TiO2-Based Photocatalytic Coatings on Glass Substrates for Environmental Applications
Abstract
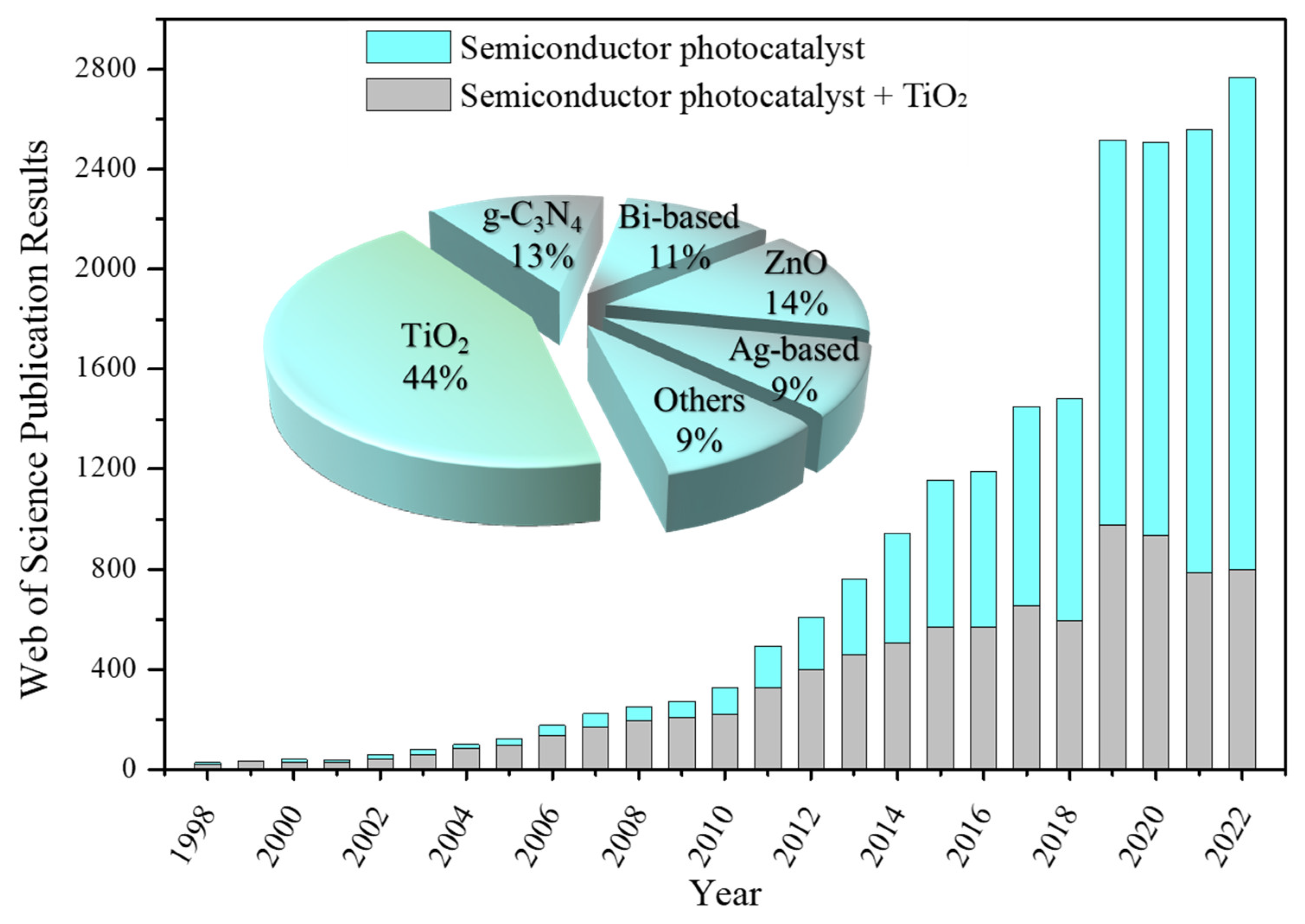
:1. Introduction
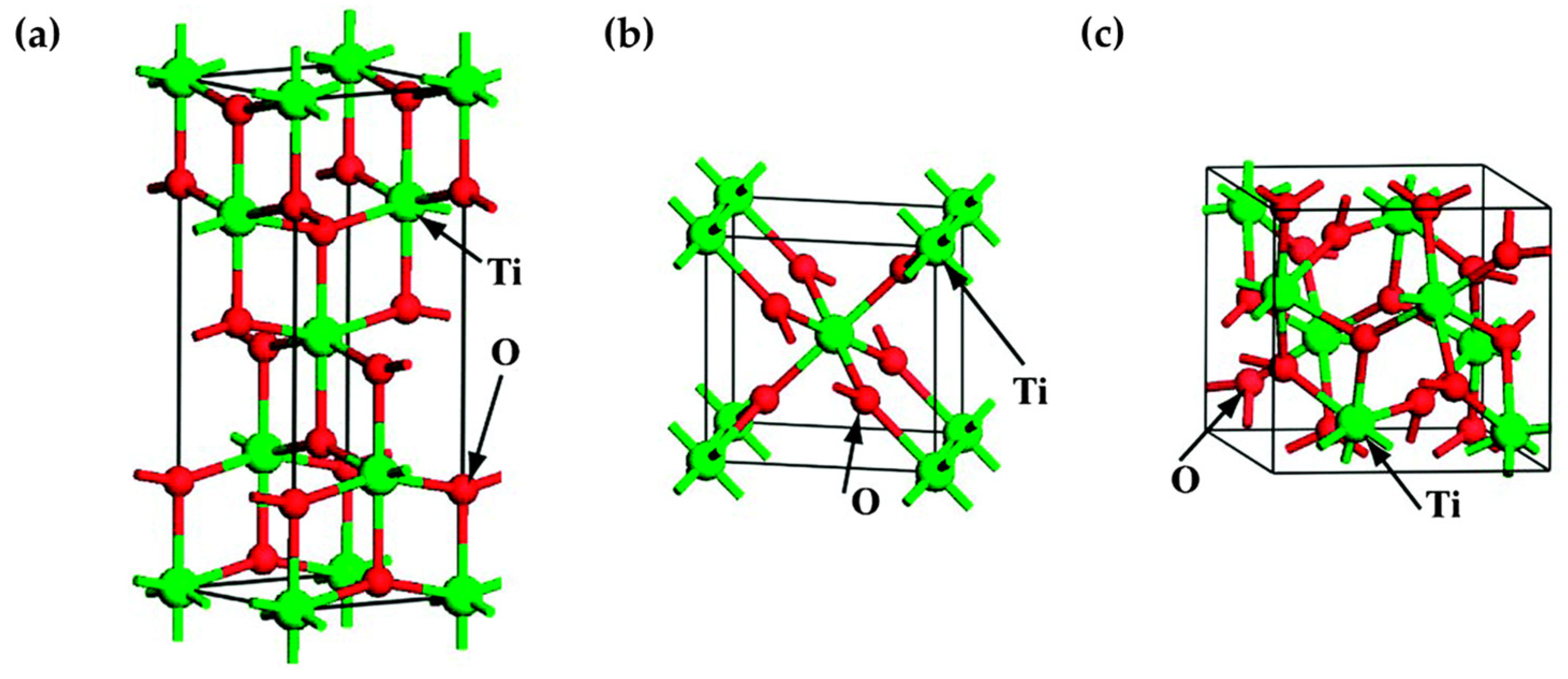
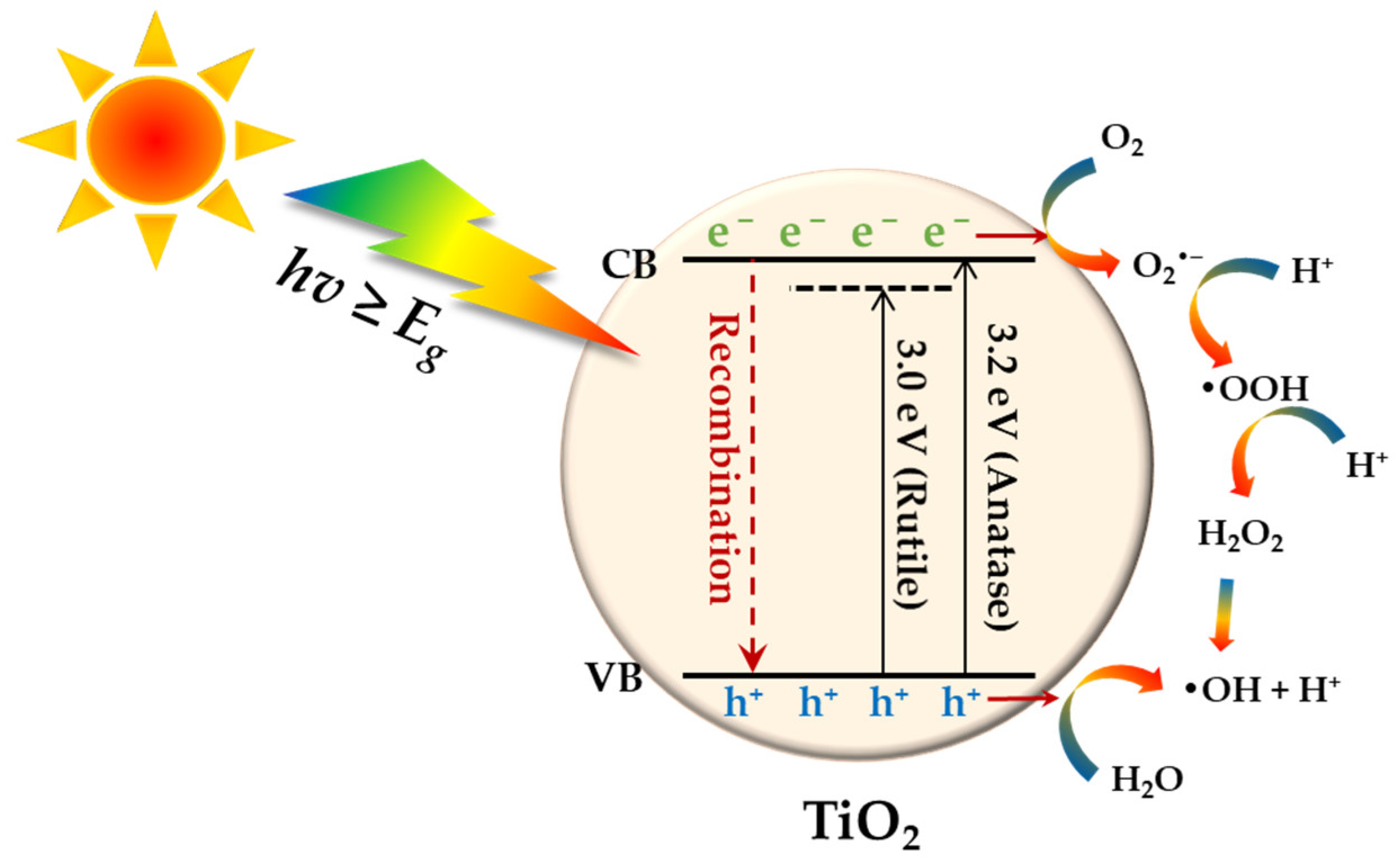
2. The Basic Principle of TiO2 Photocatalysis
3. Preparations and Applications of TiO2-Based Photocatalytic Coatings
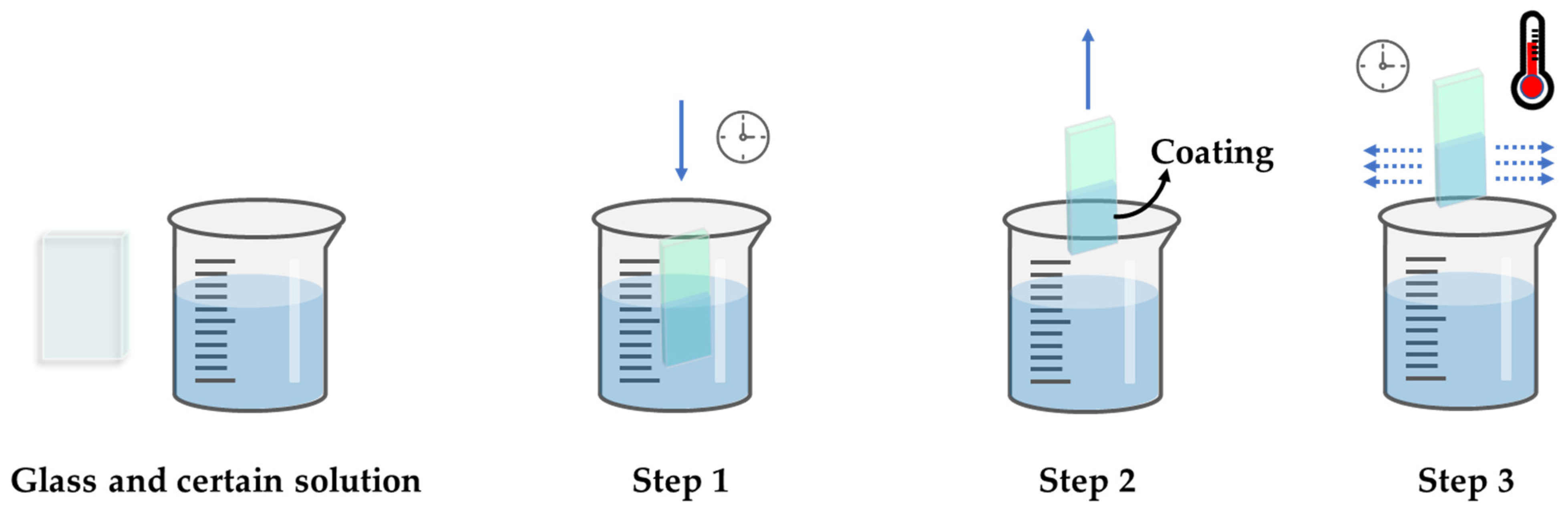
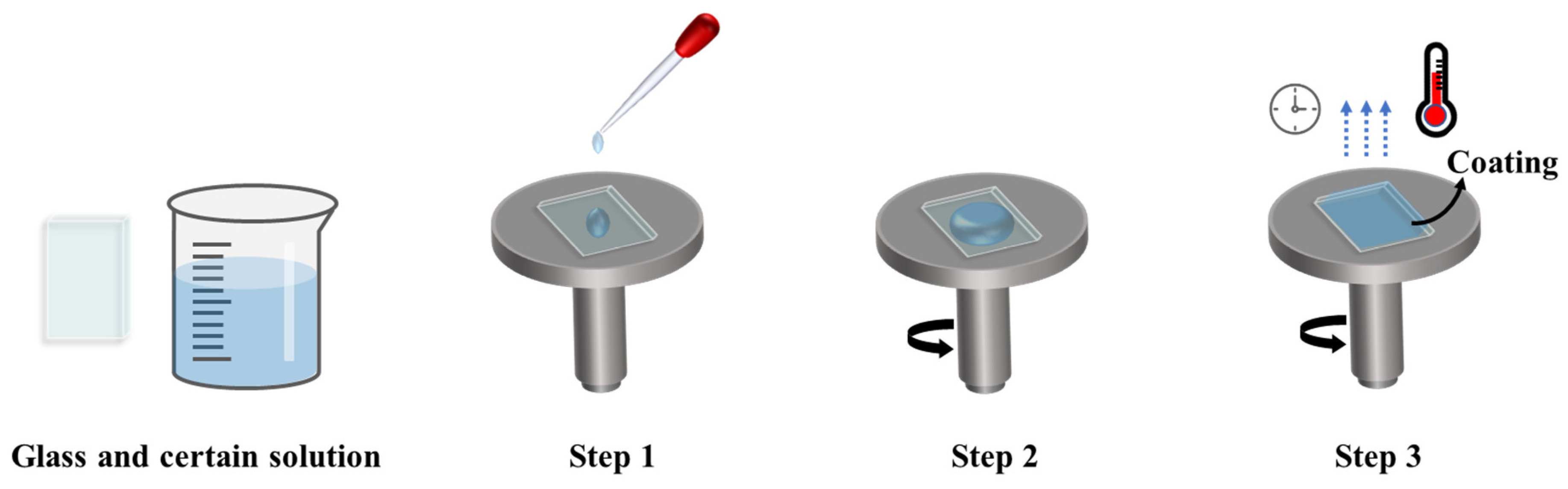
3.1. Wet Chemical Deposition
3.1.1. Dip Coating
3.1.2. Spin Coating
3.1.3. Spray Coating
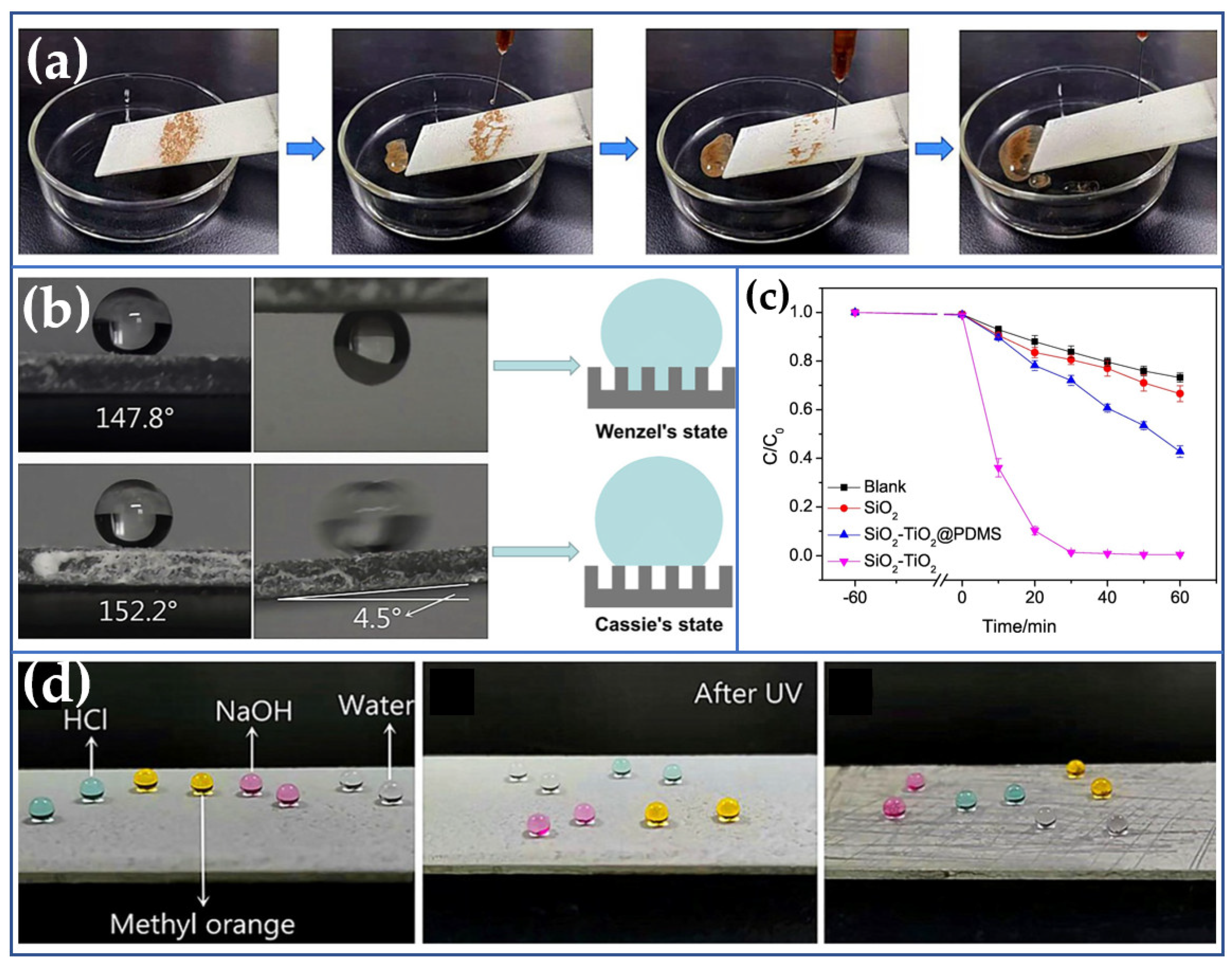
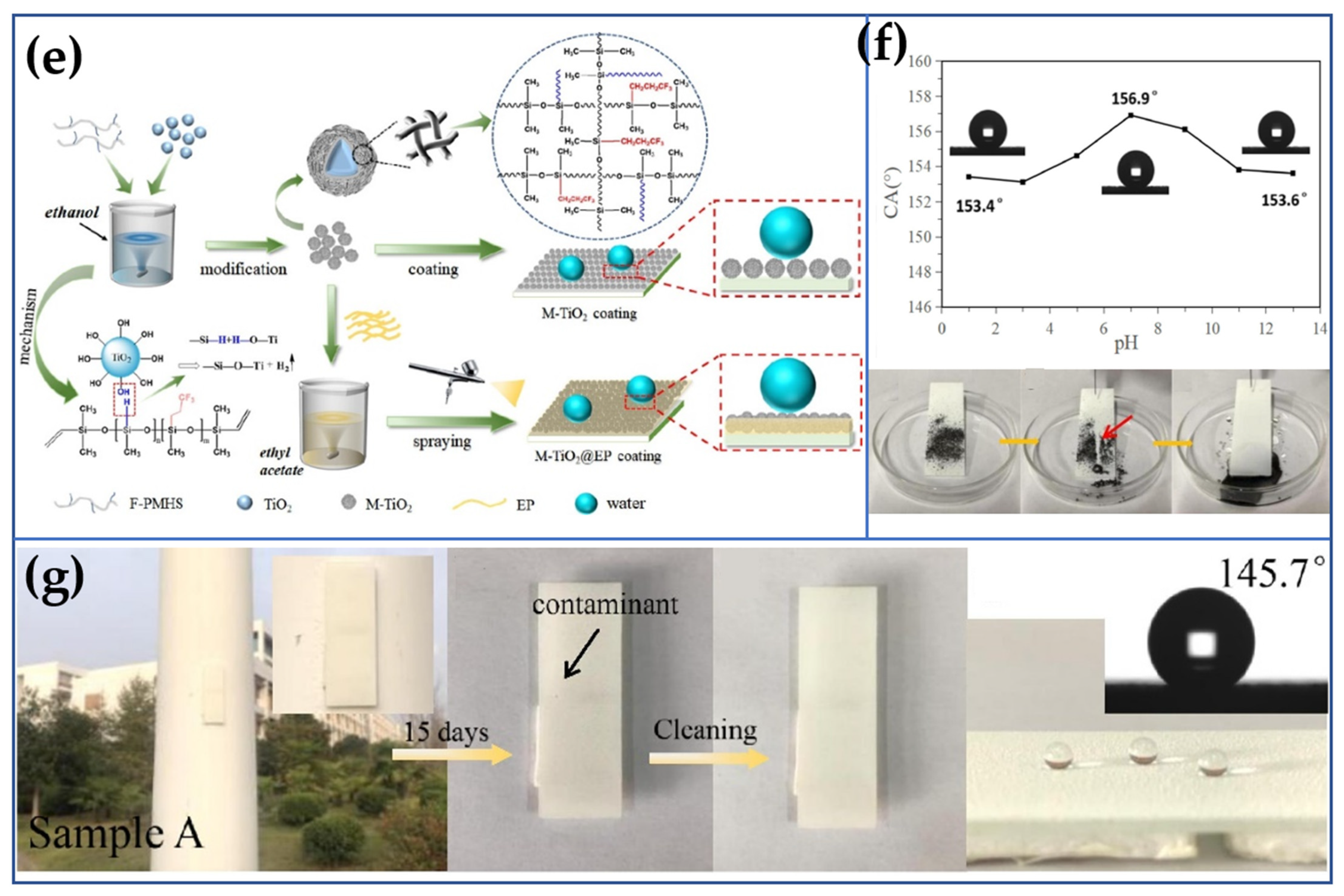
3.2. Electrodeposition
3.3. Physical Vapor Deposition (PVD)
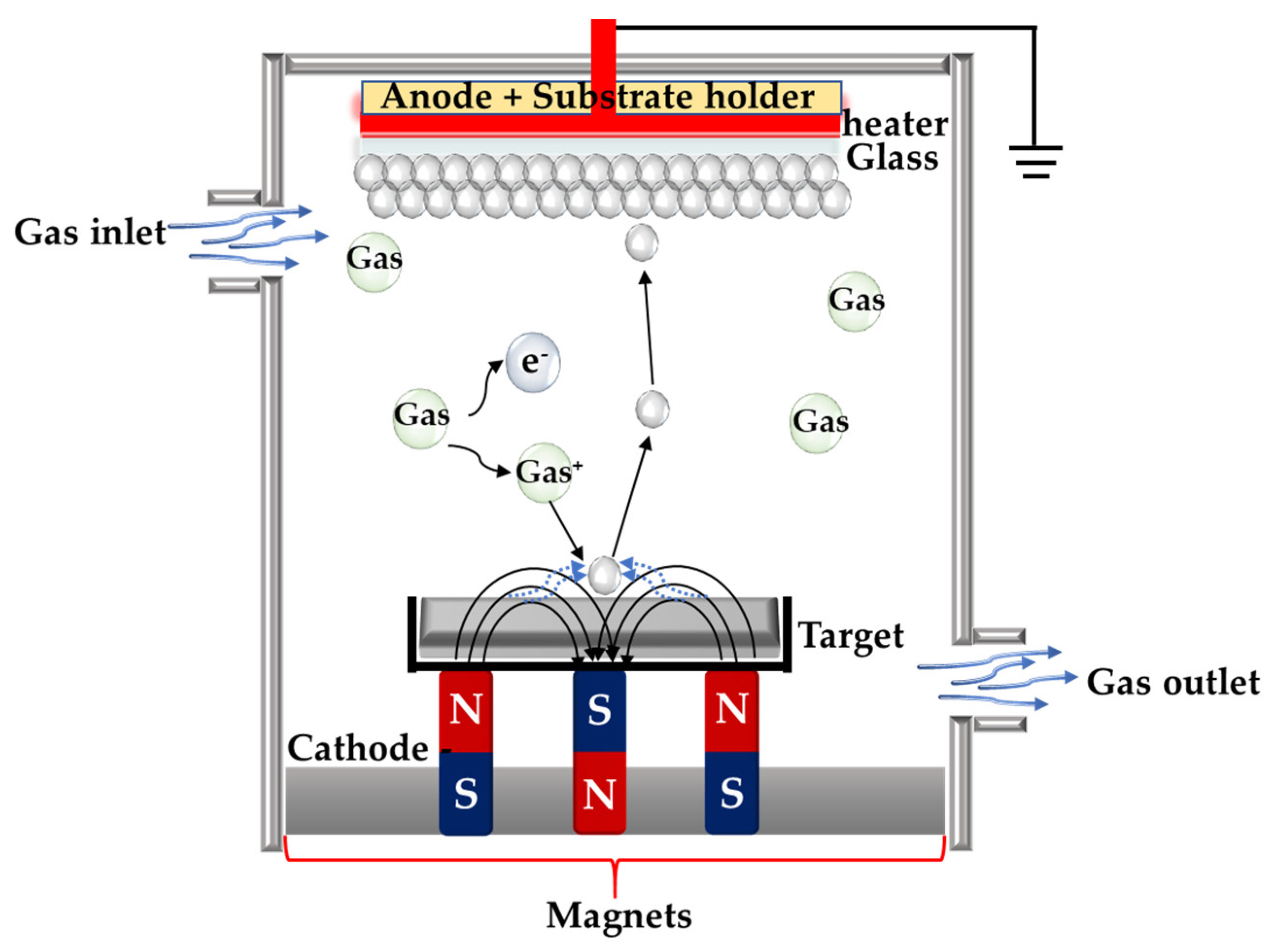
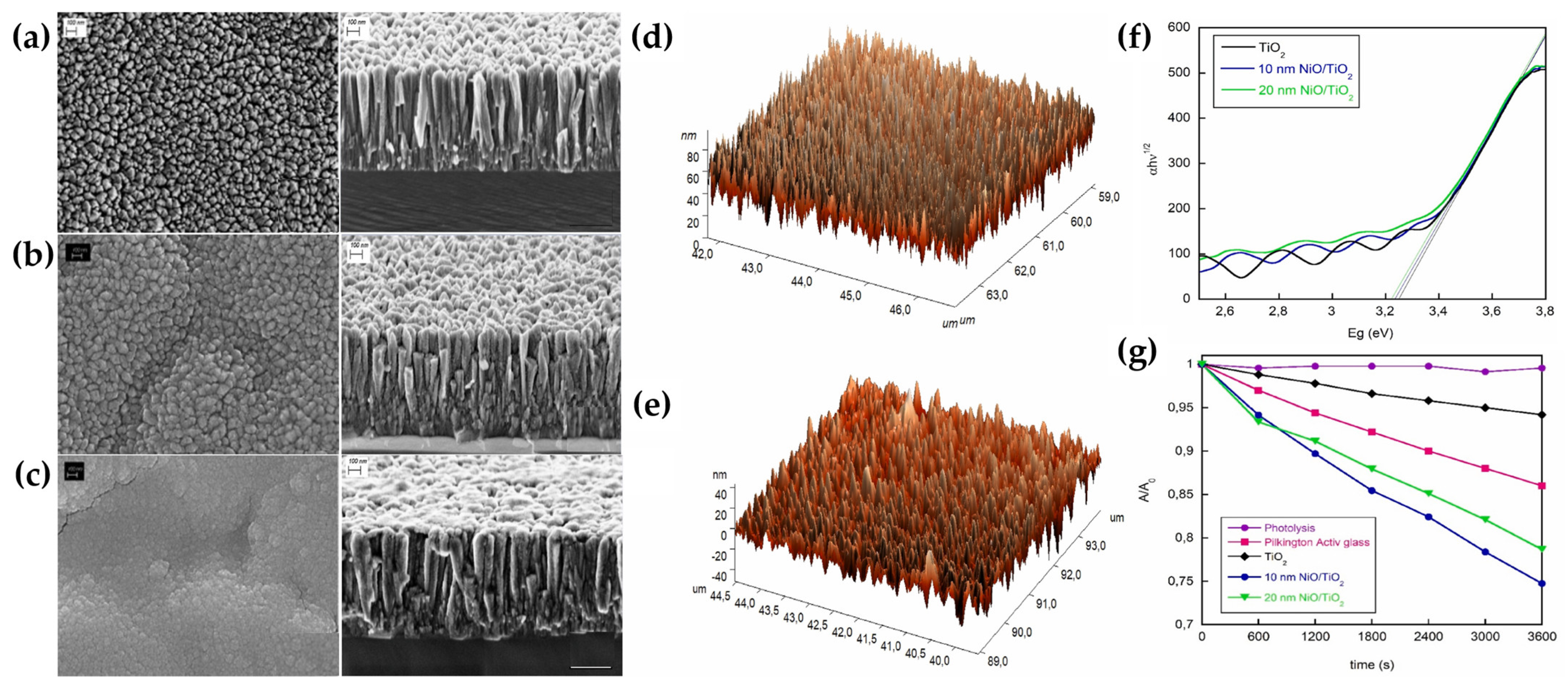
3.3.1. Magnetron Sputtering
3.3.2. Pulsed Laser Deposition
3.4. Chemical Vapor Deposition (CVD)

3.5. Other Techniques
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- He, Z.; Zhang, J.; Li, X.; Guan, S.; Dai, M.; Wang, S. 1D/2D heterostructured photocatalysts: From design and unique properties to their environmental applications. Small 2020, 16, 2005051. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, M.T.H.; Jones, E.R.; Flörke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global water scarcity including surface water quality and expansions of clean water technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Dong, F. Two dimensional photocatalytic materials. Acta Phys.-Chim. Sin. 2021, 37, 2101002. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Liu, S.; Sun, Y.; Xu, Y.-J. Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, M.; Zhang, S.; Dai, M.; Zhang, P.; Wang, S.; He, Z. Recent rrogress on carbon-nanotube-based materials for photocatalytic applications: A review. Sol. RRL 2022, 6, 2200243. [Google Scholar] [CrossRef]
- Xiao, L.H.; Li, X.; Zhang, J.; He, Z.L. MgB4 MXene-like nanosheets for photocatalytic hydrogen evolution. ACS Appl. Nano Mater. 2021, 4, 12779–12787. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Zhang, Y.; Qiu, L.; Xing, Y. A 0D/2D Bi4V2O11/g-C3N4 S-scheme heterojunction with rapid interfacial charges migration for photocatalytic antibiotic degradation. Acta Phys.-Chim. Sin. 2022, 38, 2112027. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Zhang, P.; Li, X.; Wang, S. ZnWO4-ZnIn2S4 S-scheme heterojunction for enhanced photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 122, 231–242. [Google Scholar] [CrossRef]
- Sun, T.; Li, C.; Bao, Y.; Fan, J.; Liu, E. S-scheme MnCo2S4/g-C3N4 heterojunction photocatalyst for H2 production. Acta Phys.-Chim. Sin. 2023, 39, 2212009. [Google Scholar] [CrossRef]
- Dai, M.; Yu, H.; Chen, W.; Qu, K.-A.; Zhai, D.; Liu, C.; Zhao, S.; Wang, S.; He, Z. Boosting photocatalytic activity of CdLa2S4/ZnIn2S4 S-scheme heterojunctions with spatial separation of photoexcited carries. Chem. Eng. J. 2023, 470, 144240. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Chen, S.; Wang, H.; Huo, P. Fabricating Ag/CN/ZnIn2S4 S-scheme heterojunctions with plasmonic effect for enhanced light-driven photocatalytic CO2 reduction. Acta Phys.-Chim. Sin. 2023, 39, 2211051. [Google Scholar] [CrossRef]
- Cao, W.R.; He, Z.L.; Dai, M.; Wang, G.Z.; Huang, G.H.; Wang, S.G. Electronic structure modulation of bimetallic sulfides for efficient sacrificial-agent-free photocatalytic H2 evolution. ACS Appl. Energy Mater. 2023, 6, 4715–4723. [Google Scholar] [CrossRef]
- Li, X.; Chang, J.; Zhang, S.; Xiao, L.; Wu, X.; He, Z. Microcystis@TiO2 nanoparticles for photocatalytic reduction reactions: Nitrogen fixation and hydrogen evolution. Catalysts 2021, 11, 1443. [Google Scholar] [CrossRef]
- Yu, H.; Xu, S.; Zhang, S.; Wang, S.; He, Z. In-Situ construction of core–shell structured TiB2-TiO2@g-C3N4 for efficient photocatalytic degradation. Appl. Surf. Sci. 2022, 579, 152201. [Google Scholar] [CrossRef]
- Zhang, S.J.; He, Z.L.; Xu, S.S.; Li, X.; Zhang, J.; Zhan, X.P.; Dai, M.; Wang, S.G. In Situ liquid-phase growth strategies of g-C3N4 solar-driven heterogeneous catalysts for environmental applications. Sol. RRL 2021, 5, 2100233. [Google Scholar] [CrossRef]
- Toloman, D.; Stefan, M.; Macavei, S.; Barbu-Tudoran, L.; Popa, A. Photocatalytic self-cleaning PVDF membrane blended with MWCNT-ZnO nanocomposites for RhB removal. Coatings 2023, 13, 594. [Google Scholar] [CrossRef]
- Flores-Carrasco, G.; Rodriguez-Pena, M.; Urbieta, A.; Fernandez, P.; Rabanal, M.E. ZnO nanoparticles with controllable Ce content for efficient photocatalytic degradation of MB synthesized by the polyol method. Catalysts 2021, 11, 71. [Google Scholar] [CrossRef]
- Lv, Y.-R.; Liu, C.-J.; He, R.-K.; Li, X.; Xu, Y.-H. BiVO4/TiO2 heterojunction with enhanced photocatalytic activities and photoelectochemistry performances under visible light illumination. Mater. Res. Bull. 2019, 117, 35–40. [Google Scholar] [CrossRef]
- Liu, X.; Huang, W.-Y.; Zhou, Q.; Chen, X.-R.; Yang, K.; Li, D.; Dionysiou, D.D. Ag-decorated 3D flower-like Bi2MoO6/rGO with boosted photocatalytic performance for removal of organic pollutants. Rare Met. 2020, 40, 1086–1098. [Google Scholar] [CrossRef]
- Chen, W.; Dai, M.; Xiang, L.; Zhao, S.; Wang, S.; He, Z. Assembling S-scheme heterojunction between basic bismuth nitrate and bismuth tungstate with promoting charges’ separation for accelerated photocatalytic sulfamethazine degradation. J. Mater. Sci. Technol. 2023, in press. [Google Scholar] [CrossRef]
- Yu, C.L.; Wei, L.F.; Chen, J.C.; Zhou, W.Q.; Fan, Q.Z.; Yu, J. Novel AgCl/Ag2CO3 heterostructured photocatalysts with enhanced photocatalytic performance. Rare Met. 2016, 35, 475–480. [Google Scholar] [CrossRef]
- Ping, M.M.; Qiu, S.J.; Wei, G.J.; Liu, J.X.; Wang, Z.J.; Wang, S.T.; An, C.H. Monolith free-standing plasmonic PAN/Ag/AgX (X = Br, I) nanofiber mat as easily recoverable visible-light-driven photocatalyst. Rare Met. 2019, 38, 361–368. [Google Scholar] [CrossRef]
- He, Z.L.; Que, W.X.; He, Y.C. Enhanced photocatalytic performance of sensitized mesoporous TiO2 nanoparticles by carbon mesostructures. RSC Adv. 2014, 4, 3332–3339. [Google Scholar] [CrossRef]
- He, Z.; Kim, C.; Jeon, T.H.; Choi, W. Hydrogenated heterojunction of boron nitride and titania enables the photocatalytic generation of H2 in the absence of noble metal catalysts. Appl. Catal. B Environ. 2018, 237, 772–782. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, M.; Guo, J.; Wang, G.; Wang, S.; He, Z. Stable Ti3+ in B-TiO2/BN based hybrids for efficient photocatalytic reduction. Chem. Eng. J. Adv. 2022, 11, 100333. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, S.; Chen, J.; Sun, X.; Zhao, S.; Chang, J.; He, Z. Ti3C2@g-C3N4/TiO2 ternary heterogeneous photocatalyst for promoted photocatalytic degradation activities. Coatings 2023, 13, 655. [Google Scholar] [CrossRef]
- Ji, H.; Ni, J.; Zhao, D.; Liu, W. Application of titanate nanotubes for photocatalytic decontamination in water: Challenges and prospects. ACS EST Eng. 2022, 2, 1015–1038. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, S.; Cai, J.; Ma, J.; Zhao, G. Perspective on photoelectrocatalytic removal of refractory organic pollutants in water systems. ACS EST Eng. 2022, 2, 1001–1014. [Google Scholar] [CrossRef]
- Yu, H.; Dai, M.; Zhang, J.; Chen, W.; Jin, Q.; Wang, S.; He, Z. Interface engineering in 2D/2D heterogeneous photocatalysts. Small 2023, 19, 2205767. [Google Scholar] [CrossRef]
- He, Z.; Que, W.; Chen, J.; Yin, X.; He, Y.; Ren, J. Photocatalytic degradation of methyl orange over nitrogen-fluorine codoped TiO2 nanobelts prepared by solvothermal synthesis. ACS Appl. Mater. Interfaces 2012, 4, 6816–6826. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Hu, H.; Zeng, G.; Li, C. Recovering hydrogen energy from photocatalytic treatment of pharmaceutical-contaminated water using Co3O4 modified {001}/{101}-TiO2 nanosheets. ACS EST Eng. 2021, 1, 603–611. [Google Scholar] [CrossRef]
- Amoros-Perez, A.; Lillo-Rodenas, M.A.; Roman-Martinez, M.D.; Garcia-Munoz, P.; Keller, N. TiO2 and TiO2-carbon hybrid photocatalysts for Diuron removal from water. Catalysts 2021, 11, 457. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.Q.; Liu, Y.F.; Zhao, Q.H.; Li, X.; Zhou, Q.Y.; Chen, Y.F.; Wang, S.F. Construction of bronze TiO2/Ti3C2 MXene/Ag3PO4 ternary composite photocatalyst toward high photocatalytic performance. Catalysts 2022, 12, 599. [Google Scholar] [CrossRef]
- Wang, L.B.; Fei, X.G.; Zhang, L.Y.; Yu, J.G.; Cheng, B.; Ma, Y.H. Solar fuel generation over nature-inspired recyclable TiO2/g-C3N4 S-scheme hierarchical thin-film photocatalyst. J. Mater. Sci. Technol. 2022, 112, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, Y.; Yu, H.; Yu, K.; Yu, H.B. Interfacial defective Ti3+ on Ti/TiO2 as visible-light responsive sites with promoted charge transfer and photocatalytic performance. J. Mater. Sci. Technol. 2022, 106, 139–146. [Google Scholar] [CrossRef]
- Weon, S.; Suh, M.J.; Chu, C.; Huang, D.; Stavitski, E.; Kim, J.H. Site-selective loading of single-atom Pt on TiO2 for photocatalytic oxidation and reductive hydrodefluorination. ACS EST Eng. 2021, 1, 512–522. [Google Scholar] [CrossRef]
- Rosales, A.; Maury-Ramirez, A.; Mejia-De Gutierrez, R.; Guzman, C.; Esquivel, K. SiO2@TiO2 coating: Synthesis, physical characterization and photocatalytic evaluation. Coatings 2018, 8, 120. [Google Scholar] [CrossRef]
- Belle, U.; Pelizzari, F.; Lucotti, A.; Castiglioni, C.; Ormellese, M.; Pedeferri, M.; Diamanti, M.V. Immobilized nano-TiO2 photocatalysts for the degradation of three organic dyes in single and multi-dye solutions. Coatings 2020, 10, 919. [Google Scholar] [CrossRef]
- Qu, K.-A.; Chen, W.; Guo, J.; He, Z. A mini-review on preparation of functional composite fibers and their based devices. Coatings 2022, 12, 473. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Guo, J.; Zhou, G.H.; Xiang, L.; Wang, S.G.; He, Z.L. Novel TiO2/TPU composite fiber-based smart textiles for photocatalytic applications. Mater. Adv. 2022, 3, 1518–1526. [Google Scholar] [CrossRef]
- Rashid, M.M.; Forte Tavčer, P.; Tomšič, B. Influence of Titanium Dioxide Nanoparticles on Human Health and the Environment. Nanomaterials 2021, 11, 2354. [Google Scholar] [CrossRef] [PubMed]
- Fiordaliso, F.; Bigini, P.; Salmona, M.; Diomede, L. Toxicological impact of titanium dioxide nanoparticles and food-grade titanium dioxide (E171) on human and environmental health. Environ. Sci. Nano 2022, 9, 1199–1211. [Google Scholar] [CrossRef]
- Garlisi, C.; Trepci, E.; Li, X.; Al Sakkaf, R.; Al-Ali, K.; Nogueira, R.P.; Zheng, L.; Azar, E.; Palmisano, G. Multilayer thin film structures for multifunctional glass: Self-cleaning, antireflective and energy-saving properties. Appl. Energy 2020, 264, 114697. [Google Scholar] [CrossRef]
- Ratova, M.; Marcelino, R.B.P.; de Souza, P.P.; Amorim, C.C.; Kelly, P.J. Reactive magnetron sputter deposition of bismuth tungstate coatings for water treatment applications under natural sunlight. Catalysts 2017, 7, 283. [Google Scholar] [CrossRef]
- Liu, Y.; Choi, C.-H. Superhydrophobic sands for the preservation and purification of water. Coatings 2021, 11, 151. [Google Scholar] [CrossRef]
- Dufner, L.; Ott, F.; Otto, N.; Lembcke, T.; Kern, F. Immobilization of TiO2 photocatalysts for water treatment in geopolymer based coatings. Catalysts 2023, 13, 898. [Google Scholar] [CrossRef]
- Rtimi, S. Indoor light enhanced photocatalytic ultra-thin films on flexible non-heat resistant substrates reducing bacterial infection risks. Catalysts 2017, 7, 57. [Google Scholar] [CrossRef]
- Raditoiu, V.; Raditoiu, A.; Raduly, M.F.; Amariutei, V.; Gifu, I.C.; Anastasescu, M. Photocatalytic behavior of water-based styrene-acrylic coatings containing TiO2 sensitized with metal-phthalocyanine Tetracarboxylic acids. Coatings 2017, 7, 229. [Google Scholar] [CrossRef]
- Truppi, A.; Luna, M.; Petronella, F.; Falcicchio, A.; Giannini, C.; Comparelli, R.; Mosquera, M.J. Photocatalytic activity of TiO2/AuNRs-SiO2 nanocomposites applied to building materials. Coatings 2018, 8, 296. [Google Scholar] [CrossRef]
- Rosales, A.; Escalante, K.E. SiO2@TiO2 composite synthesis and its hydrophobic applications: A review. Catalysts 2020, 10, 171. [Google Scholar] [CrossRef]
- Panniello, A.; Curri, M.L.; Diso, D.; Licciulli, A.; Locaputo, V.; Agostiano, A.; Comparelli, R.; Mascolo, G. Nanocrystalline TiO2 based films onto fibers for photocatalytic degradation of organic dye in aqueous solution. Appl. Catal. B Environ. 2012, 121–122, 190–197. [Google Scholar] [CrossRef]
- Navabpour, P.; Cooke, K.; Sun, H. Photocatalytic properties of doped TiO2 coatings deposited using reactive magnetron sputtering. Coatings 2017, 7, 10. [Google Scholar] [CrossRef]
- Watte, J.; Van Zele, M.; De Buysser, K.; Van Driessche, I. Recent advances in low-temperature deposition methods of transparent, photocatalytic TiO2 coatings on polymers. Coatings 2018, 8, 131. [Google Scholar] [CrossRef]
- Ma, J.; Tian, Z.; Li, L.; Lu, Y.; Xu, X.; Hou, J. Loading nano-CuO on TiO2 nanomeshes towards efficient photodegradation of Methylene Blue. Catalysts 2022, 12, 383. [Google Scholar] [CrossRef]
- Dell’Edera, M.; Lo Porto, C.; De Pasquale, I.; Petronella, F.; Curri, M.L.; Agostiano, A.; Comparelli, R. Photocatalytic TiO2-based coatings for environmental applications. Catal. Today 2021, 380, 62–83. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Quispe, E.; Yang, H.; Sanfiorenzo, C.; Rogers, S.W.; Wang, K.; Yang, Y.; Hoffmann, M.R. Removal of antibiotic resistant bacteria and genes by UV-assisted electrochemical oxidation on degenerative TiO2 nanotube arrays. ACS EST Eng. 2021, 1, 612–622. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- He, Z.; Que, W.; Chen, J.; He, Y.; Wang, G. Surface chemical analysis on the carbon-doped mesoporous TiO2 photocatalysts after post-thermal treatment: XPS and FTIR characterization. J. Phys. Chem. Solids 2013, 74, 924–928. [Google Scholar] [CrossRef]
- Feng, X.; Guo, H.; Patel, K.; Zhou, H.; Lou, X. High performance, recoverable Fe3O4-ZnO nanoparticles for enhanced photocatalytic degradation of phenol. Chem. Eng. J. 2014, 244, 327–334. [Google Scholar] [CrossRef]
- Kim, B.C.; Jeong, E.; Kim, E.; Hong, S.W. Bio-organic–inorganic hybrid photocatalyst, TiO2 and glucose oxidase composite for enhancing antibacterial performance in aqueous environments. Appl. Catal. B Environ. 2019, 242, 194–201. [Google Scholar] [CrossRef]
- Velázquez-Palenzuela, A.; Ulusoy, B.; Dam-Johansen, K.; Christensen, J.M. Photo-oxidation of nitrite anions in aqueous solution for the benchmarking of nano-TiO2 photocatalytic coatings. Prog. Org. Coat. 2023, 175, 107380. [Google Scholar] [CrossRef]
- Khatibnezhad, H.; Ambriz-Vargas, F.; Ben Ettouil, F.; Moreau, C. Role of phase content on the photocatalytic performance of TiO2 coatings deposited by suspension plasma spray. J. Eur. Ceram. Soc. 2022, 42, 2905–2920. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Yu, H.; Jiang, X.; Sun, D. A heterogeneous TiO2/SrTiO3 coating on titanium alloy with excellent photocatalytic antibacterial, osteogenesis and tribocorrosion properties. Surf. Coat. Technol. 2022, 431, 128008. [Google Scholar] [CrossRef]
- Sansotera, M.; Kheyli, S.G.M.; Baggioli, A.; Bianchi, C.L.; Pedeferri, M.P.; Diamanti, M.V.; Navarrini, W. Absorption and photocatalytic degradation of VOCs by perfluorinated ionomeric coating with TiO2 nanopowders for air purification. Chem. Eng. J. 2019, 361, 885–896. [Google Scholar] [CrossRef]
- Özcan, M.; Birol, B.; Kaya, F. Investigation of photocatalytic properties of TiO2 nanoparticle coating on fly ash and red mud based porous ceramic substrate. Ceram. Int. 2021, 47, 24270–24280. [Google Scholar] [CrossRef]
- Zhao, S.; Kim, S.Y.; Saqlain, S.; Cha, B.J.; Seo, H.O.; Kim, Y.D. Photocatalytic NO abatement by cement blocks covered with Fe–TiO2 and surface hardening agents: Impacts of Fe–TiO2 contents of coating solutions. J. Phys. Chem. Solids 2023, 174, 111159. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lin, W.-X. A simple method to prepare g-C3N4-TiO2/waste zeolites as visible-light-responsive photocatalytic coatings for degradation of indoor formaldehyde. J. Hazard. Mater. 2019, 368, 468–476. [Google Scholar] [CrossRef]
- Monmaturapoj, N.; Thepsuwan, W.; Mai-Ngam, K.; Ngernpimai, S.; Klinsukhon, W.; Prahsarn, C. Preparation and properties of hydroxyapatite/titania composite for microbial filtration application. Adv. Appl. Ceram. 2014, 113, 267–274. [Google Scholar] [CrossRef]
- Xu, F.; Wang, T.; Chen, H.; Bohling, J.; Maurice, A.M.; Wu, L.; Zhou, S. Preparation of photocatalytic TiO2-based self-cleaning coatings for painted surface without interlayer. Prog. Org. Coat. 2017, 113, 15–24. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Moghaddam, S.G.; Modirshahla, N.; Shokri, M. Investigation of the effect of heat attachment method parameters at photocatalytic activity of immobilized ZnO nanoparticles on glass plate. Desalination 2009, 249, 1371–1376. [Google Scholar] [CrossRef]
- Joseph, A.; Vijayanandan, A. Review on support materials used for immobilization of nano-photocatalysts for water treatment applications. Inorganica Chim. Acta 2023, 545, 121284. [Google Scholar] [CrossRef]
- Lu, B.-J.; Lin, K.-T.; Kuo, Y.-M.; Tsai, C.-H. Preparation of high-transparency, superhydrophilic visible photo-induced photocatalytic film via a rapid plasma-modification process. Coatings 2021, 11, 784. [Google Scholar] [CrossRef]
- Mittal, T. Self-cleaning smart photocatalytic coatings for water treatment. Mater. Today Proc. 2023, 78, 891–894. [Google Scholar] [CrossRef]
- Najafidoust, A.; Allahyari, S.; Rahemi, N.; Tasbihi, M. Uniform coating of TiO2 nanoparticles using biotemplates for photocatalytic wastewater treatment. Ceram. Int. 2020, 46, 4707–4719. [Google Scholar] [CrossRef]
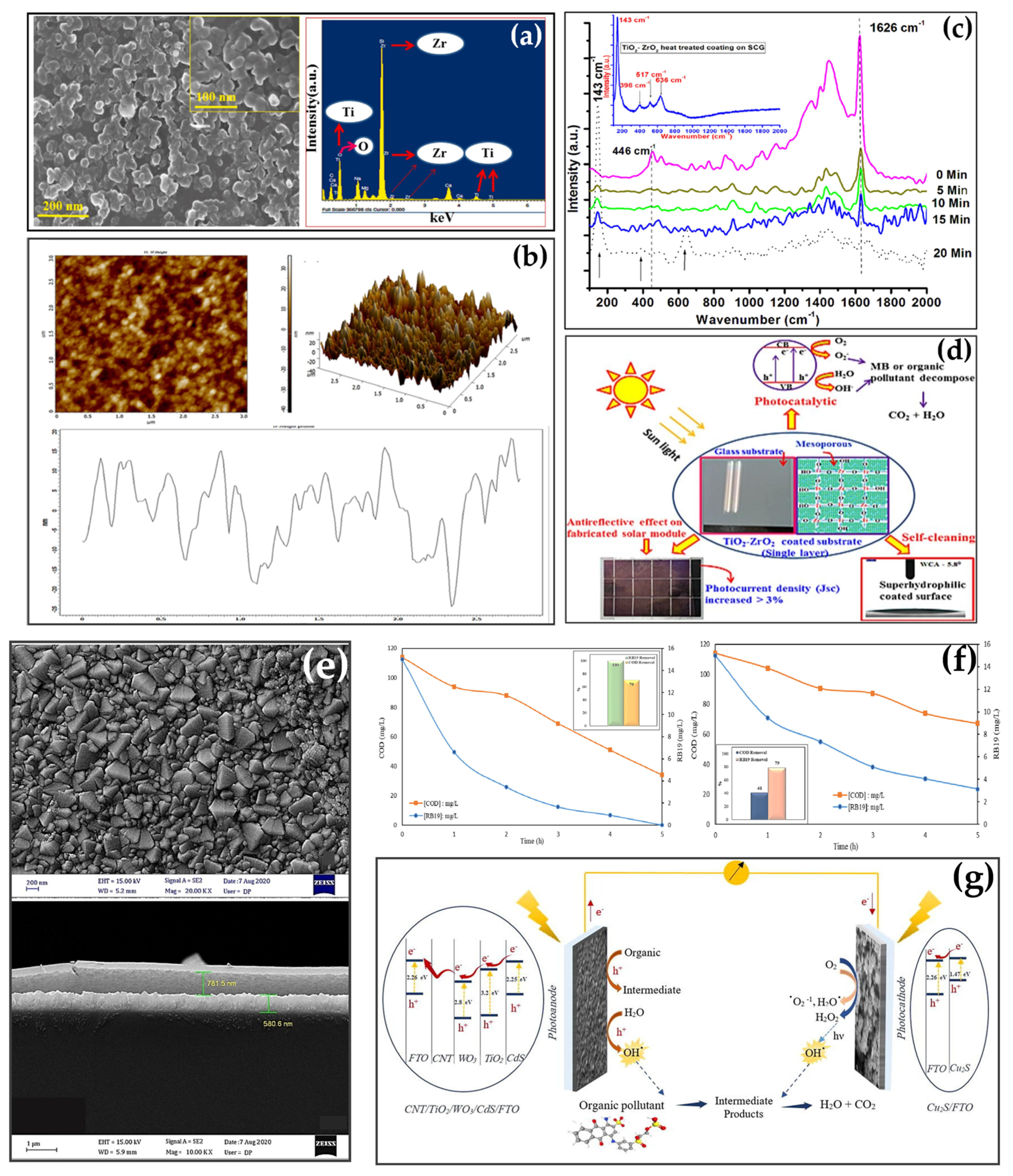
- Manna, S.; Adak, D.; Manna, S.; Maity, S.; Jana, S.; Bhattacharya, R.; Medda, S.K. Antireflection cum photocatalytic with superhydrophilic based durable single layer mesoporous TiO2-ZrO2 coating surface for efficient solar photovoltaic application. Sustain. Energy Technol. Assess. 2023, 57, 103236. [Google Scholar] [CrossRef]
- Ohya, Y.; Mishina, J.; Matsuda, T.; Ban, T.; Takahashi, Y. Crystallization and microstructure development of sol-gel derived titanium dioxide thin films with single and multiple layers. J. Am. Ceram. Soc. 1999, 82, 2601–2606. [Google Scholar] [CrossRef]
- Rajendran, K.; Senthil Kumar, V.; Anitha Rani, K. Synthesis and characterization of immobilized activated carbon doped TiO2 thin films. Optik 2014, 125, 1993–1996. [Google Scholar] [CrossRef]
- Rapsomanikis, A.; Apostolopoulou, A.; Stathatos, E.; Lianos, P. Cerium-modified TiO2 nanocrystalline films for visible light photocatalytic activity. J. Photochem. Photobiol. A 2014, 280, 46–53. [Google Scholar] [CrossRef]
- Bagheri, M.; Farhadian, M.; Vesali-Naseh, M. Performance evaluation of a novel visible-driven CNT/TiO2/WO3/CdS heterojunction in photocatalytic fuel cell: Photodegradation of Reactive Blue 19 and electricity production. Adv. Powder Technol. 2023, 34, 104088. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Ngqoloda, S.; Pescetelli, S.; Di Carlo, A.; Dinu, M.; Vladescu, A.; Parau, A.C.; Agresti, A.; Braic, M.; Arendse, C.J.; et al. Spin coating immobilisation of C-N-TiO2 co-doped nano catalyst on glass and application for photocatalysis or as electron transporting layer for perovskite solar cells. Coatings 2020, 10, 1029. [Google Scholar] [CrossRef]
- Huong, H.T.; Nhu, T.T.Q.; Nang, H.X.; Tuan, P.A.; Huy, P.T. Super-hydrophilic Ce3+-doped TiO2-SiO2 nanocomposite thin films with high optical transmittance by a simple spin-coating method. Thin Solid Film. 2023, 768, 139730. [Google Scholar] [CrossRef]
- Peeters, H.; Keulemans, M.; Nuyts, G.; Vanmeert, F.; Li, C.; Minjauw, M.; Detavernier, C.; Bals, S.; Lenaerts, S.; Verbruggen, S.W. Plasmonic gold-embedded TiO2 thin films as photocatalytic self-cleaning coatings. Appl. Catal. B Environ. 2020, 267, 118654. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Sivakumar, J.; Ramana Reddy, M.V.; Sayanna, R. Structural, optical properties and photocatalytic activity of Fe3+ doped TiO2 thin films deposited by sol-gel spin coating. Surf. Interfaces 2019, 17, 100368. [Google Scholar] [CrossRef]
- Romero-Morán, A.; Sánchez-Salas, J.L.; Molina-Reyes, J. Influence of selected reactive oxygen species on the photocatalytic activity of TiO2/SiO2 composite coatings processed at low temperature. Appl. Catal. B Environ. 2021, 291, 119685. [Google Scholar] [CrossRef]
- Hernandez-Del Castillo, P.C.; Oliva, J.; Robledo-Trujillo, G.; Rodriguez-Gonzalez, V. Enhancing the eosin-yellowish dye degradation in drinking water by using TiO2 coatings co-doped with Ni and In. Environ. Sci. Pollut. Res. Int. 2023, 30, 5258–5266. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Liu, M.; Liu, X.Z.; Deng, C.M.; Zhou, K.S. Deposition of photocatalytic TiO2 coating by modifying the solidification pathway in plasma spraying. Coatings 2017, 7, 169. [Google Scholar] [CrossRef]
- Suligoj, A.; Pliekhova, O.; Vodisek, N.; Mihelcic, M.; Surca, A.K.; Kunic, R.; Subic, B.; Starman, J.; Ugovsek, A.; Lavrencic Stangar, U. Field test of self-cleaning Zr-modified-TiO2-SiO2 films on glass with a demonstration of their anti-fogging effect. Materials 2019, 12, 2196. [Google Scholar] [CrossRef]
- Dundar, I.; Krichevskaya, M.; Katerski, A.; Krunks, M.; Acik, I.O. Photocatalytic degradation of different VOCs in the gas-phase over TiO2 thin films prepared by ultrasonic spray pyrolysis. Catalysts 2019, 9, 915. [Google Scholar] [CrossRef]
- Zong, L.; Wu, Y.; Li, X.; Jiang, B. The preparation of superhydrophobic photocatalytic fluorosilicone/SiO2–TiO2 coating and its self-cleaning performance. J. Coat. Technol. Res. 2021, 18, 1245–1259. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Sun, S.; Zhang, H.; Zhou, R.; Li, Y.; Liang, Y.; Wang, J. Preparation of a temperature-sensitive superhydrophobic self-cleaning SiO2-TiO2@PDMS coating with photocatalytic activity. Surf. Coat. Technol. 2021, 408, 126853. [Google Scholar] [CrossRef]
- Wu, B.R.; Lyu, J.J.; Peng, C.Y.; Jiang, D.Z.; Yang, J.; Yang, J.S.; Xing, S.L.; Sheng, L.P. Inverse infusion processed hierarchical structure towards superhydrophobic coatings with ultrahigh mechanical robustness. Chem. Eng. J. 2020, 387, 124066. [Google Scholar] [CrossRef]
- Li, H.; Lai, J.; Yao, M.; Leng, Y.M.; Wu, Z.D.; Zhang, J.; Peng, H.L.; Qiu, Z.M. Development of high performance superhydrophobic coating with excellent corrosion resistance, durability, and self-cleaning properties using M-TiO2@EP composites. Appl. Surf. Sci. 2022, 601, 154109. [Google Scholar] [CrossRef]
- Gent, E.; Taffa, D.H.; Wark, M. Multi-layered mesoporous TiO2 thin films: Photoelectrodes with improved activity and stability. Coatings 2019, 9, 625. [Google Scholar] [CrossRef]
- Rosolymou, E.; Spanou, S.; Zanella, C.; Tsoukleris, D.S.; Kohler, S.; Leisner, P.; Pavlatou, E.A. Electrodeposition of photocatalytic Sn-Ni matrix composite coatings embedded with doped TiO2 particles. Coatings 2020, 10, 775. [Google Scholar] [CrossRef]
- Becker, M.; Yezerska, O. Review—Recent progress in low temperature dynthesis of crystalline TiO2 photocatalytic films by highly controllable electrodeposition. J. Electrochem. Soc. 2022, 169, 052507. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Zhi, H.; Dong, J.; Hao, J.; Zhang, X.; Wang, J.; Li, D.; Liu, B. Synthesis of the porous ZnO nanosheets and TiO2/ZnO/FTO composite films by a low-temperature hydrothermal method and their applications in photocatalysis and electrochromism. Coatings 2022, 12, 695. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, T.; Meng, F.; Yang, M.; Kawi, S. Recent advances in ZnIn2S4-based materials towards photocatalytic purification, solar fuel production and organic transformations. J. Mater. Chem. C 2022, 10, 5400–5424. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Zhu, L.H.; Li, J.; Tang, H.Q. Electrochemical preparation of TiO2/SiO2 composite film and its high activity toward the photoelectrocatalytic degradation of methyl orange. J. Appl. Electrochem. 2009, 39, 1745–1753. [Google Scholar] [CrossRef]
- Lu, C.L.; Zhang, L.; Zhang, Y.W.; Liu, S.Y. Electrodeposition of TiO2/CdSe heterostructure films and photocatalytic degradation of methylene blue. Mater. Lett. 2016, 185, 342–345. [Google Scholar] [CrossRef]
- Vasile, E.; Sima, M.; Sima, A.; Logofatu, C. TiO2/Fe2O3 photoanodes for solar water oxidation prepared via electrodeposition of amorphous precursors. Mater. Res. Bull. 2020, 121, 110623. [Google Scholar] [CrossRef]
- de Oliveira, C.V.; Alhussein, A.; Creus, J.; Schuster, F.; Schlegel, M.L.; Dong, Z.; Jimenez, C.; Sanchette, F. Bifunctional TiO2/AlZr thin films on steel substrate combining corrosion resistance and photocatalytic properties. Coatings 2019, 9, 564. [Google Scholar] [CrossRef]
- Vahl, A.; Veziroglu, S.; Henkel, B.; Strunskus, T.; Polonskyi, O.; Aktas, O.C.; Faupel, F. Pathways to tailor photocatalytic performance of TiO2 thin films deposited by reactive magnetron sputtering. Materials 2019, 12, 2840. [Google Scholar] [CrossRef]
- Kelly, P.J.; West, G.T.; Ratova, M.; Fisher, L.; Ostovarpour, S.; Verran, J. Structural formation and photocatalytic activity of magnetron sputtered titania and doped-titania coatings. Molecules 2014, 19, 16327–16348. [Google Scholar] [CrossRef]
- Cui, K.X.; Zhang, Y. High-entropy alloy films. Coatings 2023, 13, 635. [Google Scholar] [CrossRef]
- Mazur, M.; Wojcieszak, D.; Kaczmarek, D.; Domaradzki, J.; Song, S.; Gibson, D.; Placido, F.; Mazur, P.; Kalisz, M.; Poniedzialek, A. Functional photocatalytically active and scratch resistant antireflective coating based on TiO2 and SiO2. Appl. Surf. Sci. 2016, 380, 165–171. [Google Scholar] [CrossRef]
- Villamayor, A.; Pomone, T.; Perero, S.; Ferraris, M.; Barrio, V.L.; G-Berasategui, E.; Kelly, P. Development of photocatalytic nanostructured TiO2 and NiO/TiO2 coatings by DC magnetron sputtering for photocatalytic applications. Ceram. Int. 2023, 49, 19309–19317. [Google Scholar] [CrossRef]
- Ratova, M.; Klaysri, R.; Praserthdam, P.; Kelly, P.J. Pulsed DC magnetron sputtering deposition of crystalline photocatalytic titania coatings at elevated process pressures. Mater. Sci. Semicon. Proc. 2017, 71, 188–196. [Google Scholar] [CrossRef]
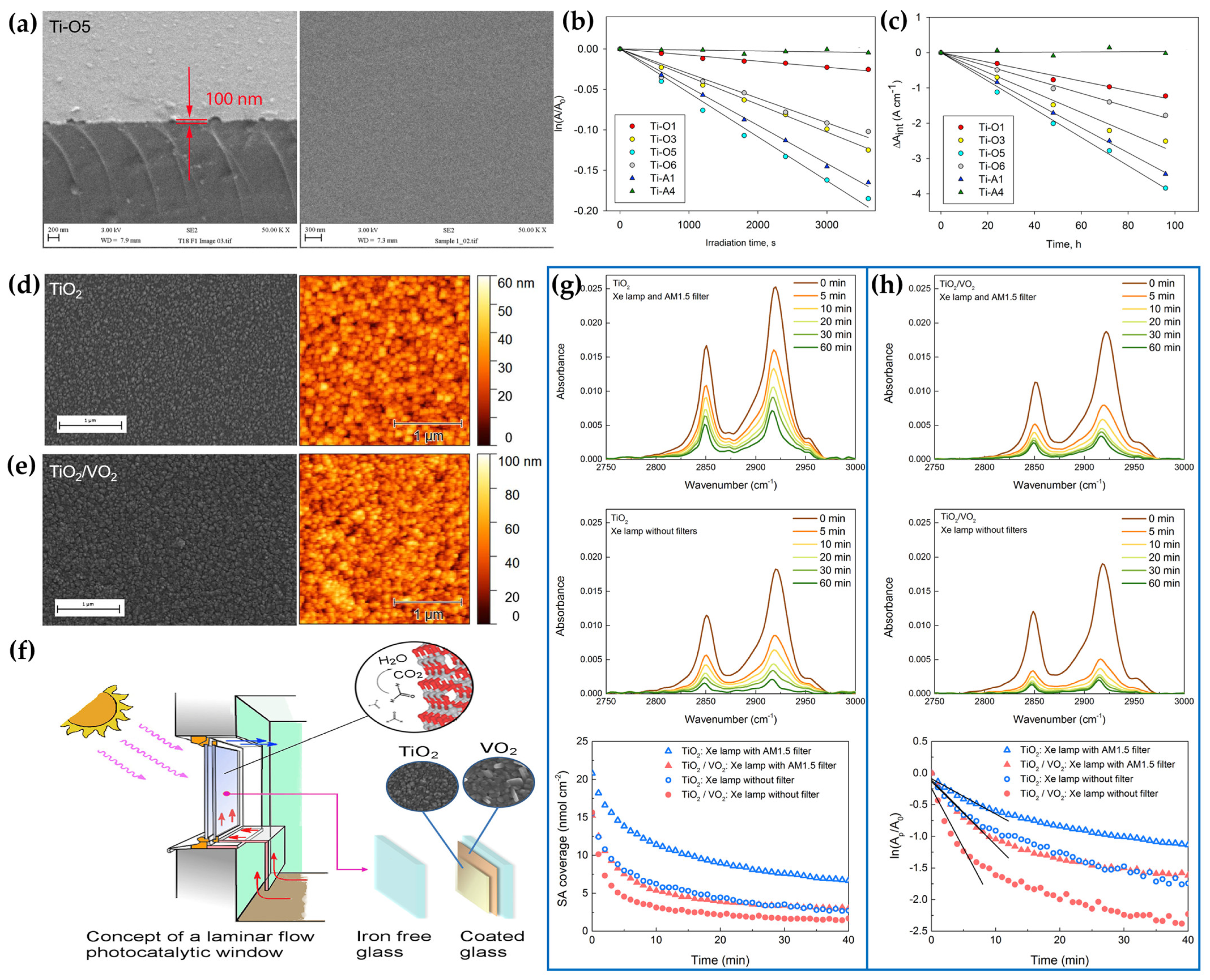
- Ji, Y.; Mattsson, A.; Niklasson, G.A.; Granqvist, C.G.; Österlund, L. Synergistic TiO2/VO2 window coating with thermochromism, enhanced luminous transmittance, and photocatalytic activity. Joule 2019, 3, 2457–2471. [Google Scholar] [CrossRef]
- Ding, R.; Wang, K.; Hong, K.; Zhang, Y.; Cui, Y. Hierarchical core-shell tungsten oxide/TiO2 nanowires as an effective photocatalyst. Chem. Phys. Lett. 2019, 714, 156–159. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 porous layers by pulsed laser deposition for surface acoustic wave H2 gas sensor. Nanomaterials 2020, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Gondal, M.A.; Ilyas, A.M.; Fasasi, T.A.; Dastageer, M.A.; Seddigi, Z.S.; Qahtan, T.F.; Faiz, M.; Khattak, G.D. Synthesis of green TiO2/ZnO/CdS hybrid nano-catalyst for efficient light harvesting using an elegant pulsed laser ablation in liquids method. Appl. Surf. Sci. 2015, 357, 2217–2222. [Google Scholar] [CrossRef]
- Bricchi, B.R.; Ghidelli, M.; Mascaretti, L.; Zapelli, A.; Russo, V.; Casari, C.S.; Terraneo, G.; Alessandri, I.; Ducati, C.; Li Bassi, A. Integration of plasmonic Au nanoparticles in TiO2 hierarchical structures in a single-step pulsed laser co-deposition. Mater. Design. 2018, 156, 311–319. [Google Scholar] [CrossRef]
- Kadri, L.; Abderrahmane, A.; Bulai, G.; Carlescu, A.; Doroftei, C.; Motrescu, I.; Gurlui, S.; Leontie, L.; Adnane, M. Optical and structural analysis of TiO2-SiO2 nanocomposite thin films fabricated via pulsed laser deposition technique. Nanomaterials 2023, 13, 1632. [Google Scholar] [CrossRef]
- Linnik, O.; Shestopal, N.; Smirnova, N.; Eremenko, A.; Korduban, O.; Kandyba, V.; Kryshchuk, T.; Socol, G.; Stefan, N.; Popescu-Pelin, G.; et al. Correlation between electronic structure and photocatalytic properties of non-metal doped TiO2/ZrO2 thin films obtained by pulsed laser deposition method. Vacuum 2015, 114, 166–171. [Google Scholar] [CrossRef]
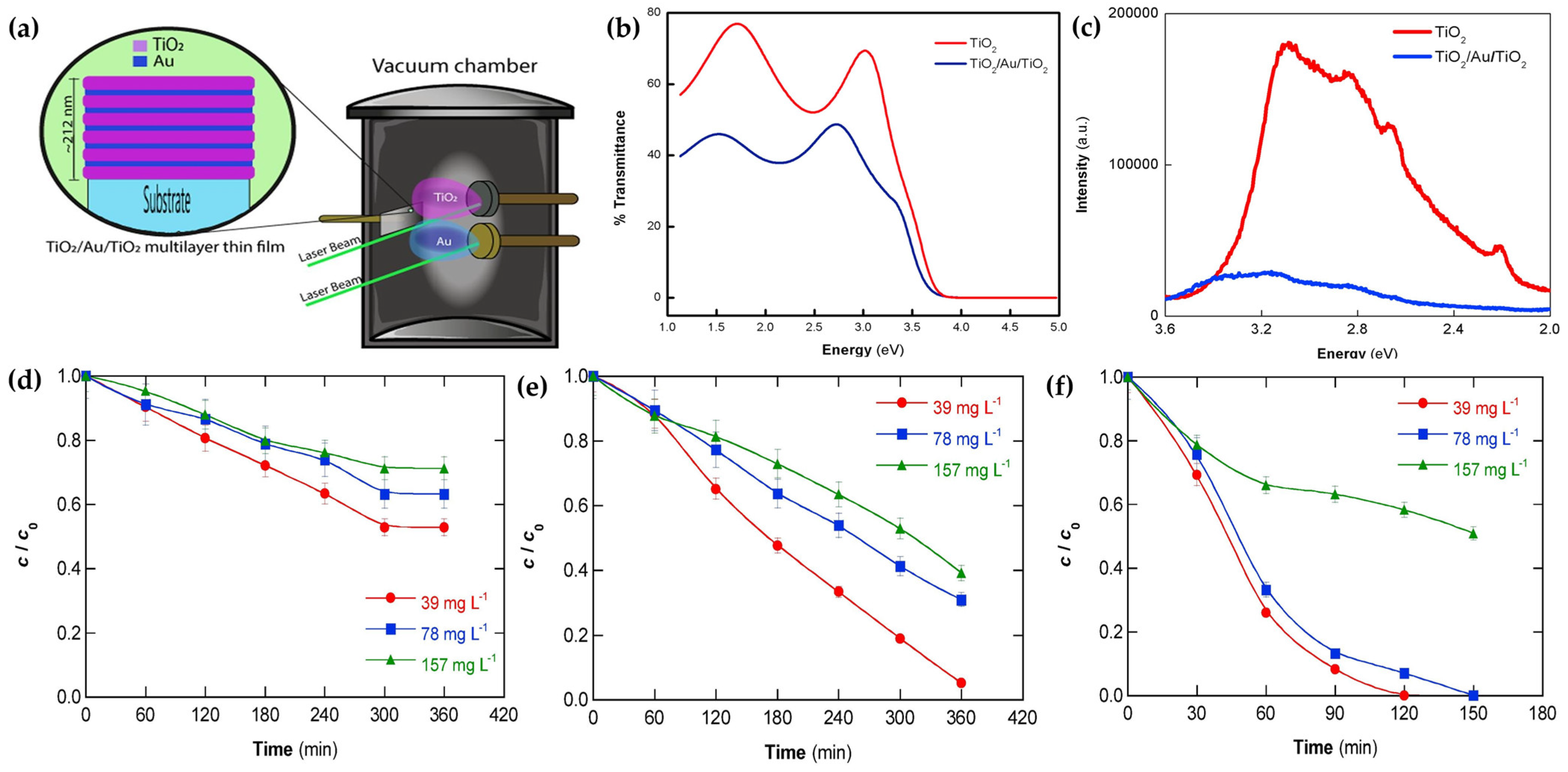
- Olvera-Rodríguez, I.; Hernández, R.; Medel, A.; Guzmán, C.; Escobar-Alarcón, L.; Brillas, E.; Sirés, I.; Esquivel, K. TiO2/Au/TiO2 multilayer thin-film photoanodes synthesized by pulsed laser deposition for photoelectrochemical degradation of organic pollutants. Sep. Purif. Technol. 2019, 224, 189–198. [Google Scholar] [CrossRef]
- Lin, L.; Deng, B.; Sun, J.; Peng, H.; Liu, Z. Bridging the gap between reality and ideal in chemical vapor deposition growth of graphene. Chem. Rev. 2018, 118, 9281–9343. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hsu, Y.-L.; Zheng, Y.X. Investigation of oxygen deficiency-rich/oxygen deficiency-poor stacked TiO2 based resistive random access memory by mist chemical vapor deposition. Ceram. Int. 2022, 48, 28881–28888. [Google Scholar] [CrossRef]
- Yang, K.; Zhong, S.; Zhou, X.; Tang, S.; Qiao, K.; Ma, K.; Song, L.; Yue, H.; Liang, B. Controllable Al2O3 coating makes TiO2 photocatalysts active under visible light by pulsed chemical vapor deposition. Chem. Eng. Sci. 2023, 277, 118792. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Sathasivam, S.; Parkin, I.P. Aerosol assisted chemical vapour deposition of a ZrO2–TiO2 composite thin film with enhanced photocatalytic activity. RSC Adv. 2015, 5, 67944–67950. [Google Scholar] [CrossRef]
- Quesada-Gonzalez, M.; Boscher, N.D.; Carmalt, C.J.; Parkin, I.P. Interstitial boron-doped TiO2 thin films: The significant effect of boron on TiO2 coatings grown by atmospheric pressure chemical vapor deposition. ACS Appl. Mater. Interfaces 2016, 8, 25024–25029. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Vazquez, C.; Noor, N.; Kafizas, A.; Quesada-Cabrera, R.; Scanlon, D.O.; Taylor, A.; Durrant, J.R.; Parkin, I.P. Multifunctional P-doped TiO2 films: A new approach to self-cleaning, transparent conducting oxide materials. Chem. Mater. 2015, 27, 3234–3242. [Google Scholar] [CrossRef]
- Lang, J.H.; Takahashi, K.; Kubo, M.; Shimada, M. Ag-doped TiO2 composite films prepared using aerosol-assisted, plasma-enhanced chemical vapor deposition. Catalysts 2022, 12, 365. [Google Scholar] [CrossRef]
- Villardi de Oliveira, C.; Petitbois, J.; Faÿ, F.; Sanchette, F.; Schuster, F.; Alhussein, A.; Chaix-Pluchery, O.; Deschanvres, J.-L.; Jiménez, C. Marine antibiofouling properties of TiO2 and Ti-Cu-O films deposited by aerosol-assisted chemical vapor deposition. Coatings 2020, 10, 779. [Google Scholar] [CrossRef]
- Assaker, I.B.; Gannouni, M.; Naceur, J.B.; Almessiere, M.A.; Al-Otaibi, A.L.; Ghrib, T.; Shen, S.; Chtourou, R. Electrodeposited ZnIn2S4 onto TiO2 thin films for semiconductor-sensitized photocatalytic and photoelectrochemical applications. Appl. Surf. Sci. 2015, 351, 927–934. [Google Scholar] [CrossRef]
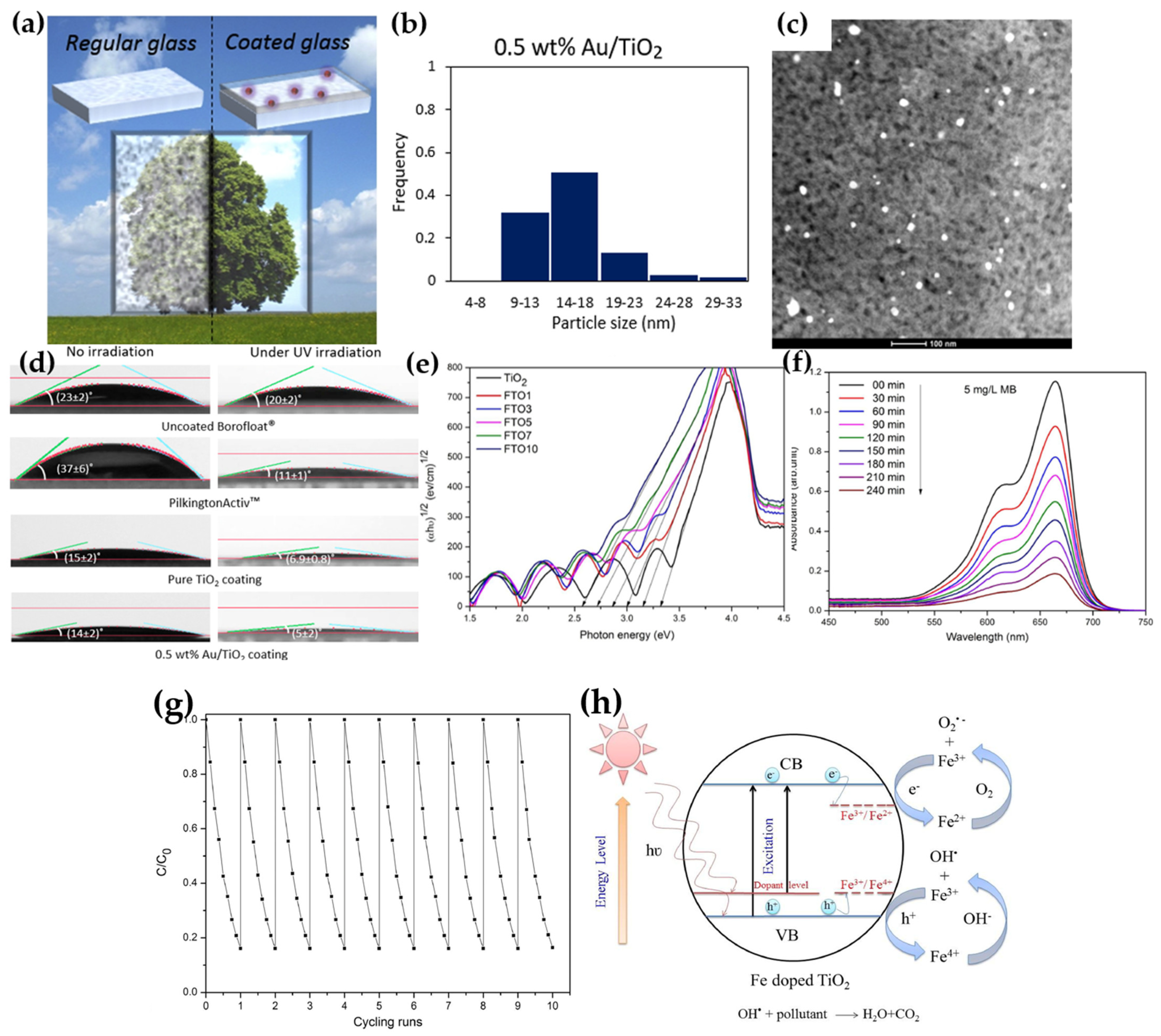

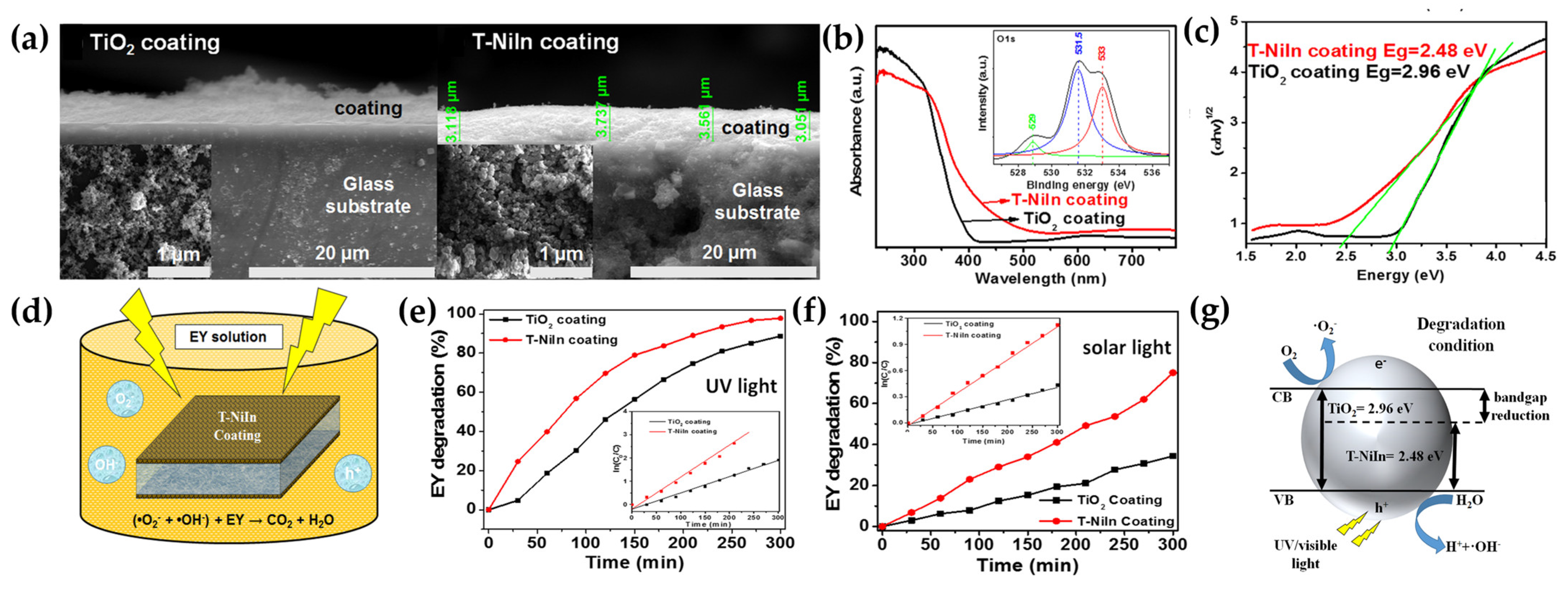
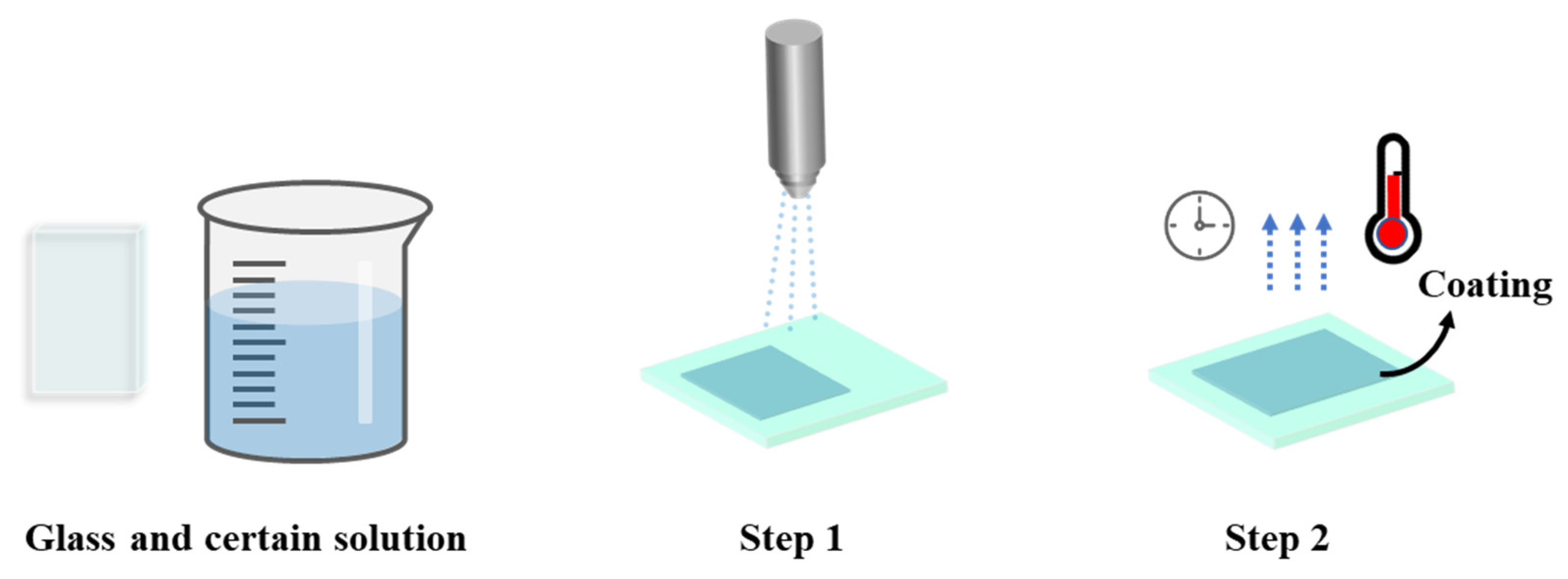

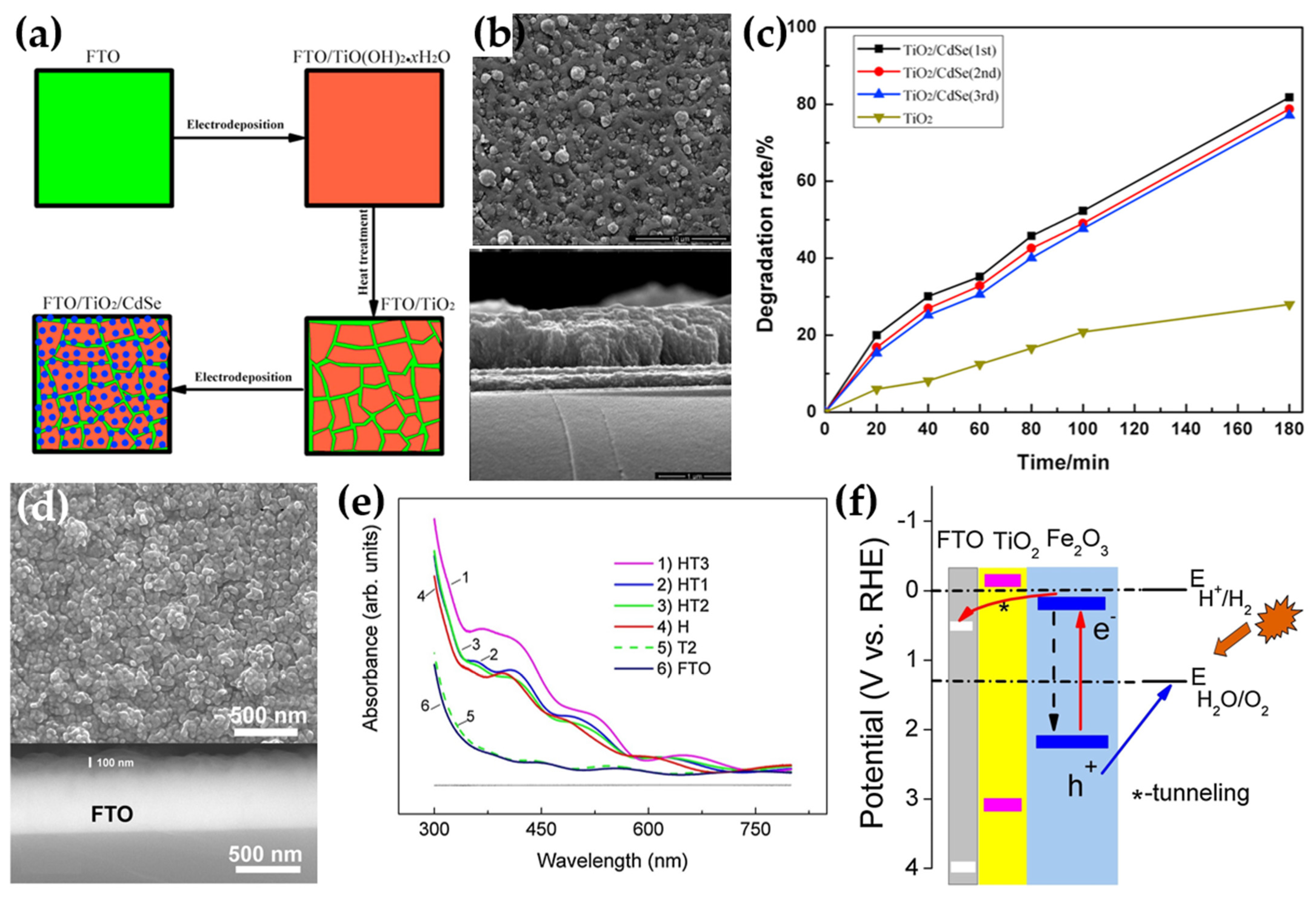

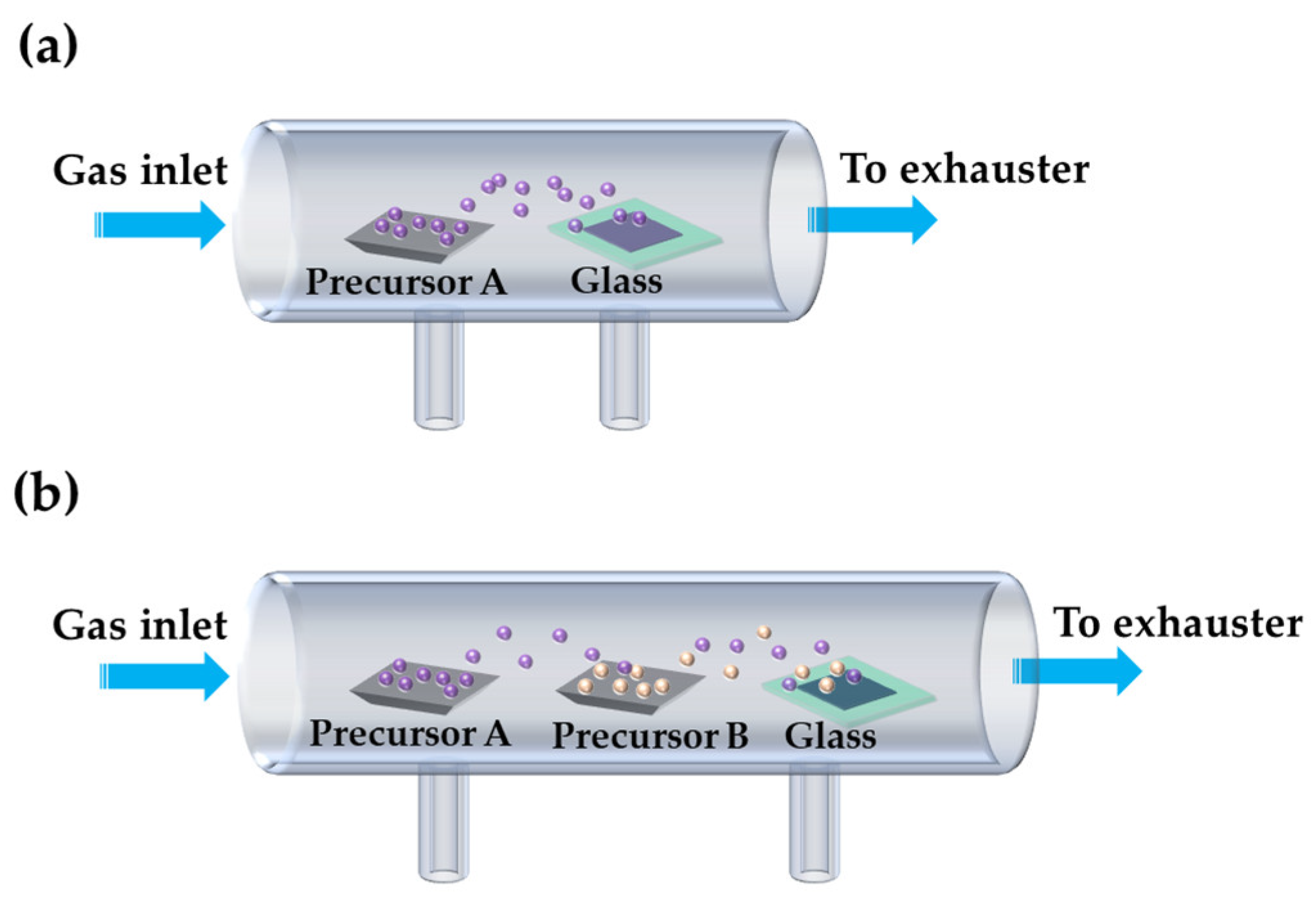
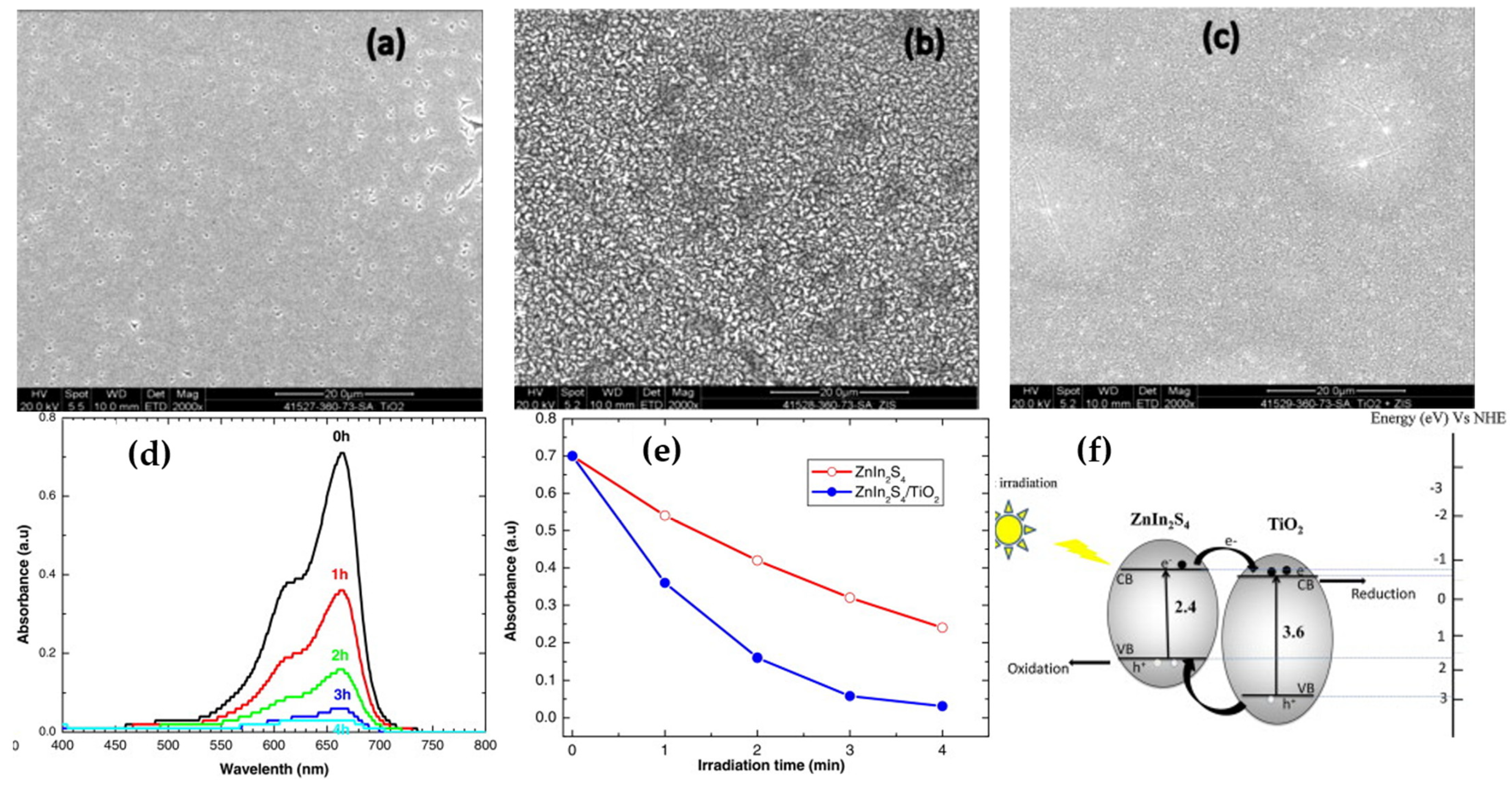
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| WCD | Simplicity Cost-effectiveness Versatility Larger-scale production | Difficulty in controlling the uniformity of the film layer Low material density and poor adhesion Possible severe cracking Requires post-deposition thermal annealing | [75,83,89] |
| Electro- deposition | Low cost Environmentally friendly Controllable operation Low-temperature synthesis | Slow dispersion of surface charges and particles in the electrolyte Conductive substrate required Inability to deposit above 80 °C Requires post-deposition thermal annealing | [97,99] |
| PVD | Impurity-free High adhesion or durability No harsh chemicals and limited wastes Does not require complex chemical reactions | High temperature and pressure required Often need post-deposition thermal annealing | [108,115] |
| CVD | Simple equipment Diversified precursor Controllable operation | High temperature (400–900 °C) Disposal and storage of highly corrosive precursors or by-products High cost of high-purity chemicals High toxicity of produced waste gases Often need post-deposition thermal annealing | [119,120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Feng, Y.; Zheng, Z.; He, Z. TiO2-Based Photocatalytic Coatings on Glass Substrates for Environmental Applications. Coatings 2023, 13, 1472. https://doi.org/10.3390/coatings13081472
Tian S, Feng Y, Zheng Z, He Z. TiO2-Based Photocatalytic Coatings on Glass Substrates for Environmental Applications. Coatings. 2023; 13(8):1472. https://doi.org/10.3390/coatings13081472
Chicago/Turabian StyleTian, Shuang, Yuxiao Feng, Ziye Zheng, and Zuoli He. 2023. "TiO2-Based Photocatalytic Coatings on Glass Substrates for Environmental Applications" Coatings 13, no. 8: 1472. https://doi.org/10.3390/coatings13081472






