Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production and Characterization of Plant Extracts
2.2. Evaluation of the Bioactive Potential of the Extracts Obtained through the Optimization Process
2.2.1. Antioxidant Analysis
2.2.2. Evaluation of Antimicrobial Activity
2.3. Preparation of Alginate Coatings/Films Incorporated with Plant Extracts
2.4. Antimicrobial Activity of the Films/Coatings
2.4.1. Antibacterial Activity
2.4.2. Antifungal Activity
2.5. Antioxidant Activity of the Films
2.6. Statistical Analysis
3. Results
3.1. Characterization of Plant Extracts
3.2. Evaluation of the Bioactive Potential of the Extracts
3.2.1. Antioxidant Activity
3.2.2. Antimicrobial Activity
3.3. Alginate Coatings and Films with Plant Extracts
3.4. Evaluation of the Bioactive Potential of the Coatings/Films
3.4.1. Antibacterial Activity
3.4.2. Antifungal Activity
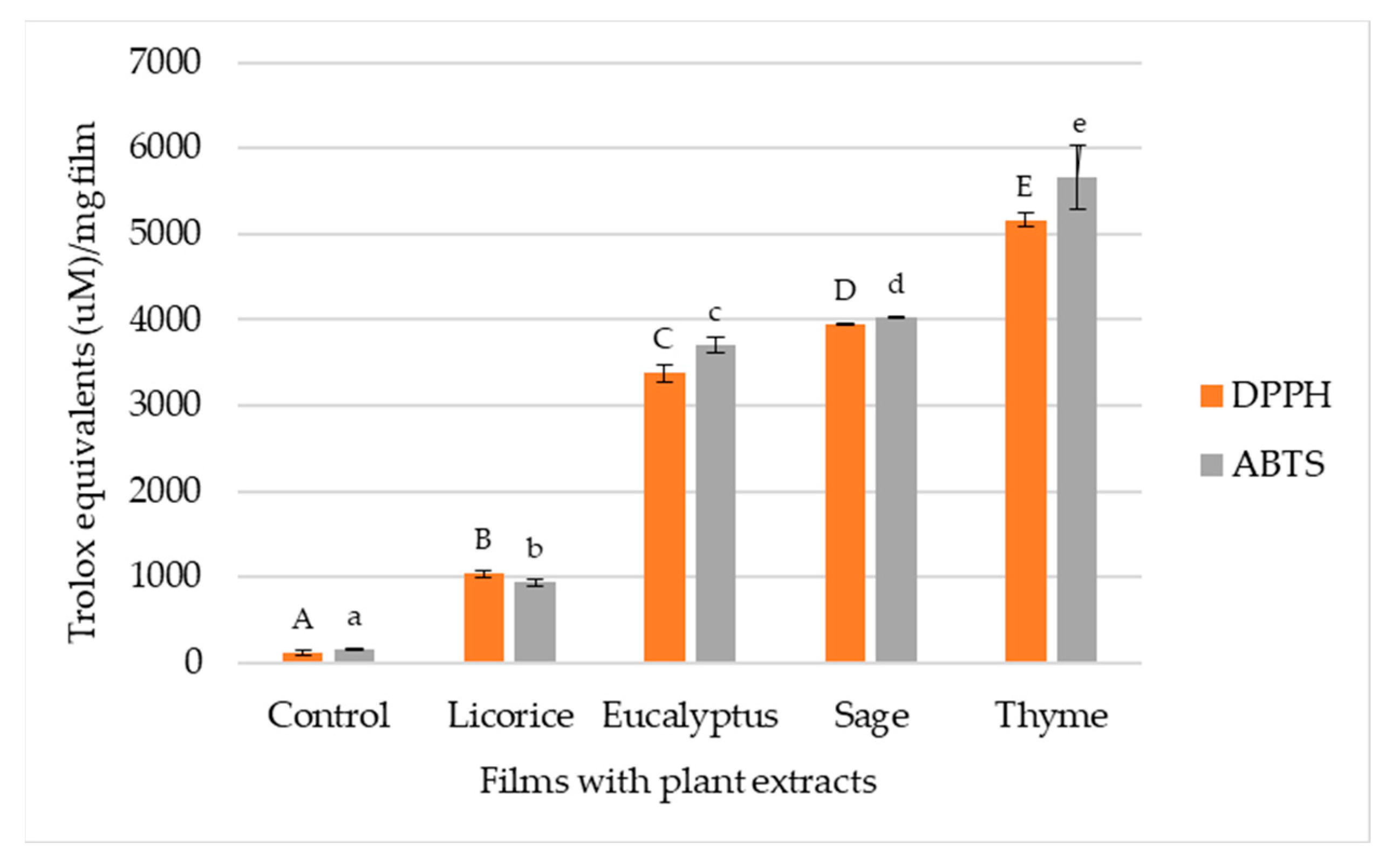
3.4.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aitboulahsen, M.; El Galiou, O.; Laglaoui, A.; Bakkali, M.; Hassani Zerrouk, M. Effect of Plasticizer Type and Essential Oils on Mechanical, Physicochemical, and Antimicrobial Characteristics of Gelatin, Starch, and Pectin-Based Films. J. Food Process. Preserv. 2020, 44, e14480. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and Characterization of Sodium Alginate Based Active Edible Films Incorporated with Essential Oils of Some Medicinal Plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Development of Essential Oil Incorporated Active Film Based on Biodegradable Blends of Poly (Lactide)/Poly (Butylene Adipate-Co-Terephthalate) for Food Packaging Application. J. Packag. Technol. Res. 2020, 4, 235–245. [Google Scholar] [CrossRef]
- Amalraj, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Preparation, Characterization and Antimicrobial Activity of Polyvinyl Alcohol/Gum Arabic/Chitosan Composite Films Incorporated with Black Pepper Essential Oil and Ginger Essential Oil. Int. J. Biol. Macromol. 2020, 151, 366–375. [Google Scholar] [CrossRef] [PubMed]
- López de Dicastillo, C.; Bustos, F.; Guarda, A.; Galotto, M.J. Cross-Linked Methyl Cellulose Films with Murta Fruit Extract for Antioxidant and Antimicrobial Active Food Packaging. Food Hydrocoll. 2016, 60, 335–344. [Google Scholar] [CrossRef]
- Augusto, A.; Dias, J.R.; Campos, M.J.; Alves, N.M.; Pedrosa, R.; Silva, S.F.J. Influence of Codium tomentosum Extract in the Properties of Alginate and Chitosan Edible Films. Foods 2018, 7, 53. [Google Scholar] [CrossRef]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria, A.M.; Labidi, J.; Abdulqadir, A.H.; Deligöz, E. Antioxidative and Antimicrobial Edible Chitosan Films Blended with Stem, Leaf and Seed Extracts of Pistacia terebinthus for Active Food Packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P.M. Edible Polymers: Challenges and Opportunities. J. Polym. 2014, 2014, 427259. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased Materials for Active Food Packaging: A Review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Bashir, A.; Jabeen, S.; Gull, N.; Islam, A.; Sultan, M.; Ghaffar, A.; Khan, S.; Sagar, S.; Jamil, T. Co-Concentration Effect of Silane with Natural Extract on Biodegradable Polymeric Films for Food Packaging. Int. J. Biol. Macromol. 2018, 106, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, L.; Gong, X.; Chi, F.; Ma, Z. Fabrication and Comparison of Active Films from Chitosan Incorporating Different Spice Extracts for Shelf Life Extension of Refrigerated Pork. LWT 2021, 135, 110181. [Google Scholar] [CrossRef]
- Kaur, N.; Bains, A.; Kaushik, R.; Dhull, S.B.; Melinda, F.; Chawla, P. A Review on Antifungal Efficiency of Plant Extracts Entrenched Polysaccharide-Based Nanohydrogels. Nutrients 2021, 13, 2055. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial Hydrogels: Promising Materials for Medical Application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Özkahraman, B.; Özbaş, Z.; Yaşayan, G.; Tamahkar, E.; Alarçin, E. Advancements and Future Directions in the Antibacterial Wound Dressings—A Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109B, 703–716. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, Mechanical and Barrier Properties of Corn Starch Films Incorporated with Plant Essential Oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, Mechanical and Antibacterial Properties of Alginate Film: Effect of the Crosslinking Degree and Oregano Essential Oil Concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Prieto, M.A.; Bento, A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a Natural Preservative Obtained from Male Chestnut Flowers: Optimization of a Heat-Assisted Extraction Technique. Food Funct. 2019, 10, 1352–1363. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and Chemical Characterization in Hydrophilic and Lipophilic Compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Profiles of Cultivated, in Vitro Cultured and Commercial Samples of Melissa officinalis L. Infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C.F.R. Melissa officinalis L. Decoctions as Functional Beverages: A Bioactive Approach and Chemical Characterization. Food Funct. 2015, 6, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Piccirillo, C.; Pullar, R.C.; Castro, P.M.L.; Pintado, M.M.E. Characterization and Antimicrobial Properties of Food Packaging Methylcellulose Films Containing Stem Extract of Ginja Cherry. J. Sci. Food Agric. 2014, 94, 2097–2103. [Google Scholar] [CrossRef]
- Guimarães, A.; Ramos, O.; Cerqueira, M.; Venâncio, A.; Abrunhosa, L. Active Whey Protein Edible Films and Coatings Incorporating Lactobacillus buchneri for Penicillium nordicum Control in Cheese. Food Bioprocess Technol. 2020, 13, 1074–1086. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Barreira, J.C.M.; Calhelha, R.C.; Soković, M.; Fernández-Ruiz, V.; Buelga, C.S.; Morales, P.; Ferreira, I.C.F.R. Basil as Functional and Preserving Ingredient in “Serra Da Estrela” Cheese. Food Chem. 2016, 207, 51–59. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takano, F.; Takata, T.; Niiyama, M.; Ohta, T. Bioactive Monoterpene Glycosides Conjugated with Gallic Acid from the Leaves of Eucalyptus globulus. Phytochemistry 2008, 69, 747–753. [Google Scholar] [CrossRef]
- Kitagawa, I.; Chen, W.Z.; Hori, K.; Harada, E.; Yasuda, N.; Yoshikawa, M.; Ren, J. Chemical Studies of Chinese Licorice-Roots. I. Elucidation of Five New Flavonoid Constituents from the Roots of Glycyrrhiza glabra L. Collected in Xinjiang. Chem. Pharm. Bull. 1994, 42, 1056–1062. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of Phenolic Compounds and Antioxidant Properties of Glycyrrhiza glabra L. Rhizomes and Roots. RSC Adv. 2015, 5, 26991–26997. [Google Scholar] [CrossRef]
- Schmid, C.; Dawid, C.; Peters, V.; Hofmann, T. Saponins from European Licorice Roots (Glycyrrhiza glabra). J. Nat. Prod. 2018, 81, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.; Jiddah-kazeem, B.; Fatoki, T.H.; Ibukun, E.O.; Akinmoladun, A.C. Antioxidant Property of Eucalyptus globulus Labill. Extracts and Inhibitory Activities on Carbohydrate Metabolizing Enzymes Related to Type-2 Diabetes. Biocatal. Agric. Biotechnol. 2021, 36, 102111. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; He, Z.; Chen, Y.; Li, Z.; Meng, T.; Li, Y.; Cao, Y. In Vitro and in Vivo Antioxidant Activity of Eucalyptus Leaf Polyphenols Extract and Its Effect on Chicken Meat Quality and Cecum Microbiota. Food Res. Int. 2020, 136, 109302. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Antonio, A.L.; Cabo Verde, S.; Santos-Buelga, C.; Ferreira, I.C.F.R. Infusions from Thymus vulgaris L. Treated at Different Gamma Radiation Doses: Effects on Antioxidant Activity and Phenolic Composition. LWT 2016, 74, 34–39. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Matkowski, A.; Wysokińska, H. Antioxidant Activity of Extracts from in Vitro Cultures of Salvia officinalis L. Food Chem. 2007, 104, 536–541. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Hadian, J.; Saharkhiz, M.J.; Nejad Ebrahimi, S. Variation in the Phytochemical Contents and Antioxidant Activity of Glycyrrhiza glabra Populations Collected in Iran. Ind. Crops Prod. 2019, 137, 248–259. [Google Scholar] [CrossRef]
- Etchepare, M.A.; de Menezes, M.F.D.S.C.; Rodrigues, L.Z.; Codevilla, C.; Menezes, C. Microencapsulação de Compostos Bioativos Pelo Método de Extrusão. Ciência E Nat. 2015, 37, 97–105. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant Edible Films Based on Chitosan and Starch Containing Polyphenols from Thyme Extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the Functionality of Chitosan- and Alginate-Based Active Edible Coatings/Films for the Preservation of Fruits and Vegetables: A Review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Bisht, A.S.; Alam, M.S.; Bhatia, S.; Gupta, S.K. Studies on Development and Evaluation of Glycerol Incorporated Cellulose and Alginate Based Edible Films. Indian J. Agric. Biochem. 2017, 30, 67–72. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J.; Jakubowska, E. Characteristics of Ascorbic Acid Release from TPP-Crosslinked Chitosan/Alginate Polyelectrolyte Complex Membranes. Prog. Chem. Appl. Chitin Its Deriv. 2018, XXIII, 76–87. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Sánchez-González, L.; Muneret, L.; Jeandel, C.; Hamdi, M.; Desobry, S. Alginate Coatings Containing Grapefruit Essential Oil or Grapefruit Seed Extract for Grapes Preservation. Int. J. Food Sci. Technol. 2014, 49, 952–959. [Google Scholar] [CrossRef]
- Hasan, K.; Islam, R.; Hasan, M.; Sarker, S.H.; Biswas, M.H. Effect of Alginate Edible Coatings Enriched with Black Cumin Extract for Improving Postharvest Quality Characteristics of Guava (Psidium guajava L.) Fruit. Food Bioprocess Technol. 2022, 15, 2050–2064. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible Alginate-Based Coating as Carrier of Antimicrobials to Improve Shelf-Life and Safety of Fresh-Cut Melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jia, Y.; Hu, Y.; Xia, X.; Li, Y.; Zhou, J.; Liu, Y. Effect of Citrus Wilsonii Tanaka Extract Combined with Alginate-Calcium Coating on Quality Maintenance of White Shrimps (Litopenaeus vannamei Boone). Food Control 2016, 68, 83–91. [Google Scholar] [CrossRef]
- Aydin, G.; Zorlu, E.B. Characterisation and Antibacterial Properties of Novel Biodegradable Films Based on Alginate and Roselle (Hibiscus sabdariffa L.) Extract. Waste Biomass Valoriz. 2022, 13, 2991–3002. [Google Scholar] [CrossRef]
- Engin, M.S.; Zamahay, F.; Kalkan, S.; Otağ, M.R. Physical, Mechanical, and Bioactive Properties of Edible Film Based on Sodium Alginate Enriched with Lythrum salicaria L. Extract. J. Food Process. Preserv. 2022, 46, e16620. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Tarning, J.; Cho, T.Z.A.; Anal, A.K. Activities and Possible Modes of Action of Acacia nilotica (L.) Del. against Multidrug-Resistant Escherichia coli and Salmonella. Molecules 2017, 22, 47. [Google Scholar] [CrossRef]
- Chew, Y.L.; Mahadi, A.M.; Wong, K.M.; Goh, J.K. Anti-Methicillin-Resistance Staphylococcus Aureus (MRSA) Compounds from Bauhinia kockiana Korth. And Their Mechanism of Antibacterial Activity. BMC Complement. Altern. Med. 2018, 18, 70. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The Antimicrobial Capacity of Cistus salviifolius and Punica granatum Plant Extracts against Clinical Pathogens Is Related to Their Polyphenolic Composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Z.-H.; Wang, D.-M.; Li, D.-W.; Yang, L.-N.; Wang, W. Chemical Composition, Antibacterial Activity and Related Mechanism of Valonia and Shell from Quercus variabilis Blume (Fagaceae) against Salmonella paratyphi a and Staphylococcus aureus. BMC Complement. Altern. Med. 2019, 19, 271. [Google Scholar] [CrossRef]
- Xu, M.; Xue, H.; Li, X.; Zhao, Y.; Lin, L.; Yang, L.; Zheng, G. Chemical Composition, Antibacterial Properties, and Mechanism of Smilax china L. Polyphenols. Appl. Microbiol. Biotechnol. 2019, 103, 9013–9022. [Google Scholar] [CrossRef]
- Mombeshora, M.; Mukanganyama, S. Antibacterial Activities, Proposed Mode of Action and Cytotoxicity of Leaf Extracts from Triumfetta welwitschii against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2019, 19, 315. [Google Scholar] [CrossRef]
- Roshan, N.; Riley, T.V.; Knight, D.R.; Steer, J.H.; Hammer, K.A. Natural Products Show Diverse Mechanisms of Action against Clostridium difficile. J. Appl. Microbiol. 2019, 126, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Characterization of Pea Starch-Guar Gum Biocomposite Edible Films Enriched by Natural Antimicrobial Agents for Active Food Packaging. Food Bioprod. Process. 2017, 105, 51–63. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium Alginate-Based Green Packaging Films Functionalized by Guava Leaf Extracts and Their Bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef]
- Lim, L.I.; Tan, H.L.; Pui, L.P. Development and Characterization of Alginate-Based Edible Film Incorporated with Hawthorn berry (Crataegus pinnatifida) Extract. J. Food Meas. Charact. 2021, 15, 2540–2548. [Google Scholar] [CrossRef]
- Santos, L.G.; Silva, G.F.A.; Gomes, B.M.; Martins, V.G. A Novel Sodium Alginate Active Films Functionalized with Purple Onion Peel Extract (Allium cepa). Biocatal. Agric. Biotechnol. 2021, 35, 102096. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A. Development and Characterization of an Antimicrobial Edible Film from Basil Seed (Ocimum basilicum L.) Mucilage and Sodium Alginate. Biocatal. Agric. Biotechnol. 2022, 44, 102450. [Google Scholar] [CrossRef]
- Amankwaah, C.; Li, J.; Lee, J.; Pascall, M.A. Antimicrobial Activity of Chitosan-Based Films Enriched with Green Tea Extracts on Murine norovirus, Escherichia coli, and Listeria innocua. Int. J. Food Sci. 2020, 2020, 3941924. [Google Scholar] [CrossRef]
- Türe, H.; Eroğlu, E.; Soyer, F.; Özen, B. Antifungal Activity of Biopolymers Containing Natamycin and Rosemary Extract against Aspergillus niger and Penicillium roquefortii. Int. J. Food Sci. Technol. 2008, 43, 2026–2032. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, Optical and Mechanical Properties of Chitosan–Starch Films with Natural Extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and Antimicrobial Properties of Ethylene Vinyl Alcohol Copolymer Films Based on the Release of Oregano Essential Oil and Green Tea Extract Components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Lourenço, R.V.; Balestra, F.; Bittante, A.M.Q.B.; Sobral, P.J.d.A.; Rosa, M.D. Influence of Pitanga (Eugenia uniflora L.) Leaf Extract and/or Natamycin on Properties of Cassava Starch/Chitosan Active Films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, J.; Dong, H.; Hou, H.; Guo, P. Properties and Antimicrobial Activities of Starch–Sodium Alginate Composite Films Incorporated with Sodium Dehydroacetate or Rosemary Extract. J. Appl. Polym. Sci. 2013, 127, 1951–1958. [Google Scholar] [CrossRef]
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel Composite Films Based on Sodium Alginate and Gallnut Extract with Enhanced Antioxidant, Antimicrobial, Barrier and Mechanical Properties. Food Hydrocoll. 2021, 113, 106508. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Yáñez-Fernández, J.; Montañez-Barragán, B.; Huerta, B.E.B. Valorization of Coffee Parchment Waste (Coffea arabica) as a Source of Caffeine and Phenolic Compounds in Antifungal Gellan Gum Films. LWT 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Norajit, K.; Kim, K.M.; Ryu, G.H. Comparative Studies on the Characterization and Antioxidant Properties of Biodegradable Alginate Films Containing Ginseng Extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Bojorges, H.; Ríos-Corripio, M.A.; Hernández-Cázares, A.S.; Hidalgo-Contreras, J.V.; Contreras-Oliva, A. Effect of the Application of an Edible Film with Turmeric (Curcuma longa L.) on the Oxidative Stability of Meat. Food Sci. Nutr. 2020, 8, 4308–4319. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and Antioxidant Properties of Active Alginate Edible Films Containing Phenolic Extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, Physicochemical and Antioxidant Properties of Sodium Alginate Isolated from a Tunisian Brown Seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical Properties and Antioxidant Activity of Gelatin-Sodium Alginate Edible Films with Tea Polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Romani, V.P.; Hernández, C.P.; Martins, V.G. Pink Pepper Phenolic Compounds Incorporation in Starch/Protein Blends and Its Potential to Inhibit Apple Browning. Food Packag. Shelf Life 2018, 15, 151–158. [Google Scholar] [CrossRef]

 ), alginate + TPP + licorice coating (
), alginate + TPP + licorice coating ( ), alginate + TPP + eucalyptus coating (
), alginate + TPP + eucalyptus coating ( ), alginate + TPP + sage coating (
), alginate + TPP + sage coating ( ) and alginate + TPP + thyme coating (
) and alginate + TPP + thyme coating ( ). The coating with alginate and TPP (no extract) is the control. Bacterial strains: (a) S. aureus, (b) B. cereus, (c) L. monocytogenes, (d) E. coli and (e) P. aeruginosa.
). The coating with alginate and TPP (no extract) is the control. Bacterial strains: (a) S. aureus, (b) B. cereus, (c) L. monocytogenes, (d) E. coli and (e) P. aeruginosa.
 ), alginate + TPP + licorice coating (
), alginate + TPP + licorice coating ( ), alginate + TPP + eucalyptus coating (
), alginate + TPP + eucalyptus coating ( ), alginate + TPP + sage coating (
), alginate + TPP + sage coating ( ) and alginate + TPP + thyme coating (
) and alginate + TPP + thyme coating ( ). The coating with alginate and TPP (no extract) is the control. Bacterial strains: (a) S. aureus, (b) B. cereus, (c) L. monocytogenes, (d) E. coli and (e) P. aeruginosa.
). The coating with alginate and TPP (no extract) is the control. Bacterial strains: (a) S. aureus, (b) B. cereus, (c) L. monocytogenes, (d) E. coli and (e) P. aeruginosa.
 ), alginate + TPP + licorice film (
), alginate + TPP + licorice film ( ) alginate + TPP + eucalyptus film (
) alginate + TPP + eucalyptus film ( ), alginate + TPP + sage film (
), alginate + TPP + sage film ( ) and alginate + TPP + thyme film (
) and alginate + TPP + thyme film ( ). The film with alginate and TPP (no extract) is the control. Bacterial strains: (a) S. aureus, (b) B. cereus, (c) L. monocytogenes, (d) E. coli and (e) P. aeruginosa.
). The film with alginate and TPP (no extract) is the control. Bacterial strains: (a) S. aureus, (b) B. cereus, (c) L. monocytogenes, (d) E. coli and (e) P. aeruginosa.
 ), alginate + TPP + licorice film (
), alginate + TPP + licorice film ( ) alginate + TPP + eucalyptus film (
) alginate + TPP + eucalyptus film ( ), alginate + TPP + sage film (
), alginate + TPP + sage film ( ) and alginate + TPP + thyme film (
) and alginate + TPP + thyme film ( ). The film with alginate and TPP (no extract) is the control. Bacterial strains: (a) S. aureus, (b) B. cereus, (c) L. monocytogenes, (d) E. coli and (e) P. aeruginosa.
). The film with alginate and TPP (no extract) is the control. Bacterial strains: (a) S. aureus, (b) B. cereus, (c) L. monocytogenes, (d) E. coli and (e) P. aeruginosa.
| Standard Compound | Calibration Curve | R2/LOD/LOQ |
|---|---|---|
| S. officinalis | ||
| Apigenin-6-C-glucoside | y = 107025x + 61531 | R2 = 0.9989; LOD = 0.19 µg/mL; LOQ = 0.63 µg/mL |
| Rosmarinic Acid | y = 191291x − 652903 | R2 = 0.999; LOD = 0.15 µg/mL; LOQ = 0.68 µg/mL |
| Apigenin-7-O-glucoside | y = 10683x − 45794 | R2 = 0.999; LOD = 0.10 μg/mL; LOQ = 0.53 μg/mL |
| E. obliqua | ||
| Catechin | y = 84950x − 23200 | R2 = 0.9999; LOD 0.17 μg/mL; LOQ 0.68 μg/mL |
| Caffeic Acid | y = 388345x + 406369 | R2 = 0.994; LOD = 0.78 μg/mL; LOQ = 1.97 μg/mL |
| Apigenin-7-O-glucoside | y = 10683x − 45794 | R2 = 0.999; LOD = 0.10 μg/mL; LOQ = 0.53 μg/mL |
| Apigenin-6-C-glucoside | y = 107025x + 61531 | R2 = 0.9989; LOD = 0.19 µg/mL; LOQ = 0.63 µg/mL |
| Naringenin | y = 18433x + 78903 | R2 = 0.9998; LOD = 0.17 µg/mL; LOQ = 0.81 µg/mL |
| Quercetin-3-O-glucoside | y = 34843x − 160173 | R2 = 0.9998; LOD 0.21 μg/mL; LOQ 0.71 μg/mL |
| Rosmarinic Acid | y = 191291x − 652903 | R2 = 0.999; LOD = 0.15 µg/mL; LOQ = 0.68 µg/mL |
| Naringenin | y = 18433x + 78903 | R2 = 0.9998; LOD = 0.17 µg/mL; LOQ = 0.81 µg/mL |
| G. glaba | ||
| Naringenin | y = 18433x + 78903 | R2 = 0.9998; LOD = 0.17 µg/mL; LOQ = 0.81 µg/mL |
| Apigenin-6-C-glucoside | y = 107025x + 61531 | R2 = 0.9989; LOD = 0.19 µg/mL; LOQ = 0.63 µg/mL |
| Rosmarinic Acid | y = 191291x − 652903 | R2 = 0.999; LOD = 0.15 µg/mL; LOQ = 0.68 µg/mL |
| p-hydroxybenzoic Acid | y = 208604x + 173056 | R2 = 0.9988 LOD = 0.27 µg/mL; LOQ = 0.91 µg/mL |
| Isoliquiritigenin | y = 42820x + 184902 | R2 = 0.9899; LOD = 0.85 µg/mL; LOQ = 0.98 µg/mL |
| S. officinalis (Sage) | ||||||
|---|---|---|---|---|---|---|
| Peak | Rt (min) | Λmax (nm) | [M–H]− m/z | MS2 | Tentative Identification | Quantification (mg/g) |
| 1 | 4.87 | 325 | 473 | 311(46), 293(17), 179(81), 149(100), 135(12) | Caftaric Acid Hexoside | 0.54 ± 0.02 |
| 2 | 5.15 | 324 | 341 | 179(100) | Caffeic Acid Hexoside Isomer I | 0.52 ± 0.02 |
| 3 | 5.76 | 315 | 341 | 179(100) | Caffeic Acid Hexoside Isomer II | 0.46 ± 0.01 |
| 4 | 6.65 | 282sh313 | 447 | 401(71), 269(100) | Apigenin-O-glucuronide | 1.84 ± 0.10 |
| 5 | 7.40 | 287sh324 | 387 | 369(26), 207(100), 163(47) | Caffeic Acid Acetylhexoside | 0.41 ± 0.03 |
| 6 | 8.75 | 325 | 593 | 473(100), 383(22), 353(41) | Apigenin-C-Hexoside-O-Hexoside | 6.9 ± 0.2 |
| 7 | 10.88 | 324 | 537 | 519(84), 341(10), 179(32), 161(48), 135(10) | Salvianolic Acid I | 1.21 ± 0.01 |
| 8 | 12.80 | 328 | 637 | 351(100), 285(47) | Luteolin-O-diglucuronide | 1.4 ± 0.1 |
| 9 | 13.70 | 327 | 533 | 489(100), 285(18) | Luteolin-O-malonylhexoside Isomer I | 2.5 ± 0.1 |
| 10 | 14.65 | 339 | 533 | 489(100), 285(21) | Luteolin-O-malonylhexoside Isomer II | 2.1 ± 0.1 |
| 11 | 14.84 | 337 | 533 | 489(100), 285(19) | Luteolin-O-malonylhexoside Isomer III | 1.97 ± 0.10 |
| 12 | 16.07 | 326 | 521 | 359(100), 197(22), 179(34), 161(74) | Rosmarinic Acid Hexoside | 2.38 ± 0.01 |
| 13 | 16.82 | 331 | 593 | 285(100) | Luteolin-O-rutinoside | 1.72 ± 0.04 |
| 14 | 17.57 | 343 | 461 | 285(100) | Luteolin-7-O-glucuronide | 27 ± 1 |
| 15 | 20.10 | 328 | 719 | 539(9), 521(5), 359(100), 197(8) | Sagerinic Acid | 6.9 ± 0.1 |
| 16 | 20.31 | 335 | 359 | 197(19), 179(23), 161(100) | Rosmarinic Acid | 51 ± 1 |
| 17 | 21.65 | 331 | 401 | 269(100) | Apigenin-O-pentoside | 5.3 ± 0.3 |
| 18 | 22.50 | 331 | 533 | 489(100), 285(15) | Luteolin-O-malonylhexoside Isomer IV | 5.1 ± 0.1 |
| 19 | 23.83 | 331 | 769 | 285(100) | Methyl-Luteolin-O-Deoxyhexoside-O-glucoside-C-glucoside | 3.0 ± 0.1 |
| 20 | 25.82 | 327 | 563 | 545(22), 503(41), 473(100), 443(80), 383(95), 353(74) | Apigenin 6-C-pentosyl-8-C-hexoside | 2.4 ± 0.1 |
| 21 | 27.96 | 326 | 563 | 545(21), 503(34), 473(100), 443(78), 383(94), 353(81) | Apigenin-8-C-pentosyl-6-C-hexoside | 4.93 ± 0.01 |
| TPA | 63 ± 1 | |||||
| TF | 66 ± 2 | |||||
| TPC | 130 ± 3 | |||||
| E. obliqua (Eucalyptus) | ||||||
| 22 | 4.96 | 262 | 783 | 481(13), 301(27) | Bis-HHDP-glucose | 37.4 ± 1.2 |
| 23 | 5.45 | 281sh328 | 353 | 191(100), 179(81), 173(8), 135(45) | 4-O-Caffeoylquinic Acid | 13.2 ± 0.1 |
| 24 | 6.36 | 280sh329 | 353 | 191(100), 179(32), 161(15), 135(5) | 5-O-Caffeoylquinic Acid | 11 ± 2 |
| 25 | 6.76 | 280 | 785 | 615(21), 463(5), 301(66) | Digalloyl-HHDP-hexose Isomer I | 8.1 ± 0.3 |
| 26 | 8.75 | 278 | 633 | 481(2), 463(14), 301(100) | Galloyl-HHDP-glucose | 5.7 ± 1.3 |
| 27 | 10.08 | 272 | 785 | 615(12), 463(3), 301(54) | Digalloyl-HHDP-hexose Isomer II | 7.6 ± 1.2 |
| 28 | 11.16 | 310 | 337 | 191(13), 173(6), 163(96), 155(6) | 3-p-Coumaroylquinic Acid | 1.0 ± 0.2 |
| 29 | 14.84 | 360 | 787 | 635(28), 483(84), 465(100), 447(6), 423(73), 313(10), 169(5) | Tetragalloyl-glucose | 4.5 ± 0.4 |
| 30 | 15.97 | 278 | 499 | 377(100), 273(52), 163(23) | Caffeoyl-coumaroyl-quinic Acid | 2.47 ± 0.01 |
| 31 | 16.53 | 355 | 609 | 301(100) | Quercetin-3-O-rutinoside | 1.3 ± 0.1 |
| 32 | 17.13 | 353 | 477 | 315(68), 301(19) | Methyl Ellagic Acid Hexoside | 6.3 ± 0.1 |
| 33 | 18.21 | 358 | 497 | 313(45), 169(100) | Eucaglobulin/Globulusin B Isomer I | 5.3 ± 0.1 |
| 34 | 19.60 | 360 | 497 | 313(49), 169(100) | Eucaglobulin/Globulusin B Isomer II | 4.4 ± 0.3 |
| 35 | 20.94 | 344 | 447 | 301(100) | Quercetin-O-rhamnoside | 1.1 ± 0.1 |
| 36 | 21.76 | 349 | 447 | 315(100), 300(32) | Methyl ellagic acid pentoside sómer I | 3.1 ± 0.3 |
| 37 | 22.62 | 363 | 447 | 315(100), 300(12) | Methyl Ellagic Acid Pentoside Isomer II | 2.3 ± 0.1 |
| 38 | 23.82 | 350 | 461 | 315(95), 300(41) | Methyl Ellagic Acid Deoxyhexoside | 2.2 ± 0.1 |
| 39 | 29.20 | 345 | 463 | 301(100) | Quercetin-3-O-glucoside | 0.65 ± 0.03 |
| 40 | 29.85 | 345 | 895 | 447(100), 301(6), 273(97), 179(2) | Luteolin-O-dihydrogalloyl-glucosyl-C-pentosyl-glucoside | 1.0 ± 0.1 |
| 41 | 30.42 | 342 | 933 | 631(17), 301(33) | Castalagin/Vescalagin | 1.33 ± 0.02 |
| TPA | 27.7 ± 2.1 | |||||
| THT | 88 ± 6 | |||||
| TF | 4.0 ± 0.2 | |||||
| TPC | 120.1 ± 8.2 | |||||
| T. vulgaris (Thyme) | ||||||
| 42 | 4.94 | 263 | 305 | 219(45), 179(41), 125(100) | Gallocatechin | 3.5 ± 0.1 |
| 43 | 5.92 | 314 | 341 | 179(100) | Caffeic Acid Hexoside | 0.48 ± 0.02 |
| 44 | 6.66 | 315 | 387 | 369(25), 207(100), 163(47) | Caffeic Acid Acetylhexoside | 0.47 ± 0.02 |
| 45 | 8.72 | 321 | 593 | 473(100), 455(9), 383(18), 353(26) | Apigenin 6-C-glucose-8-C-Glucose | 1.4 ± 0.01 |
| 46 | 9.29 | 321 | 593 | 503(21), 473(100), 383(17), 353(32) | Apigenin-C-Hexoside-O-Hexoside | 2.5 ± 0.1 |
| 47 | 12.04 | 330 | 637 | 351(100), 285(47) | Luteolin-O-diglucuronide | 1.0 ± 0.1 |
| 48 | 12.85 | 316 | 563 | 473(93), 443(100), 383(27), 353(31), 287(5) | Apigenin 6-C-pentosyl-8-C-hexoside | 0.519 ± 0.003 |
| 49 | 13.99 | 325 | 595 | 287(100) | Eriodictyol-7-O-rutinoside | 0.14 ± 0.01 |
| 50 | 15.01 | 339 | 463 | 301(100) | Quercetin-3-O-galactoside | 0.81 ± 0.03 |
| 51 | 15.82 | 322 | 521 | 359(100) | Rosmarinic Acid Hexoside | 2.5 ± 0.1 |
| 52 | 17.01 | 343 | 461 | 285(100) | Luteolin-7-O-glucuronide | 6.3 ± 0.3 |
| 53 | 17.83 | 340 | 447 | 285(100) | Luteolin-6-C-glucoside | 2.9 ± 0.2 |
| 54 | 19.07 | 330 | 555 | 493(100), 359(85) | Salvianolic Acid K | 0.8 ± 0.05 |
| 55 | 19.67 | 328 | 359 | 197(15), 179(21), 161(100) | cis-Rosmarinic Acid | 20.6 ± 0.2 |
| 56 | 20.98 | 330 | 359 | 197(19), 179(23), 161(100) | trans-Rosmarinic Acid | 11.4 ± 0.3 |
| 57 | 22.11 | 328 | 539 | 377(100), 307(92), 275(61) | Yunnaneic Acid D | 0.758 ± 0.004 |
| 58 | 22.69 | 333 | 537 | 493(100), 359(22), 179(3) | Lithospermic Acid A | 0.97 ± 0.05 |
| 59 | 23.13 | 326 | 537 | 493(100), 359(12) | Lithospermic Acid A Isomer | 0.93 ± 0.05 |
| 60 | 29.64 | 328 | 637 | 351(6), 285(5), 283(100) | Luteolin-7-O-diglucuronide | 1.06 ± 0.04 |
| TPA | 39 ± 1 | |||||
| TF | 17 ± 1 | |||||
| TF3O | 3.5 ± 0.1 | |||||
| TPC | 59 ± 1 | |||||
| G. glaba (Licorice) | ||||||
| 61 | 4.66 | 276 | 209 | 191(100), 85(13) | Glucaric Acid | 3.8 ± 0.2 |
| 62 | 6.62 | 273 | 711 | 549(100) | Glucoliquiritin Apioside | 0.33 ± 0.02 |
| 63 | 8.79 | 271sh331 | 593 | 473(100), 383(23),353(45) | Apigenin-6,8-di-C-glycoside | 2.08 ± 0.05 |
| 64 | 10.41 | 323 | 565 | 445(100), 271(93) | Naringenin-O-apiosylglucoside Isomer I | 0.035 ± 0.002 |
| 65 | 12.54 | 272sh331 | 563 | 443(13), 413(4), 323(4), 311(3), 293(3) | Apigenin-O-pentosyl-6-C-Hexoside | 1.42 ± 0.003 |
| 66 | 12.92 | 272sh333 | 563 | 443(13), 413(4), 323(4), 311(3), 293(3) | Apigenin-O-pentosyl-6-C-hexoside Isomer | 2.01 ± 0.05 |
| 67 | 14.26 | 312 | 549 | 429(69), 255(100) | Liquiritin Apioside | 2.36 ± 0.01 |
| 68 | 15.41 | 274/322 | 549 | 429(9), 255(100) | Isoliquiritin Apioside | 4.06 ± 0.05 |
| 69 | 15.99 | 325 | 577 | 457(100), 383(35), 353(43) | (Iso)violanthin | 3.1 ± 0.2 |
| 70 | 18.29 | 330 | 565 | 445(100), 427(33), 313(14), 271(59) | Naringenin-O-apiosylglucoside Isomer II | 1.1 ± 0.1 |
| 71 | 19.70 | 288sh32 | 359 | 197(41), 179(28), 161(100) | Rosmarinic Acid | 1.9 ± 0.1 |
| 72 | 23.52 | 252sh300 | 561 | 267(100), 252(52) | Formononetin-7-O-apiosylglucoside | 1.21 ± 0.03 |
| 73 | 24.97 | 361 | 549 | 417(18), 297(23), 311(8), 255(100), 191(3) | Liquiritigenin Apiosyl-glucoside | 2.4 ± 0.1 |
| 74 | 25.86 | 370 | 549 | 429(80), 297(11), 255(100) | Liquiritigenin Apiosyl-glucoside Isomer I | 5.1 ± 0.2 |
| 75 | 26.62 | 367 | 549 | 429(80), 255(100) | Liquiritigenin Apiosyl-glucoside Isomer II | 0.36 ± 0.01 |
| 76 | 27.55 | 280 | 695 | 549(100), 531(52), 255(11) | Licorice Glycoside D1/ D | 2.5 ± 0.1 |
| 77 | 29.34 | 285 | 1025 | 991(100), 946(12) | 22-Acetoxyl-rhaoglycyrrhizin | 1.2 ± 0.1 |
| 78 | 32.04 | 325 | 837 | 351(100), 289(5) | Licorice Saponin G2 | 0.9 ± 0.1 |
| 79 | 32.79 | 325 | 695 | 549(100), 531(80), 255(12) | Licorice Glycoside B | 2 ± 0.1 |
| 80 | 39.48 | 283 | 837 | 351(100), 289(5) | 24-hydroxyl-glycyrrhizin | 0.87 ± 0.04 |
| 81 | 40.28 | 251 | 821 | 351(100), 193(5) | Glycyrrhizin | 3.92 ± 0.01 |
| TPA | 5.7 ± 0.3 | |||||
| TF | 25.5 ± 0.2 | |||||
| TSAP | 11.35 ± 0.01 | |||||
| TPC | 42.6 ± 0.1 | |||||
| Eucalyptus Extract | Sage Extract | Thyme Extract | Licorice Extract | Positive Control | |
|---|---|---|---|---|---|
| Trolox | |||||
| DPPH (EC50, mg/mL) | 0.11 ± 0.01 d | 0.36 ± 0.01 b | 0.21 ± 0.01 c | 3.6 ± 0.1 a | |
| Reducing Power (EC50, mg/mL) | 0.092 ± 0.001 d | 0.271 ± 0.001 b | 0.168 ± 0.001 c | 0.945 ± 0.005 a | |
| TBARS (EC50, mg/mL) | 0.136 ± 0.003 d | 0.51 ± 0.01 c | 1.44 ± 0.04 b | 3.03 ± 0.13 a | 5.4 ± 0.3 |
| CAA (% inhibition [] max tested) | >2000 | 23 ± 2 c | 31 ± 1 b | 50 ± 4 a |
| Eucalyptus Extract | Sage Extract | Thyme Extract | Licorice Extract | Commercial Food Preservatives | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E211 | E224 | |||||||||||
| Antibacterial Activity (mg/mL) | ||||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. aureus | 0.5 | 1 | 0.25 | 0.5 | 1 | 2 | <0.25 | <0.25 | 4 | 4 | 1 | 1 |
| B. cereus | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | <0.25 | <0.25 | 0.5 | 0.5 | 2 | 4 |
| L. monocytogenes | 0.5 | 1 | 0.25 | 0.5 | 1 | 2 | <0.25 | <0.25 | 1 | 2 | 0.5 | 1 |
| E. coli | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | <0.25 | <0.25 | 1 | 2 | 0.5 | 1 |
| S. typhimurium | 0.5 | 1 | 0.25 | 0.5 | 1 | 2 | <0.25 | <0.25 | 1 | 2 | 1 | 1 |
| E. cloacae | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | <0.25 | <0.25 | 2 | 4 | 0.5 | 0.5 |
| Antifungal Activity (mg/mL) | ||||||||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| A. fumigatus | 4 | 8 | 1 | 2 | 2 | 4 | 2 | 4 | 1 | 2 | 1 | 1 |
| A. niger | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 4 | 1 | 2 | 1 | 1 |
| A. versicolor | 2 | 4 | 1 | 2 | 2 | 4 | 4 | 8 | 2 | 2 | 1 | 1 |
| P. funiculosum | 2 | 4 | 1 | 2 | 2 | 4 | 1 | 2 | 1 | 2 | 0.5 | 0.5 |
| T. viride | 1 | 2 | 0.5 | 1 | 1 | 2 | 1 | 2 | 2 | 4 | 1 | 1 |
| P. verrucosum var. cyclopium | 2 | 4 | 2 | 4 | 2 | 4 | 1 | 2 | 1 | 2 | 0.5 | 0.5 |
| Inhibition (%) | ||||
|---|---|---|---|---|
| Fungus | Eucalyptus | Licorice | Sage | Thyme |
| A. niger | 33 ± 7.1 a | 47 ± 0 b | 48 ± 5.9 b | 21 ± 7.9 c |
| P. expansum | NI | NI | 13 ± 3.7 a | NI |
| F. verticillioides | 40 ± 0 a | NI | 83 ± 0 b | NI |
| Cladosporium sp. | 100 ± 0 a | NI | 100 ± 0 a | NI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.I.; Melo, A.; Caleja, C.; Pereira, E.; Finimundy, T.C.; Afonso, T.B.; Silva, S.; Ivanov, M.; Soković, M.; Tavaria, F.K.; et al. Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts. Coatings 2023, 13, 1487. https://doi.org/10.3390/coatings13091487
Lopes AI, Melo A, Caleja C, Pereira E, Finimundy TC, Afonso TB, Silva S, Ivanov M, Soković M, Tavaria FK, et al. Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts. Coatings. 2023; 13(9):1487. https://doi.org/10.3390/coatings13091487
Chicago/Turabian StyleLopes, Ana I., Adma Melo, Cristina Caleja, Eliana Pereira, Tiane C. Finimundy, Tiago B. Afonso, Sara Silva, Marija Ivanov, Marina Soković, Freni K. Tavaria, and et al. 2023. "Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts" Coatings 13, no. 9: 1487. https://doi.org/10.3390/coatings13091487
APA StyleLopes, A. I., Melo, A., Caleja, C., Pereira, E., Finimundy, T. C., Afonso, T. B., Silva, S., Ivanov, M., Soković, M., Tavaria, F. K., Barros, L., & Pintado, M. (2023). Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts. Coatings, 13(9), 1487. https://doi.org/10.3390/coatings13091487