Effect of Cu and Ni Inclusion on Tribological Performance of Tribocatalytically Active Coatings in Hydrocarbon Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CoP-Based Tribocatalytic Coatings
2.2. Tribological Analysis
2.3. Characterization
3. Results
3.1. Characterization of the Deposited Coatings
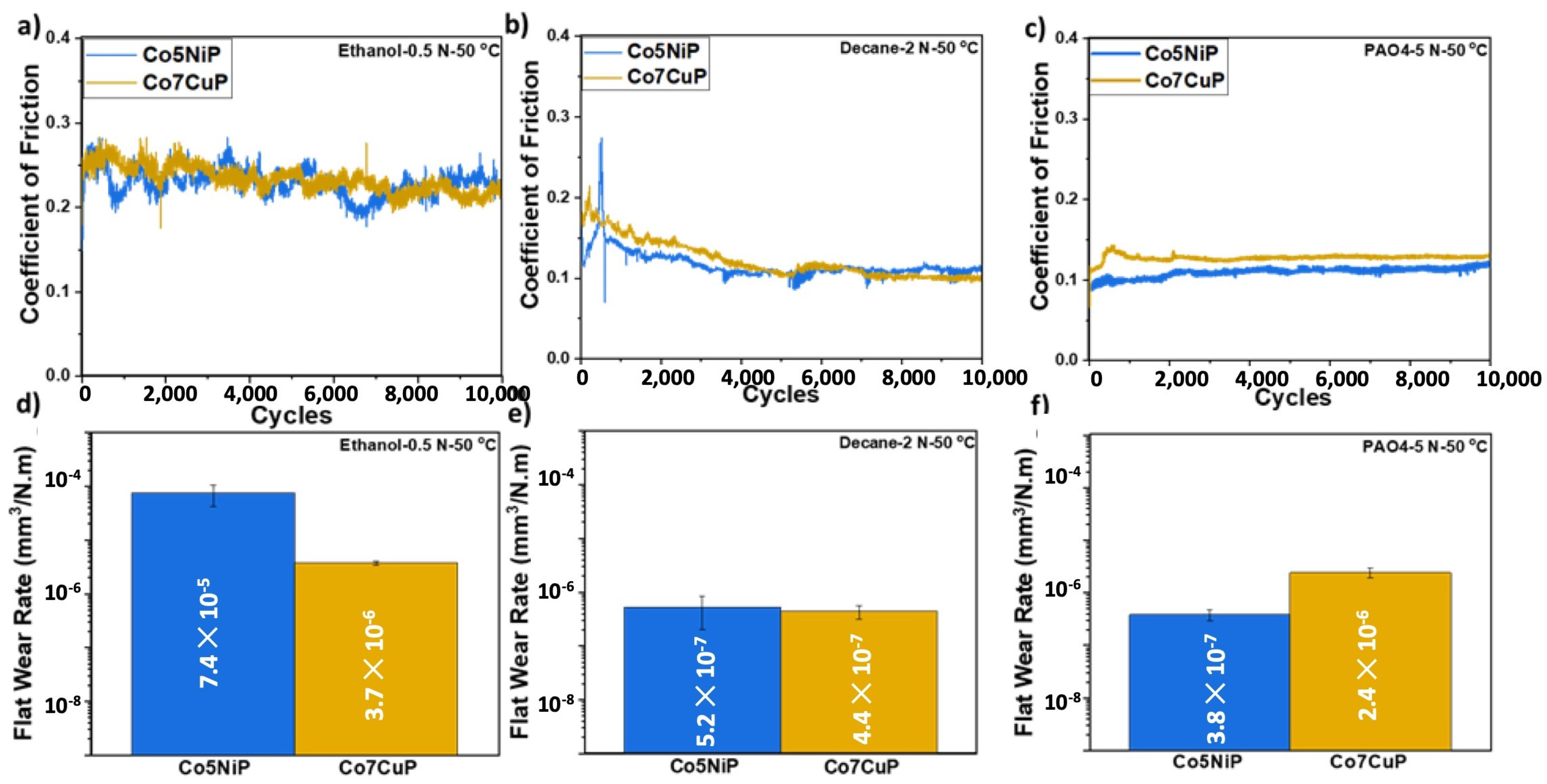
3.2. Effect of the Composition and Environment on Tribological Behavior
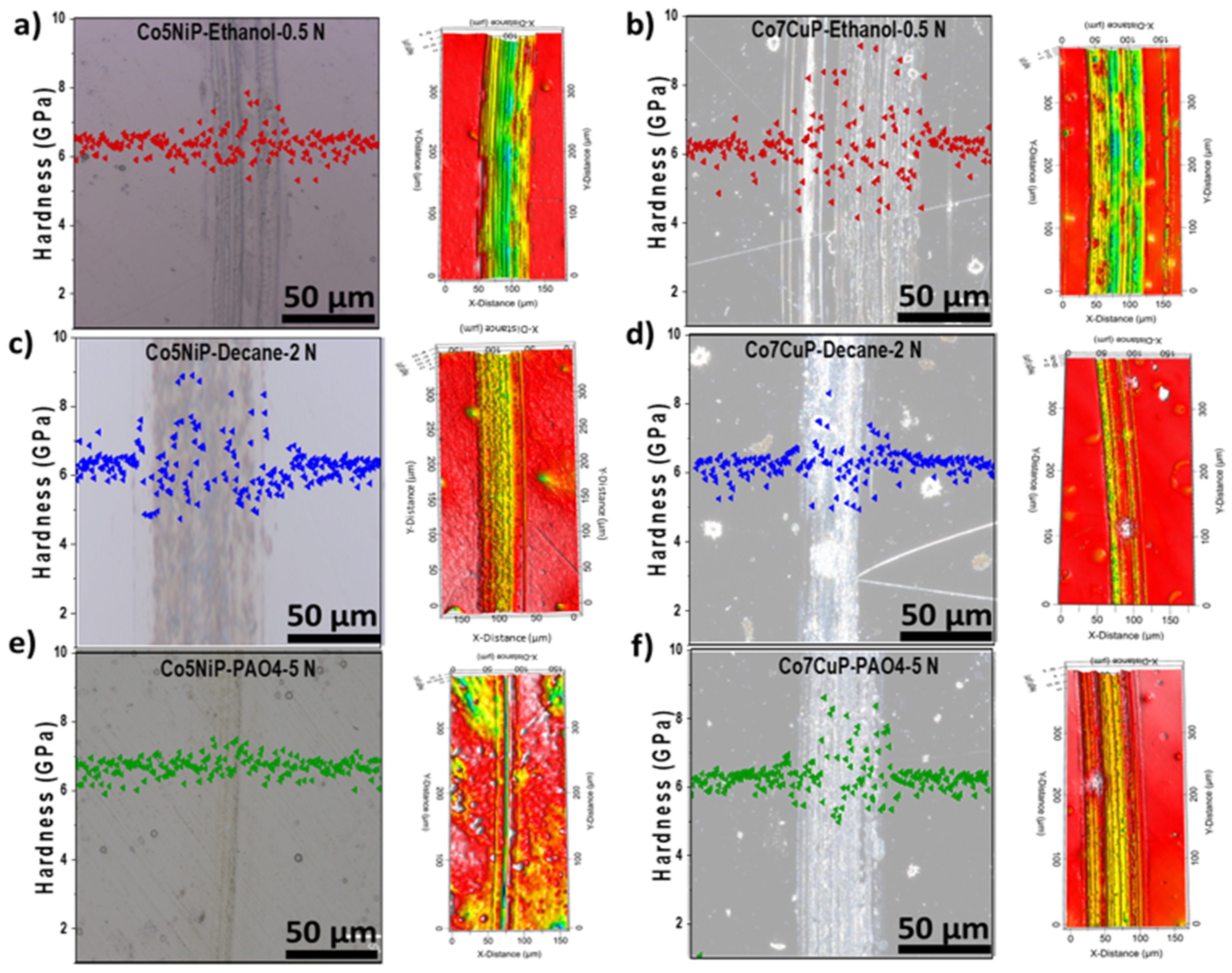
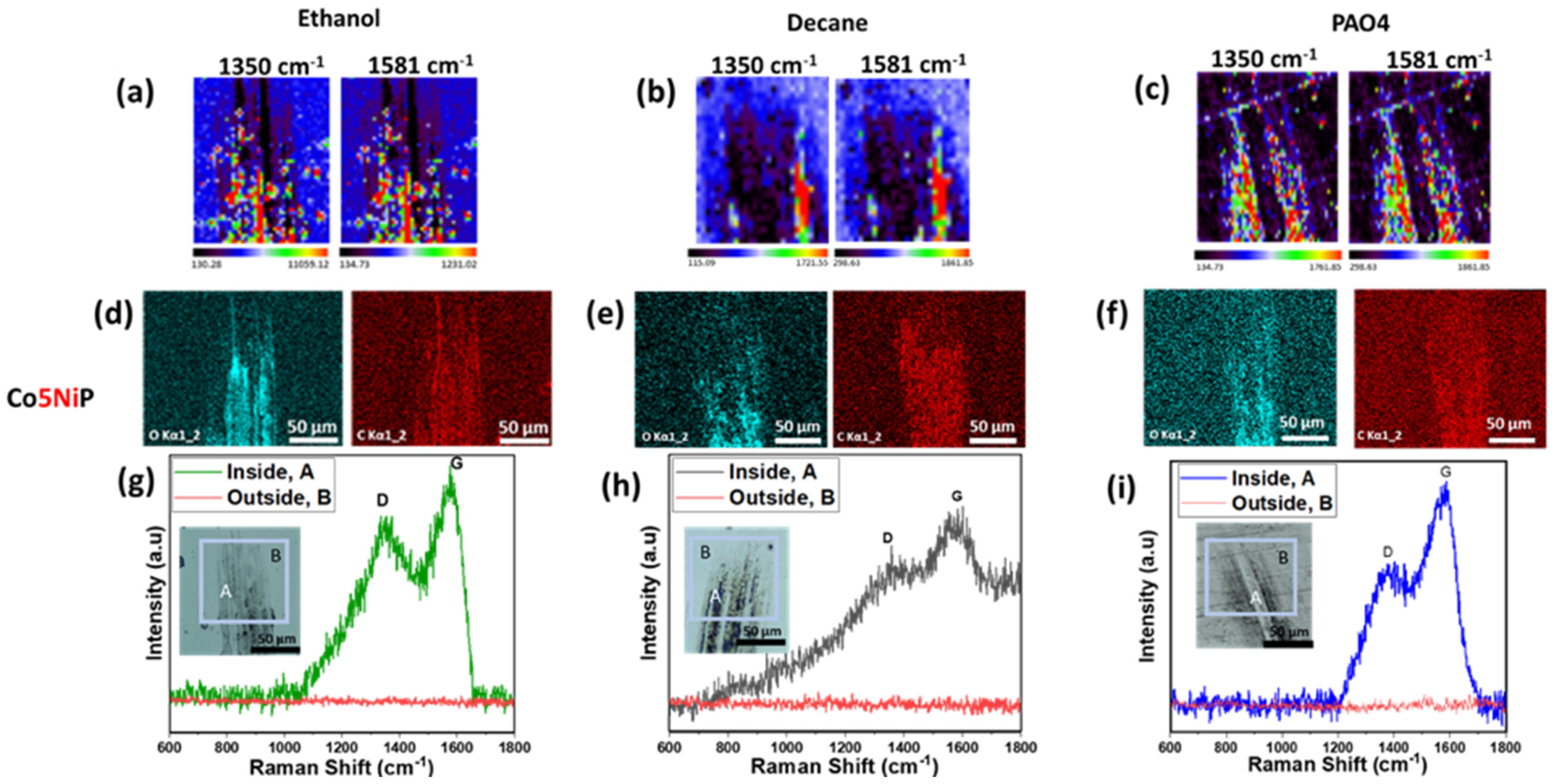
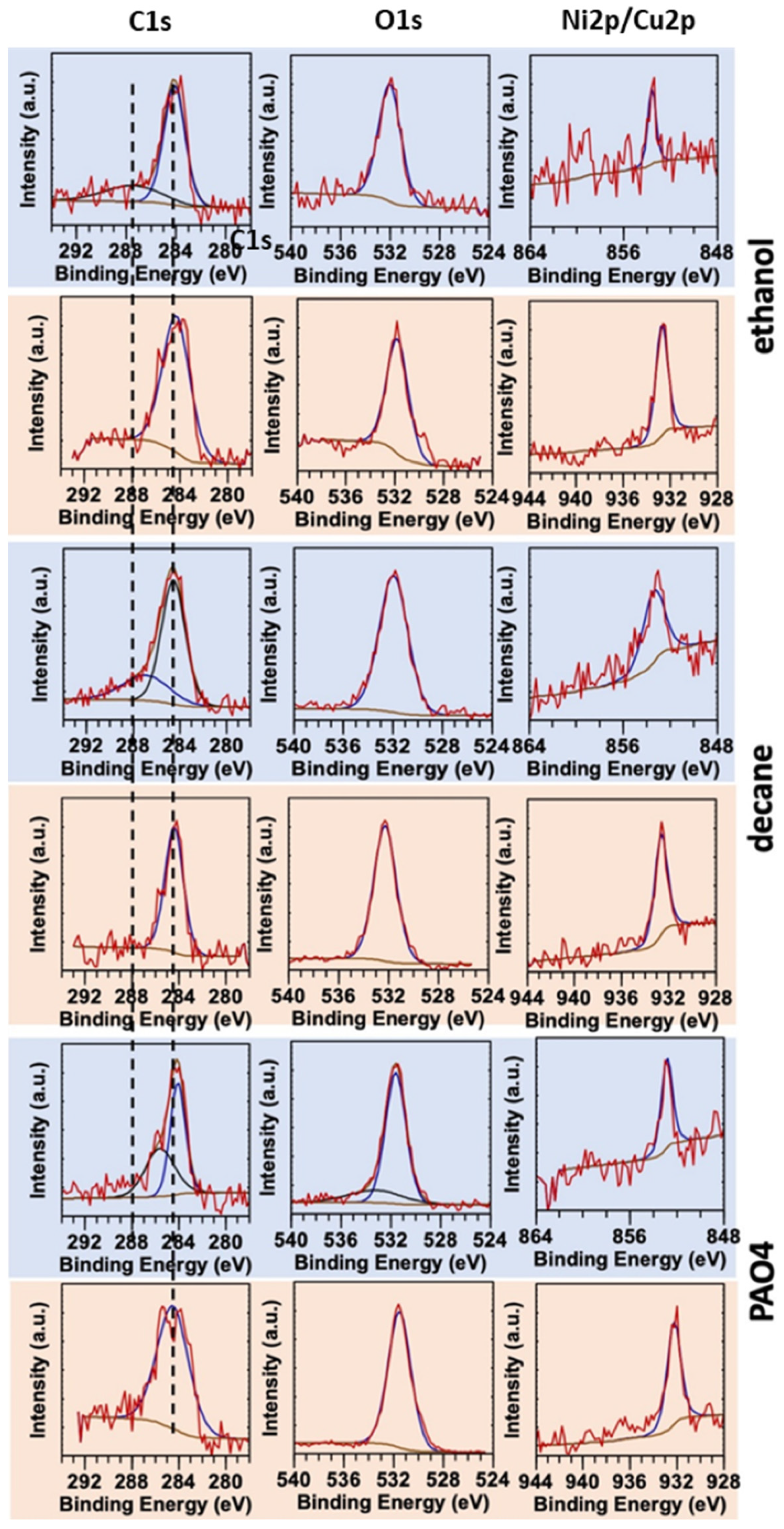
3.3. Comparative Analysis of Co5NiP and Co7CuP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Al Sulaimi, R.; Macknojia, A.; Eskandari, M.; Shirani, A.; Gautam, B.; Park, W.; Whitehead, P.; Alonso, A.P.; Sedbrook, J.C.; Chapman, K.D.; et al. Evaluating the effects of very long chain and hydroxy fatty acid content on tribological performance and thermal oxidation behavior of plant-based lubricants. Tribol. Int. 2023, 185, 108576. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; Macfarlane, D.R.; Forsyth, M. A review of ionic liquid lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Sunil, T.; Sandeep, M.; Kumaraswami, R.; Shravan, A. A critical review on solid lubricants. Int. J. Mech. Eng. Technol. 2016, 7, 193–199. [Google Scholar]
- Macknojia, A.; Ayyagari, A.; Zambrano, D.; Rosenkranz, A.; Shevchenko, E.V.; Berman, D. Macroscale Superlubricity Induced by MXene/MoS2 Nanocomposites on Rough Steel Surfaces under High Contact Stresses. ACS Nano 2023, 17, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Breton, O.K.; Handzel, E.J.; Tennant, J.M. Process for Making Wear-Resistant Coatings. U.S. Patent 6,649,682, 18 November 2003. [Google Scholar]
- Mroczkowski, S.J. Multi-Layer Wear Resistant Coatings. Ausz. Aus Den Eur. Patentanmeldungen Teil I 1990, 6, 2595. [Google Scholar]
- Chu, J.P.; Jang, J.; Huang, J.; Chou, H.; Yang, Y.; Ye, J.; Wang, Y.; Lee, J.; Liu, F.; Liaw, P.; et al. Thin film metallic glasses: Unique properties and potential applications. Thin Solid Films 2012, 520, 5097–5122. [Google Scholar] [CrossRef]
- Awang, M.; Khalili, A.A.; Pedapati, S.R. A review: Thin protective coating for wear protection in high-temperature application. Metals 2019, 10, 42. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A. Achieving Ultralow Friction and Wear by Tribocatalysis: Enabled by In-Operando Formation of Nanocarbon Films. ACS Nano 2021, 15, 18865–18879. [Google Scholar] [CrossRef]
- Ashby, M.F.; Abulawi, J.; Kong, H.S. Temperature Maps for Frictional Heating in Dry Sliding. Tribol. Trans. 1991, 34, 577–587. [Google Scholar] [CrossRef]
- Junbin, Y.; Junxiu, D. Tribocatalysis reaction during antiwear synergism between borates and Sn(IV) compounds in boundary lubrication. Tribol. Int. 1996, 29, 429–432. [Google Scholar] [CrossRef]
- Shirani, A.; Li, Y.; Smith, J.; Curry, J.; Lu, P.; Wilson, M.; Chandross, M.; Argibay, N.; Berman, D. Mechanochemically driven formation of protective carbon films from ethanol environment. Mater. Today Chem. 2022, 26, 101112. [Google Scholar] [CrossRef]
- Erdemir, A.; Ramirez, G.; Eryilmaz, O.L.; Narayanan, B.; Liao, Y.; Kamath, G.; Sankaranarayanan, S.K.R.S. Carbon-based tribofilms from lubricating oils. Nature 2016, 536, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Jacques, K.; Shirani, A.; Smith, J.; Scharf, T.W.; Walck, S.D.; Berkebile, S.; Eryilmaz, O.L.; Voevodin, A.A.; Aouadi, S.; Berman, D. MoVN-Cu Coatings for In Situ Tribocatalytic Formation of Carbon-Rich Tribofilms in Low-Viscosity Fuels. ACS Appl. Mater. Interfaces 2023, 15, 30070–30082. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.; Eryilmaz, O.L.; Fatti, G.; Righi, M.C.; Wen, J.; Erdemir, A. Tribochemical Conversion of Methane to Graphene and Other Carbon Nanostructures: Implications for Friction and Wear. ACS Appl. Nano Mater. 2020, 3, 8060–8067. [Google Scholar] [CrossRef]
- Hsu, S.M.; Zhang, J.; Yin, Z. The Nature and Origin of Tribochemistry. Tribol. Lett. 2002, 13, 131–139. [Google Scholar] [CrossRef]
- Argibay, N.; Babuska, T.; Curry, J.; Dugger, M.; Lu, P.; Adams, D.; Nation, B.; Doyle, B.; Pham, M.; Pimentel, A.; et al. In-situ tribochemical formation of self-lubricating diamond-like carbon films. Carbon 2018, 138, 61–68. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, Properties and Potential of Graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Chen, X.; Li, J. Superlubricity of carbon nanostructures. Carbon 2020, 158, 1–23. [Google Scholar] [CrossRef]
- Ayyagari, A.; Alam, K.I.; Berman, D.; Erdemir, A. Progress in Superlubricity Across Different Media and Material Systems—A Review. Front. Mech. Eng. 2022, 8, 908497. [Google Scholar] [CrossRef]
- Tyagi, A.; Walia, R.; Murtaza, Q.; Pandey, S.M.; Tyagi, P.K.; Bajaj, B. A critical review of diamond like carbon coating for wear resistance applications. Int. J. Refract. Met. Hard Mater. 2019, 78, 107–122. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, X.; Li, J. Graphene lubrication. Appl. Mater. Today 2020, 20, 100662. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, S.; Hou, K.; Li, Z.; Wang, J. Graphene-based nanomaterials as lubricant additives: A review. Lubricants 2022, 10, 273. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, M.; Mayer, M.; Rappen, M.; Schmidt, F.; Saure, R.; Grätz, S.; Borchardt, L. From Inert to Catalytically Active Milling Media: Galvanostatic Coating for Direct Mechanocatalysis. Angew. Chem. Int. Ed. 2022, 61, e202212694. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.M.; Gates, R.S. Effect of materials on tribochemical reactions between hydrocarbons and surfaces. J. Phys. D Appl. Phys. 2006, 39, 3128–3137. [Google Scholar] [CrossRef]
- Ta, T.D.; Tieu, A.K.; Tran, B.H. Influences of iron and iron oxides on ultra-thin carbon-based tribofilm lubrication. Tribol. Int. 2022, 173, 107665. [Google Scholar] [CrossRef]
- Shirani, A.; Li, Y.; Eryilmaz, O.L.; Berman, D. Tribocatalytically-activated carbon-based triboilm formation for wear reduction in alkane environment. Sci. Rep. 2021, 11, 20643. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, H.; Kosasih, B.; Tieu, A.K. A molecular dynamics simulation of boundary lubrication: The effect of n-alkanes chain length and normal load. Wear 2013, 301, 62–69. [Google Scholar] [CrossRef]
- Vijayan, S.; Luo, N.; Aindow, M. Microstructural stability and phase transformations in electrodeposited cobalt-phosphorus coatings. J. Alloys Compd. 2017, 719, 142–150. [Google Scholar] [CrossRef]
- Yang, B.; Qin, G.; Pei, W.; Ren, Y.; Xiao, N. Sputtered amorphous Co–Pt–P thin films for soft underlayer of perpendicular magnetic recording. J. Magn. Magn. Mater. 2010, 322, 1854–1858. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, Q.; Ma, J. The hydrogen generation from alkaline NaBH4 solution by using electroplated amorphous Co–Ni–P film catalysts. Appl. Surf. Sci. 2013, 273, 253–256. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Knudsen, J.; Nilekar, A.U.; Vang, R.T.; Schnadt, J.; Kunkes, E.L.; Dumesic, J.A.; Mavrikakis, M.; Besenbacher, F. A Cu/Pt Near-Surface Alloy for Water−Gas Shift Catalysis. J. Am. Chem. Soc. 2007, 129, 6485–6490. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xiao, Y.-L.; Zhang, X. Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via Cross-Coupling with Difluoroalkyl Halides. Accounts Chem. Res. 2018, 51, 2264–2278. [Google Scholar] [CrossRef]
- Takenaka, S.; Ogihara, H.; Yamanaka, I.; Otsuka, K. Decomposition of methane over supported-Ni catalysts: Effects of the supports on the catalytic lifetime. Appl. Catal. A Gen. 2001, 217, 101–110. [Google Scholar] [CrossRef]
- Shirani, A.; Al Sulaimi, R.; Macknojia, A.Z.; Eskandari, M.; Berman, D. Tribocatalytically-active nickel/cobalt phosphorous films for universal protection in a hydrocarbon-rich environment. Sci. Rep. 2023, 13, 10914. [Google Scholar] [CrossRef]
- Pingale, A.D.; Owhal, A.; Katarkar, A.S.; Belgamwar, S.U.; Rathore, J.S. Recent researches on Cu-Ni alloy matrix composites through electrodeposition and powder metallurgy methods: A review. Mater. Today Proc. 2021, 47, 3301–3308. [Google Scholar] [CrossRef]
- Mahajan, C.; Hasannaeimi, V.; Pole, M.; Kautz, E.; Gwalani, B.; Mukherjee, S. Corrosion mechanisms in model binary metallic glass coatings on mild steel and correlation with electron work function. Corros. Sci. 2022, 207, 110578. [Google Scholar] [CrossRef]
- Saidi, M.; Pasc, A.; El Moujahid, C.; Canilho, N.; Badawi, M.; Delgado-Sanchez, C.; Celzard, A.; Fierro, V.; Peignier, R.; Kouitat-Njiwa, R.; et al. Improved tribological properties, thermal and colloidal stability of poly-α-olefins based lubricants with hydrophobic MoS2 submicron additives. J. Colloid Interface Sci. 2020, 562, 91–101. [Google Scholar] [CrossRef]
- Liu, Y.; DiFoggio, R.; Sanderlin, K.; Perez, L.; Zhao, J. Measurement of density and viscosity of dodecane and decane with a piezoelectric tuning fork over 298–448K and 0.1–137.9 MPa. Sens. Actuators A Phys. 2011, 167, 347–353. [Google Scholar] [CrossRef]
- Zeegers-Huyskens, T.; Huyskens, P. Intermolecular forces. In Intermolecular Forces: An Introduction to Modern Methods and Results; Springer: Berlin/Heidelberg, Germany, 1991; pp. 1–30. [Google Scholar]
- Morriss, G.P.; Daivis, P.J.; Evans, D.J. The rheology of n alkanes: Decane and eicosane. J. Chem. Phys. 1991, 94, 7420–7433. [Google Scholar] [CrossRef]
- Patel, S.; Azad, A.; Khan, M. Numerical investigation for predicting diesel engine performance and emission using different fuels. Energy Procedia 2019, 160, 834–841. [Google Scholar] [CrossRef]
- Yi, Y.; Xing, J.; Wan, M.; Yu, L.; Lu, Y.; Jian, Y. Effect of Cu on microstructure, crystallography and mechanical properties in Fe-B-C-Cu alloys. Mater. Sci. Eng. A 2017, 708, 274–284. [Google Scholar] [CrossRef]
- Yi, Y.; Xing, J.; Lu, Y.; Gao, Y.; Fu, H.; Yu, L.; Wan, M.; Zheng, Q. Effect of normal load on two-body abrasive wear of an Fe-B-Cr-C based alloy with minor Cu and Ni additions. Wear 2018, 408–409, 160–170. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Wang, C.; Zhang, X. Corrosion and wear behavior of Ni60CuMoW coatings fabricated by combination of laser cladding and mechanical vibration processing. J. Alloys Compd. 2015, 621, 357–363. [Google Scholar] [CrossRef]
- Alanazi, N.M.; El-Sherik, A.; Alamar, S.H.; Shen, S. Influence of residual stresses on corrosion and wear behavior of electrodeposited nanocrystalline cobalt-phosphorus coatings. Int. J. Electrochem. Sci. 2013, 8, 10350–10358. [Google Scholar] [CrossRef]
- Rochefort, A.; Vernisse, L.; Gómez-Herrero, A.C.; Sánchez-Sánchez, C.; Martín-Gago, J.A.; Chérioux, F.; Clair, S.; Coraux, J.; Martínez, J.I. Role of the Structure and Reactivity of Cu and Ag Surfaces in the Formation of a 2D Metal–Hexahydroxytriphenylene Network. J. Phys. Chem. C 2021, 125, 17333–17341. [Google Scholar] [CrossRef]
- Sinfelt, J.; Carter, J.; Yates, D. Catalytic hydrogenolysis and dehydrogenation over copper-nickel alloys. J. Catal. 1972, 24, 283–296. [Google Scholar] [CrossRef]
- Khan, A.M.; Ahmed, J.; Liu, S.; Martin, T.; Berkebile, S.; Chung, Y.-W.; Wang, Q.J. Formation of Wear-Protective Tribofilms on Different Steel Surfaces During Lubricated Sliding. Tribol. Lett. 2023, 71, 101089. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]




| NiCl2 (g/L) | CuCl2 (g/L) | Ni (wt%) | Cu (wt%) | P (wt%) | |
|---|---|---|---|---|---|
| CoP | 0 | 0 | 0 | 0 | 12.5 |
| Co5NiP | 10 | 0 | 4.9 | 0 | 12.2 |
| Co7NiP | 20 | 0 | 7.4 | 0 | 11.3 |
| Co5CuP | 0 | 0.5 | 0 | 4.8 | 11.6 |
| Co7CuP | 0 | 1 | 0 | 6.8 | 11.2 |
| Lubricant | Ethanol | Decane | PAO4 |
|---|---|---|---|
| Viscosity at 50 °C (mPA·s) | 1.14 | 0.85 | 32.0 |
| Lubricant | Coating | D-Band FWHM, cm−1 | G-Band FWHM, cm−1 |
|---|---|---|---|
| Ethanol | Co5NiP | 100 ± 8 | 104 ± 12 |
| Ethanol | Co7CuP | 47 ± 6 | 26 ± 7 |
| Decane | Co5NiP | 101 ± 6 | 103 ± 12 |
| Decane | Co7CuP | 53 ± 7 | 68 ± 8 |
| PAO4 | Co5NiP | 98 ± 12 | 102 ± 9 |
| PAO4 | Co7CuP | 70 ± 14 | 78 ± 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Sulaimi, R.; Eskandari, M.; Shirani, A.; Macknojia, A.Z.; Miller, W.; Berman, D. Effect of Cu and Ni Inclusion on Tribological Performance of Tribocatalytically Active Coatings in Hydrocarbon Environments. Coatings 2024, 14, 61. https://doi.org/10.3390/coatings14010061
Al Sulaimi R, Eskandari M, Shirani A, Macknojia AZ, Miller W, Berman D. Effect of Cu and Ni Inclusion on Tribological Performance of Tribocatalytically Active Coatings in Hydrocarbon Environments. Coatings. 2024; 14(1):61. https://doi.org/10.3390/coatings14010061
Chicago/Turabian StyleAl Sulaimi, Rawan, Mohammad Eskandari, Asghar Shirani, Ali Zayaan Macknojia, Wesley Miller, and Diana Berman. 2024. "Effect of Cu and Ni Inclusion on Tribological Performance of Tribocatalytically Active Coatings in Hydrocarbon Environments" Coatings 14, no. 1: 61. https://doi.org/10.3390/coatings14010061






