Thickness and Humidity on Proton Conductivity in MOF-508 Thin Film by Twin-Zinc-Source Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zinc Substrate
2.3. Preparation of MOF-508a Thin Film by “Twin-Zinc-Source” Method
2.4. Preparation of MOF-508a Thin Film by the Solvent Thermal Method
2.5. Characterization
2.6. The Method of the Conductivity
3. Result and Discussion
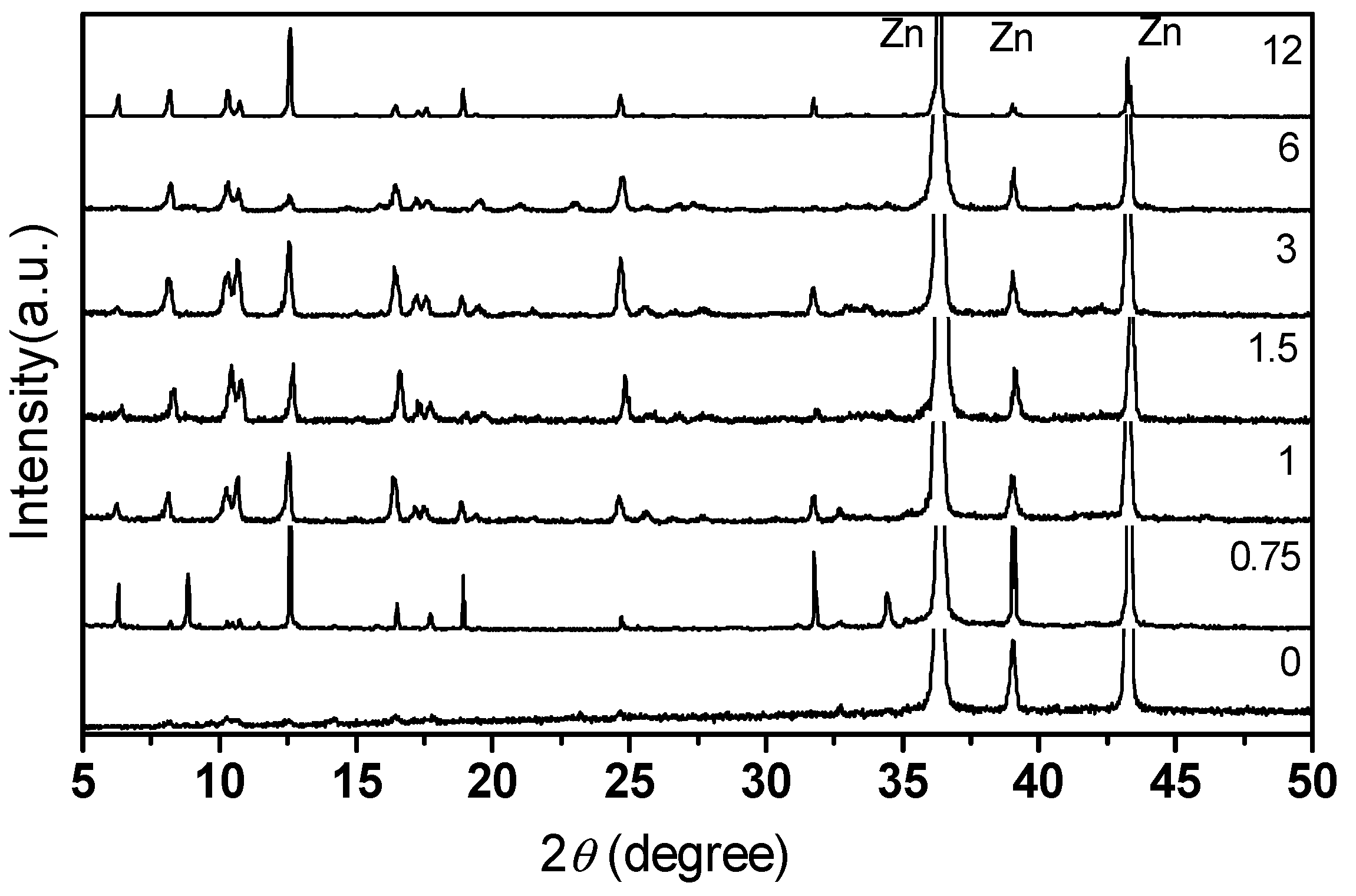
3.1. PXRD Pattern of the MOF-508a Thin Film
3.2. The Effect of Zn2+ Concentration on the Thickness of MOF-508a Film
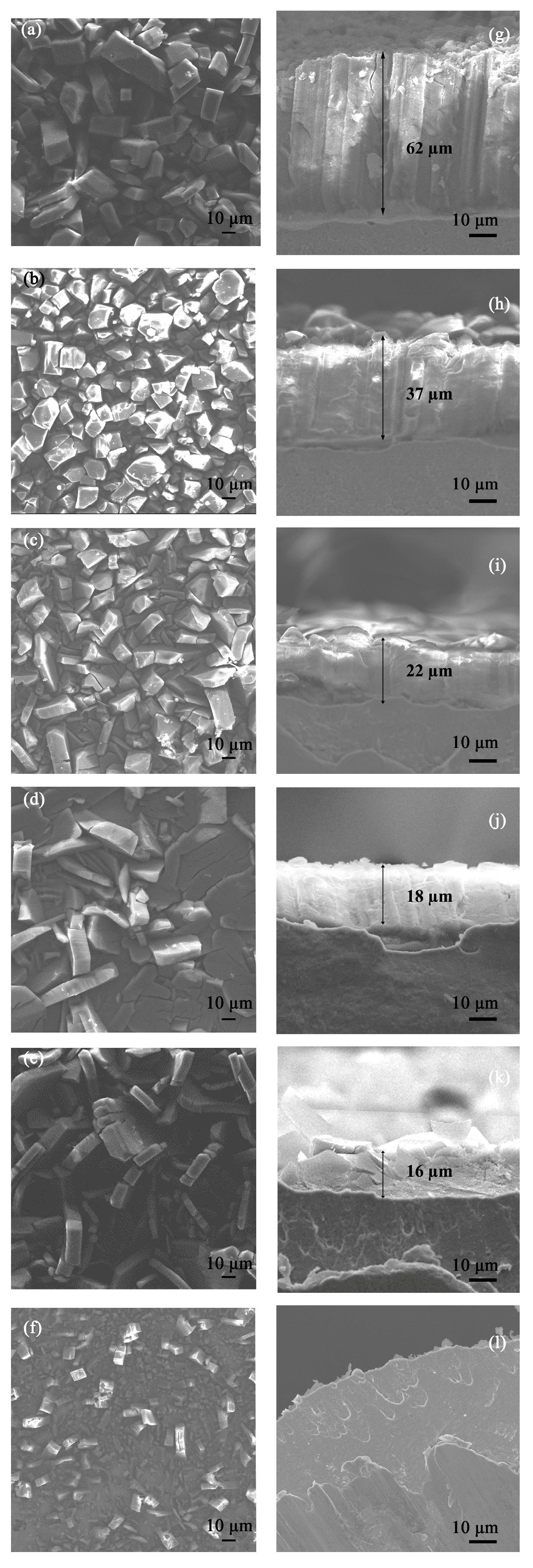
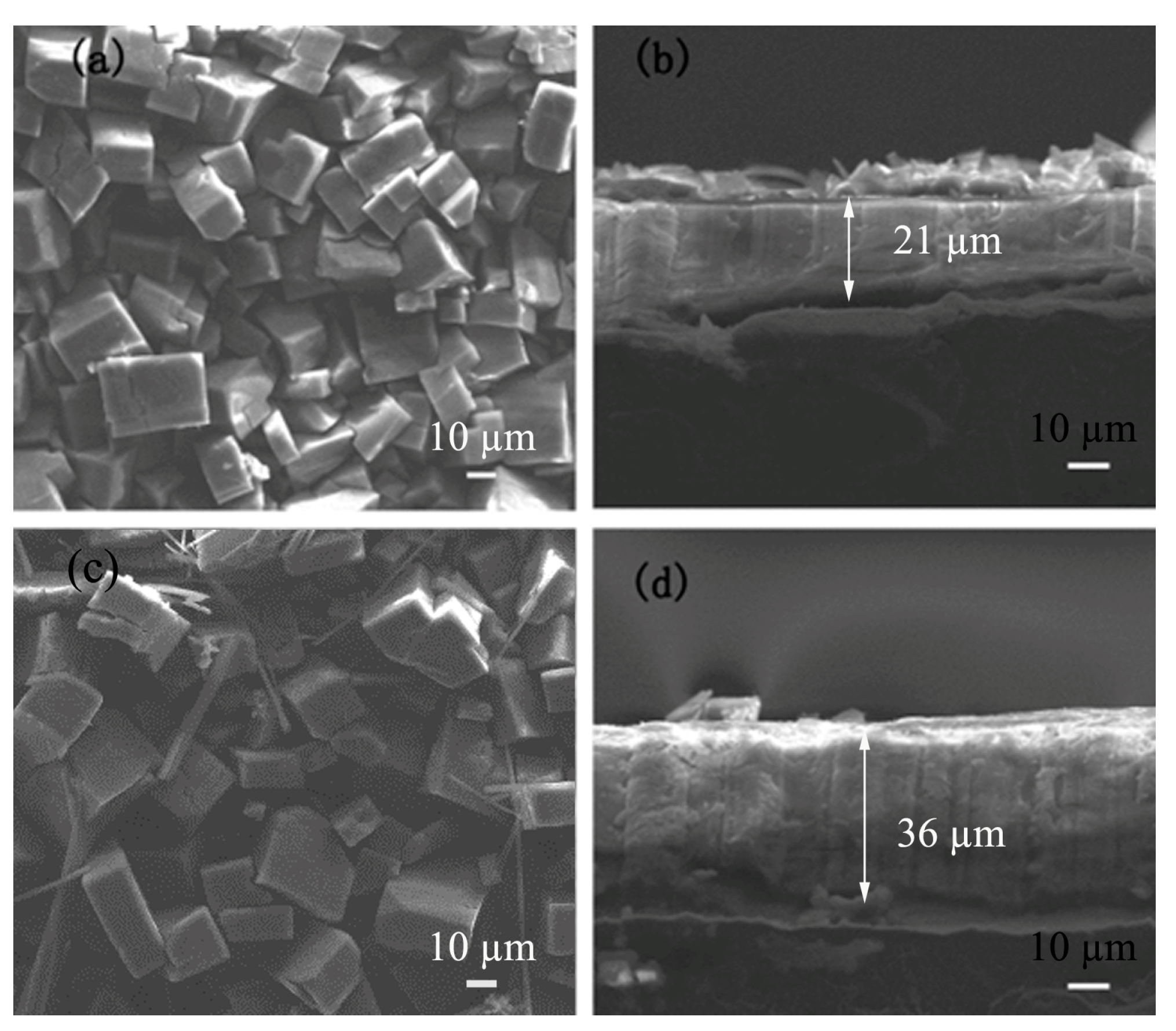
3.3. The SEM Images of the MOF-508a/Zn Thin Films Obtained under Different Zn2+ Concentrations
3.4. The Growth Mechanism of MOF-508 Crystals on the Zinc Foil
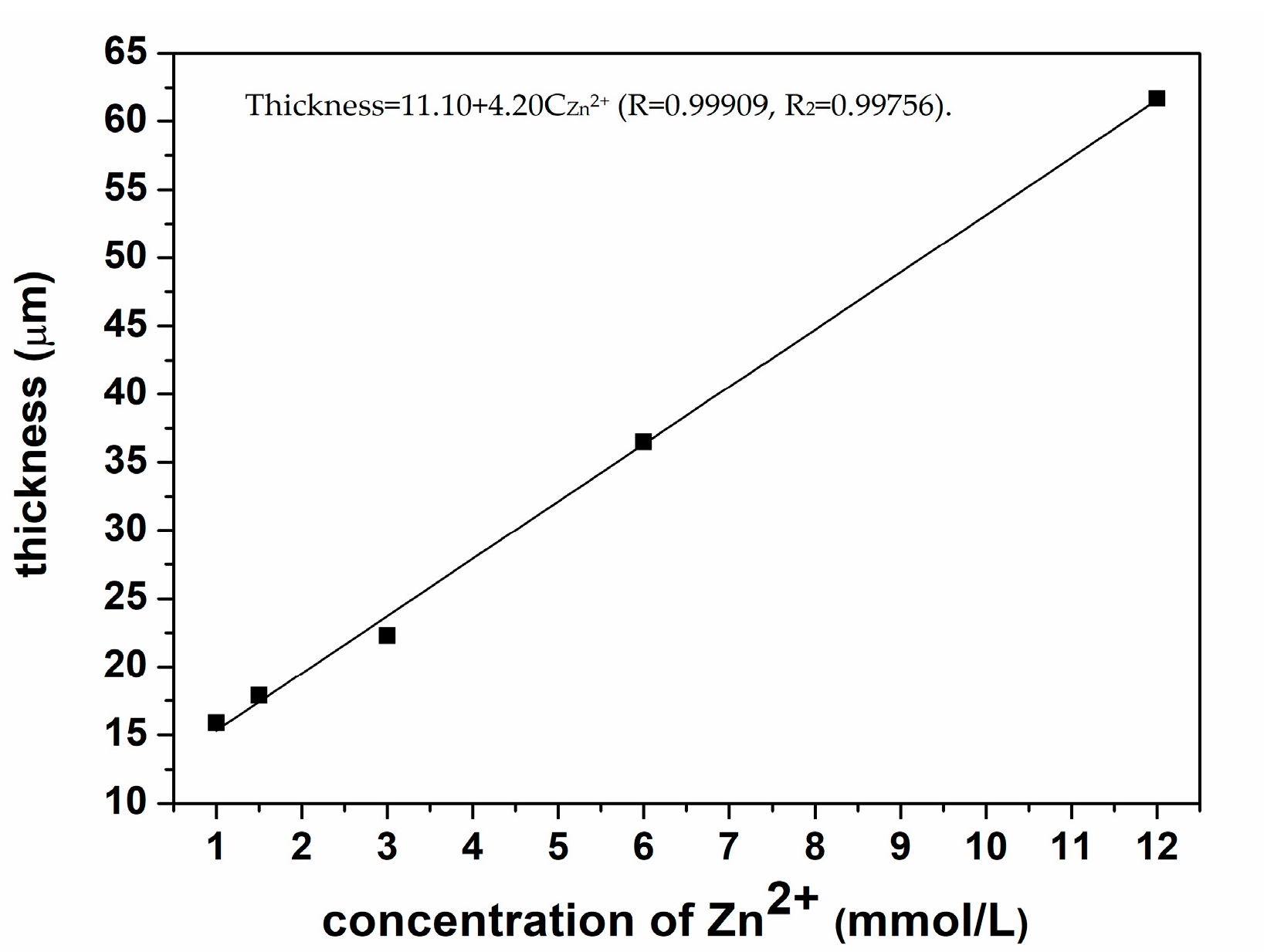
3.5. The Linear Relationship of the Zn2+ Concentration and the Thickness of MOF-508a/Zn Thin Film
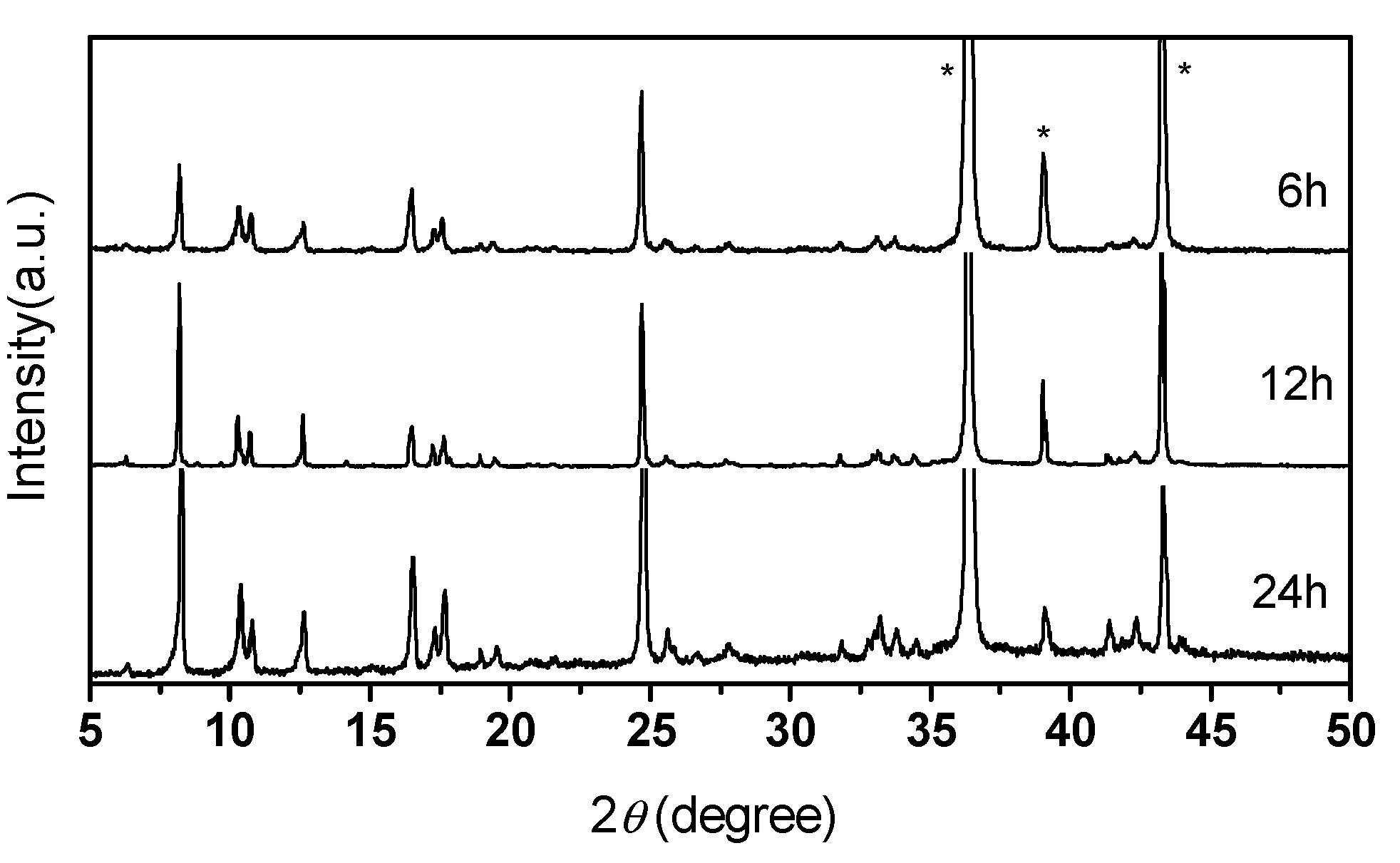
3.6. The Effects of Different Reaction Times on the MOF-508/Zn Thin Film
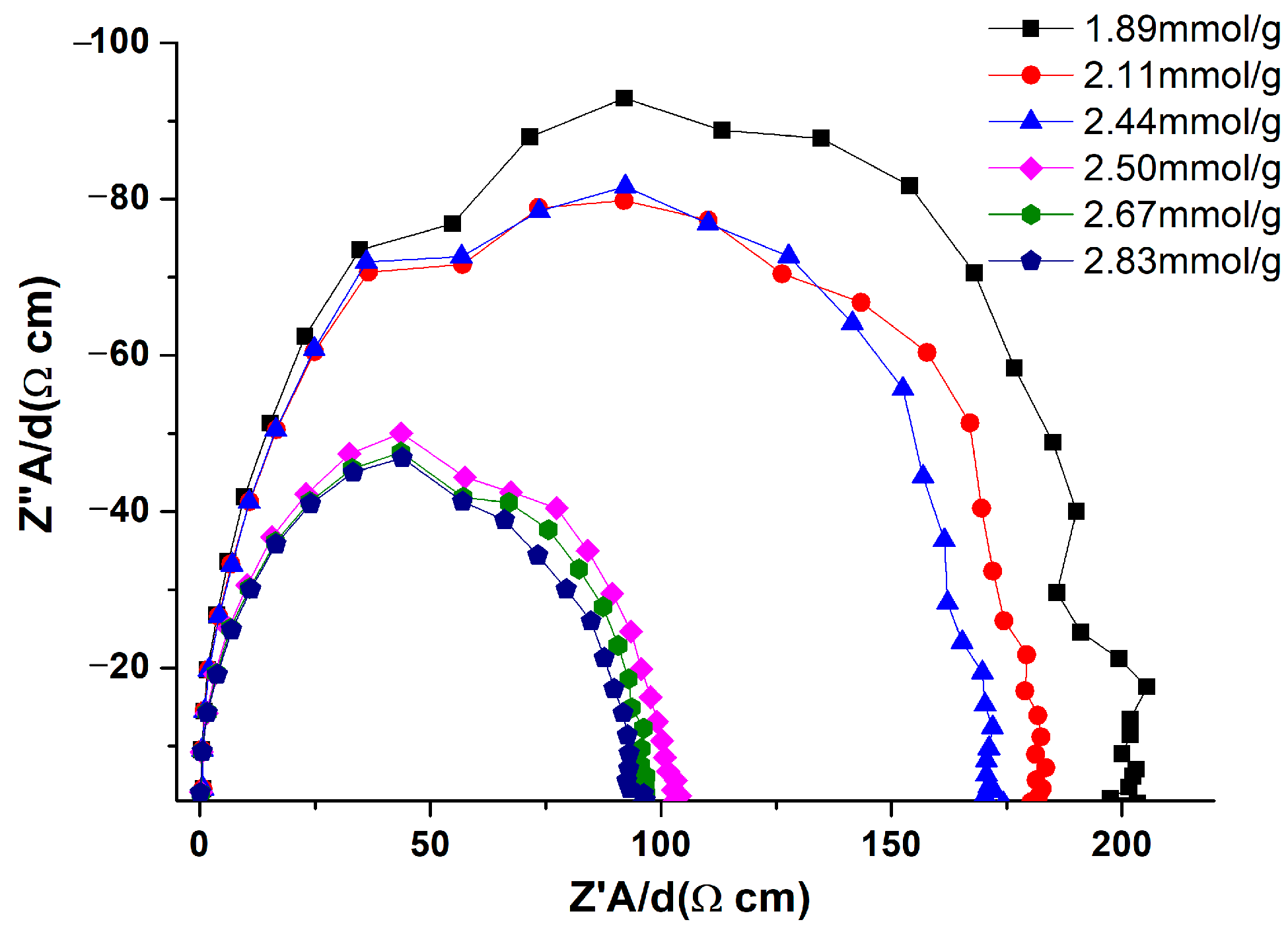
3.7. The Effect of the Thickness on the Resistivity of the MOF-508a/Zn Thin Film
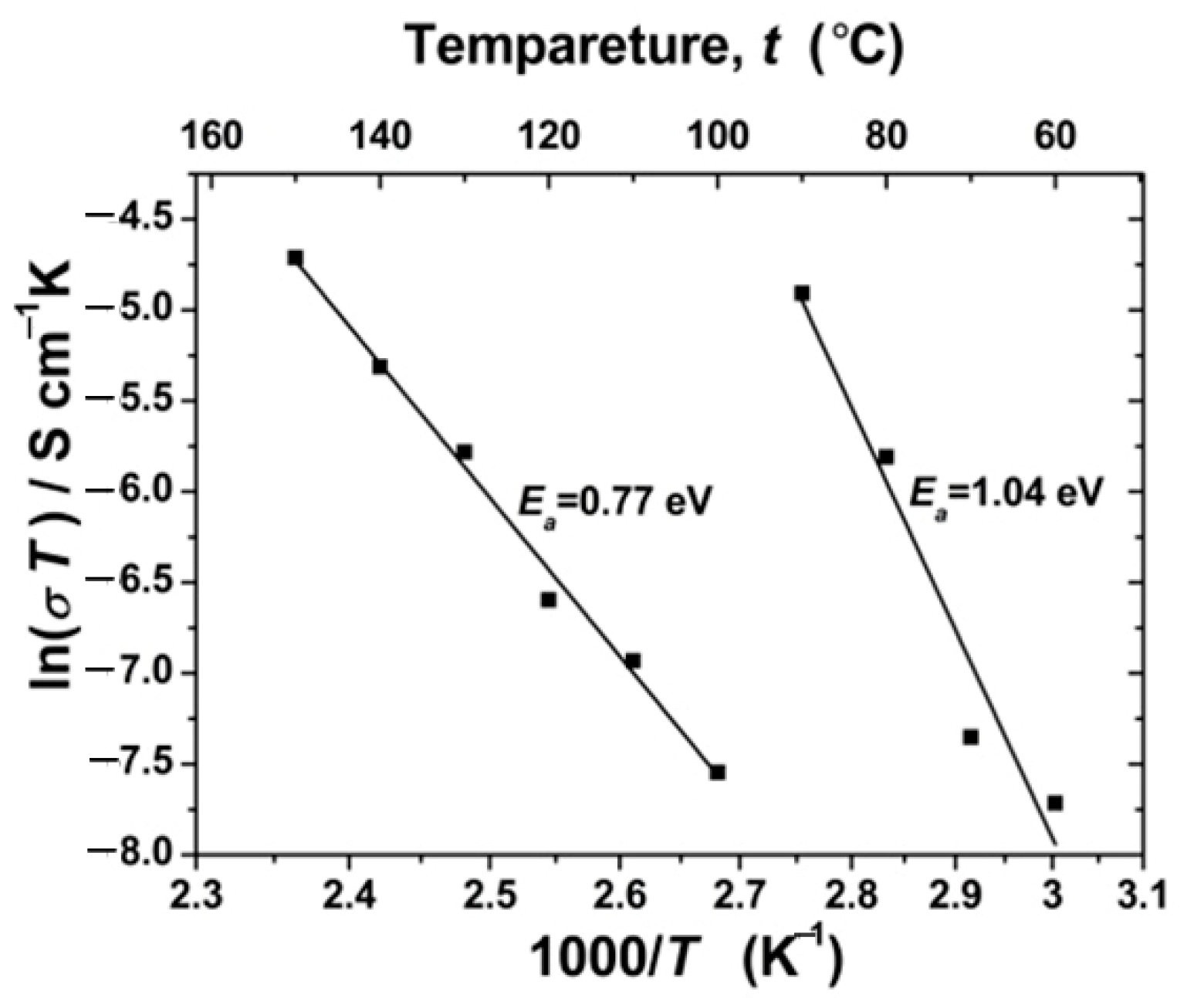
3.8. Arrhenius-Type Plot of MOF-508a Sample at Various Temperatures
3.9. The Effect of H2O Molecules on the Resistivity of the MOF-508b/Zn Thin Film
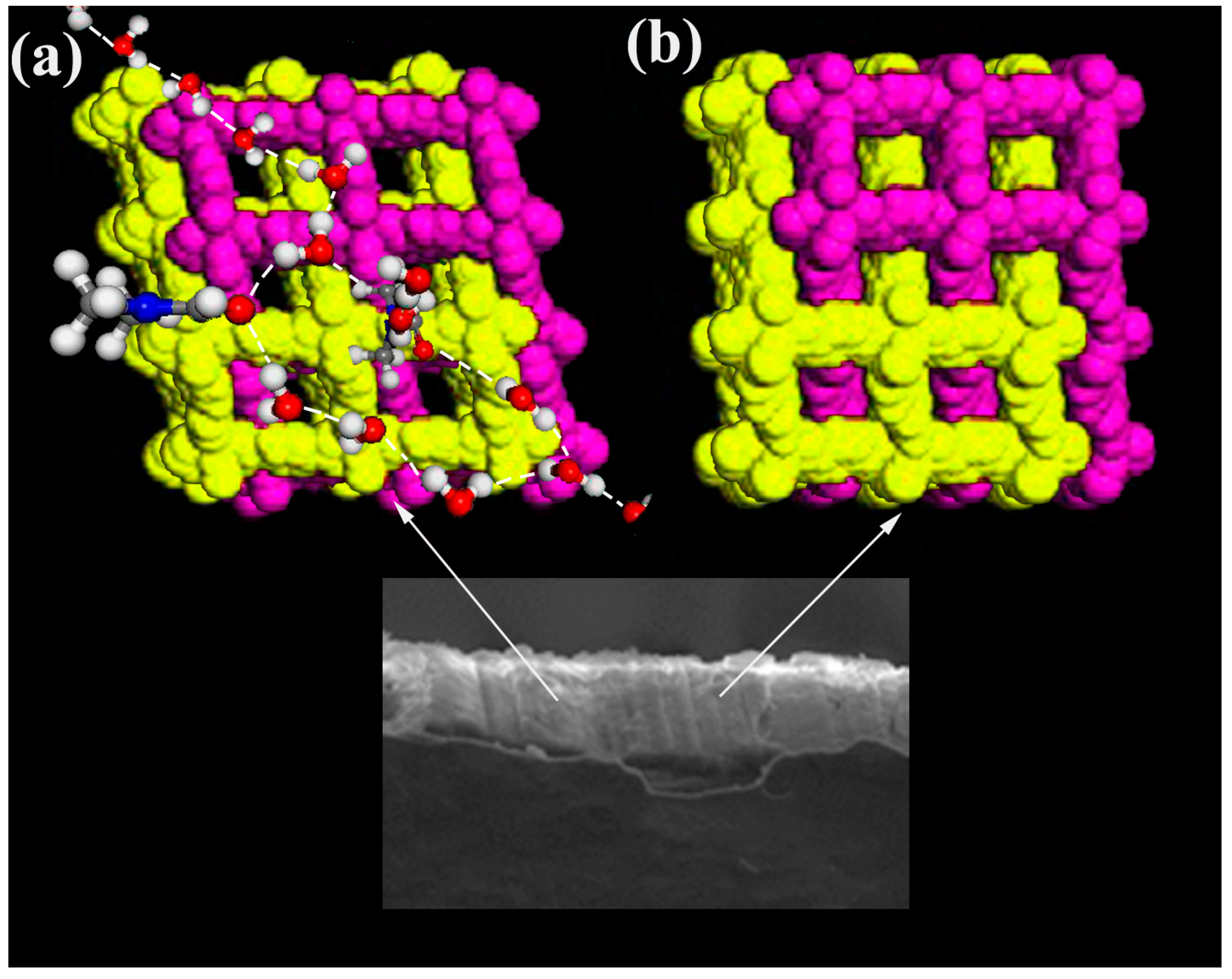
3.10. The Mechanism of Proton Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ye, Y.; Gong, L.; Xiang, S.; Zhang, Z.; Chen, B. Metal–Organic Frameworks as a Versatile Platform for Proton Conductors. Adv. Mater. 2020, 32, e1907090. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.F.; Barbosa, P.; Domingues, E.M.; Silva, P.; Figueiredo, F.; Paz, F.A.A. Enhanced proton conductivity in a layered coordination polymer. Chem. Sci. 2020, 11, 6305–6311. [Google Scholar] [CrossRef] [PubMed]
- Aluru, N.R.; Aydin, F.; Bazant, M.Z.; Blankschtein, D.; Brozena, A.H.; de Souza, J.P.; Elimelech, M.; Faucher, S.; Fourkas, J.T.; Koman, V.B.; et al. Fluids and Electrolytes under Confinement in Single-Digit Nanopores. Chem. Rev. 2023, 123, 2737–2831. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gu, Y.; Liu, C.; Liu, S.; Li, X.; Ma, J.; Ding, M. Missing-Linker 2D Conductive Metal Organic Frameworks for Rapid Gas Detection. ACS Sens. 2021, 6, 429–438. [Google Scholar] [CrossRef]
- Dutta, S.; More, Y.D.; Fajal, S.; Mandal, W.; Dam, G.K.; Ghosh, S.K. Ionic metal–organic frameworks (iMOFs): Progress and prospects as ionic functional materials. Chem. Commun. 2022, 58, 13676–13698. [Google Scholar] [CrossRef]
- Que, Z.; Ye, Y.; Yang, Y.; Xiang, F.; Chen, S.; Huang, J.; Li, Y.; Liu, C.; Xiang, S.; Zhang, Z. Solvent-Assisted Modification to Enhance Proton Conductivity and Water Stability in Metal Phosphonates. Inorg. Chem. 2020, 59, 3518–3522. [Google Scholar] [CrossRef]
- Beaubras, F.; Rue, J.-M.; Perez, O.; Veillon, F.; Caignaert, V.; Jean-François, L.; Cardin, J.; Rogez, G.; Jestin, C.; Couthon, H.; et al. M(H2O)(PO3C10H6OH)·(H2O)0.5 (M = Co, Mn, Zn, Cu): A new series of layered metallophosphonate compounds obtained from 6-hydroxy-2-naphthylphosphonic acid. Dalton Trans. 2020, 49, 3877–3891. [Google Scholar] [CrossRef]
- Lim, D.W.; Kitagawa, H. Rational strategies for proton-conductive metal-organic frameworks. Chem. Soc. Rev. 2021, 50, 6349–6368. [Google Scholar] [CrossRef]
- Monjezi, B.H.; Okur, S.; Limbach, R.; Chandresh, A.; Sen, K.; Hashem, T.; Schwotzer, M.; Wondraczek, L.; Wöll, C.; Knebel, A. Fast Dynamic Synthesis of MIL-68(In) Thin Films in High Optical Quality for Optical Cavity Sensing. ACS Nano 2023, 17, 6121–6130. [Google Scholar] [CrossRef]
- Okada, K.; Mashita, R.; Fukatsu, A.; Takahashi, M. Polarization-dependent plasmonic heating in epitaxially grown multilayered metal–organic framework thin films embedded with Ag nanoparticles. Nanoscale Adv. 2023, 5, 1795–1801. [Google Scholar] [CrossRef]
- Berry, J.F.; Lu, C.C. Metal-Metal Bonds: From Fundamentals to Applications. Inorg. Chem. 2017, 56, 7577–7581. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Ejsmont, A.; Freund, R.; Goscianska, J.; Schmidt, B.V.K.J.; Wuttke, S. Controlling the morphology of metal–organic frameworks and porous carbon materials: Metal oxides as primary architecture-directing agents. Chem. Soc. Rev. 2020, 49, 3348–3422. [Google Scholar] [CrossRef] [PubMed]
- Dadashi, J.; Khaleghian, M.; Hanifehpour, Y.; Mirtamizdoust, B.; Woo Joo, S. Lead(II)-Azido Metal-Organic Coordination Polymers: Synthesis, Structure and Application in PbO Nanomaterials Preparation. Nanomaterials 2022, 12, 2257. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lustig, W.P.; Li, J. Sensing and capture of toxic and hazardous gases and vapors by metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 4729–4756. [Google Scholar] [CrossRef]
- Han, B.-X.; Jiang, Y.-F.; Sun, X.-R.; Li, Z.-F.; Li, G. Proton conductive N-heterocyclic metal–organic frameworks. Coord. Chem. Rev. 2021, 432, 213754. [Google Scholar] [CrossRef]
- Siman, P.; Trickett, C.A.; Furukawa, H.; Yaghi, O.M. l-Aspartate links for stable sodium metal–organic frameworks. Chem. Commun. 2015, 51, 17463–17466. [Google Scholar] [CrossRef]
- Hwang, K.; Ahn, J.; Cho, I.; Kang, K.; Kim, K.; Choi, J.; Polychronopoulou, K.; Park, I. Microporous Elastomer Filter Coated with Metal Organic Frameworks for Improved Selectivity and Stability of Metal Oxide Gas Sensors. ACS App. Mater. Inter. 2020, 12, 13338–13347. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Bian, Y.; Liu, Y.; Zhang, X.; Chen, M.; Hu, B.; Jin, Q. Tuning luminescence of the fluorescent molecule 2-(2-hydroxyphenyl)-1H-benzimidazole via zeolitic imidazolate framework-8. RSC Adv. 2022, 12, 9342–9350. [Google Scholar] [CrossRef]
- Chen, B.; Liang, C.; Yang, J.; Contreras, D.S.; Clancy, Y.L.; Lobkovsky, E.B.; Yaghi, O.M.; Dai, S. A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angew. Chem. Int. Ed. Engl. 2006, 45, 1390–1393. [Google Scholar] [CrossRef]
- Hailing, G.G.Z.; Ian, J.H.; Qiu, S. “Twin Copper Source” Growth of Metal-Organic Framework Membrane: Cu3(BTC)2 with High Permeability and Selectivity for Recycling H2. J. Am. Chem. Soc. 2009, 131, 1646. [Google Scholar]
- Sarango-Ramírez, M.K.; Lim, D.-W.; Kolokolov, D.I.; Khudozhitkov, A.E.; Stepanov, A.G.; Kitagawa, H. Superprotonic Conductivity in Metal–Organic Framework via Solvent-Free Coordinative Urea Insertion. J. Am. Chem. Soc. 2020, 142, 6861–6865. [Google Scholar] [CrossRef] [PubMed]
- Kolokolov, D.I.; Lim, D.; Kitagawa, H. Characterization of Proton Dynamics for the Understanding of Conduction Mechanism in Proton Conductive Metal-Organic Frameworks. Chem. Rec. 2020, 20, 1297–1313. [Google Scholar] [CrossRef]
- Carotenuto, A.; Dell’Isola, M. An experimental verification of saturated salt solution-based humidity fixed points. Int. J. Thermophys. 1996, 17, 1423–1439. [Google Scholar] [CrossRef]
- Arman Kuzubasoglu, B. Recent Studies on the Humidity Sensor: A Mini Review. ACS App. Elec. Mater. 2022, 4, 4797–4807. [Google Scholar] [CrossRef]
- Fontanella, J.J.W.M.; Wainright, J.S.; Savinell, R.F.; Litt, M. High pressure electrical conductivity studies of acid doped polybenzimidazole. Electrochim Acta 1998, 43, 1289–1294. [Google Scholar] [CrossRef]
- Biemmi, C.S.E.; Bein, T. Oriented Growth of the Metal Organic Framework Cu3(BTC)2(H2O)3·xH2O Tunable with Functionalized Self-Assembled Monolayers. J. Am. Chem. Soc. 2007, 129, 8054–8055. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, S. In Situ Fabrication of Inorganic Nanowire Arrays Grown from and Aligned on Metal Substrates. Acc. Chem. Res. 2009, 42, 1617–1627. [Google Scholar] [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Senz, S.; Gösele, U. Silicon Nanowires: A Review on Aspects of their Growth and their Electrical Properties. Adv. Mater. 2009, 21, 2681–2702. [Google Scholar] [CrossRef]
- Saravanabharathi, D.; Obulichetty, M.; Rameshkumar, S.; Kumaravel, M. New 2-methyl benzimidazole based zinc carboxylates: Supramolecular structures, biomimetic proton conductivities and luminescent properties. Inorganica Chim. Acta 2015, 437, 167–176. [Google Scholar] [CrossRef]
- Colomban, P. Proton Conductors; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Ponomareva, V.G.; Kovalenko, K.A.; Chupakhin, A.P.; Dybtsev, D.N.; Shutova, E.S.; Fedin, V.P. Imparting High Proton Conductivity to a Metal–Organic Framework Material by Controlled Acid Impregnation. J. Am. Chem. Soc. 2012, 134, 15640–15643. [Google Scholar] [CrossRef]
- Peterson, G.W.W.; Balboa, A.; Mahle, J.; Sewell, T.; Karwacki, C.J. Ammonia Vapor Removal by Cu3(BTC)2 and Its Characterization by MAS NMR. J. Phys. Chem. C 2009, 113, 13906–13917. [Google Scholar] [CrossRef]
- Morikawa, S.; Yamada, T.; Kitagawa, H. Crystal Structure and Proton Conductivity of a One-dimensional Coordination Polymer, {Mn(DHBQ)(H2O)2}. Chem. Let. 2009, 38, 654–655. [Google Scholar] [CrossRef]
- Prestipino, C.R.L.; Vitillo, J.G.; Bonino, F.; Damin, A.; Lamberti, C.; Zecchina, A.; Solari, P.L.; Kongshaug, K.O.; Bordiga, S. Local Structure of Framework Cu(II) in HKUST-1 Metallorganic Framework: Spectroscopic Characterization upon Activation and Interaction with Adsorbates. Chem. Mater. 2006, 18, 1337–1346. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Chen, C.; Lin, C. Investigation of humidity sensor based on Au modified ZnO nanosheets via hydrothermal method and first principle. Sens. Actuators B Chem. 2019, 287, 526–534. [Google Scholar] [CrossRef]
- Duan, Z.; Xu, M.; Li, T.; Zhang, Y.; Zou, H. Super-fast response humidity sensor based on La0.7Sr0.3MnO3 nanocrystals prepared by PVP-assisted sol-gel method. Sens. Actuators B Chem. 2018, 258, 527–534. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, T.; Qi, R.; Dai, J.; Liu, S.; Fei, T.; Lu, G. Humidity sensor based on solution processible microporous silica nanoparticles. Sens. Actuators B Chem. 2018, 266, 131–138. [Google Scholar] [CrossRef]
- Cao, J.; Ma, W.; Lyu, K.; Zhuang, L.; Cong, H.; Deng, H. Twist and sliding dynamics between interpenetrated frames in Ti-MOF revealing high proton conductivity. Chem. Sci. 2020, 11, 3978–3985. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhao, Q.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B Chem. 2020, 317, 128204. [Google Scholar] [CrossRef]
- Yang, L.; Qian, S.; Wang, X.; Cui, X.; Chen, B.; Xing, H. Energy-efficient separation alternatives: Metal-organic frameworks and membranes for hydrocarbon separation. Chem. Soc. Rev. 2020, 49, 5359–5406. [Google Scholar] [CrossRef]
- Li, X.M.; Dong, L.Z.; Liu, J.; Ji, W.X.; Li, S.L.; Lan, Y.Q. Intermediate-Temperature Anhydrous High Proton Conductivity Triggered by Dynamic Mo-lecular Migration in Trinuclear Cluster Lattice. Chem 2020, 6, 2272–2282. [Google Scholar] [CrossRef]
- Marsh, K.N. (Ed.) Recommended Reference Material for the Realization of Physicochemical Properties; Blackwell Scientific Publications: Oxford, UK, 1987; pp. 157–162. [Google Scholar]


| Thickness of Film/μm | Resistivity × 104 Ω cm |
|---|---|
| 62 | -- |
| 37 | 27.88 |
| 22 | 6.21 |
| 18 | 0.40 |
| 16 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Wang, C.; Yang, F.; Li, J.; Yan, S.; Qi, Y. Thickness and Humidity on Proton Conductivity in MOF-508 Thin Film by Twin-Zinc-Source Method. Coatings 2023, 13, 1520. https://doi.org/10.3390/coatings13091520
Zhang K, Wang C, Yang F, Li J, Yan S, Qi Y. Thickness and Humidity on Proton Conductivity in MOF-508 Thin Film by Twin-Zinc-Source Method. Coatings. 2023; 13(9):1520. https://doi.org/10.3390/coatings13091520
Chicago/Turabian StyleZhang, Kun, Chunxia Wang, Feng Yang, Jing Li, Shuguang Yan, and Yue Qi. 2023. "Thickness and Humidity on Proton Conductivity in MOF-508 Thin Film by Twin-Zinc-Source Method" Coatings 13, no. 9: 1520. https://doi.org/10.3390/coatings13091520
APA StyleZhang, K., Wang, C., Yang, F., Li, J., Yan, S., & Qi, Y. (2023). Thickness and Humidity on Proton Conductivity in MOF-508 Thin Film by Twin-Zinc-Source Method. Coatings, 13(9), 1520. https://doi.org/10.3390/coatings13091520





