Study on the Effects and Mechanism of Temperature and AO Flux Density in the AO Interaction with Upilex-S Using the ReaxFF Method
Abstract
1. Introduction
2. Computational Details
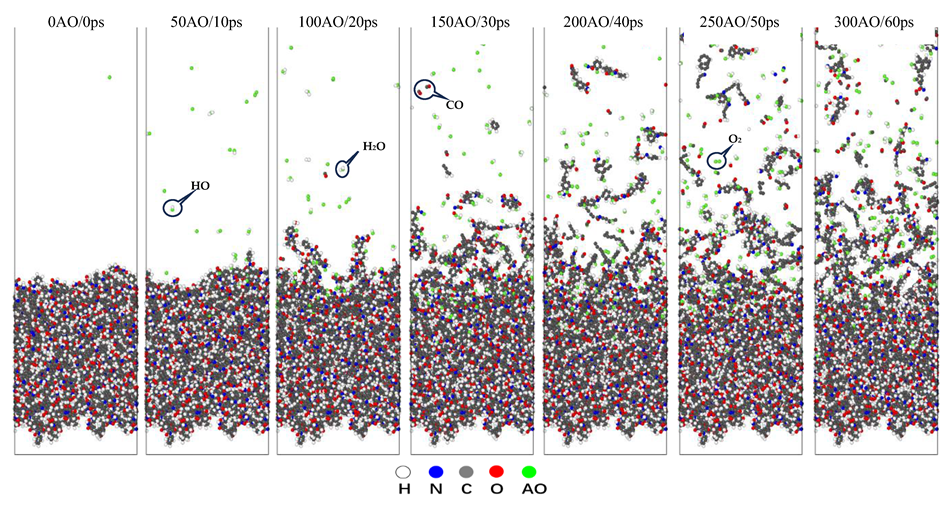
3. Results and Discussion
3.1. The Temperature Effects on the AO Interaction with Upilex-S
3.1.1. Temperature Variation
3.1.2. Mass Loss
3.1.3. Reaction Products and Erosion Yield
3.2. Effects of Flux Density on the AO Interaction with Upilex-S
3.2.1. Temperature Variation
3.2.2. Mass Loss
3.2.3. Reaction Products and Erosion Yield
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banks, B.A.; de Groh, K.K.; Miller, S.K. Low Earth Orbital Atomic Oxygen Interactions with Spacecraft Materials. MRS Proc. 2004, 851, NN8-1. [Google Scholar] [CrossRef]
- Samwel, S. Low Earth Orbital Atomic Oxygen Erosion Effect on Spacecraft Materials. Space Res. J. 2014, 7, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Dong, S.; Tubi, M.; Ye, Z. The effect of atomic oxygen on spacecraft materials and the protection measures. Spacecr. Environ. Eng. 2012, 29, 185–190. [Google Scholar] [CrossRef]
- Qiao, S.; Jiang, H.; Liu, Y.; Li, T.; Jiao, Z.; Cui, N.; Sun, J.; Jiang, L. Overview of atomic oxygen effect to space materials based on molecular dynamics ReaxFF method. Spacecr. Environ. Eng. 2023, 40, 319–329. [Google Scholar]
- Duo, S.W.; Song, M.M.; Liu, T.Z.; Hu, C.Y.; Li, M.S. Effect of Atomic Oxygen Exposure on Polyhedral Oligomeric Silsesquioxane/Polyimide Hybrid Materials in Low Earth Orbit Environment. Key Eng. Mater. 2011, 492, 521–524. [Google Scholar] [CrossRef]
- Zhao, W.; Li, W.; Liu, H.; Zhu, L. Erosion of a Polyimide Material Exposed to Simulated Atomic Oxygen Environment. Chin. J. Aeronaut. 2010, 23, 268–273. [Google Scholar]
- Banks, B.A.; Mirtich, M.J.; Rutledge, S.K.; Swec, D.M. Sputtered coatings for protection of spacecraft polymers. Thin Solid Film. 1985, 127, 107–114. [Google Scholar] [CrossRef]
- Jiao, Z.; Jiang, H.; Jiang, L.; Zhai, R.; Yang, Y. The test conditions in the study of synergistic effects of atomic oxygen and far ultraviolet irradiation in low Earth orbit. Spacecr. Environ. Eng. 2022, 39, 230–236. [Google Scholar]
- Sechkar, E.; Tollis, G.; Dever, J.; Miller, S.; Messer, R. Exposure of polymer film thermal control materials on the Materials International Space Station Experiment (MISSE). In Proceedings of the 2001 Conference and Exhibit on International Space Station Utilization, Cape Canaveral, FL, USA, 15–18 October 2001. [Google Scholar] [CrossRef]
- Gorreta, S.; Pons-Nin, J.; López, G.; Figueras, E.; Jové-Casulleras, R.; Araguz, C.; Via, P.; Camps, A.; Domínguez-Pumar, M. A CubeSAT payload for in-situ monitoring of pentacene degradation due to atomic oxygen etching in LEO. Acta Astronaut. 2016, 126, 456–462. [Google Scholar] [CrossRef]
- Stambler, A.H.; Inoshita, K.E.; Roberts, L.M.; Barbagallo, C.E.; de Groh, K.K.; Banks, B.A.; Kleiman, J.I. Ground-Laboratory to In-Space Atomic Oxygen Correlation for the PEACE Polymers. AIP Conf. Proc. 2009, 1087, 51–66. [Google Scholar] [CrossRef]
- Miller, S.K.; Banks, B.A.; Waters, D.L. Investigation into the Differences Between Atomic Oxygen Erosion Yields of Materials in Ground-Based Facilities and LEO. High Perform. Polym. 2008, 20, 523–534. [Google Scholar] [CrossRef]
- Aghaei, A.A.; Eshaghi, A.; Karami, E. Silicon solar cell performance deposited by diamond like carbon thin film “Atomic oxygen effects”. Acta Astronaut. 2017, 138, 369–373. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Senftle, T.P.; Hong, S.; Islam, M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF Reactive Force-Field: Development, Applications and Future Directions. Comput. Mater. 2016, 2, 15011. [Google Scholar] [CrossRef]
- Nayir, N.; Mao, Q.; Wang, T.; Kowalik, M.; Zhang, Y.; Wang, M.; Dwivedi, S.; Jeong, G.-U.; Shin, Y.K.; van Duin, A. Modeling and simulations for 2D materials: A ReaxFF perspective. 2d Mater. 2023, 10, 032002. [Google Scholar] [CrossRef]
- Chaney, H.; Lu, K. ReaxFF Simulation of Pyrolysis Behaviors of Polysiloxane Precursors with Different Carbon Content. Chem. Mater. 2023, 35, 3902–3910. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Zhou, Q.; Yang, T.; Li, S.; Zhou, Q. Simulation strategies for ReaxFF molecular dynamics in coal pyrolysis applications: A review. J. Anal. Appl. Pyrolysis 2023, 170, 105882. [Google Scholar] [CrossRef]
- Jung, C.K.; Braunwarth, L.; Jacob, T. Grand Canonical ReaxFF Molecular Dynamics Simulations for Catalytic Reactions. J. Chem. Theory Comput. 2019, 15, 5810–5816. [Google Scholar] [CrossRef]
- Song, L.; Zhao, F.-Q.; Xu, S.-Y.; Ju, X.-H.; Ye, C.-C. Onset of catalytic activity of graphene nanosheets in reaction with energetic materials evaluated by ReaxFF molecular dynamics simulation. Surf. Interfaces 2022, 31, 102024. [Google Scholar] [CrossRef]
- Romine, D.; Sakidja, R. Modeling atomic layer deposition of alumina using reactive force field molecular dynamics. MRS Adv. 2022, 7, 185–189. [Google Scholar] [CrossRef]
- Kowalik, M.; Ashraf, C.; Damirchi, B.; Akbarian, D.; Rajabpour, S.; van Duin, A.C.T. Atomistic Scale Analysis of the Carbonization Process for C/H/O/N-Based Polymers with the ReaxFF Reactive Force Field. J. Phys. Chem. B 2019, 123, 5357–5367. [Google Scholar] [CrossRef]
- Mao, Q.; Rajabpour, S.; Kowalik, M.; van Duin, A.C. Predicting cost-effective carbon fiber precursors: Unraveling the functionalities of oxygen and nitrogen-containing groups during carbonization from ReaxFF simulations. Carbon 2019, 159, 25–36. [Google Scholar] [CrossRef]
- Rajabpour, S.; Mao, Q.; Gao, Z.; Talkhoncheh, M.K.; Zhu, J.; Schwab, Y.; Kowalik, M.; Li, X.; van Duin, A.C. Low-temperature carbonization of polyacrylonitrile/graphene carbon fibers: A combined ReaxFF molecular dynamics and experimental study. Carbon 2020, 174, 345–356. [Google Scholar] [CrossRef]
- Mao, Q.; Rajabpour, S.; Talkhoncheh, M.K.; Zhu, J.; Kowalik, M.; van Duin, A.C.T. Cost-effective carbon fiber precursor selections of polyacrylonitrile-derived blend polymers: Carbonization chemistry and structural characterizations. Nanoscale 2022, 14, 6357–6372. [Google Scholar] [CrossRef]
- Zhang, L.; Kowalik, M.; Mao, Q.; Damirchi, B.; Zhang, Y.; Bradford, P.D.; Li, Q.; van Duin, A.C.T.; Zhu, Y.T. Joint Theoretical and Experimental Study of Stress Graphitization in Aligned Carbon Nanotube/Carbon Matrix Composites. ACS Appl. Mater. Interfaces 2023, 15, 32656–32666. [Google Scholar] [CrossRef] [PubMed]
- Rahnamoun, A.; van Duin, A.C.T. Reactive Molecular Dynamics Simulation on the Disintegration of Kapton, POSS Polyimide, Amorphous Silica, and Teflon during Atomic Oxygen Impact Using the Reaxff Reactive Force-Field Method. J. Phys. Chem. A 2014, 118, 2780–2787. [Google Scholar] [CrossRef]
- Zeng, F.; Peng, C.; Liu, Y.; Qu, J. Reactive Molecular Dynamics Simulations on the Disintegration of PVDF, FP-POSS, and Their Composite during Atomic Oxygen Impact. J. Phys. Chem. A 2015, 119, 8359–8368. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Nouranian, S.; Li, X.; Al-Ostaz, A. Reactive Molecular Simulation of the Damage Mitigation Efficacy of POSS-, Graphene-, and Carbon Nanotube-Loaded Polyimide Coatings Exposed to Atomic Oxygen Bombardment. ACS Appl. Mater. Interfaces 2017, 9, 12802–12811. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Peng, F.; Zhang, C.; Sun, C.; Zeng, Z.; Fang, G. Multi-faceted simulation of atomic oxygen erosion of deorbit sail for cleaning space debris. Acta Astronaut. 2021, 187, 61–69. [Google Scholar] [CrossRef]
- De Groh, K.K.; Banks, B.A.; Mccarthy, C.E.; Rucker, R.N.; Roberts, L.M.; Berger, L.A. MISSE 2 PEACE Polymers Atomic Oxygen Erosion Experiment on the International Space Station. High Perform. Polym. 2008, 20, 388–409. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J. Thermal ablation mechanism of polyimide reinforced with POSS under atomic oxygen bombardment. Appl. Surf. Sci. 2021, 567, 150578. [Google Scholar] [CrossRef]
- Evans, D.J.; Holian, B.L. The Nose–Hoover thermostat. J. Chem. Phys. 1985, 83, 4069–4074. [Google Scholar] [CrossRef]
- Štich, I.; Car, R.; Parrinello, M.; Baroni, S. Conjugate gradient minimization of the energy functional: A new method for electronic structure calculation. Phys. Rev. B 1989, 39, 4997–5004. [Google Scholar] [CrossRef]
- Pisacane, V. Fundamentals of Space Systems; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Duo, S.W.; Li, M.S.; Zhang, Y.M.; Zhou, Y.C. Mass change and erosion mechanism of the polyimide film during atomic oxygen exposure. Chin. J. Mater. Res. 2005, 19, 337–342. [Google Scholar]


























| Parameter | Upilex-S Model | AO Energy (eV) | Initial Temperature (K) | Dose Rate (Atoms/ps) | AO (Atoms) | Time (ps) | |
|---|---|---|---|---|---|---|---|
| Job | |||||||
| 1 | (C22H12O4N2)240 | 4.5 | 103 | 5 | 300 | 60 | |
| 2 | 200 | ||||||
| 3 | 300 | ||||||
| 4 | 396 | ||||||
| 5 | 500 | ||||||
| 6 | 600 | ||||||
| Parameter | Upilex-S Model | Upilex-S Temperature (K) | AO Energy (eV) | Dose Rate (Atoms/ps) | AO (Atoms) | Time (ps) | |
|---|---|---|---|---|---|---|---|
| Job | |||||||
| 1 | (C22H12O4N2)240 | 300 | 4.5 | 5 | 150 | 30 | |
| 2 | 10 | 300 | 30 | ||||
| 3 | 15 | 450 | 30 | ||||
| 4 | 20 | 600 | 30 | ||||
| 5 | 25 | 750 | 30 | ||||
| 6 | 30 | 900 | 30 | ||||
| 7 | 35 | 1050 | 30 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, S.; Jiang, H.; Zhai, R.; Liu, Y.; Li, T.; Xu, Y.; Jiang, L. Study on the Effects and Mechanism of Temperature and AO Flux Density in the AO Interaction with Upilex-S Using the ReaxFF Method. Coatings 2023, 13, 1586. https://doi.org/10.3390/coatings13091586
Qiao S, Jiang H, Zhai R, Liu Y, Li T, Xu Y, Jiang L. Study on the Effects and Mechanism of Temperature and AO Flux Density in the AO Interaction with Upilex-S Using the ReaxFF Method. Coatings. 2023; 13(9):1586. https://doi.org/10.3390/coatings13091586
Chicago/Turabian StyleQiao, Shiying, Haifu Jiang, Ruiqiong Zhai, Yuming Liu, Tao Li, Yanlin Xu, and Lixiang Jiang. 2023. "Study on the Effects and Mechanism of Temperature and AO Flux Density in the AO Interaction with Upilex-S Using the ReaxFF Method" Coatings 13, no. 9: 1586. https://doi.org/10.3390/coatings13091586
APA StyleQiao, S., Jiang, H., Zhai, R., Liu, Y., Li, T., Xu, Y., & Jiang, L. (2023). Study on the Effects and Mechanism of Temperature and AO Flux Density in the AO Interaction with Upilex-S Using the ReaxFF Method. Coatings, 13(9), 1586. https://doi.org/10.3390/coatings13091586






