.; Kang, H.; Teat, S.J.; Su, G.M.; Pao, C.-W.; Liu, Y.-S.; Zaia, E.W.; Guo, J.; Chen, J.-L.; Meihaus, K.R.; et al. A nature-inspired hydrogen-bonded supramolecular complex for selective copper ion removal from water. Nat. Commun. 2020, 11, 3947. [Google Scholar] [CrossRef] [PubMed]
- Karikalan, N.; Karthik, R.; Chen, S.-M.; Karuppiah, C.; Elangovan, A. Sonochemical Synthesis of Sulfur Doped Reduced Graphene Oxide Supported CuS Nanoparticles for the Non-Enzymatic Glucose Sensor Applications. Sci. Rep. 2017, 7, 2494. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, S.; Duan, C.; Mao, J.; Dong, Y.; Dong, K.; Wang, Z.; Luo, S.; Liu, Y.; Qi, X. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J. Alloys Compd. 2020, 844, 156158. [Google Scholar] [CrossRef]
- Alex, C.; Sarma, S.C.; Peter, S.C.; John, N.S. Competing Effect of Co3+ Reducibility and Oxygen-Deficient Defects Toward High Oxygen Evolution Activity in Co3O4 Systems in Alkaline Medium. ACS Appl. Energy Mater. 2020, 3, 5439–5447. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Patra, J.; Chang, J.-K.; Ting, J.-M. High entropy spinel oxide nanoparticles for superior lithiation-delithiation performance. J. Mater. Chem. 2020, 8, 18963–18973. [Google Scholar] [CrossRef]
- Rajan, A.; Sharma, M.; Sahu, N.K. Assessing magnetic and inductive thermal properties of various surfactants functionalised Fe3O4 nanoparticles for hyperthermia. Sci. Rep. 2020, 10, 15045. [Google Scholar] [CrossRef]
- Xiao, B.; Wu, G.; Wang, T.; Wei, Z.; Sui, Y.; Shen, B.; Qi, J.; Wei, F.; Zheng, J. High-entropy oxides as advanced anode materials for long-life lithium-ion Batteries. Nano Energy. 2022, 95, 106962. [Google Scholar] [CrossRef]
- Cabanel, R.; Barral, G.; Diard, J.P.; Le Gorrec, B.; Montella, C. Determination of the diffusion coefficient of an inserted species by impedance spectroscopy: Application to the H/HxNb2O5 system. J. Appl. Electrochem. 1993, 23, 93–97. [Google Scholar] [CrossRef]
- Ramya, R.; Sivasubramanian, R.; Sangaranarayanan, M.V. Conducting polymers-based electrochemical supercapacitors-Progress and prospects. Electrochim. Acta 2013, 101, 109–129. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, S.; Kumar, N.; Krishnaiah, M.; Jin, S.H. Self-discharge mitigated supercapacitors via hybrid CuO-nickel sulfide heterostructure for energy efficient, wireless data storage application. J. Mater. Sci. Technol. 2023, 147, 77–90. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Tsai, C.-C.; Patra, J.; Clemens, O.; Chang, J.-K.; Ting, J.-M. Co-free high entropy spinel oxide anode with controlled morphology and crystallinity for outstanding charge/discharge performance in Lithium-ion batteries. Chem. Eng. J. 2022, 430, 132658. [Google Scholar] [CrossRef]
- Patra, J.; Nguyen, T.X.; Tsai, C.-C.; Clemens, O.; Li, J.; Pal, P.; Chan, W.K.; Lee, C.-H.; Chen, H.-Y.T.; Ting, J.-M.; et al. Effects of Elemental Modulation on Phase Purity and Electrochemical Properties of Co-free High-Entropy Spinel Oxide Anodes for Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2110992. [Google Scholar] [CrossRef]
- Mahmood, A.; Tezcan, F.; Kardaş, G. Photoelectrochemical characteristics of CuO films with different electrodeposition time. Int. J. Hydrogen Energy 2017, 42, 23268–23275. [Google Scholar] [CrossRef]
- Yadav, P.; Manivannan, S.; Kim, H.-S.; Pandey, K.; Kim, K.; Kim, J. Electrochemical Properties of Highly Sensitive and Selective CuO Nanostructures Based Neurotransmitter Dopamine Sensor. Electroanalysis 2017, 29, 2106–2113. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Uke, S.J.; Mardikar, S.P.; Bambole, D.R.; Kumar, Y.; Chaudhari, G.N. Sol-gel citrate synthesized Zn doped MgFe2O4 nanocrystals: A promising supercapacitor electrode material. Mater. Sci. 2020, 3, 446–455. [Google Scholar] [CrossRef]
- Ansari, M.S.; Kim, H. Enhanced electrocatalytic oxygen evolution reaction kinetics using dual-phase engineering of self-supported hierarchical NiCoV(OH)x nanowire arrays. Fuel 2021, 304, 121309. [Google Scholar] [CrossRef]
- Allagui, A.; Freeborn, T.J.; Elwakil, A.S.; Maundy, B.J. Reevaluation of Performance of Electric Double-layer Capacitors from Constant-current Charge/Discharge and Cyclic Voltammetry. Sci. Rep. 2016, 6, 38568. [Google Scholar] [CrossRef]
- Adewinbi, S.A.; Taleatu, B.A.; Busari, R.A.; Maphiri, V.M.; Oyedotun, K.O.; Manyala, N. Synthesis and electrochemical characterization of pseudocapacitive α-MoO3 thin film as transparent electrode material in optoelectronic and energy storage devices. Mater. Chem. Phys. 2021, 264, 124468. [Google Scholar] [CrossRef]
- Huang, J.; Sullivan, N.P.; Zakutayev, A.; O’Hayre, R. How reliable is distribution of relaxation times (DRT) analysis? A dual regression-classification perspective on DRT estimation, interpretation, and accuracy. Electrochim. Acta 2023, 443, 141879. [Google Scholar] [CrossRef]
- Ciucci, F.; Chen, C. Analysis of Electrochemical Impedance Spectroscopy Data Using the Distribution of Relaxation Times: A Bayesian and Hierarchical Bayesian Approach. Electrochim. Acta 2015, 167, 439–454. [Google Scholar] [CrossRef]
- Effat, M.B.; Ciucci, F. Bayesian and Hierarchical Bayesian Based Regularization for Deconvolving the Distribution of Relaxation Times from Electrochemical Impedance Spectroscopy Data. Electrochim. Acta 2017, 247, 1117–1129. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, D.; Zhuang, H.; Le, J.; Kuang, Y. High-performance bulk heterojunction-based photocathode with facile architecture for photoelectrochemical water splitting. Chin. Chem. Lett. 2023, 34, 107480. [Google Scholar] [CrossRef]
- Schutjajew, K.; Tichter, T.; Schneider, J.; Antonietti, M.; Roth, C.; Oschatz, M. Insights into the sodiation mechanism of hard carbon-like materials from electrochemical impedance spectroscopy. Phys. Chem. Chem. Phys. 2021, 23, 11488–11500. [Google Scholar] [CrossRef]
- Attias, R.; Vijaya Sankar, K.; Dhaka, K.; Moschkowitsch, W.; Elbaz, L.; Caspary Toroker, M.; Tsur, Y. Optimization of Ni-Co-Fe-Based Catalysts for Oxygen Evolution Reaction by Surface and Relaxation Phenomena Analysis. ChemSusChem 2021, 14, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.; Du, X.; Zhang, X. Promoted electrocatalytic water splitting by regulating the concentration of oxygen vacancies. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar] [CrossRef]
- Song, Z.; Wang, K.; Sun, Q.; Zhang, L.; Li, J.; Li, D.; Sze, P.W.; Liang, Y.; Sun, X.; Fu, X.Z. High-Performance Ammonium Cobalt Phosphate Nanosheet Electrocatalyst for Alkaline Saline Water Oxidation. Adv. Sci. 2021, 8, 2100498. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.; Sun, Z.; Ren, Y.; Zhang, X.; Du, P. Self-supported copper oxide electrocatalyst for water oxidation at low overpotential and confirmation of its robustness by Cu K-edge X-ray absorption spectroscopy. J. Phys. Chem. 2016, 120, 831–840. [Google Scholar] [CrossRef]
- Hong, Y.R.; Kim, K.M.; Ryu, J.H.; Mhin, S.; Kim, J.; Ali, G.; Chung, K.Y.; Kang, S.; Han, H. Dual-phase engineering of nickel boride-hydroxide nanoparticles toward high-performance water oxidation electrocatalysts. Adv. Funct. Mater. 2020, 30, 2004330. [Google Scholar] [CrossRef]
- Ran, J.; Wu, J.-F.; Hu, Y.; Shakouri, M.; Xia, B.; Gao, D. Atomic-level coupled spinel@ perovskite dual-phase oxides toward enhanced performance in Zn-air batteries. J. Mater. Chem. 2022, 10, 1506–1513. [Google Scholar] [CrossRef]
- Einert, M.; Waheed, A.; Lauterbach, S.; Mellin, M.; Rohnke, M.; Wagner, L.Q.; Gallenberger, J.; Tian, C.; Smarsly, B.M.; Jaegermann, W. Sol-Gel-Derived Ordered Mesoporous High Entropy Spinel Ferrites and Assessment of Their Photoelectrochemical and Electrocatalytic Water Splitting Performance. Small 2023, 19, 2205412. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.K.; Kulkarni, P.; Maiti, U.N.; Balakrishna, R.G.; Mukherjee, P.; Jung, H.Y.; Samanta, K.; Ghosh, D. Realizing Favourable Oxygen Electrocatalytic Activity with Compositionally Complex Metal Molybdates. Sustain. Energy Fuels 2023. [Google Scholar] [CrossRef]


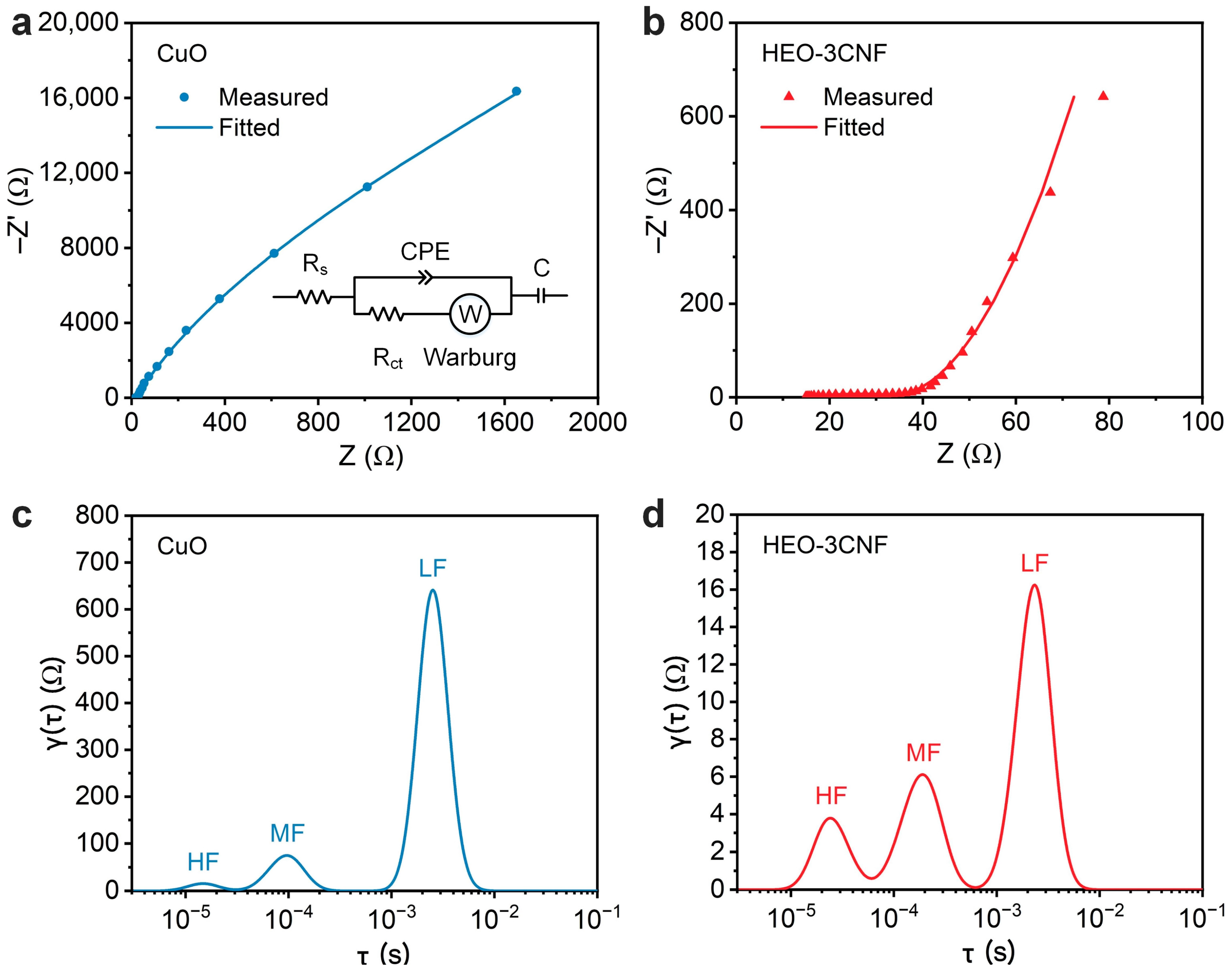

| Electrode | Rs (Ω) | Rct (Ω) | Q (F × s(α − 1)) | α | CPE (F) | W (Ω/s1/2) | C (F) |
|---|---|---|---|---|---|---|---|
| CuO | 16.1 | 2.7 × 104 | 1.3 × 10−4 | 0.92 | 1.4 × 10−4 | 1169 | 6.4 × 10−5 |
| HEO-3CNF | 15.7 | 18.7 | 1.2 × 10−4 | 0.80 | 2.6 × 10−5 | 30.4 | 2.6 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.-J.; Yin, T.-H.; Lin, Y.-W.; Chang, C.-W.; Yu, H.-C.; Lim, Y.; Lee, H.; Choi, C.; Tsai, M.-K.; Choi, Y. A Cost-Effective, Nanoporous, High-Entropy Oxide Electrode for Electrocatalytic Water Splitting. Coatings 2023, 13, 1461. https://doi.org/10.3390/coatings13081461
Liu B-J, Yin T-H, Lin Y-W, Chang C-W, Yu H-C, Lim Y, Lee H, Choi C, Tsai M-K, Choi Y. A Cost-Effective, Nanoporous, High-Entropy Oxide Electrode for Electrocatalytic Water Splitting. Coatings. 2023; 13(8):1461. https://doi.org/10.3390/coatings13081461
Chicago/Turabian StyleLiu, Bu-Jine, Tai-Hsin Yin, Yu-Wei Lin, Chun-Wei Chang, Hsin-Chieh Yu, Yongtaek Lim, Hyesung Lee, Changsik Choi, Ming-Kang Tsai, and YongMan Choi. 2023. "A Cost-Effective, Nanoporous, High-Entropy Oxide Electrode for Electrocatalytic Water Splitting" Coatings 13, no. 8: 1461. https://doi.org/10.3390/coatings13081461






