In Situ Synthesis of Graphene Oxide-Sealed LDHs Coatings: A Novel Approach to Enhancing Corrosion Resistance and Tribological Performance on Magnesium Alloys
Abstract
:1. Introduction
2. Experimental
2.1. Materials
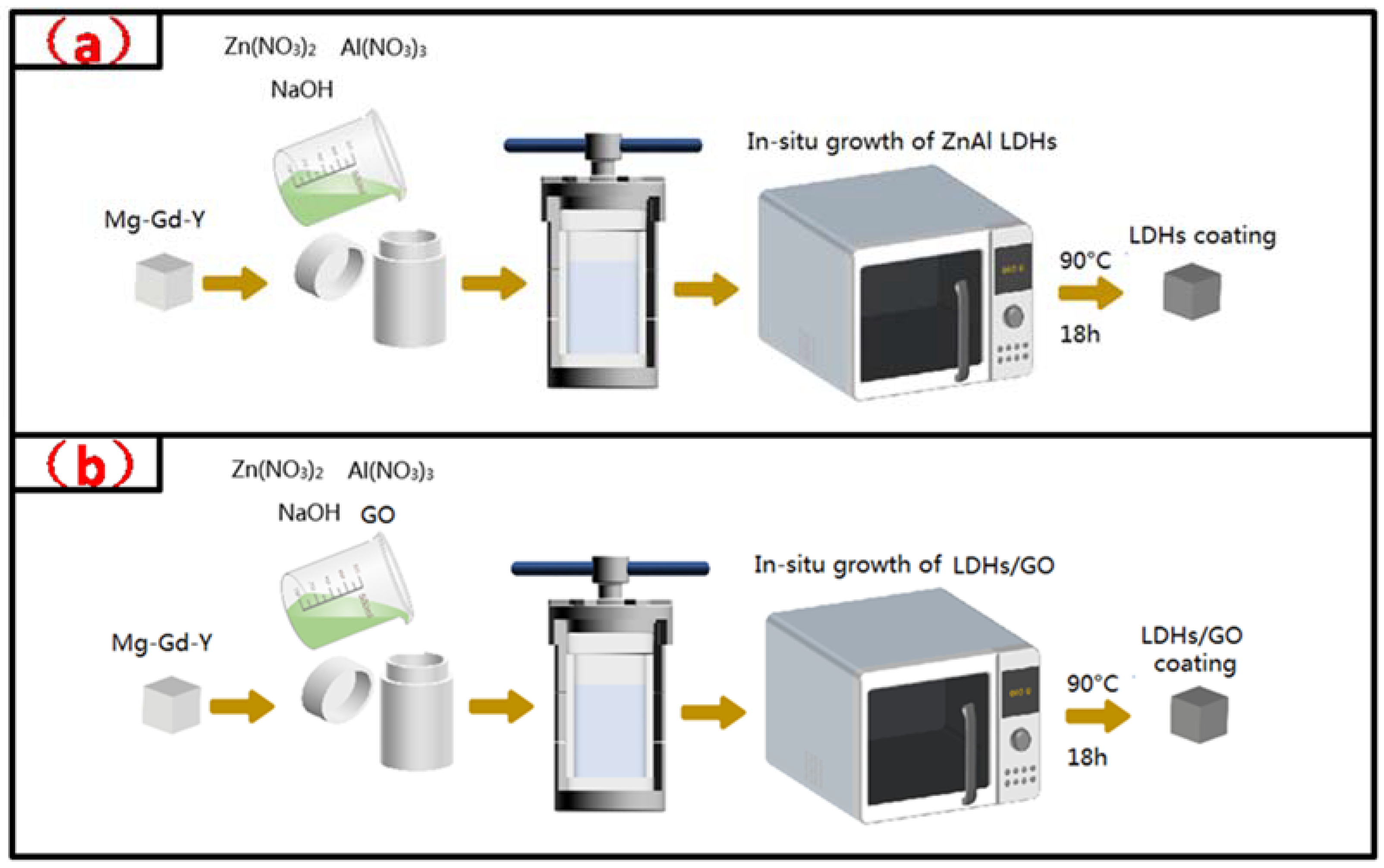
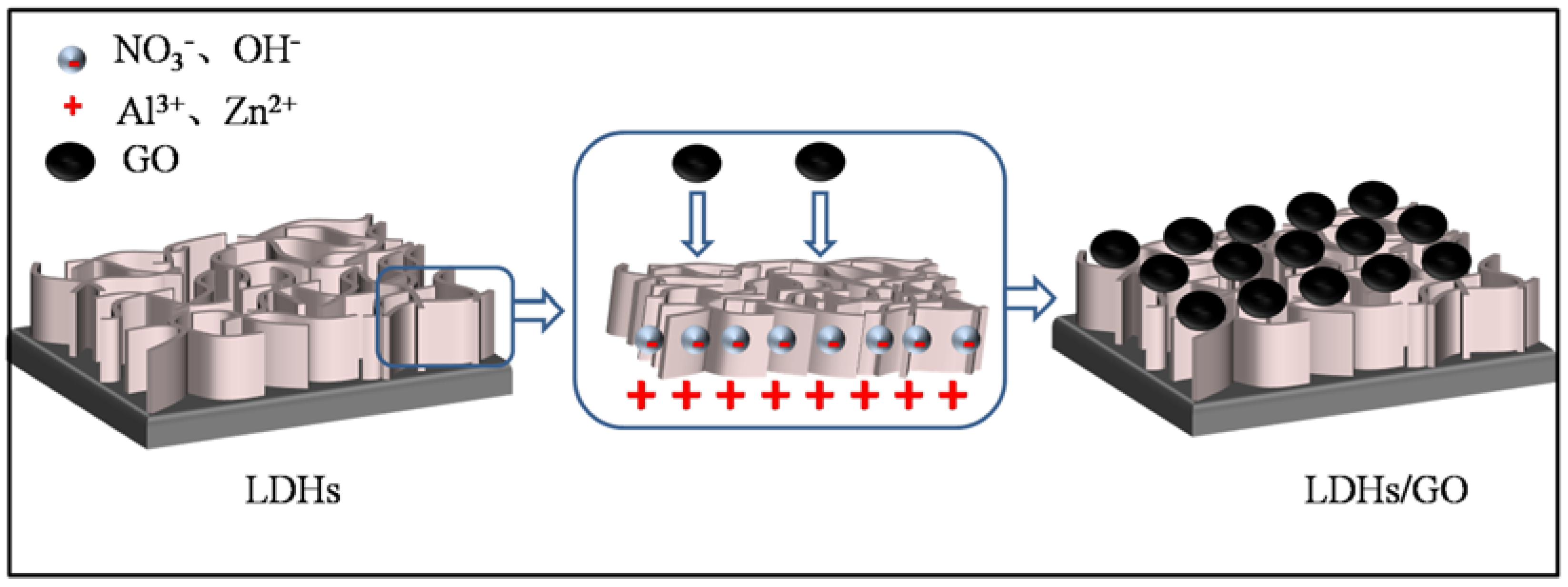
2.2. Sample Preparation
2.3. Characterization
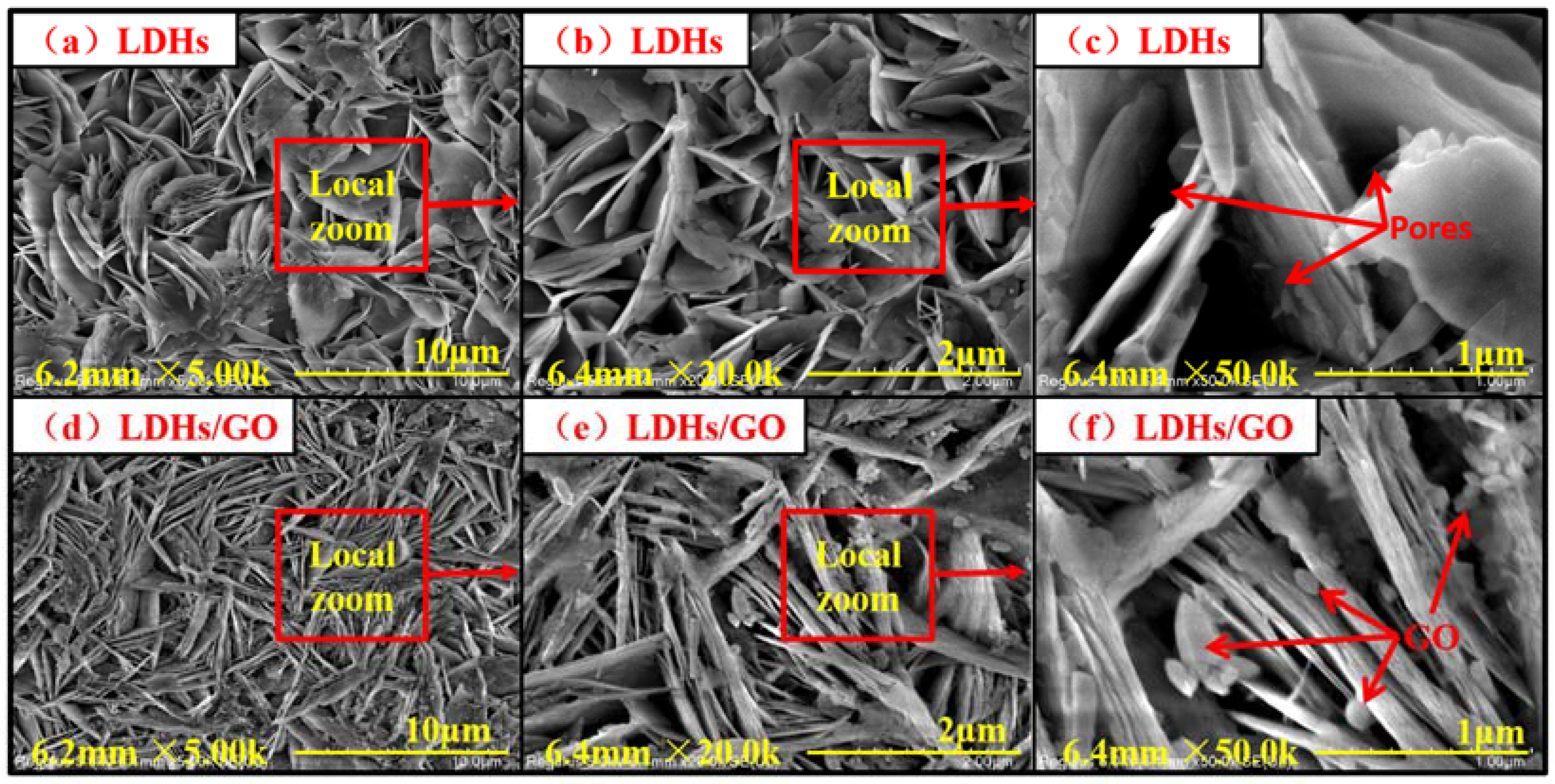
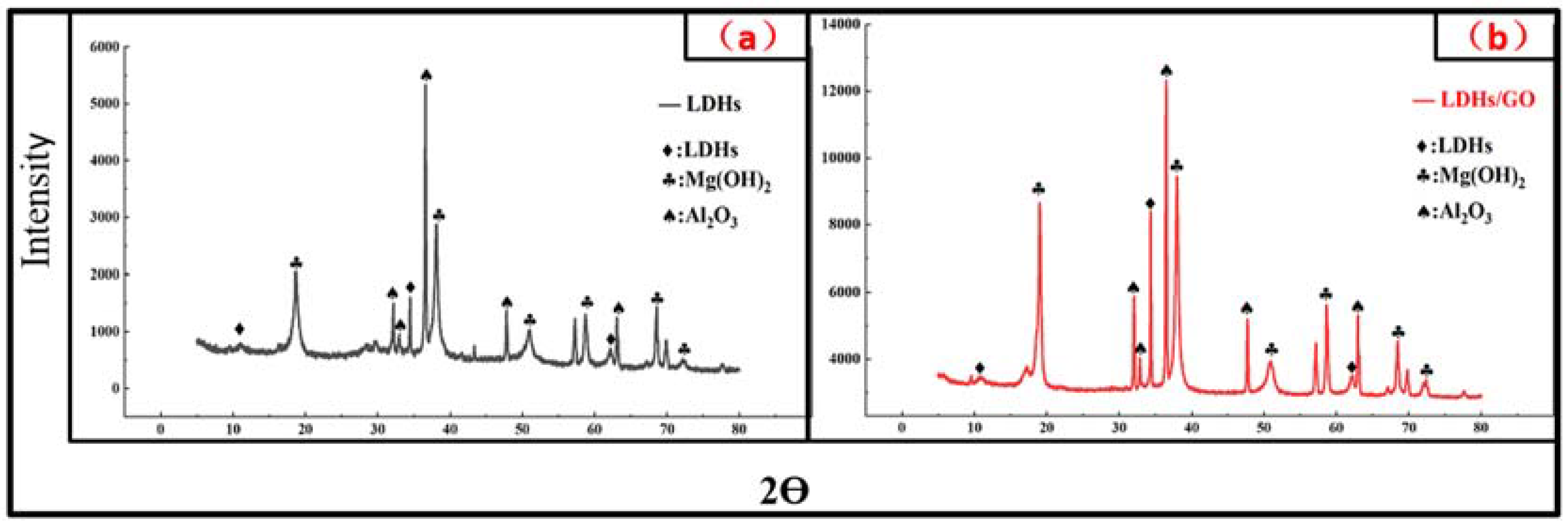
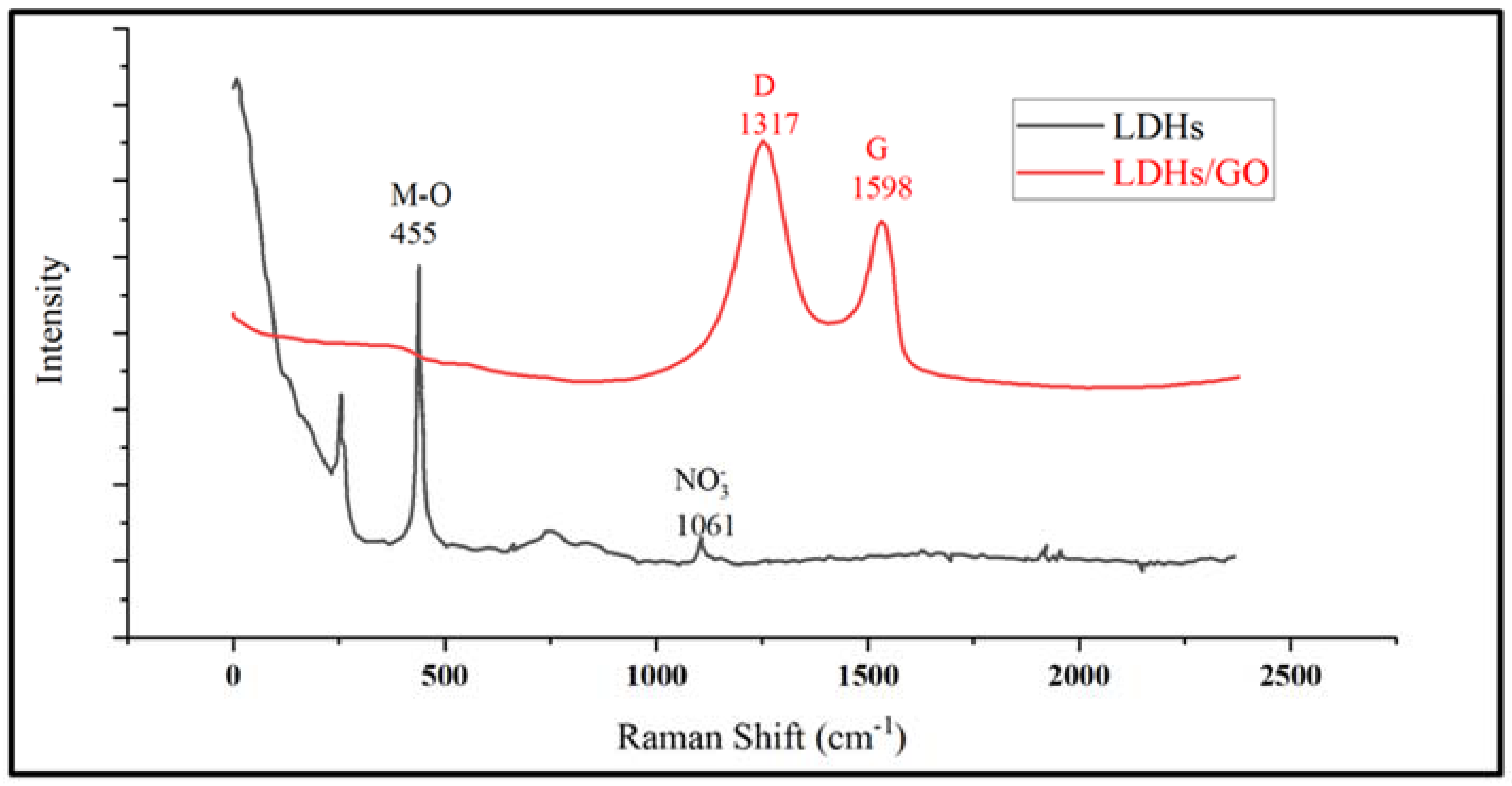
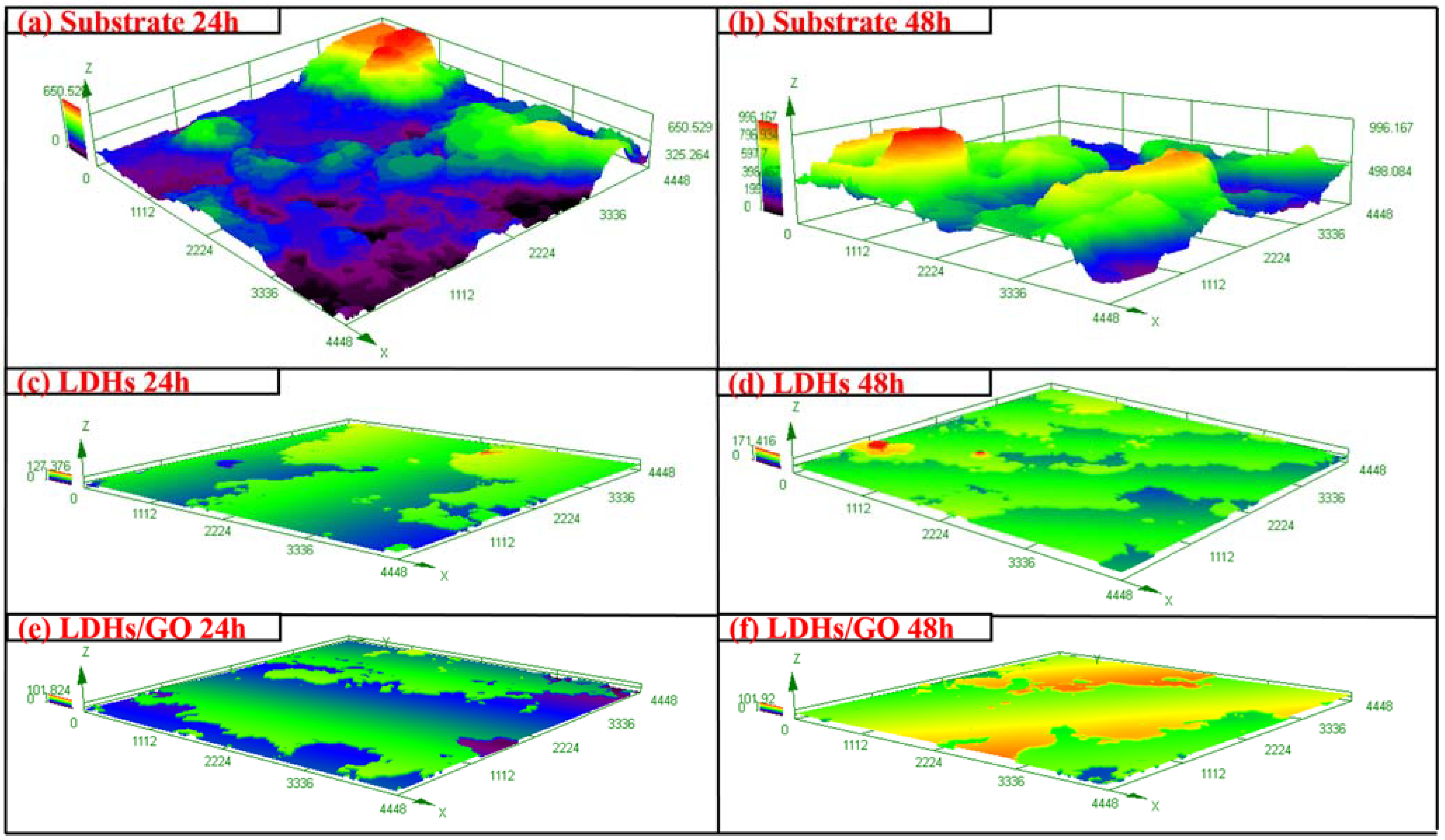
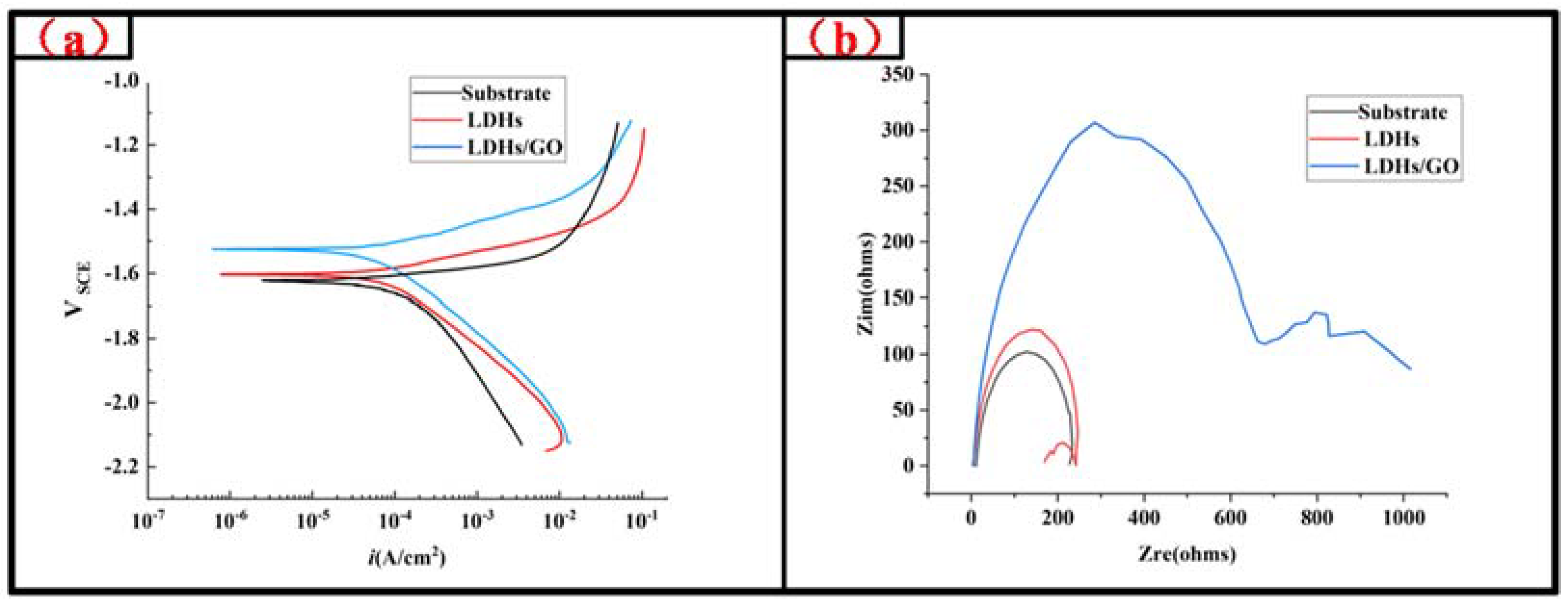
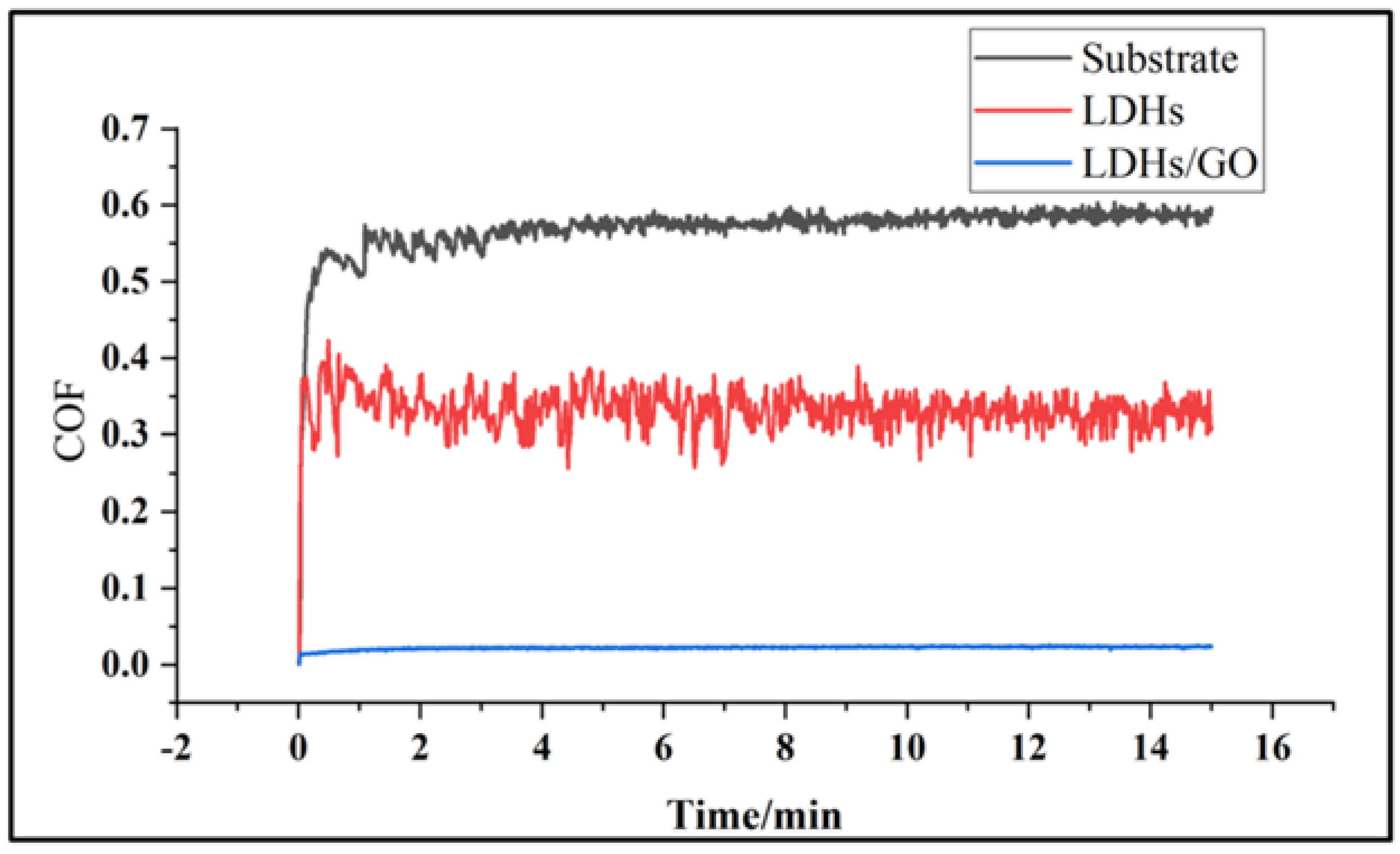
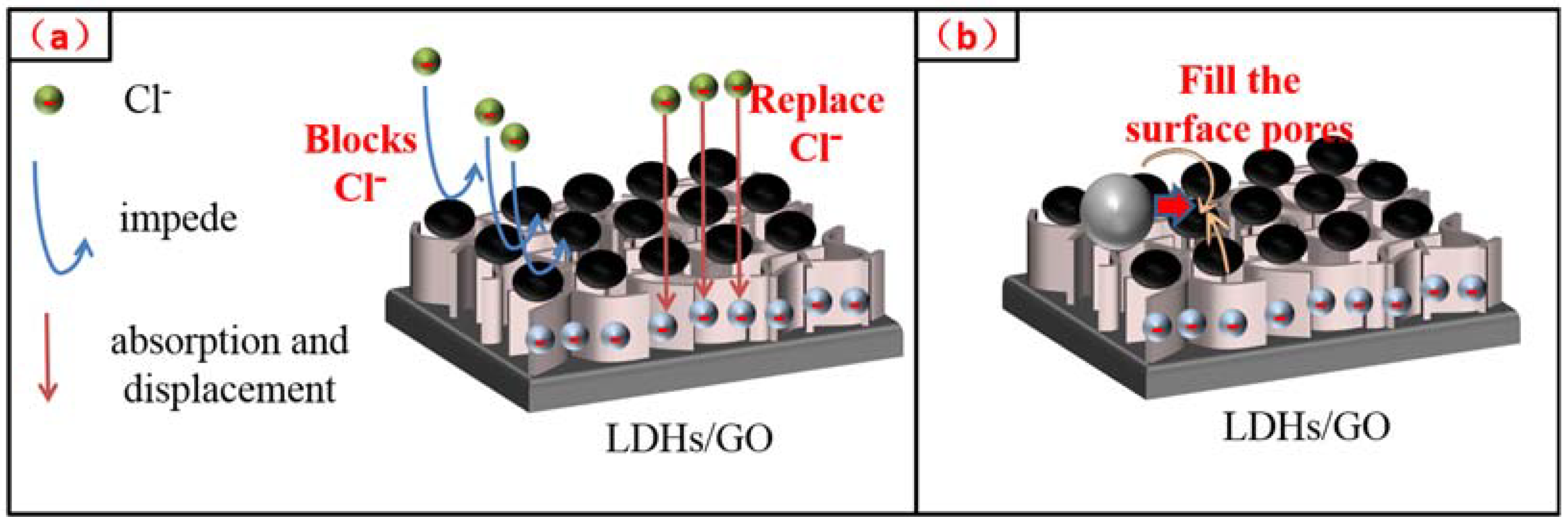
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.Q.; Li, F.; Kang, F.W.; Du, H.Q.; Chen, Z.Y. Recent research and advances in extrusion forming of magnesium alloys: A review. J. Alloys Compd. 2023, 953, 170080. [Google Scholar] [CrossRef]
- Pang, H.; Li, Q.; Chen, X.; Chen, P.; Li, X.; Tan, J. Hot deformation behavior and microstructure evolution of Mg-Gd-Y(-Sm)-Zr alloys. J. Alloys Compd. 2022, 920, 165937. [Google Scholar] [CrossRef]
- Prabhu, D.B.; Dhamotharan, S.; Sathishkumar, G.; Gopalakrishnan, P.; Ravi, K. Stress corrosion cracking of biodegradable Mg-4Zn alloy in simulated body fluid at different strain rates—A fractographic investigation. Mater. Sci. Eng. A 2018, 730, 223–231. [Google Scholar] [CrossRef]
- Li, P.; Ma, J.; Kang, S.; Chen, S. Copper-loaded chitosan coating for improved in-vitro corrosion resistance and endothelialization of magnesium alloy stents. Mater. Chem. Phys. 2023, 205, 127931. [Google Scholar] [CrossRef]
- Atrens, A.; Shi, Z.; Mehreen, S.U.; Johnston, S.; Song, G.-L.; Chen, X.; Pan, F. Review of Mg alloy corrosion rates. J. Magnes. Alloys 2020, 8, 989–998. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H. Smart stannate based self-healing coatings for corrosion protection of magnesium alloys. In Handbook of Smart Coatings for Materials Protection; Woodhead Publishing: Sawston, UK, 2014. [Google Scholar]
- Atrens, A.; Song, G.-L.; Cao, F.; Shi, Z.; Bowen, P.K. Advances in Mg corrosion and research suggestions. J. Magnes. Alloys 2013, 1, 177–200. [Google Scholar] [CrossRef]
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- He, M.; Chen, L.; Yin, M.; Xu, S.; Liang, Z. Review on magnesium and magnesium-based alloys as biomaterials for bone immobilization. J. Mater. Sci. Technol. 2023, 23, 4396–4419. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Weng, B.; Atrens, A.; Ma, S.; Liu, L.; Pan, F. Sealing of anodized magnesium alloy AZ31 with MgAl layered double hydroxides layers. RSC Adv. 2018, 8, 2248–2259. [Google Scholar] [CrossRef]
- Wu, L.; Ding, X.; Zheng, Z.; Tang, A.; Zhang, G.; Atrens, A.; Pan, F. Doubly-doped Mg-Al-Ce-V2O74-LDH composite film on magnesium alloy AZ31 for anticorrosion. J. Mater. Sci. Technol. 2021, 64, 66–72. [Google Scholar] [CrossRef]
- Chen, J.; Wu, L.; Ding, X.; Liu, Q.; Dai, X.; Song, J.; Jiang, B.; Atrens, A.; Pan, F. Effects of deformation processes on morphology, microstructure and corrosion resistance of LDHs films on magnesium alloy AZ31. J. Mater. Sci. Technol. 2021, 64, 10–20. [Google Scholar] [CrossRef]
- Salak, A.N.; Tedim, J.; Kuznetsova, A.I.; Ribeiro, J.L.; Vieira, L.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Comparative X-ray diffraction and infrared spectroscopy study of Zn-Al layered double hydroxides: Vanadate vs. nitrate. Chem. Phys. 2012, 397, 102–108. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, L.; Gu, Z.; Zheng, Z.; Liu, Y.; Tan, G.; Jie, X. Zinc aluminum-layered double hydroxide (LDH)-graphene oxide (GO) lubricating and corrosion-resistant composite coating on the surface of magnesium alloy. Surf. Coat. Technol. 2022, 437, 128354. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Long, S.; Zhang, L.; Jie, X. Tribological behavior of graphene anchored Mg-Al layered double hydroxide film on mg alloy pre-sprayed Al coating. Appl. Surf. Sci. 2020, 530, 146536. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Zhang, Y.; Long, S.; Jie, X. Corrosion behavior of Mg-Al LDH film in-situ assembled with graphene on Mg alloy pre-sprayed Al layer. J. Alloys Compd. 2020, 834, 155107. [Google Scholar] [CrossRef]
- Wen, T.; Wu, X.; Tan, X.; Wang, X.; Xu, A. One-pot synthesis of water-swellable mg-Al layered double hydroxides and graphene oxide nanocomposites for efficient removal of As(V) from aqueous solutions. ACS Appl. Mater. Interfaces 2013, 5, 3304–3311. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, A.S.; Tran, B.A.; Vu, K.O.; Tran, D.L.; Phan, T.T.; Scharnagl, N.; Zheludkevich, M.L.; To, T.X.H. Molybdate intercalated hydrotalcite/graphene oxide composite as corrosion inhibitor for carbon steel. Surf. Coat. Technol. 2020, 399, 126165. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Z.; Liu, Y.; Zhu, X.; Long, R.; Li, X. Electrostatic self-assembly method to prepare intercalated graphene oxide composite membrane to improve hydrophilicity and flux. Diam. Relat. Mater. 2021, 117, 108492. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B. Self-assembly of graphene oxide aerogels by layered double hydroxides cross-linking and their application in water purification. J. Mater. Chem. A 2014, 2, 8941–8951. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, P.; Wang, J.; Li, Y.; Chen, F.; Wei, K.; Zuo, Y. LDHs/graphene film on aluminum alloys for active protection. Appl. Surf. Sci. 2018, 433, 927–933. [Google Scholar] [CrossRef]
- Yu, D.; Wen, S.; Yang, J.; Wang, J.; Chen, Y.; Luo, J.; Wu, Y. RGO modified ZnAl-LDH as epoxy nanostructure filler: A novel synthetic approach to anticorrosive waterborne coating. Surf. Coat. Technol. 2017, 326, 207–215. [Google Scholar] [CrossRef]
- Kwon, Y.; Hong, H.-G. Electrodeposition of graphene-Zn/Al layered double hydroxide (LDH) composite for selective determination of hydroquinone. Bull. Kor. Chem. Soc. 2013, 34, 1755–1762. [Google Scholar] [CrossRef]
- Liang, S.Y.; Ren, W.W.; Lin, W.X.; Zou, L.C.; Cui, X.P.; Chen, J.F. Magnesium alloy microarc oxidation coating surface in-situ prepared MgCr-LDH nano-layer and its corrosion resistance mechanism. Rare Met. Mater. Eng. 2020, 49, 2830–2838. [Google Scholar]
- Zhou, D.; Cai, Z.; Lei, X.; Tian, W.; Bi, Y.; Jia, Y.; Han, N.; Gao, T.; Zhang, Q.; Kuang, Y.; et al. NiCoFe-layered double hydroxides/N-doped graphene oxide array colloid composite as an efficient bifunctional catalyst for oxygen electrocatalytic reactions. Adv. Energy Mater. 2017, 8, 1701905. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, L.; Ling, H.; Diao, Y.; Pang, X.; Wang, Y.; Gao, K. Design and fabrication of enhanced corrosion resistance Zn-Al layered double hydroxides films based anion-exchange mechanism on magnesium alloys. J. Therm. Spray Technol. 2017, 404, 246–253. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Zhang, S.; Yuan, B.; Zheng, Z.; Pan, F. A Novel Approach to Fabricate Protective Layered Double Hydroxide Films on the Surface of Anodized Mg-Al Alloy. Adv. Mater. Interfaces 2017, 4, 1700163. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]


| Element | Gd | Y | Zr | Zn | Mg |
|---|---|---|---|---|---|
| Content (%) | 9.0 | 3.0 | 0.5 | 0.6 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, P.; Wang, S.; Duan, Z.; Liu, L.; Wang, Y.; Xu, M. In Situ Synthesis of Graphene Oxide-Sealed LDHs Coatings: A Novel Approach to Enhancing Corrosion Resistance and Tribological Performance on Magnesium Alloys. Coatings 2023, 13, 1544. https://doi.org/10.3390/coatings13091544
Li W, Wang P, Wang S, Duan Z, Liu L, Wang Y, Xu M. In Situ Synthesis of Graphene Oxide-Sealed LDHs Coatings: A Novel Approach to Enhancing Corrosion Resistance and Tribological Performance on Magnesium Alloys. Coatings. 2023; 13(9):1544. https://doi.org/10.3390/coatings13091544
Chicago/Turabian StyleLi, Weiming, Ping Wang, Shaoqing Wang, Zhihong Duan, Lele Liu, Yimeng Wang, and Min Xu. 2023. "In Situ Synthesis of Graphene Oxide-Sealed LDHs Coatings: A Novel Approach to Enhancing Corrosion Resistance and Tribological Performance on Magnesium Alloys" Coatings 13, no. 9: 1544. https://doi.org/10.3390/coatings13091544
APA StyleLi, W., Wang, P., Wang, S., Duan, Z., Liu, L., Wang, Y., & Xu, M. (2023). In Situ Synthesis of Graphene Oxide-Sealed LDHs Coatings: A Novel Approach to Enhancing Corrosion Resistance and Tribological Performance on Magnesium Alloys. Coatings, 13(9), 1544. https://doi.org/10.3390/coatings13091544


.jpg)


