The Influence of Chitosan on Water Absorption and Solubility of Calcium Phosphate Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Absorption and Solubility Tests
2.3. NMR Relaxometry Experiments
2.4. Microstructural Analysis of Cements
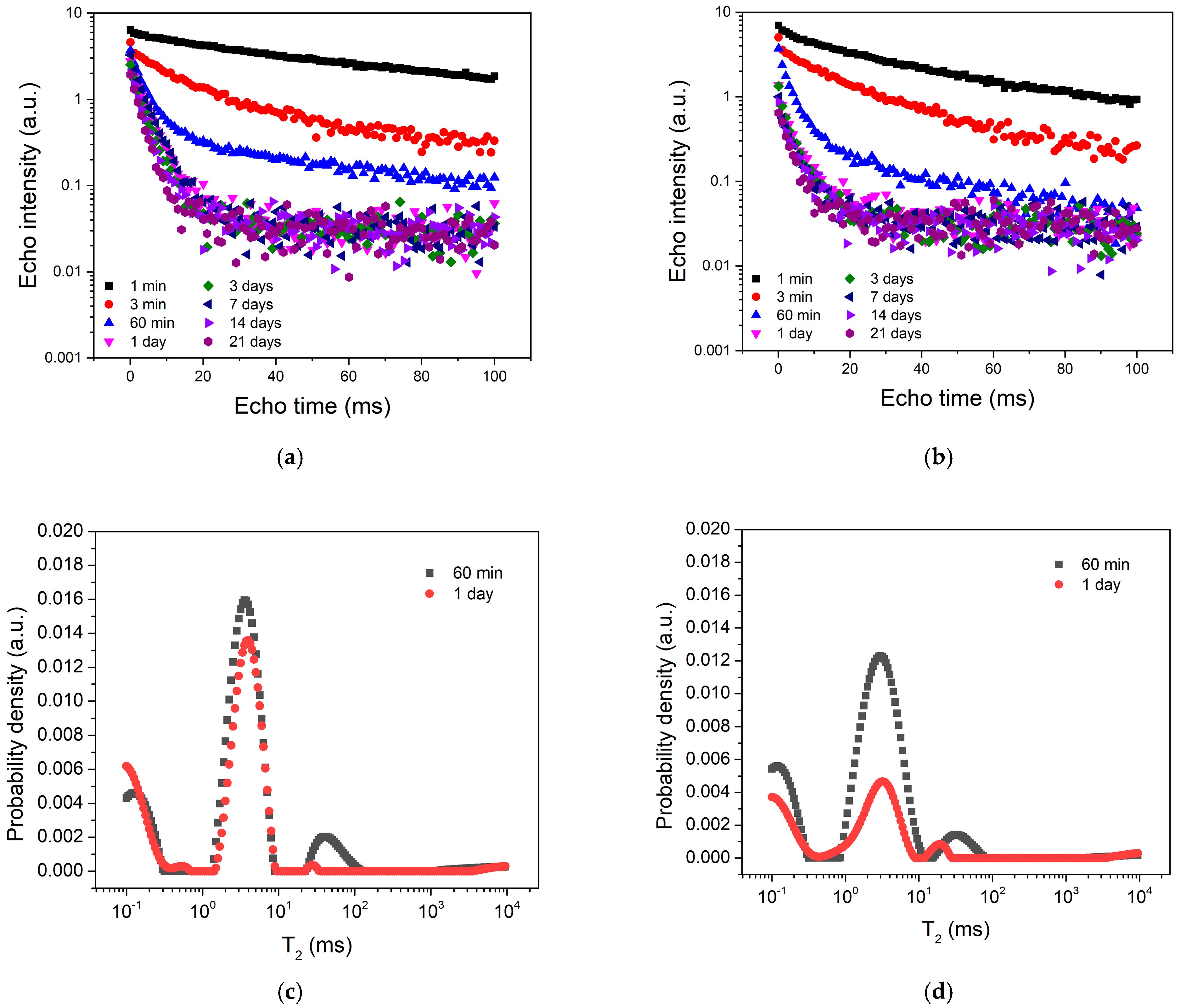
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goh, K.W.; Wong, Y.H.; Ramesh, S.; Chandran, H.; Krishnasamy, S.; Sidhu, A.; Teng, W. Effect of pH on the properties of eggshell-derived hydroxyapatite bioceramic synthesized by wet chemical method assisted by microwave irradiation. Ceram. Int. 2021, 47, 8879–8887. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Wong, S.K.; Wong, Y.H.; Chin, K.-Y.; Ima-Nirwana, S. A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers 2021, 13, 3075. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Montufar, E.B. Cements as Bone Repair Materials; Woodhead Publishing Series in Biomaterials: Cambridge, UK, 2019; pp. 233–271. [Google Scholar] [CrossRef]
- Destainville, A.; Champion, E.; Bernache-Assollant, D.; Laborde, E. Synthesis, characterization and thermal behavior of apatitic tricalcium phosphate. Mater. Chem. Phys. 2003, 80, 269–277. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; Beucken, J.v.D.; Jansen, J. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2020, 119, 1–12. [Google Scholar] [CrossRef]
- Mirtchi, A.A.; Lemaitre, J.; Terao, N. Calcium phosphate cements: Study of the β-tricalcium phosphate—Monocalcium phosphate system. Biomaterials 1989, 10, 475–480. [Google Scholar] [CrossRef]
- Hurle, K.; Christel, T.; Gbureck, U.; Moseke, C.; Neubauer, J.; Goetz-Neunhoeffer, F. Reaction kinetics of dual setting α-tricalcium phosphate cements. J. Mater. Sci. Mater. Med. 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Hurle, K.; Oliveira, J.; Reis, R.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped Brushite Cements for Bone Regeneration. Acta Biomater. 2021, 123, 51–71. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D.; Bertrand, G.; Soulié, J. 1.11 Bioactive Calcium Phosphate Compounds: Physical Chemistry. Compr. Biomater. II 2017, 1, 187–221. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Fang, C.-H.; Lin, Y.-W.; Sun, J.-S.; Lin, F.-H. The chitosan/tri-calcium phosphate bio-composite bone cement promotes better osteo-integration: An in vitro and in vivo study. J. Orthop. Surg. Res. 2019, 14, 162. [Google Scholar] [CrossRef]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and Characteristics of Chitosan Sponge as a Tissue Engineering Scaffold. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Neoh, K.; Kang, E.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Jessel, B.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.H.K.; Takagi, S.; Chow, L.C. Fast setting calcium phosphate cement-chitosan composite: Mechanical properties and dissolution rates. J. Biomater. Appl. 2007, 21, 299–315. [Google Scholar] [CrossRef]
- Pelletier, M.H.; Lau, A.C.B.; Smitham, P.J.; Nielsen, G.; Walsh, W.R. Pore distribution and material properties of bone cement cured at cifferent temperatures. Acta Biomater. 2010, 6, 886–891. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms; IntechOpen: Rijeka, Croatia, 2017; pp. 109–127. [Google Scholar] [CrossRef]
- Lim, J. Stress Corrosion Cracking (SCC) in Polymer Composites; Woodhead Publishing: Cambridge, UK, 2011; pp. 485–536. [Google Scholar] [CrossRef]
- Slane, J.; Vivanco, J.; Meyer, J.; Ploeg, H.-L.; Squire, M. Modification of acrylic bone cement with mesoporous silica nanoparticles: Effects on mechanical, fatigue and absorption properties. J. Mech. Behav. Biomed. Mater. 2014, 29, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Pascual, B.; Gurruchaga, M.; Ginebra, M.; Gil, F.; Planell, J.; Goñi, I. Influence of the modification of P/L ratio on a new formulation of acrylic bone cement. Biomaterials 1999, 20, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Fantazzini, P.; Testa, C.; Bortolotti, V.; Baruffaldi, F.; Kogan, F.; Brizi, L. Characterization of Structural Bone Properties through Portable Single-Sided NMR Devices: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 7318. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; King, J.D.; Wang, X. The characterization of human compact bone structure changes by low-field nuclear magnetic resonance. Meas. Sci. Technol. 2004, 15, 58–66. [Google Scholar] [CrossRef]
- Pop, A.; Ardelean, I. Monitoring the size evolution of capillary pores in cement paste during the early hydration via diffusion in internal gradients. Cem. Concr. Res. 2015, 77, 76–81. [Google Scholar] [CrossRef]
- Lacan, I.; Moldovan, M.; Sarosi, C.; Ardelean, I. Chitosan Effect on Hardening Dynamics of Calcium Phosphate Cement: Low-Field NMR Relaxometry Investigations. Polymers 2022, 14, 3042. [Google Scholar] [CrossRef]
- Lv, S. High-performance superplasticizer based on chitosan. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 131–150. [Google Scholar] [CrossRef]
- Craciun, A.; Prodan, D.; Constantiniuc, M.; Ispas, A.; Filip, M.; Moldovan, M.; Badea, M.; Petean, I.; Crisan, M. Stability of Dental Composites in Water and Artificial Saliva. Mater. Plast. 2020, 57, 57–66. [Google Scholar] [CrossRef]
- Handal, J.A.; Tiedeken, N.C.; Gershkovich, G.E.; Kushner, J.A.; Dratch, B.; Samuel, S.P. Polyethylene glycol improves elution properties of polymethyl methacrylate bone cements. J. Surg. Res. 2015, 194, 161–166. [Google Scholar] [CrossRef]
- Müller, J.A.; Rohr, N.; Fischer, J. Evaluation of ISO 4049: Water sorption and water solubility of resin cements. Eur. J. Oral Sci. 2017, 125, 141–150. [Google Scholar] [CrossRef]
- Labban, N.; AlSheikh, R.; Lund, M.; Matis, B.A.; Moore, B.K.; Cochran, M.A.; Platt, J.A. Evaluation of the Water Sorption and Solubility Behavior of Different Polymeric Luting Materials. Polymers 2021, 13, 2851. [Google Scholar] [CrossRef]
- Venkataramanan, L.; Song, Y.Q.; Hurlimann, M.D. Solving Fredholm integrals of the first kind with tensor product structure in 2 and 2.5 dimensions. IEEE Trans. Signal Process. 2002, 50, 1017–1026. [Google Scholar] [CrossRef]
- Huang, C.; Fu, S.; Zhang, Y.; Lauke, B.; Li, L.; Ye, L. Cryogenic properties of SiO2/epoxy nanocomposites. Cryogenics 2006, 45, 450–454. [Google Scholar] [CrossRef]
- Ali, M. Synthesis and Characterization of the Composite Material PVA/Chitosan/5% Sorbitol with Different Ratio of Chitosan. Int. J. Mech. Mechatron. Eng. 2017, 17, 15–28. [Google Scholar]
- Espanol, M.; Perez, R.; Montufar, E.; Marichal, C.; Sacco, A.; Ginebra, M. Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater. 2009, 5, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef] [PubMed]
- Beruto, D.T.; Mezzasalma, S.A.; Capurro, M.; Botter, R.; Cirillo, P. Use of -tricalcium phosphate (TCP) as powders and as an aqueous dispersion to modify processing, microstructure, and mechanical properties of polymethylmethacrylate (PMMA) bone cements and to produce bone-substitute compounds. J. Biomed. Mater. Res. 1999, 49, 498–505. [Google Scholar] [CrossRef]
- Jayasuriya, A.C.; Mauch, K.J. In vitro degradation behavior of chitosan based hybrid microparticles. J. Biomed. Sci. Eng. 2011, 04, 383–390. [Google Scholar] [CrossRef]
- Devi, D.A.; Smitha, B.; Sridhar, S.; Aminabhavi, T. Pervaporation separation of isopropanol/water mixtures through crosslinked chitosan membranes. J. Membr. Sci. 2005, 262, 91–99. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Beppu, M.M.; Torres, M.A.; Aimoli, C.G.; Goulart, G.A.S.; Santana, C.C. In vitro mineralization on chitosan using solutions with excess of calcium and phosphate ions. J. Mater. Res. 2005, 20, 3303–3311. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Taylor, D.; Lee, T.C. The effect of bone microstructure on the initiation and growth of microcracks. J. Orthop. Res. 2005, 23, 475–480. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacan, I.; Moldovan, M.; Ardelean, I. The Influence of Chitosan on Water Absorption and Solubility of Calcium Phosphate Cement. Coatings 2023, 13, 1641. https://doi.org/10.3390/coatings13091641
Lacan I, Moldovan M, Ardelean I. The Influence of Chitosan on Water Absorption and Solubility of Calcium Phosphate Cement. Coatings. 2023; 13(9):1641. https://doi.org/10.3390/coatings13091641
Chicago/Turabian StyleLacan, Ioana, Mărioara Moldovan, and Ioan Ardelean. 2023. "The Influence of Chitosan on Water Absorption and Solubility of Calcium Phosphate Cement" Coatings 13, no. 9: 1641. https://doi.org/10.3390/coatings13091641
APA StyleLacan, I., Moldovan, M., & Ardelean, I. (2023). The Influence of Chitosan on Water Absorption and Solubility of Calcium Phosphate Cement. Coatings, 13(9), 1641. https://doi.org/10.3390/coatings13091641






