A Novel Magnetic Fluorescent Fe3O4@ZnS@MPS Nanosensor for Highly Sensitive Determination and Removal of Ag+
Abstract
:1. Introduction
2. Materials and Methods
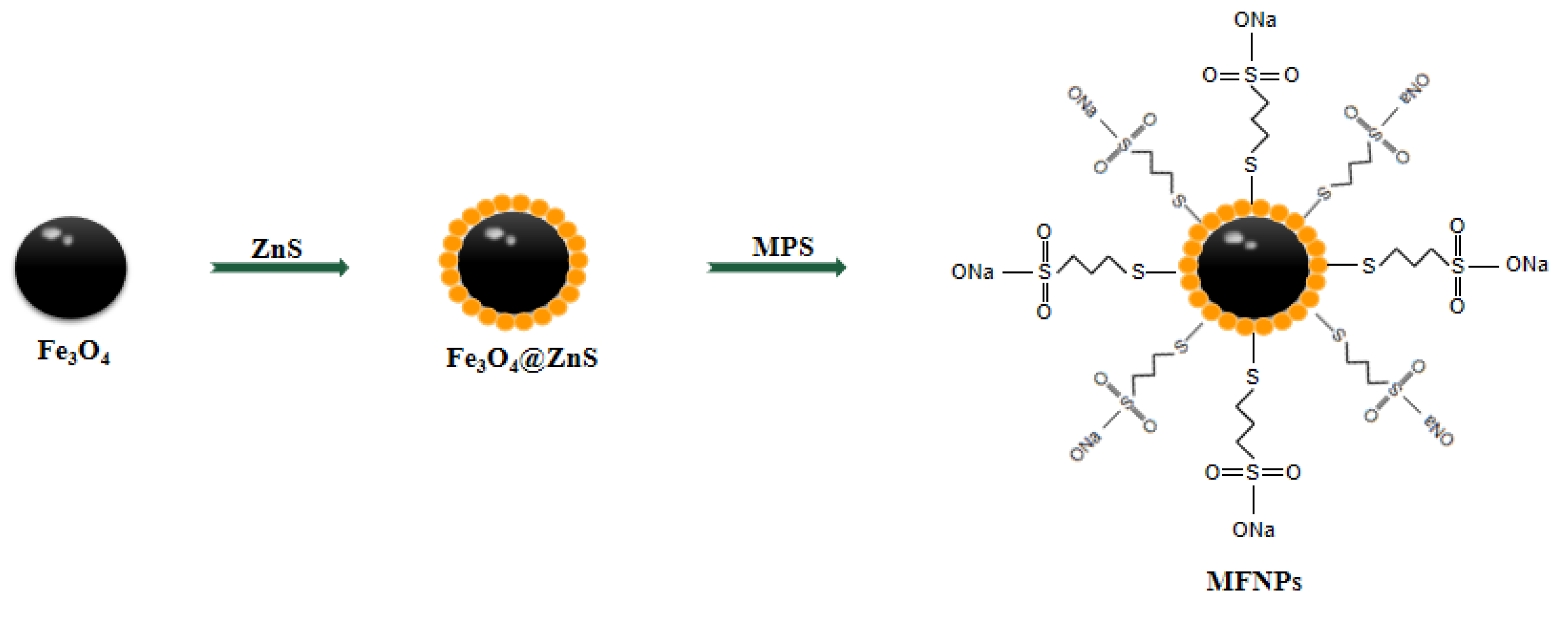
2.1. Preparation of Fe3O4 Magnetic Nanoparticle
2.2. Preparation of Fe3O4@ZnS
2.3. Preparation of Fe3O4@ZnS@MPS
2.4. Instrumentation
2.5. Materials
2.6. Procedure for the Fluorescent Detection of Ag+
2.7. Removal and Adoption Capacity Performance of Ag+
3. Results and Discussion
3.1. Characterization of MFNS
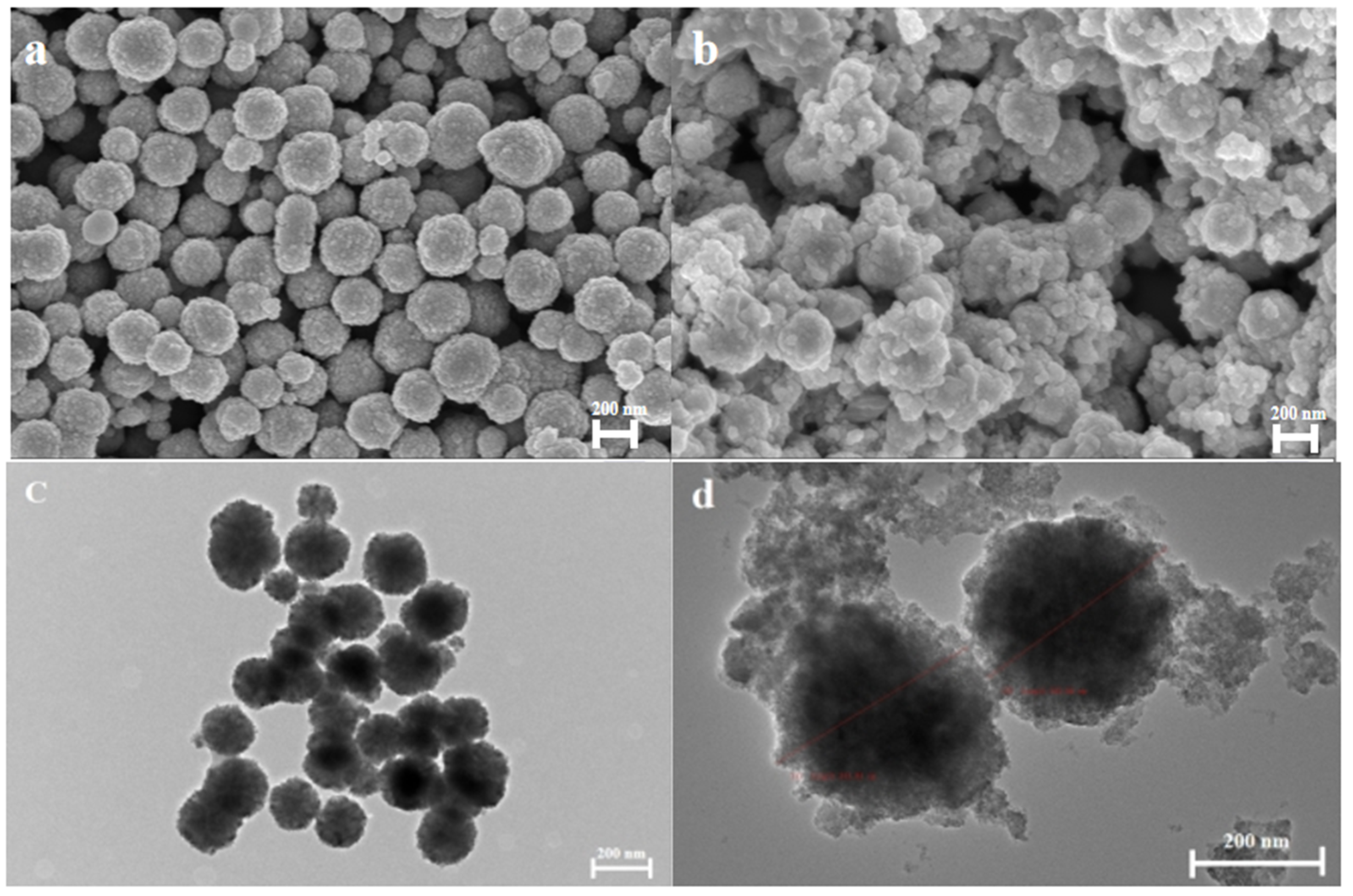
3.1.1. Morphology Analysis
3.1.2. XRD Analysis
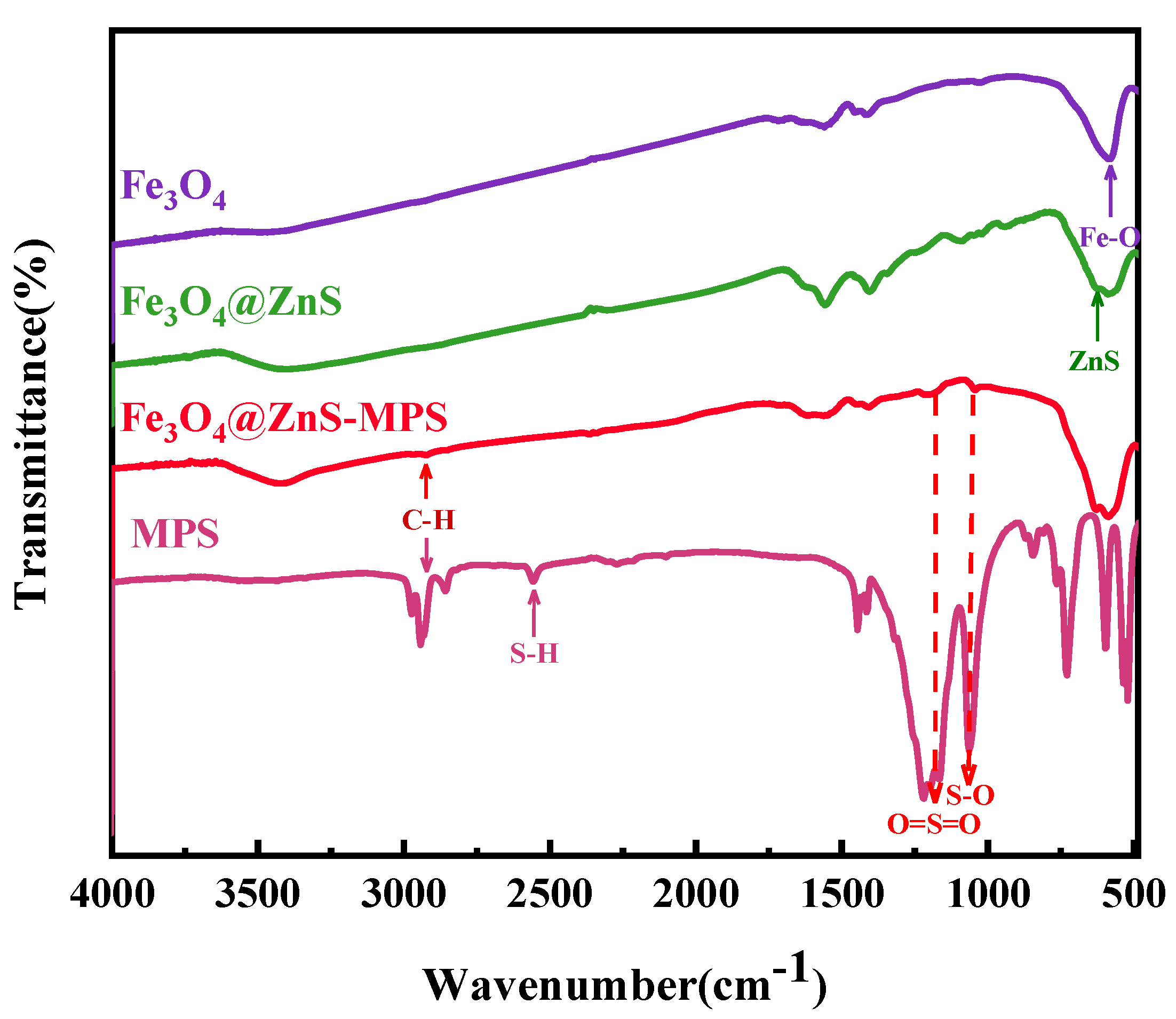
3.1.3. FT−IR Analysis
3.1.4. XPS Analysis
3.1.5. Hysteresis Curve
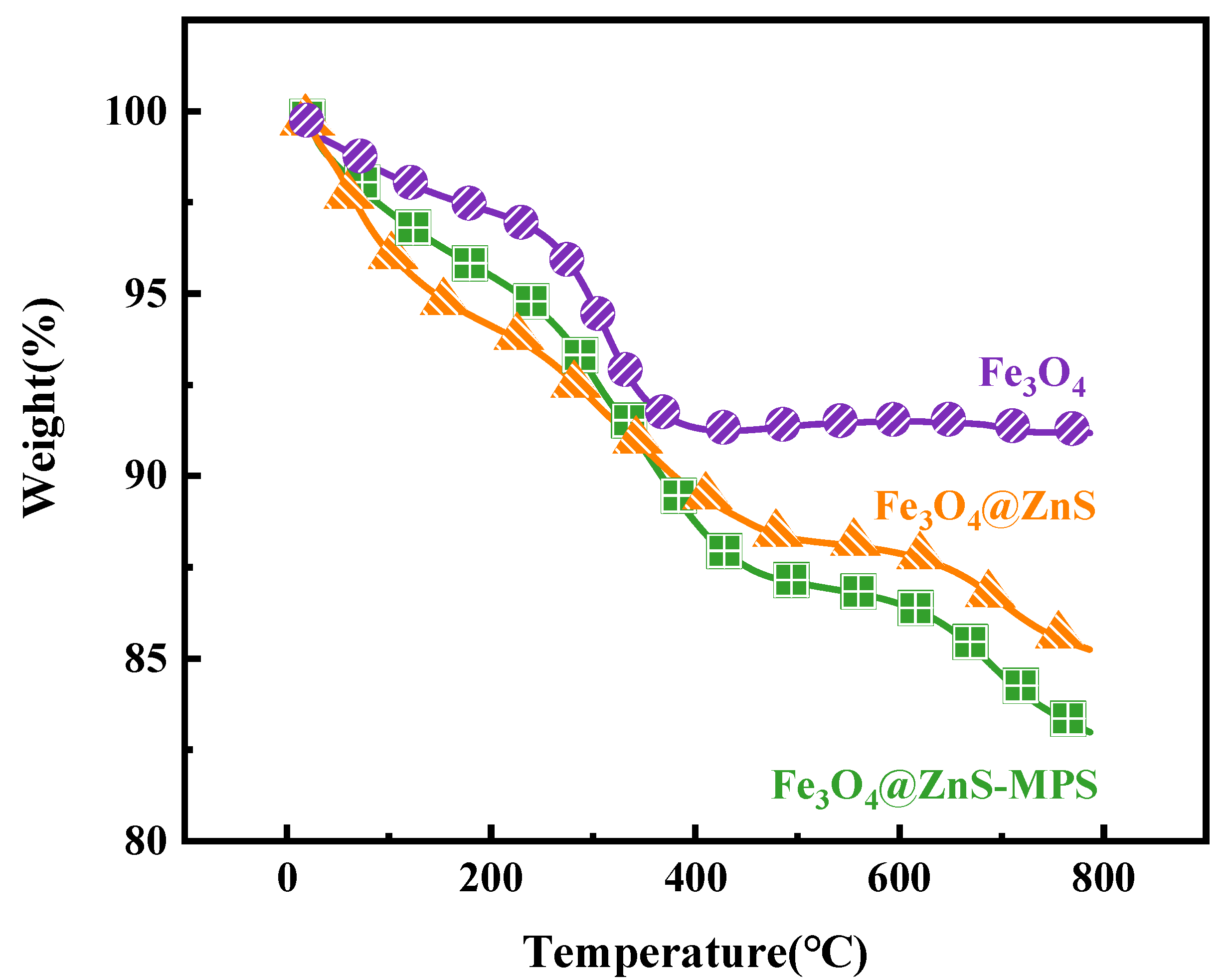
3.1.6. Thermogravimetric Analysis
3.2. Detection Performance Study of MFNPs
3.2.1. Performance Analysis of Magnetic Fluorescence Nanosensor MFNPs
3.2.2. The Effect of pH on the Fluorescence of MFNPs
3.2.3. The Response Range and Relation Curve of MFNPs to Ag+
3.2.4. Particle Selectivity and Interference Ion Determination
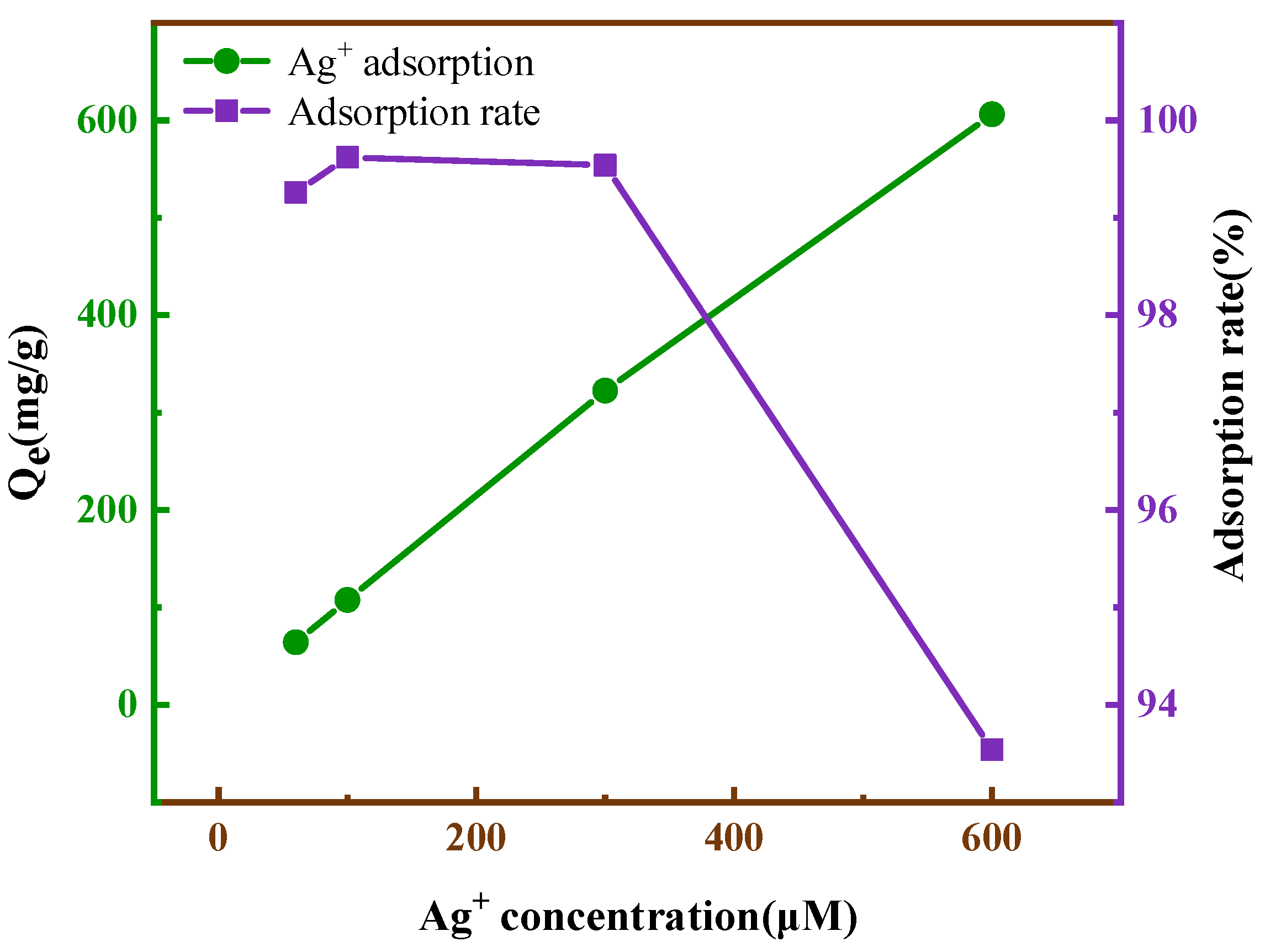
3.2.5. Removal of Ag+
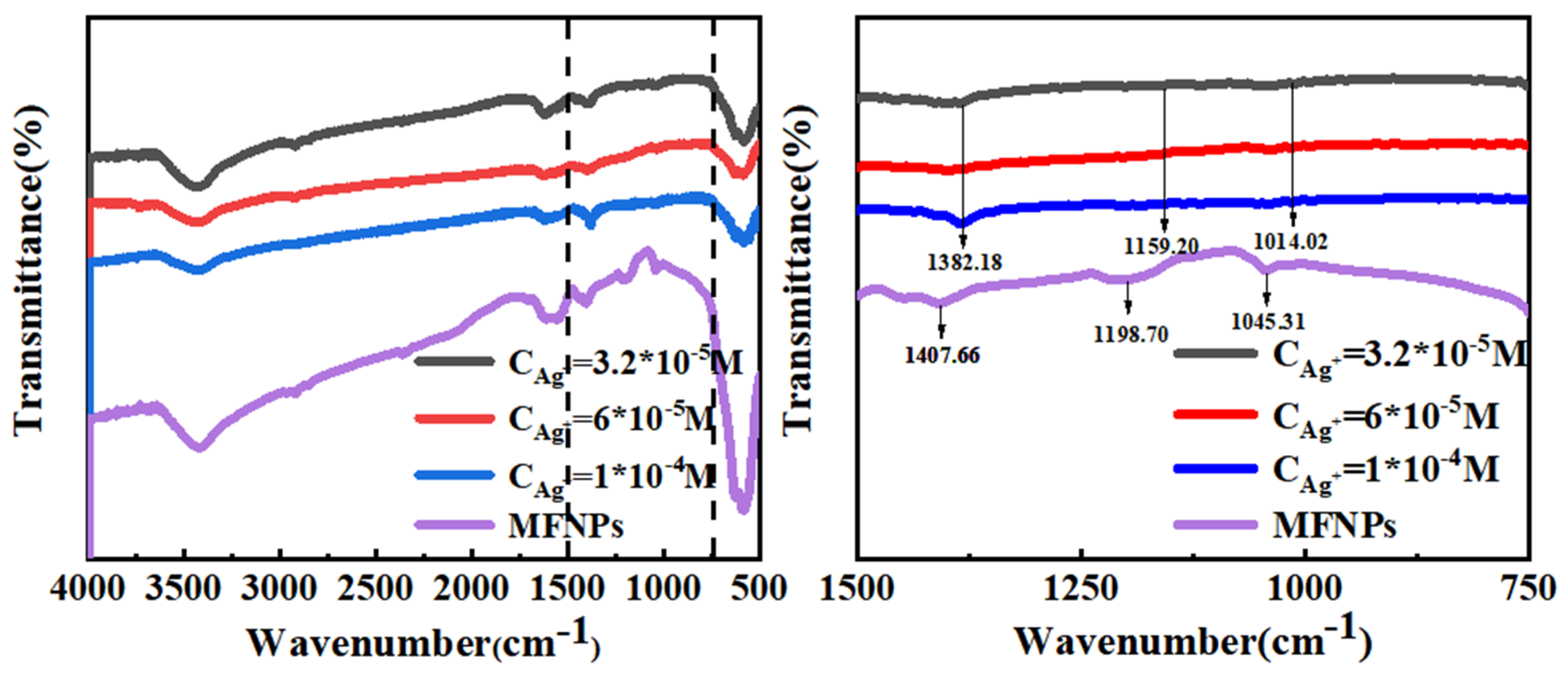
3.3. Infrared Spectroscopy Analysis before and after Complexation of MFNPs with Ag+
3.4. The Fluorescent Detection of Ag+ in Actual Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singha, S.; Kim, D.; Seo, H.; Cho, S.W.; Ahn, K.H. Fluorescence sensing systems for gold and silver species. Chem. Soc. Rev. 2015, 44, 4367–4399. [Google Scholar] [CrossRef]
- Bahareh, R.; Hossein, R.M.; Mehdi, S.K. Colorimetric/fluorometric optical chemosensors based on oxazolidine for highly selective detection of Fe3+ and Ag+ in aqueous media: Development of ionochromic security papers. J. Mol. Struct. 2023, 1271, 134021. [Google Scholar]
- Pal, C.; Majumder, S. Label-free electrochemical biosensor for ultra-low level detection of Ag(I) using ssDNA functionalized multi-walled carbon nanotube. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128927. [Google Scholar] [CrossRef]
- Han, J.S.; Zhang, X.; Zhou, Y.B.; Ning, Y.; Wu, J.; Liang, S.; Sun, H.C.; Zhang, H.; Yang, B. Fabrication of CdTe nanoparticles-based superparticles for an improved detection of Cu2+ and Ag+. J. Mater. Chem. 2012, 22, 2679–2686. [Google Scholar] [CrossRef]
- Bian, J.; Xia, Y.X.; Sang, L.Y.; Zhu, C.X.; Li, Y.X.; Li, G.Y.; Liu, X.Y.; Wang, X.; Liu, Y. A recyclable colorimetric probe: In situ fabrication of highly stable HPEI–AuNPs for selective Ag+ detection. New J. Chem. 2020, 44, 5438–5447. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M. A review on detection of heavy metal ions in water an electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Cai, C.; Cheng, H.Y.; Wang, Y.C.; Bao, H.F. Mercaptosuccinic acid modified CdTe quantum dots as a selective fluorescence sensor for Ag+ determination in aqueous solutions. RSC Adv. 2014, 4, 59157–59163. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, A.; Xu, Q.; Pandey, D.S. Zinc(II), copper(II) and cadmium(II) complexes as fluorescent chemosensors for cations. Dalton Trans. 2020, 49, 542–568. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.Y.; Wang, W.; Liu, X.Q.; Wang, L.; Yan, Y.S. A hydrophobic polymer stabilized CsPbBr3 sensor for environmental pollutant detection. New J. Chem. 2021, 45, 930–938. [Google Scholar] [CrossRef]
- Wu, P.; Yan, X.P. Doped quantum dots for chemo/biosensing and bioimaging. Chem. Soc. Rev. 2013, 42, 5489–5521. [Google Scholar] [CrossRef]
- Stefan, M.; Leostean, C.; Pana, O.; Suciu, M.; Popa, A.; Toloman, D.; Macavei, S.; Bele, C.; Tabaran, F.; Barbu-Tudoran, L. Synthesis and characterization of Fe3O4–ZnS: Mn nanocomposites for biomedical applications. Mater. Chem. Phys. 2021, 264, 124474. [Google Scholar] [CrossRef]
- Arano-Martinez, J.A.; Martínez-González, C.L.; Salazar, M.L.; Torres-Torres, C. A Framework for Biosensors Assisted by Multiphoton Effects and Machine Learning. Biosensors 2022, 12, 710. [Google Scholar] [CrossRef]
- Wang, C.W.; Shen, W.Z.; Rong, Z.; Liu, X.X.; Gu, B.; Xiao, R.; Wang, S.Q. Layer-by-layer assembly of magnetic-core dual quantum dot-shell nanocomposites for fluorescence lateral flow detection of bacteria. Nanoscale 2020, 12, 795–807. [Google Scholar] [CrossRef]
- Shah, M.T.; Balouch, A.; Alveroglu, E. Sensitive Fluorescence Detection of Ni2+ ions Using Fluorescein Functionalized Fe3O4 Nanoparticles. J. Mater. Chem. C 2018, 6, 1105–1115. [Google Scholar] [CrossRef]
- Chen, M.; Shao, L.L.; Li, J.J.; Pei, W.J.; Chen, M.K.; Xie, X.H. One-step hydrothermal synthesis of hydrophilic Fe3O4/carbon composites and their application in removing toxic chemicals. RSC Adv. 2016, 6, 35228–35238. [Google Scholar] [CrossRef]
- Dulyasucharit, R.; Wongkasemjit, S.; Nanan, S.; Intharaksa, O.; Masingboon, C. Magnetic Fe3O4/Bi2O2(OH)(NO3) as a sunlight-driven photocatalyst for rhodamine B degradation. J. Solid State Chem. 2023, 319, 123784. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Wu, D.P.; Chen, J.H.; Ma, N.; Dai, W. Boosting highly capture of trace tetracycline with a novel water-resistant and magnetic (ZIF-8)-on-(Cu-BTC@Fe3O4) composite. J. Solid State Chem. 2023, 319, 123797. [Google Scholar] [CrossRef]
- Gao, F.; Gu, H.; Wang, H.; Wang, X.; Xiang, B. Magnetic amine-functionalized polyacrylic acid-nanomagnetite for hexavalent chromium removal from polluted water. RSC Adv. 2015, 5, 60208–60219. [Google Scholar] [CrossRef]
- Duan, M.; Xia, F.F.; Li, T.L.; Shapter, J.G.; Yang, S.; Li, Y.Y.; Gao, G.; Cui, D.X. Matrix metalloproteinase-2-targeted superparamagnetic Fe3O4-PEG-G5-MMP2@Ce6 nanoprobes for dual-mode imaging and photodynamic therapy. Nanoscale 2019, 11, 18426. [Google Scholar] [CrossRef]
- Li, G.Y.; Jiang, Y.R.; Huang, K.L.; Ding, P.D.; Chen, J. Preparation and properties of magnetic Fe3O4-chitosan nanoparticles. J. Alloys Compd. 2008, 466, 451–456. [Google Scholar] [CrossRef]
- Liu, M.; Tao, Z.; Wang, H.; Zhao, F.; Sun, Q. Study on the adsorption of Hg(II) by one-pot synthesis of amino-functionalized graphene oxide decorated with Fe3O4 microspheres nanocomposite. RSC Adv. 2016, 6, 84573–84586. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Ma, M.S.; Yu, S.H.; Liu, Z.G.; Zeng, X.D. An Ethyl-Thioglycolate-Functionalized Fe3O4@ZnS Magnetic Fluorescent Nanoprobe for the Detection of Ag+ and Its Applications in Real Water Solutions. Nanomaterials 2023, 13, 1992. [Google Scholar] [CrossRef] [PubMed]
- Dafeh, S.R.; Iranmanesh, P.; Salarizadeh, P. Fabrication, optimization, and characterization of ultra-small superparamagnetic Fe3O4 and biocompatible Fe3O4@ZnS core/shell magnetic nanoparticles: Ready for biomedicine applications. Mater. Sci. Eng. C 2019, 98, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Khawla, M.; Zouhour, H.; Yves, C.; Souhaira, H.; Rym, M. ZnS quantum dots as fluorescence sensor for quantitative detection of tetracycline. Opt. Mater. 2022, 125, 112103. [Google Scholar] [CrossRef]
- Nawaz, T.; Zulfiqar, S.; Sarwar, M.I.; Iqbal, M. Synthesis of diglycolic acid functionalized core-shell silica coated Fe3O4 nanomaterials for magnetic extraction of pb(II) and cr(VI) ions. Sci. Rep. 2020, 10, 10076. [Google Scholar] [CrossRef]
- Stefan, M.; Leostean, C.; Pana, O.; Soran, M.; Suciu, R.; Gautron, E.; Chauvet, O.; Stefan, M. Synthesis and characterization of Fe3O4@ZnS and Fe3O4@Au@ZnS core–shell nanoparticles. Appl. Surf. Sci. 2014, 288, 180–192. [Google Scholar] [CrossRef]
- Zavidovskiy, I.A.; Streletskiy, O.A.; Nuriahmetov, I.F.; Nishchak, O.U.; Savchenko, N.F.; Tatarintsev, A.A.; Pavlikov, A.V. Highly Selective Polyene-Polyyne Resistive Gas Sensors: Response Tuning by Low-Energy Ion Irradiation. J. Compos. Sci. 2023, 7, 156. [Google Scholar] [CrossRef]
- Mondal, D.K.; Phukan, G.; Paul, N.; Borah, J.P. Improved self heating and optical properties of bifunctional Fe3O4/ZnS nanocomposites for magnetic hyperthermia application. J. Magn. Magn. Mater. 2021, 528, 167809. [Google Scholar] [CrossRef]
- Koc, K.; Karakus, B.; Rajar, K.; Alveroglu, E. Synthesis and Characterization of ZnS@Fe3O4 Fluorescent-Magnetic Bifunctional Nanospheres. Superlattices Microstruct. 2017, 110, 198–204. [Google Scholar] [CrossRef]
- Wen, A.; Li, G.L.; Wu, D.; Yu, Y.X.; Yang, Y.; Hu, N.; Wang, H.L.; Chen, J.; Wu, Y.N. Sulphonate functionalized covalent organic framework-based magnetic sorbent for effective solid phase extraction and determination of fluoroquinolones. J. Chromatogr. A 2020, 1612, 460651. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, D.L.; Shi, Y.L.; Pan, Q.Q.; Wang, T. Shape-selective separation of copper nanowires and copper-based nanoparticles by a ligand exchange strategy. Appl. Surf. Sci. 2023, 611, 155597. [Google Scholar] [CrossRef]
- Masoud, H.Z.; Arjomand, M.Z. Photocatalytic activity of ZrO2/TiO2/Fe3O4 ternary nanocomposite for the degradation of naproxen: Characterization and optimization using response surface methodology. Sci. Rep. 2022, 12, 10388. [Google Scholar]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir. 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Chang, C.J.; Tsai, W.C. CuS-ZnS decorated Fe3O4 nanoparticles as magnetically separable composite photocatalysts with excellent hydrogen production activity. Int. J. Hydrog. Energy 2019, 44, 20872–20880. [Google Scholar] [CrossRef]
- Sun, N.N.; Yan, B. A reliable amplified fluorescence-enhanced chemosensor (Eu-MIL-61) for the directional detection of Ag+ in an aqueous solution. Dalton Trans. 2017, 46, 875–881. [Google Scholar] [CrossRef]
- Li, J.R.; Huang, H.; Zhang, C.; Chen, X.L.; Hu, Y.J.; Huang, X.H. Dual-key-and-lock AIE probe for thiosulfate and Ag+ detection in mitochondria. Talanta 2023, 255, 124222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.T.; Shen, M.X.; Shen, J.W.; Wang, C.Z.; Wei, Y.M. Nitrogen and bromine co-doped carbon dots with red fluorescence for sensing of Ag+ and visual monitoring of glutathione in cells. Spectrochim. Acta A Mol. Biomol. Spectrosc 2023, 296, 122642. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, L.; Hao, Y.M.; Dong, W.J.; Liu, Y.; Song, S.M.; Shuang, S.M.; Dong, C.; Gong, X.J. Ratiometric fluorescent sensors for sequential on-off-on determination of riboflavin, Agt and L-cysteine based on NPCl-doped carbon quantum dots. Anal. Chim. Acta 2021, 1144, 1–13. [Google Scholar] [CrossRef]
- Luo, L.X.; Ye, Y.; Wang, H.; Liu, Z.; Li, J.Q. Rapid Detection of Ag+ in Food Using Cholesteric Chiral Artificial Receptor L5. Microchem. J. 2023, 190, 108633. [Google Scholar]
- Wang, B.X.; Wu, K.Y.; Liu, T.H.; Cheng, Z.K.; Liu, Y.; Liu, Y.F.; Niu, Y.Z. Feasible synthesis of bifunctional polysilsesquioxane microspheres for robust adsorption of Hg(II) and Ag(I): Behavior and mechanism. J. Hazard. Mater. 2023, 442, 130121. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhang, Y.Y.; Liu, Y.; Zhang, T.A. Removal of Cr(VI) and Ag(I) by grafted magnetic zeolite/chitosan for water purification: Synthesis and adsorption mechanism. Int. J. Biol. Macromol. 2022, 222, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Lin, J.; Chen, S.L.; Xie, H.D.; Qi, Y.B. Selective recovery of Ag(I) from effluent using 2,5-dimercap-to-1,3,4-thiadiazole modified apple pomace. Sustain. Chem. Pharm. 2022, 26, 100621. [Google Scholar] [CrossRef]
- Ding, X.; Yu, W.J.; Sheng, X.; Shi, H.; You, D.; Peng, M.M.; Shao, P.H.; Yang, L.M.; Liu, L.M.; Luo, X.B. Feasible fabrication of o -phenanthroline-based polymer adsorbent for selective capture of aqueous Ag(I). Chin. Chem. Lett. 2023, 34, 107485. [Google Scholar] [CrossRef]



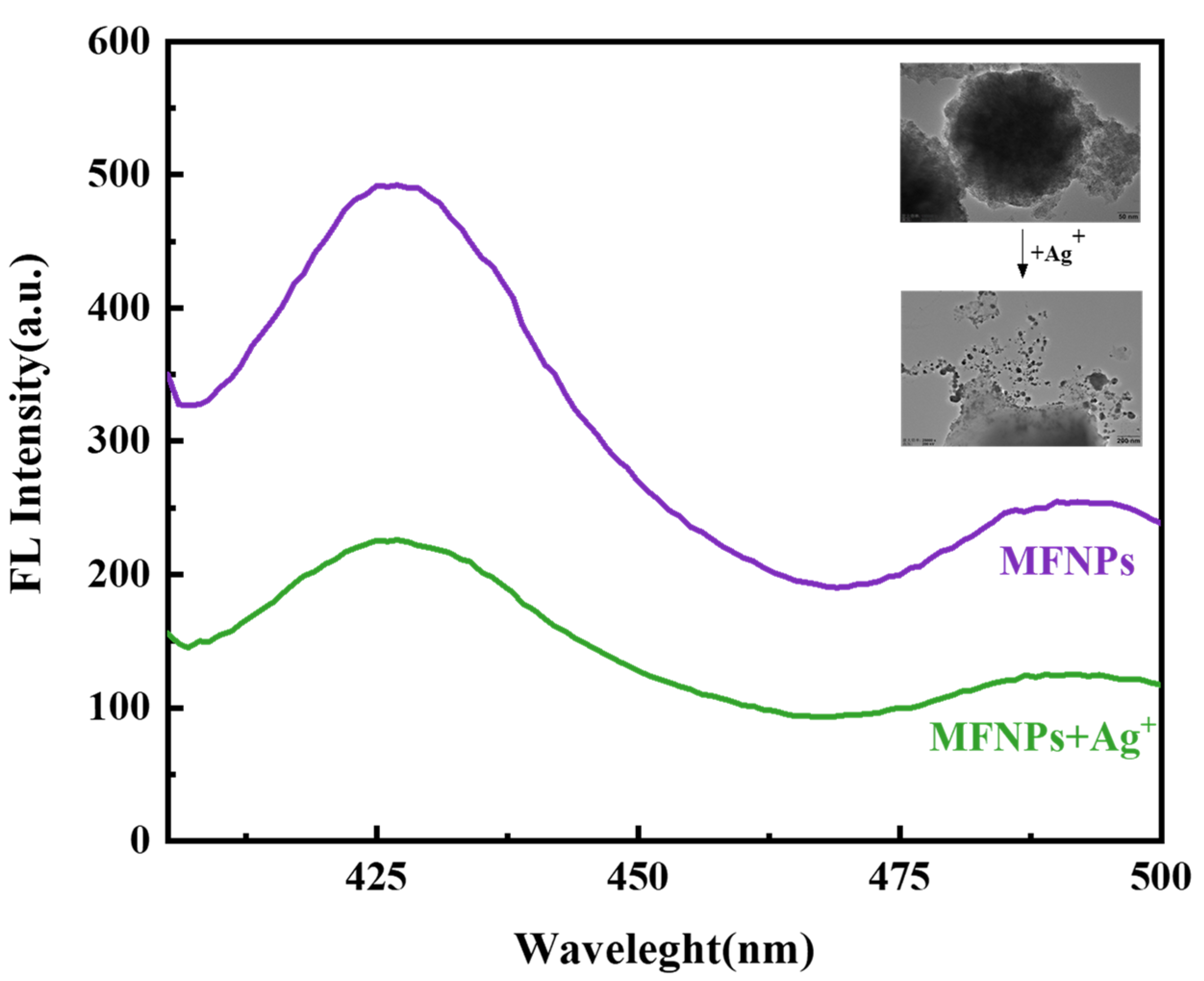
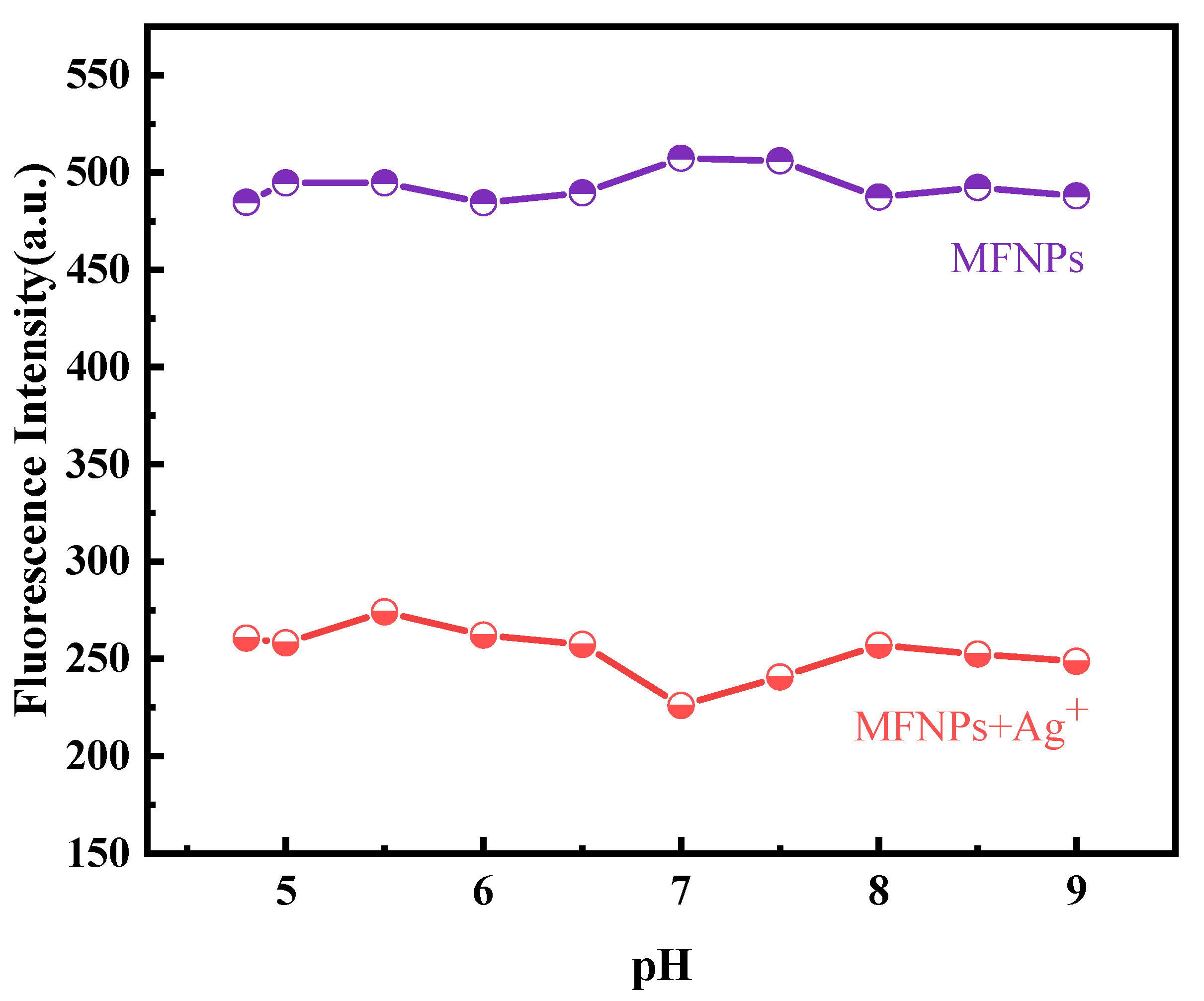
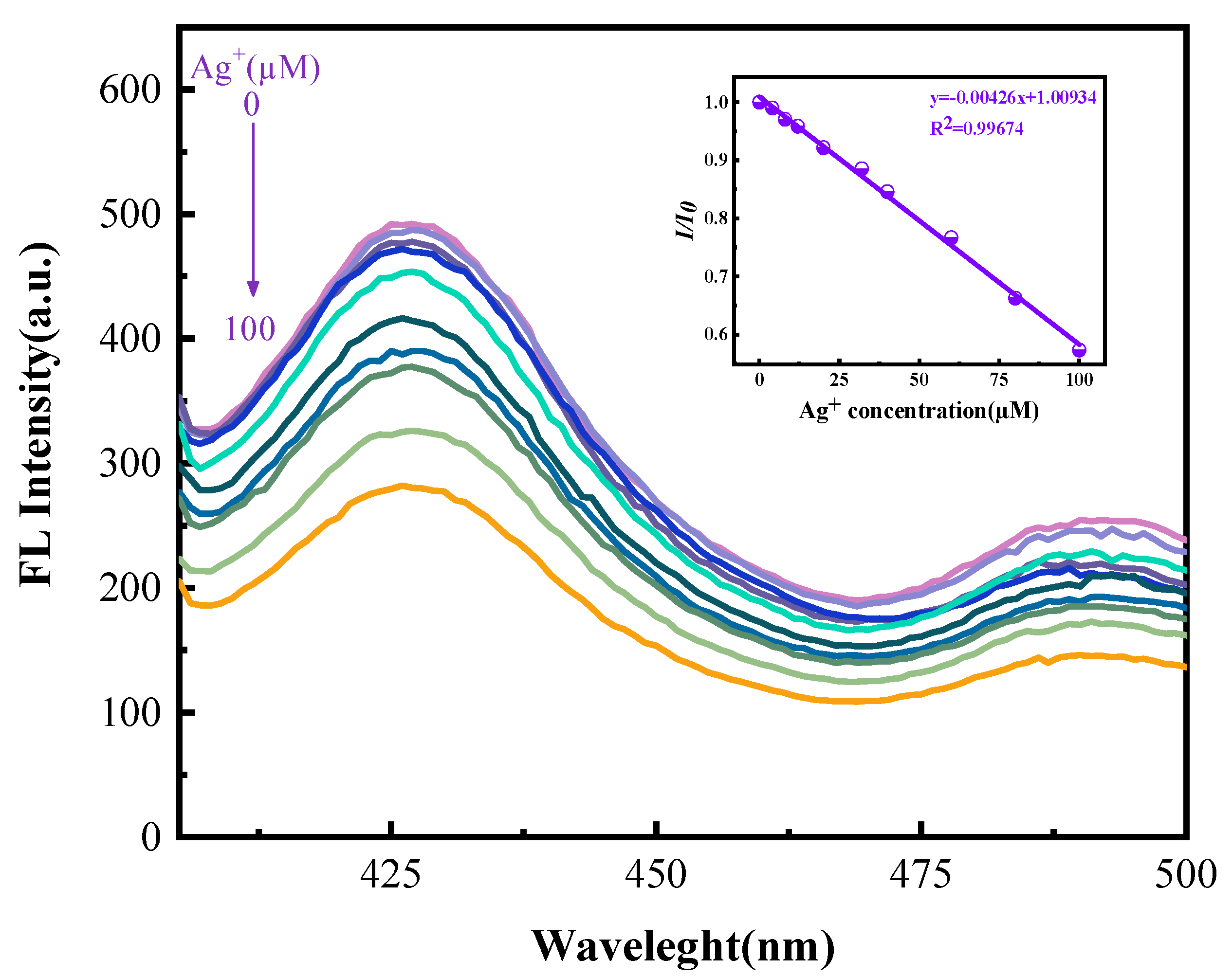
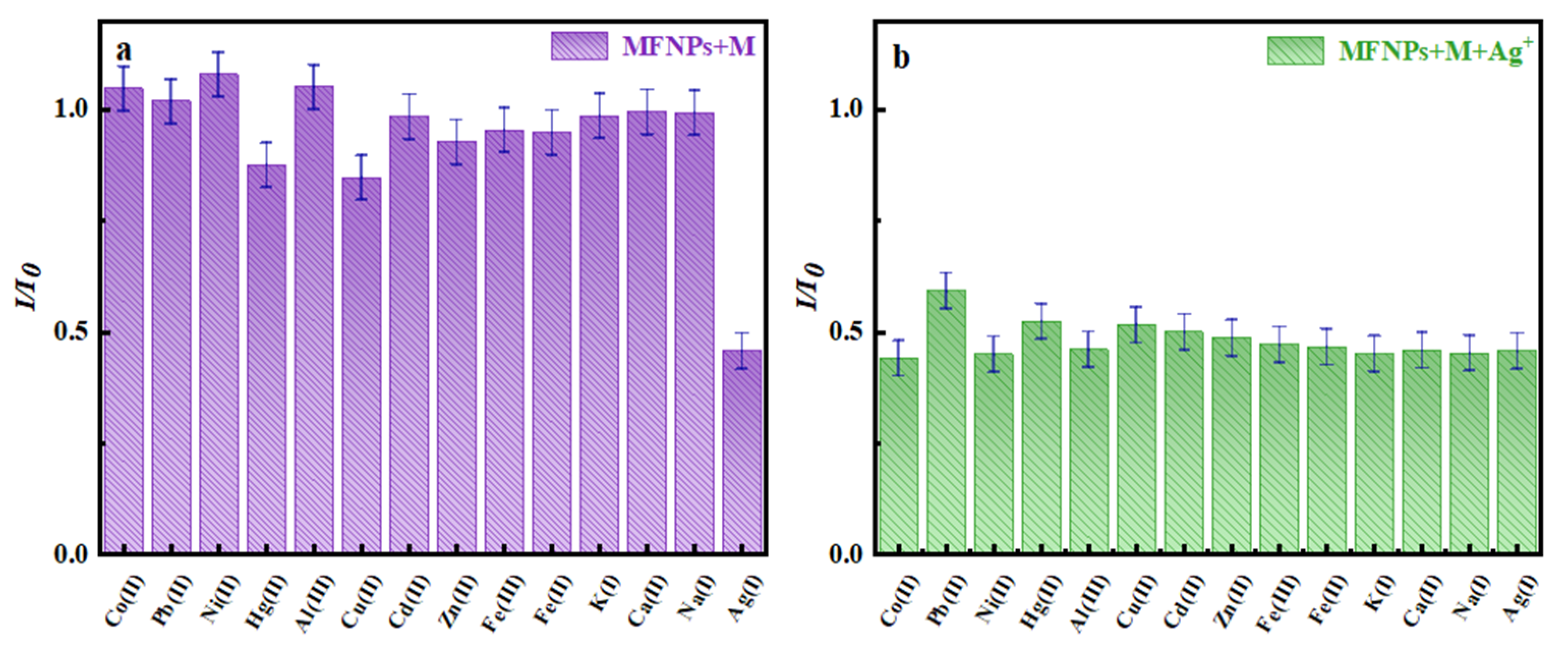
| Type of Sensor | Detection Range | LOD | Reference |
|---|---|---|---|
| Eu-MIL-61(Ga(OH)(btec)·0.5H2O) | 0–1000 μM | 0.23 μM | Dalton Transactions, 46 (2017)875 [35] |
| Triphenylamine–thiophene-pyridinium | 5.0–9.0 μM | 3.6 μM | Talanta, 255(2023)124222 [36] |
| Nitrogen and bromine co-doped carbon dots | 0–6.0 μM | 0.14 μM | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 296(2023)122642 [37] |
| NPCl-doped carbon quantum dots | 15.89–27.66 μM | 26.38 μM | Analytica Chimica Acta, 1144 (2021) 1–13 [38] |
| Cholesteric chiral artificial receptor L5 | 2–20 μM | 0.13 μM | Microchemical Journal, 190(2023)108633 [39] |
| Fe3O4@ZnS@MPS | 0–100 μM | 7.04 μM | This work |
| Type of Sensor | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| Bifunctional polysilsesquioxane microspheres | 416.88 | Journal of Hazardous Materials 442(2023) 130,121 [40] |
| ZMC-MAH-TEPA (grafted magnetic zeolite/chitosan) | 70.12 | International Journal of Biological Macromolecules 222 (2022) 2615–2627 [41] |
| DMTD-AP(2,5-dimercap-to-1,3,4-thiadiazole modified apple pomace) | 196.9 | Sustainable Chemistry and Pharmacy 26 (2022) 100,621 [42] |
| L-PRL(o -phenanthroline-based polymer) | 325.8 | Chinese Chemical Letters 34 (2023) 107,485 [43] |
| MFNPs | 395.79 | This work |
| Sample | Added (μM) | Measured (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 1 | 0.926 | 92.60 | 3.18 |
| 5.0 | 4.859 | 97.18 | 4.25 | |
| 10.0 | 10.321 | 103.21 | 3.86 | |
| River water | 1 | 1.053 | 105.30 | 4.34 |
| 5.0 | 4.987 | 99.74 | 3.89 | |
| 10.0 | 9.842 | 98.42 | 4.45 | |
| Electrolysis waste water | 1 | 0.852 | 85.20 | 5.32 |
| 5.0 | 4.320 | 86.40 | 4.96 | |
| 10.0 | 8.620 | 86.20 | 4.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Chen, X.; Xu, P.; Chen, J.; Yu, S.; Liu, Z.; Zeng, X. A Novel Magnetic Fluorescent Fe3O4@ZnS@MPS Nanosensor for Highly Sensitive Determination and Removal of Ag+. Coatings 2023, 13, 1557. https://doi.org/10.3390/coatings13091557
Gao Y, Chen X, Xu P, Chen J, Yu S, Liu Z, Zeng X. A Novel Magnetic Fluorescent Fe3O4@ZnS@MPS Nanosensor for Highly Sensitive Determination and Removal of Ag+. Coatings. 2023; 13(9):1557. https://doi.org/10.3390/coatings13091557
Chicago/Turabian StyleGao, Yan, Xin Chen, Ping Xu, Jie Chen, Shihua Yu, Zhigang Liu, and Xiaodan Zeng. 2023. "A Novel Magnetic Fluorescent Fe3O4@ZnS@MPS Nanosensor for Highly Sensitive Determination and Removal of Ag+" Coatings 13, no. 9: 1557. https://doi.org/10.3390/coatings13091557
APA StyleGao, Y., Chen, X., Xu, P., Chen, J., Yu, S., Liu, Z., & Zeng, X. (2023). A Novel Magnetic Fluorescent Fe3O4@ZnS@MPS Nanosensor for Highly Sensitive Determination and Removal of Ag+. Coatings, 13(9), 1557. https://doi.org/10.3390/coatings13091557






