Research Status of Aluminum Base Coating on Titanium Alloy
Abstract
:1. Introduction
2. Aluminide Coating
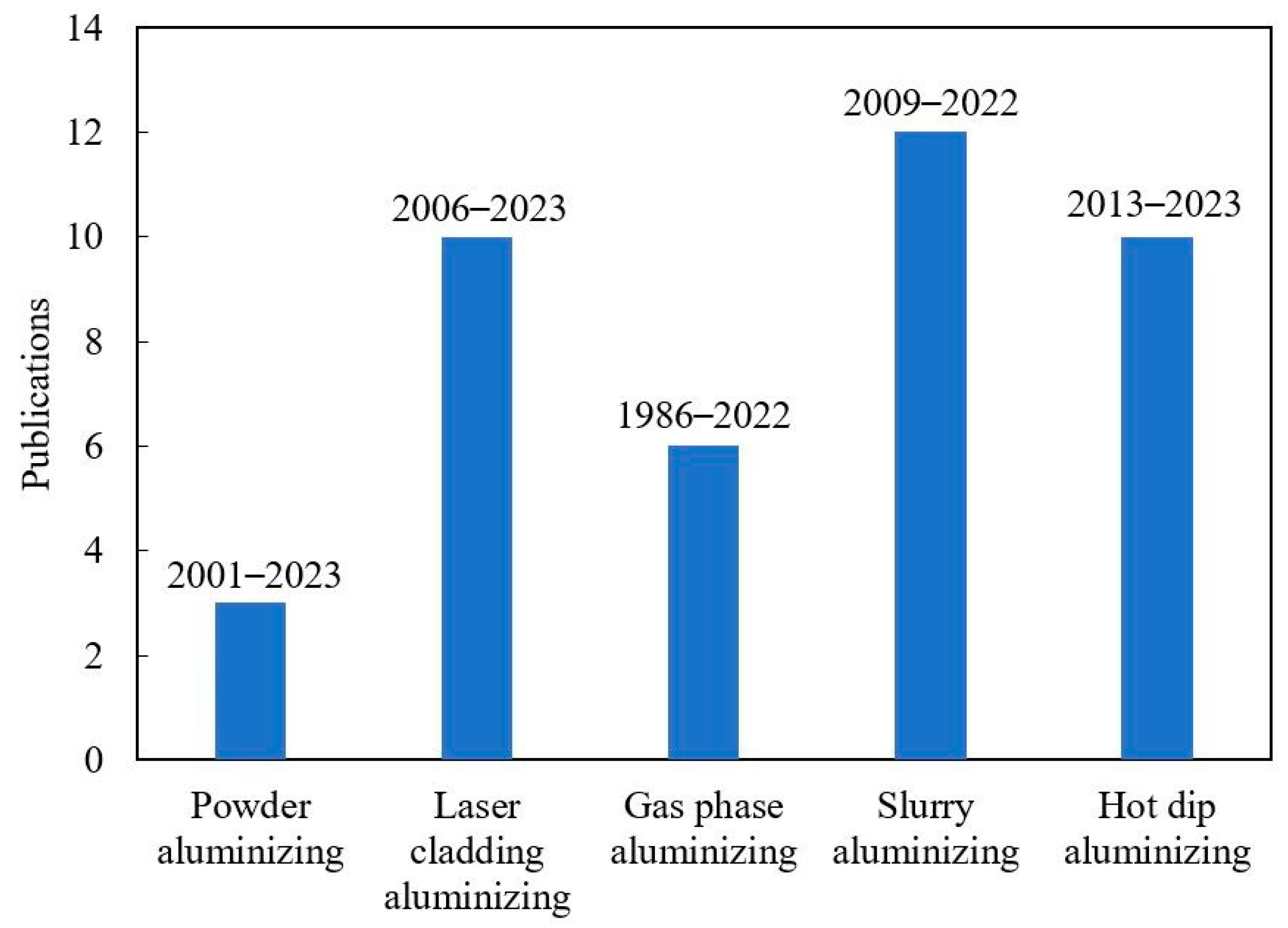
2.1. Coating Preparation Method
2.1.1. Powder Aluminizing
High-Activity Aluminizing
Low-Activity Aluminizing
2.1.2. Laser-Cladding Aluminizing
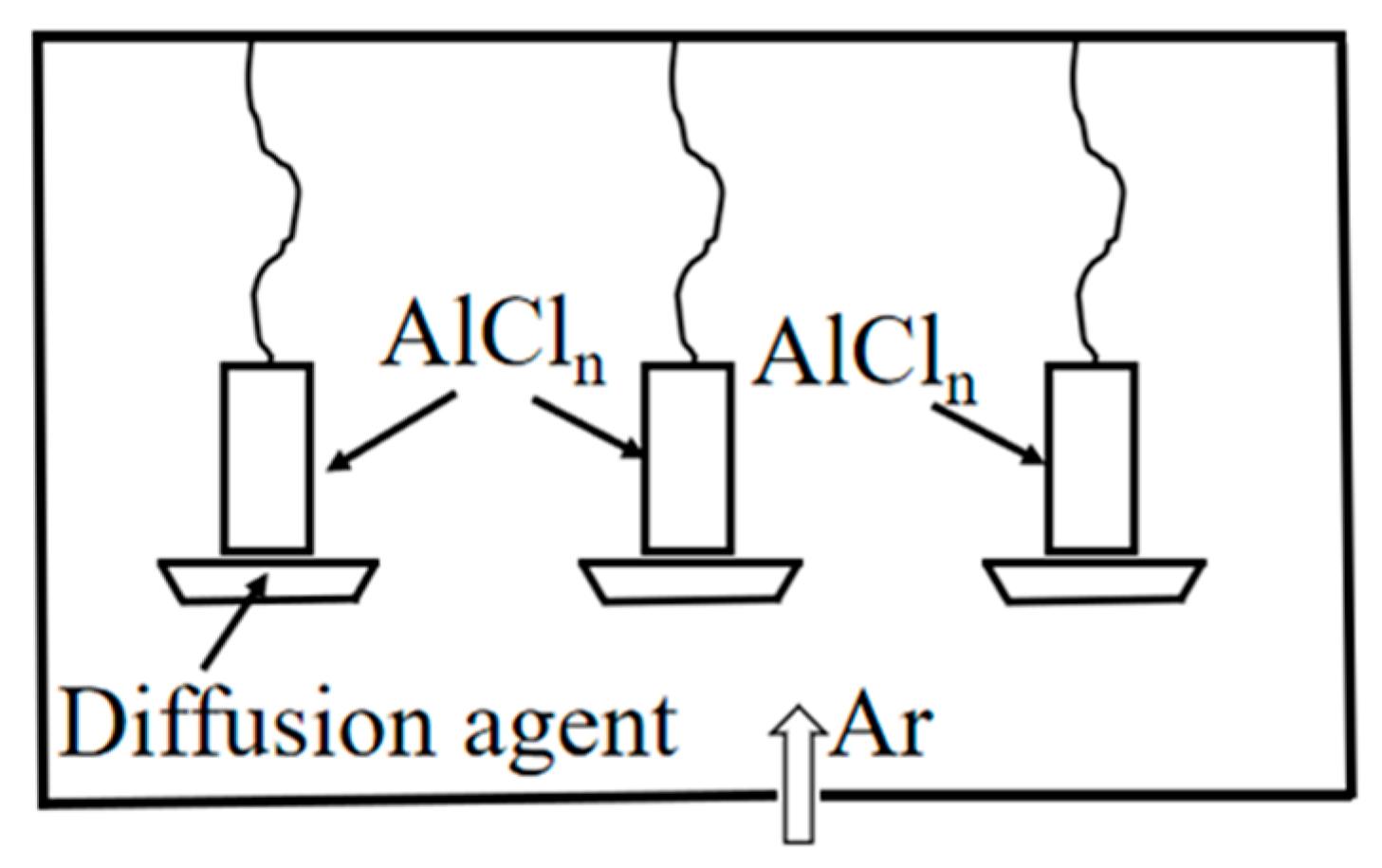
2.1.3. Gas-Phase Aluminizing
2.1.4. Slurry Aluminizing
2.1.5. Hot-Dip Aluminizing
2.2. Improved Aluminide Coating
2.2.1. Cr-Improved Aluminide Coating
2.2.2. Si-Improved Aluminide Coating
2.2.3. Nb-Improved Aluminide Coating
2.2.4. Pt-Improved Aluminide Coating
2.2.5. Rare-Earth Element-Improved Aluminide Coating
2.3. Growth Kinetics of Aluminum-Based Coatings
3. High-Temperature Oxidation-Resistant Coating
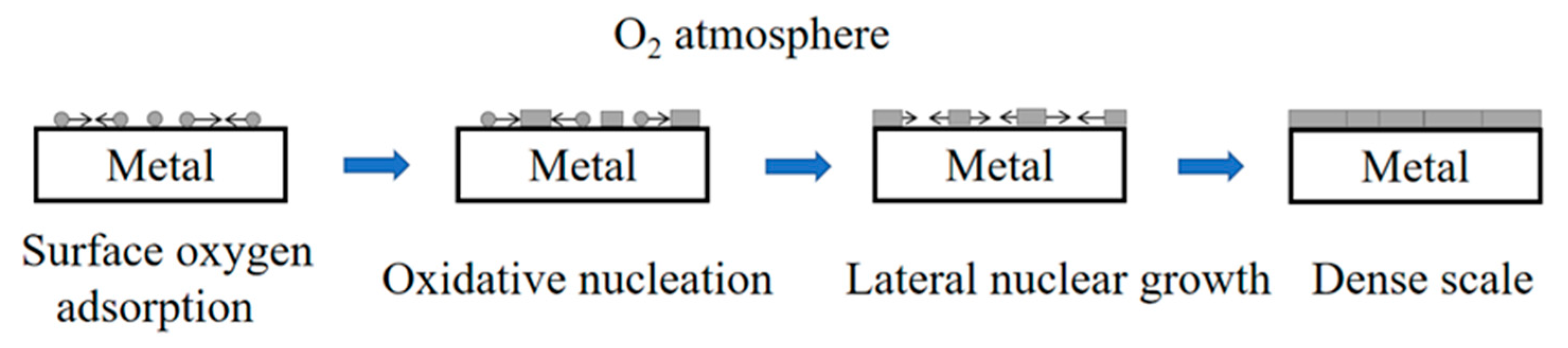
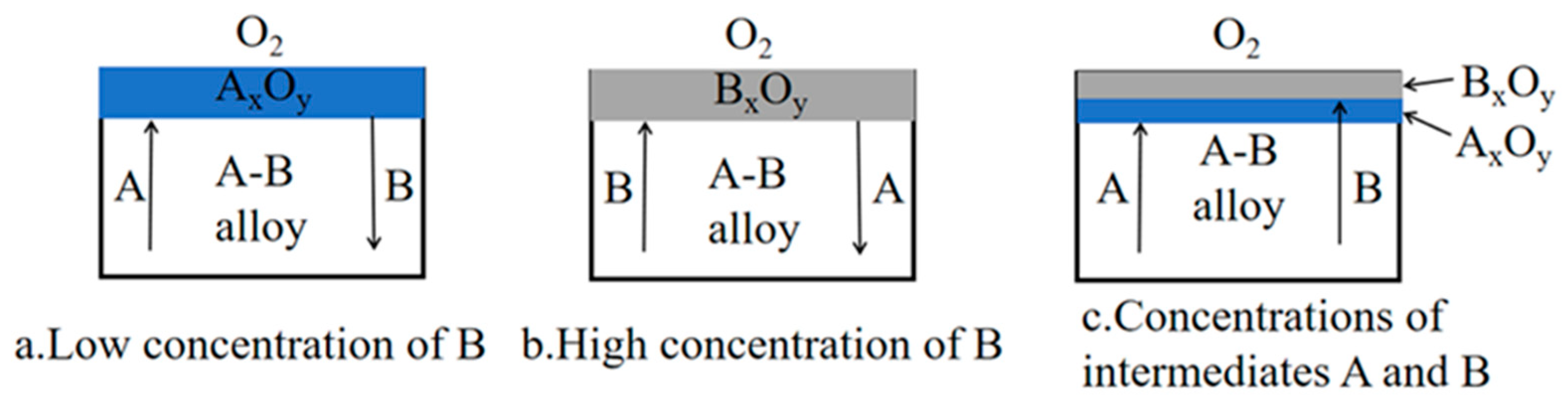
3.1. Thermodynamic Analysis of the High-Temperature Oxidation of Titanium Alloy
3.2. Kinetics Analysis of the High-Temperature Oxidation of Titanium Alloy
3.3. Improvement of the High-Temperature Oxidation Resistance of Titanium Alloy
3.3.1. Surface-Coating Technology
Aluminized Coating
Al-X Series Coating
Ti-Al-X Series Coating
Rare-Earth-Modified Aluminide Coating
3.3.2. Pre-Oxidation Treatment
4. Thermal Corrosion-Resistant Coating
4.1. Mechanism of Thermal Corrosion under the Action of Molten Salt
4.2. Improvement of the Heat Resistance and Corrosion Performance of Titanium Alloy
4.2.1. Surface-Coating Technology
Aluminized Coating
Silicon-Modified Aluminide Coating
Rare-Earth-Modified Aluminide Coating
4.2.2. Pre-Oxidation Treatment
5. Wear-Resistant Coating
5.1. Analysis of the Friction Mechanism of Titanium Alloy
5.2. Improvement to the Wear Resistance of Titanium Alloy
5.2.1. Surface-Coating Technology
Aluminized Coating
Chromium-Modified Aluminide Coating
Nb-Modified Aluminide Coating
5.2.2. Pre-Oxidation Treatment
5.2.3. Carburizing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, L.; Li, W.S.; Li, D.Y.; Xie, G.L.; Zhang, C.Z.; Zhang, C.; Huang, J.F. A review on combustion behavior and mechanism of Ti alloys for advanced aero-engine. J. Alloys Compd. 2023, 960, 170584. [Google Scholar] [CrossRef]
- Dai, J.J.; Li, S.Y.; Zhang, H.X.; Yu, H.J.; Chen, C.Z.; Li, Y. Microstructure and high-temperature oxidation resistance of Ti-Al-Nb coatings on a Ti-6Al-4V alloy fabricated by laser surface alloying. Surf. Coat. Technol. 2018, 344, 479–488. [Google Scholar] [CrossRef]
- Li, S.; Yamaguchi, T. High-temperature oxidation performance of laser-cladded amorphous TiNiSiCrCoAl high-entropy alloy coating on Ti-6Al-4V surface. Surf. Coat. Technol. 2022, 433, 128123. [Google Scholar] [CrossRef]
- Xu, Y.J.; Yao, Z.P.; Jia, F.Z.; Wang, Y.L.; Jiang, Z.H.; Bu, H.T. Preparation of PEO ceramic coating on Ti alloy and its high temperature oxidation resistance. Curr. Appl. Phys. 2010, 10, 698–702. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Peng, Y.P.; Mao, Y.L.; Pang, C.J.; Lu, L.Y. Effect of hot-dip aluminizing on the oxidation resistance of Ti–6Al–4V alloy at high temperatures. Corros. Sci. 2012, 55, 187–193. [Google Scholar] [CrossRef]
- Yao, Z.; Marek, M. NaCl-induced hot corrosion of a titanium aluminide alloy. Mater. Sci. Eng. A 1995, 192–193, 994–1000. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, B.; Ye, W.T.; Zhang, T.; Wan, J.; Zhou, Q.; Shen, J.H.; Li, J.S.; Lu, W.F.; Wang, H. Simultaneously improving mechanical, thermal, and anti-wear properties of Ti alloys using 3D-networked graphene as reinforcement. Carbon 2023, 213, 118152. [Google Scholar] [CrossRef]
- Moskal, G.; Migas, D.; Mendala, B.; Kałamarz, P.; Mikuśkiewicz, M.; Iqbal, A.; Jucha, S.; Góral, M. The Si influence on the microstructure and oxidation resistance of Ti-Al slurry coatings on Ti-48Al-2Cr-2Nb alloy. Mater. Res. Bull. 2021, 141, 111336. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Bataev, I.A.; Laptev, I.S.; Ruktuev, A.A.; Maliutina, I.N.; Golkovsky, M.G.; Bataev, A.A. Formation of Ti-Al intermetallics on a surface of titanium by non-vacuum electron beam treatment. Mater. Charact. 2017, 134, 202–212. [Google Scholar] [CrossRef]
- Gurrappa, I.; Gogia, A.K. High performance coatings for titanium alloys to protect against oxidation. Surf. Coat. Technol. 2001, 139, 216–221. [Google Scholar] [CrossRef]
- Izumi, T.; Nishimoto, T.; Narita, T. Formation of nickel aluminide coating on γ-TiAl alloy. Intermetallics 2003, 11, 841–848. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Dubey, A.K. Recent trends in laser cladding and surface alloying. Opt. Laser Technol. 2021, 134, 106619. [Google Scholar] [CrossRef]
- Liu, Y.N.; Yang, L.J.; Yang, X.J.; Zhang, T.G.; Sun, R.L. Optimization of microstructure and properties of composite coatings by laser cladding on titanium alloy. Ceram. Int. 2021, 47, 2230–2243. [Google Scholar] [CrossRef]
- Kathuria, Y.P. Some aspects of laser surface cladding in the turbine industry. Surf. Coat. Technol. 2000, 132, 262–269. [Google Scholar] [CrossRef]
- Kwok, C.T.; Man, H.C.; Cheng, F.T.; Lo, K.H. Developments in laser-based surface engineering processes: With particular reference to protection against cavitation erosion. Surf. Coat. Technol. 2016, 291, 189–204. [Google Scholar] [CrossRef]
- Moradnia, M.; Pouladi, S.; Aqib, M.; Ryou, J.-H. Thermodynamic Analysis of Group-III-Nitride Alloying with Yttrium by Hybrid Chemical Vapor Deposition. Nanomaterials 2022, 12, 4053. [Google Scholar] [CrossRef]
- Pourmohammad, H.; Bahrami, A.; Eslami, A.; Harandi, A.N. Gas-phase aluminizing of HP40Nb steel used in reformer tubes: Synthesis, characterization, and its implications for the microstructure and high-temperature creep resistance of the base alloy. Int. J. Pres.Ves. Pip. 2022, 200, 104833. [Google Scholar] [CrossRef]
- Taghipour, M.; Eslami, A.; Bahrami, A. High temperature oxidation behavior of aluminide coatings applied on HP-MA heat resistant steel using a gas-phase aluminizing process. Surf. Coa. Technol. 2022, 434, 128181. [Google Scholar] [CrossRef]
- Wang, F.H.; Lou, H.Y.; Bai, L.X.; Wu, W.T. Hot corrosion of yttrium-modified aluminide coatings. Mater. Sci. Eng. A 1989, 120–121, 387–389. [Google Scholar] [CrossRef]
- Xiong, H.P.; Mao, W.; Xie, Y.H.; Ma, W.L.; Chen, Y.F.; Li, X.H.; Li, J.P.; Cheng, Y.Y. Liquid-phase siliconizing by Al–Si alloys at the surface of a TiAl-based alloy and improvement in oxidation resistance. Acta Mater. 2004, 52, 2605–2620. [Google Scholar] [CrossRef]
- Bai, C.Y.; Luo, Y.J.; Koo, C.H. Improvement of high temperature oxidation and corrosion resistance of superalloy IN-738LC by pack cementation. Surf. Coat. Technol. 2004, 183, 74–88. [Google Scholar] [CrossRef]
- Zhou, C.G.; Xu, H.B.; Gong, S.K.; Kim, K.Y. A study of aluminide coatings on TiAl alloys by the pack cementation method. Mater. Sci. Eng. A 2003, 341, 169–173. [Google Scholar] [CrossRef]
- Gauthier, V.; Dettenwanger, F.; Schütze, M.; Shemet, V.; Quadakkers, W.J. Oxidation-Resistant Aluminide Coatings on γ-TiAl. Oxid. Met. 2003, 59, 233–255. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yakou, T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater. Sci. Eng. A 2002, 338, 44–53. [Google Scholar] [CrossRef]
- Cammarota, G.P.; Casagrande, A.; Sambogna, G. Effect of Ni, Si and Cr in the structural formation of diffusion aluminide coatings on commercial-purity titanium. Surf. Coat. Technol. 2006, 201, 230–242. [Google Scholar] [CrossRef]
- Grzegorz, M. Microstructure and oxidation behaviour of TiAlSi coatings on TiAlCrNb alloy. J. Ach. Mater. Manuf. Eng. 2007, 20, 263–266. [Google Scholar]
- Zheng, M.H.; Rapp, R.A. Simultaneous Aluminizing and Chromizing of Steels to Form (Fe, Cr)3Al Coatings. Oxid. Met. 1998, 49, 19–31. [Google Scholar] [CrossRef]
- Hu, X.Y.; Li, F.G.; Shi, D.M.; Xie, Y.; Li, Z.; Yin, F.C. A design of self-generated Ti–Al–Si gradient coatings on Ti–6Al–4V alloy based on silicon concentration gradient. J. Alloys Compd. 2020, 830, 154670. [Google Scholar] [CrossRef]
- Gogia, A.K.; Nandy, T.K.; Banerjee, D.; Carisey, T.; Strudel, J.L.; Franchet, J.M. Microstructure and mechanical properties of orthorhombic alloys in the Ti-Al-Nb system. Intermetallics 1998, 6, 741–748. [Google Scholar] [CrossRef]
- Trivedi, S.P.; Das, D.K. Microstructural aspects of plain aluminide and Pt-aluminide coatings on Ti-base alloy IMI-834. Intermetallics 2005, 13, 1122–1133. [Google Scholar] [CrossRef]
- He, Y.M.; Lu, C.Y.; Ni, C.Y.; Chen, Q.X.; Zheng, W.J.; Wang, D.H.; Wei, L.F.; Wang, L.M.; Sun, Y.; Zou, H.; et al. Tailoring microstructure and mechanical performance of the TC4 titanium alloy brazed joint through doping rare-earth element Dy into Ti-Cu-Ni filler alloy. J. Manuf. Process 2020, 50, 255–265. [Google Scholar] [CrossRef]
- Pint, P.A. On the formation of interfacial and internal voids in α-Al2O3 scales. Oxid. Met. 1997, 48, 303–328. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, F.G.; Hu, X.Y.; He, W.; Liu, Z.; Tan, Y. Preparation of Ti-Al-Si Gradient Coating Based on Silicon Concentration Gradient and Added-Ce. Coatings 2022, 12, 683. [Google Scholar] [CrossRef]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.Y.; Duan, Y.L.; Zhang, L.G.; Xu, G.F. Thermal oxidation behavior of commercial purity titanium at high temperature. Chin. J. Nonferrous Met. 2013, 23, 2190–2199. [Google Scholar]
- Zhao, A.L.; Wang, D.Y.; Wang, Y.; Gao, X.Y.; Hu, J. The comparison of thermal oxidation kinetics for pure titanium and titanium alloy. Titan. Ind. Prog. 2013, 30, 16–19. [Google Scholar]
- Jeng, S.C. Oxidation behavior and microstructural evolution of hot-dipped aluminum coating on Ti-6Al-4V alloy at 800 °C. Surf. Coat. Technol. 2013, 235, 867–874. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.G.; Li, W.; Mei, X.L.; Qin, Q.D.; Hu, S.W. Effect of Al–Si coating fusing time on the oxidation resistance of Ti–6Al–4V alloy. Mater. Sci. Eng. A 2007, 460–461, 579–586. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.M.; Shen, Y.F. Oxidation behavior of a high Si content Al–Si composite coating fabricated on Ti–6Al–4V substrate by mechanical alloying method. J. Alloys Compd. 2017, 701, 27–36. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhou, W.; Peng, X.; Liang, Y.H.; Qin, Q.D. Low oxygen pressure self-fused Al-Cr coatings formed on surface of Ti alloy and their oxidation resistance. J. Jilin Univ. Eng. Technol. Ed. 2004, 34, 521–526. [Google Scholar]
- Zhou, W.; Zhao, Y.G.; Qin, Q.D.; Li, W.; Xu, B. A new way to produce Al + Cr coating on Ti alloy by vacuum fusing method and its oxidation resistance. Mater. Sci. Eng. A 2006, 430, 254–259. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhang, F.Y.; Wang, A.M.; Yu, H.J.; Chen, C.Z. Microstructure and properties of Ti-Al coating and Ti-Al-Si system coatings on Ti-6Al-4V fabricated by laser surface alloying. Surf. Coat. Technol. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Sadeq, F.O.; Sharifitabar, M.; Afarani, M.S. Synthesis of Ti–Si–Al coatings on the surface of Ti–6Al–4V alloy via hot dip siliconizing route. Surf. Coat. Technol. 2018, 337, 349–356. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, F.; Cui, X.Y.; Wang, J.Q.; Xiong, T.Y. Long-term oxidation behavior of silicon-aluminizing coating with an in-situ formed Ti5Si3 diffusion barrier on γ-TiAl alloy. Appl. Surf. Sci. 2022, 582, 152444. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Li, F.G. Study on the Influence Law of Ce on Microstructure and High-Temperature Oxidation Resistance of Ti-Al-Si Composite Coating. Coatings 2023, 13, 1244. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Zhou, B.; Liu, N.; Chen, L.Q. Effects of Microstructure and Rare-Earth Constituent on the Oxidation Behavior of Ti–5.6Al–4.8Sn–2Zr–1Mo–0.35Si–0.7Nd Titanium Alloy. Oxid. Met. 2014, 81, 373–382. [Google Scholar] [CrossRef]
- Zhang, C.J.; Zhang, S.Z.; Liu, Z.G.; Chen, Y.Y.; Chai, L.H.; Wang, X.P. Improvement of cyclic oxidation resistance of Y-containing Ti–6Al–2.5Sn–4Zr–0.7Mo–0.3Si alloys. J. Alloys Compd. 2015, 624, 108–115. [Google Scholar] [CrossRef]
- Tan, Y.M.; Fang, H.Z.; Chen, R.R.; Liu, Y.L.; Su, Y.Q.; Guo, J.J.; Cui, H.Z.; Zhang, S.Y.; Fu, H.Z. Microalloying effects of Ho on microstructure evolution and high temperature properties of Ti46Al4Nb1Mo alloy. Intermetallics 2020, 126, 106883. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Deng, T.S.; Xiao, W.L.; Zhong, M.; Lai, Y.H.; Ojo, O.A. Effect of minor Sc modification on the high-temperature oxidation behavior of near-α Ti alloy. Corros. Sci. 2023, 217, 111122. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.G.; Qin, Q.D.; Li, W.; Xu, B. Effect of pre-oxidation on aluminized coating and their oxidation resistance of Ti alloy. Mater. Lett. 2006, 60, 414–417. [Google Scholar] [CrossRef]
- Goward, G.W.; Boone, D.H. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Das, D.K.; Joshi, S.V.; Singh, V. Evolution of aluminide coating microstructure on nickel-base cast superalloy CM-247 in a single-step high-activity aluminizing process. Metall. Mater. Trans. A 1998, 29, 2173–2188. [Google Scholar] [CrossRef]
- Jacobson, N.S. Corrosion of Silicon-Based Ceramics in Combustion Environments. J. Am. Ceram. Soc. 1993, 76, 3–28. [Google Scholar] [CrossRef]
- Say, W.C.; Wu, J.K.; Chen, W.L. Hot corrosion ofα-SiC ceramics by V2O5 melt. J. Mater. Sc. 1990, 25, 1614–1617. [Google Scholar] [CrossRef]
- Steinmetz, P.; Duret, C.; Morbioli, R. Laboratory tests for hot-corrosion studies. Mater. Sci. Technol. 1986, 2, 262–271. [Google Scholar] [CrossRef]
- Xu, H.B.; Guo, H.B.; Liu, F.S.; Gong, S.K. Development of gradient thermal barrier coatings and their hot-fatigue behavior. Surf. Coat. Technol. 2000, 130, 133–139. [Google Scholar] [CrossRef]
- Molins, R.; Hou, P.Y. Characterization of chemical and microstructural evolutions of a NiPtAl bondcoat during high temperature oxidation. Surf. Coat. Technol. 2006, 201, 3841–3845. [Google Scholar] [CrossRef]
- Wang, Y.S.; Xiong, J.; Yan, J.; Fan, H.Y.; Wang, J. Oxidation resistance and corrosion behavior of hot-dip aluminized coatings on commercial-purity titanium. Surf. Coat. Technol. 2011, 206, 1277–1282. [Google Scholar] [CrossRef]
- Rhys-Jones, T.N.; Swindells, N. The high temperature corrosion of a commercial aluminide coating on IN738-LC and MarMOO2 at 700 °C and 830 °C. Corros. Sci. 1985, 25, 559–576. [Google Scholar] [CrossRef]
- Shirvani, K.; Saremi, M.; Nishikata, A.; Tsuru, T. Electrochemical study on hot corrosion of Si-modified aluminide coated In-738LC in Na2SO4–20 wt.% NaCl melt at 750 °C. Corros. Sci. 2003, 45, 1011–1021. [Google Scholar] [CrossRef]
- Hou, P.Y.; McCarty, K.F. Surface and interface segregation in β-NiAl with and without Pt addition. Scr. Mater. 2006, 54, 937–941. [Google Scholar] [CrossRef]
- Khan, A.; Song, P.; Huang, T.H.; Zhou, Y.; Xiong, X.P.; Li, C.; Lü, J.G.; Chen, R.; Lu, J.S. Diffusion characteristics and structural stability of Pt modified β-NiAl/γ′-Ni3Al within NiCoCrAl alloy at high temperature. Appl. Surf. Sci. 2019, 476, 1096–1107. [Google Scholar] [CrossRef]
- Li, C.; Song, P.; Feng, J.; Huang, T.H.; Lü, K.Y.; Li, Q.L.; Duan, W.H.; Khan, A.; Zhai, R.X.; Lu, J.S. Alumina growth behaviour on the surface-modified NiCoCrAl alloy by Pt and Hf at high temperature. Appl. Surf. Sci. 2019, 479, 1178–1191. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, P.S. High-resolution, real-time three-dimensional shape measurement. Opti. Eng. 2006, 45, 123601. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.C.; Schütze, M. Sulphidation Behavior of a Non Harmful Water-Based Al and Al–Si Slurry Coating on CM247 Superalloy. Oxid. Met. 2013, 80, 635–649. [Google Scholar] [CrossRef]
- Zang, J.J.; Song, P.; Feng, J.; Xiong, X.P.; Chen, R.; Liu, G.L.; Lu, J.S. Oxidation behaviour of the nickel-based superalloy DZ125 hot-dipped with Al coatings doped by Si. Corros. Sci. 2016, 112, 170–179. [Google Scholar] [CrossRef]
- Fu, C.; Kong, W.K.; Cao, G.H. Microstructure and oxidation behavior of Al + Si co-deposited coatings on nickel-based superalloys. Surf. Coat. Technol. 2014, 258, 347–352. [Google Scholar] [CrossRef]
- Meng, X.X.; Yuwen, P.; Shao, W.; Qu, W.T.; Zhou, C.G. Cyclic oxidation behaviour of Co/Si co-doped β-NiAl coating on nickel based superalloys. Corros. Sci. 2018, 133, 112–119. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, R.-b.; Wu, Y.-x.; Li, S.-s.; Ma, Y.; Gong, S.-k. A comparative study of four modified Al coatings on Ni3Al-based single crystal superalloy. Prog. Nat. Sci. Mater. 2011, 21, 496–505. [Google Scholar] [CrossRef]
- Dai, P.C.; Wu, Q.; Ma, Y.; Li, S.S.; Gong, S.K. The effect of silicon on the oxidation behavior of NiAlHf coating system. Appl. Surf. Sci. 2013, 271, 311–316. [Google Scholar] [CrossRef]
- Lee, D.B.; Kim, D.J. The oxidation of Ni3Al containing decomposed SiC-particles. Intermetallics 2001, 9, 51–56. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhang, H.X.; Sun, C.X.; Li, S.Y.; Chen, C.Z.; Yang, Y. The effect of Nb and Si on the hot corrosion behaviors of TiAl coatings on a Ti-6Al-4V alloy. Corros. Sci. 2020, 168, 108578. [Google Scholar] [CrossRef]
- Lin, H.; Liang, W.P.; Jia, Y.L.; Miao, Q.; Hu, R.Y.; Ding, Z.; Yu, L.J. Effect of Al -Y gradient coating on hot corrosion resistance of γ-TiAl alloy at different temperatures. Appl. Surf. Sci. 2019, 487, 868–875. [Google Scholar] [CrossRef]
- Hu, Y.T.; Zheng, L.; Yan, H.J.; Wu, L.K.; Lin, X.J.; Cao, F.H.; Jiang, M.Y. Improving hot corrosion resistance of aluminized TiAl alloy by anodization and pre-oxidation. Trans. Nonferrous Met. Soc. China 2021, 31, 193–206. [Google Scholar] [CrossRef]
- Ceschini, L.; Lanzoni, E.; Martini, C.; Prandstraller, D.; Sambogna, G. Comparison of dry sliding friction and wear of Ti6Al4V alloy treated by plasma electrolytic oxidation and PVD coating. Wear 2008, 264, 86–95. [Google Scholar] [CrossRef]
- Molinari, A.; Straffelini, G.; Tesi, B.; Bacci, T. Dry sliding wear mechanisms of the Ti6Al4V alloy. Wear 1997, 208, 105–112. [Google Scholar] [CrossRef]
- Buckley, D.H.; Miyoshi, K. Friction and wear of ceramics. Wear 1984, 100, 333–353. [Google Scholar] [CrossRef]
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Du, Y.J.; Rao, K.P.; Chung, J.C.Y.; Han, X.D. Phase transitions in reactive formation of Ti5Si3/TiAl in situ composites. Metall. Mater. Trans. A 2000, 31, 763–771. [Google Scholar] [CrossRef]
- Gizynski, M.; Miyazaki, S.; Sienkiewicz, J.; Kuroda, S.; Araki, H.; Murakami, H.; Pakiela, Z.; Yumoto, A. Formation and subsequent phase evolution of metastable Ti-Al alloy coatings by kinetic spraying of gas atomized powders. Surf. Coat. Technol. 2017, 315, 240–249. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.S.; Ma, Y.Z.; Liang, C.P.; Liu, C.; Zhang, C.; Cai, Q.S. Microstructure and wear resistance of compositionally graded Ti Al intermetallic coating on Ti6Al4V alloy fabricated by laser powder deposition. Surf. Coat. Technol. 2018, 353, 32–40. [Google Scholar] [CrossRef]
- Zhang, W.B.; Li, W.S.; Zhai, H.M.; Wu, Y.R.; Wang, S.C.; Liang, G.; Wood, R.J.K. Microstructure and tribological properties of laser in-situ synthesized Ti3Al composite coating on Ti-6Al-4V. Surf. Coat. Technol. 2020, 395, 125944. [Google Scholar] [CrossRef]
- Lazurenko, D.; Golkovsky, M.; Stark, A.; Pyczak, F.; Bataev, I.; Ruktuev, A.; Petrov, I.; Laptev, I. Structure and Properties of Ti-Al-Ta and Ti-Al-Cr Cladding Layers Fabricated on Titanium. Metals 2021, 11, 1139. [Google Scholar] [CrossRef]
- Huang, T.-d.; Wu, S.-y.; Jiang, H.; Lu, Y.-p.; Wang, T.-m.; Li, T.-j. Effect of Ti content on microstructure and properties of TixZrVNb refractory high-entropy alloys. Int. J. Miner. Metall. Mater. 2020, 27, 1318–1325. [Google Scholar] [CrossRef]
- Xiang, K.; Chen, L.-Y.; Chai, L.J.; Guo, N.; Wang, H. Microstructural characteristics and properties of CoCrFeNiNbx high-entropy alloy coatings on pure titanium substrate by pulsed laser cladding. Appl. Surf. Sci. 2020, 517, 146214. [Google Scholar] [CrossRef]
- Li, Z.H.; Chai, L.J.; Qi, L.; Wang, Y.Y.; Liu, Y.Z.; Yang, T.; Wang, H.; Guo, N.; Zhao, Y.X. Laser-cladded Al-Cr-Ti ternary alloy coatings on Ti-4Al-2V alloy: Specific microstructure and enhanced surface performance. Surf. Coat. Technol. 2023, 452, 129073. [Google Scholar] [CrossRef]
- Li, W.S.; Zhang, W.B.; Zhai, H.M.; Wang, S.C.; Song, Q.; Wood, R.J.K.; Cheng, B.; He, D.Q.; Zhang, C.Z. Microstructure evolution and elevated temperature wear performance of in-situ laser-synthesized Ti-25Al-17Nb coating on Ti-6Al-4V. Tribol. Int. 2022, 175, 107807. [Google Scholar] [CrossRef]
- Zhao, J.H.; Shangguan, J.J.; Gao, L.S.; Gu, C.; Wang, Y.J.; Shi, Y. New Insights into Microstructure Characteristics and Tribological Property of Ti Alloy Processed by Hot-Dip Aluminizing and Heat Treatment. Metall. Mater. Trans. A 2022, 53, 1035–1050. [Google Scholar] [CrossRef]
- Yang, W.Y.; Li, F.G. Study on the Effect of Carburizing on the Microstructure and High-Temperature Oxidation Properties of Hot-Dip Aluminum Coating on Titanium Alloy. Coatings 2023, 13, 1336. [Google Scholar] [CrossRef]

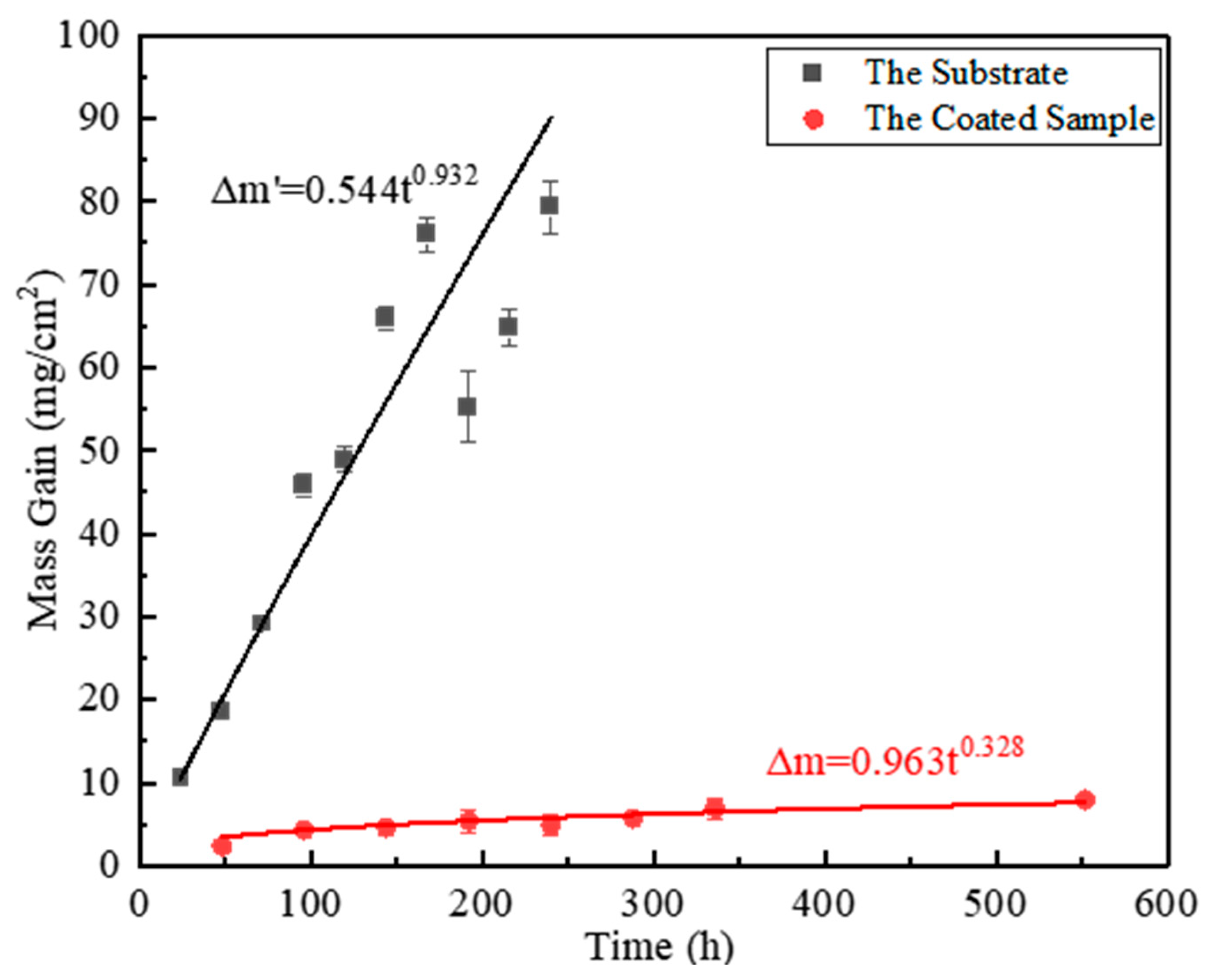
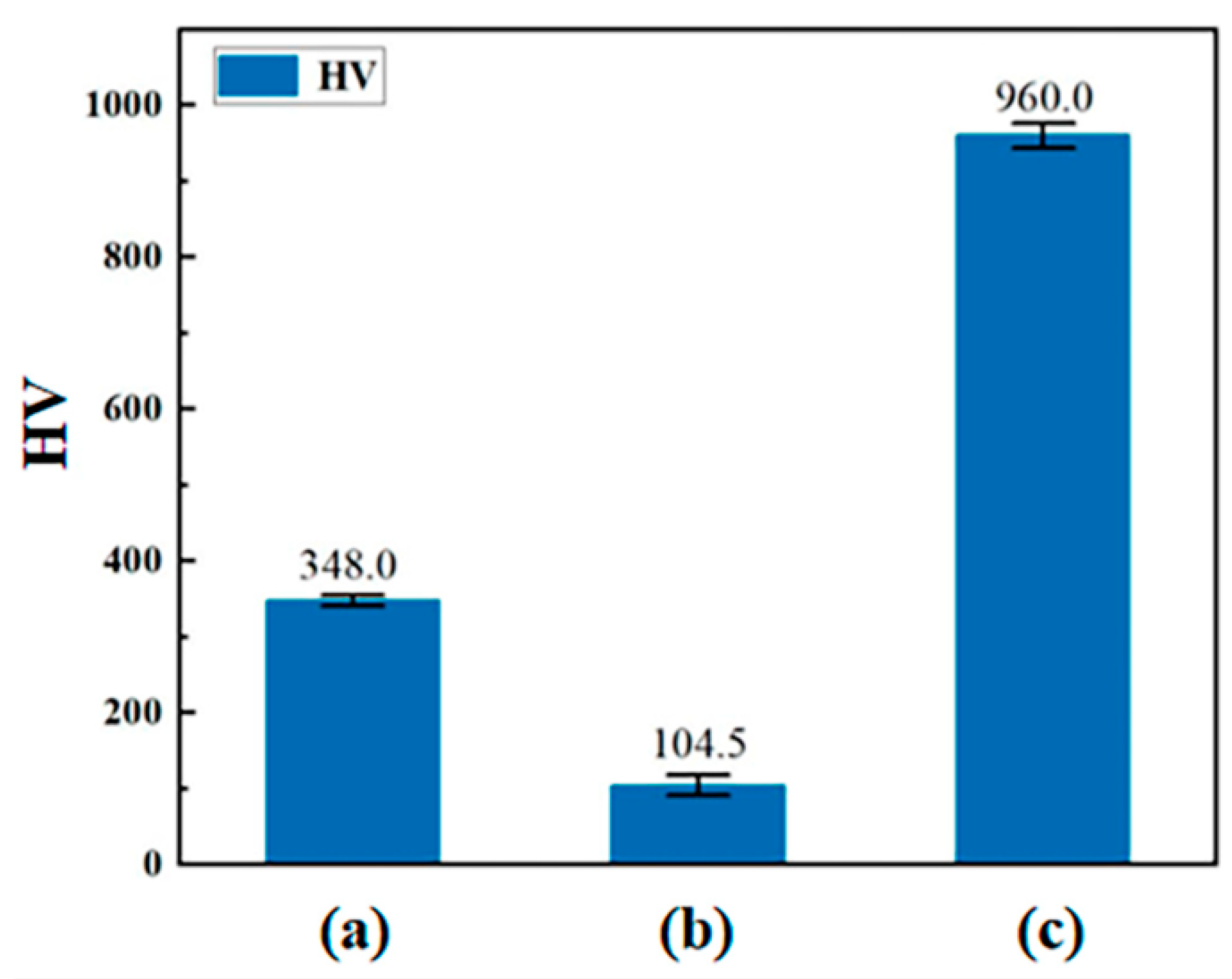
| Rare-Earth Element | Effect on High-Temperature Oxidation Resistance of Titanium Alloy. |
|---|---|
| Nb | It promoted the formation of fine Al2O3 dispersion around TiO2 scales, enhanced the density of oxide film, and thus improved the high-temperature oxidation resistance of Ti-5.6Al-4.8Sn-2Zr-1Mo-0.35Si-0.7Nd [46]. |
| Y | The adhesion between the oxide film and the substrate was significantly improved, and the diffusion of oxygen to the substrate was inhibited, so the oxidation resistance of Ti-6Al-2.5Sn-4Zr-0.7Mo-0.3Si alloy was improved [47]. |
| Ho | The formed holmium oxide promoted the θ-Al2O3 to α-Al2O3 phase transition and inhibited the inward diffusion of oxygen, thus improving the oxidation resistance of Ti46Al4Nb1Mo alloy [48]. |
| Sc | It refined the microstructure and oxide, promoted the formation of the outermost layer Al2O3, improved the density of the oxide film, inhibited the segregation of W element, and finally improved the high-temperature oxidation resistance of α titanium alloy [49]. |
| Ce | It promoted the formation of continuous Al2O3 and prevented crack propagation, thus improving the high-temperature oxidation resistance of Ti65 alloy [45]. |
| Substrate | Coating | Oxidation Condition | Substrate Weight Gain/ Coated Sample Weight Gain | Lit. |
|---|---|---|---|---|
| Ti-6Al-4V | Ti-Al-40Nb | 800 °C/1000 h | 15.02 | [2] |
| Ti-6Al-4V | Ti-Al-Si | 800 °C/120 h | 7.31 | [28] |
| Ti-6Al-4V | Al | 800 °C/96 h | 2.85 | [37] |
| Ti-6Al-4V | Al-Si | 850 °C/100 h | 5.56 | [39] |
| Ti-6Al-4V | Ti-Al-Si | 800 °C/1000 h | 24.80 | [42] |
| Ti-6Al-4V | Ti-Al-Si | 1000 °C/80 h | 6.60 | [43] |
| γ-Ti-Al | Ti(Al,Si)3 | 950 °C/1000 h | 6.33 | [44] |
| Ti65 | (Ti,Ce)(Al,Si)3 | 800 °C/552 h | 26.00 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Li, F. Research Status of Aluminum Base Coating on Titanium Alloy. Coatings 2023, 13, 1525. https://doi.org/10.3390/coatings13091525
Zeng S, Li F. Research Status of Aluminum Base Coating on Titanium Alloy. Coatings. 2023; 13(9):1525. https://doi.org/10.3390/coatings13091525
Chicago/Turabian StyleZeng, Siqi, and Faguo Li. 2023. "Research Status of Aluminum Base Coating on Titanium Alloy" Coatings 13, no. 9: 1525. https://doi.org/10.3390/coatings13091525





