Abstract
Increasing the serviceability of industrial components intended for the petrochemical industry is possible through their superficial saturation with silicon (silicon cementation). Obtaining a silicon-rich surface coating results in a considerable increase in corrosion resistance, refractoriness, and wear resistance. One of the most economically convenient options for silicon cementation is pack siliconizing in powdery solid media. This paper presents the possibility of pack siliconizing that contains ferrosilicon (FeSi75C) and a thermite mixture (SiO2 + Al) as active, silicon-providing components, in P265GH grade steel, which is frequently used in the petrochemical industry. The aim of the study was to determine the most suitable active component of the two that were analyzed and at the same time identify the processing conditions in which the siliconized coating has the greatest thickness, is free of porosity, and is in direct contact with the support. The use of experimental programming methods allowed the optimization of the operation to obtain the optimal solution. It was concluded that the thermite mixture is not compatible with pack siliconizing because it results in a superficial saturation predominantly composed of aluminum. When ferrosilicon is used as the active component, it determines the particularly intense formation kinetics of the non-porous siliconized coating with its maximum thickness being reached at high processing temperature values (over 1100 °C) with a proportion of 60% FeSi75 and, simultaneously, with the lowest possible proportion of ammonium chloride (max. 3%), which is the surface activation/cleaning component.
1. Introduction
P265GH grade steel has a wide range of applicability in industrial components. This steel was designed for vessels, boilers, and pipes which must withstand extremely elevated temperatures and pressures. However, the main field of use is in the chemical and petrochemical industry. The components selected for use in the petrochemical industry must withstand very corrosive media; HTHA (high-temperature hydrogen attack), generalized corrosion, sulfide corrosion, and stress corrosion cracking are the most common types of corrosion. The fluids that flow through these components contain large amounts of S, Cl, and H at temperatures between 425 and 540 °C. Finding metallic materials that can withstand these conditions represents a significant challenge [1]. This issue can be overcome by siliconizing the components exposed to such media. Siliconizing allows a substantial increase in corrosion resistance, refractoriness, hardness, and wear resistance. Frequently, a combination of these properties is required to achieve the operation of various categories of benchmarks [2,3]. Through the siliconizing of P265GH-grade steel, a diffusion coating is obtained (according to the Fe-Si phase diagram [4]) composed of solid solution α (more precisely, a solid solution of Si in the Feα) at the metal matrix–coating interface and the α1 phase (solid solution based on the Fe3Si-type compound) on the outside. The α1 phase represents the source of Si that will ensure the saturation of the α solid solution. Porosity usually appears in the α1 phase due to a decrease in the mobility of Fe atoms compared to those of Si and, consequently, the mobility of the Kirkendal–Frenkel effect.
The experimental results obtained by a few authors [2,3,5] in the case of pack siliconizing have demonstrated that the use of a ballast component (Al2O3 in this case) prevents the sintering of the active components concurrently with the reduction in the porous and fragile α1 (Fe3Si) phase and in the formation of an adherent coating. Also, the correct dosage of the activation component (halide) is very important, because it can lead to an equalization of the partial diffusion coefficients, thus reducing porosity, as well as corrosion of the surface and inhibition of the diffusion process, which is undesirable. The results of experimental research on siliconization in powdery solid media [2] have led to the conclusion that the minimum value of the silicon concentration in the ferrosilicon powder used as the active component of the medium must be 60%, and the temperature required for processing must exceed 1000 °C to ensure intense process kinetics. However, the possibility of using the thermite mixture (SiO2 + Al) as the active component that provides silicon in the polycomponent powdery mixtures, i.e., (SiO2 + Al) + NH4Cl (or NaF) + Al2O3, is discussed without referring to the final concentration of silicon in the obtained coating. The problem that arises if we consider the possible reactions from the medium used for siliconization is whether silicon is present or not in the coating in satisfactory concentrations.
Due to its low carbon content, P265GH grade steel can be subjected to thermochemical heat treatment, such as siliconizing. An increased carbon content decreases the temperature at which nonporous coatings can form. Currently, the only guarantee of obtaining a silicide coating without porosity is given by the absence of the α1 phase [2].
In this study, we aimed to reduce the porous phase α1 as much as possible. The effects of technological parameter variation and the powdery mixture composition of the siliconizing process on the growth kinetics of the siliconized coating (porous and adherent) were analyzed, and thus, the optimal parameters to ensure the best results were found.
2. Materials and Methods
By means of experimental research, we aimed to quantify the effect of the variation of the technological parameters of the siliconizing process and of the composition of the powdery mixture on the growth kinetics of the siliconized coating (nonporous and adherent).
The powdery mixture used for pack siliconizing was composed of the active com-ponent, powdery ferrosilicon with a high Si content (about 75%), and thermite mixture (SiO2 + Al): a mixture which, through the aluminothermic reaction, can theoretically provide the necessary Si for saturation. The mass ratio of components SiO2/Al in the thermite mixture was constant, namely 3 moles of SiO2 to 4 moles of Al. The dissipating component was alumina powder, and the activating component was ammonium chloride.
The areas of interest in the silicide coating are the adherent part, without porosity, which was defined as the thickness within the metallic matrix–layer interface, detectable metallographically, and the area where the diffusion porosity is observed, which is generated by the Kirkendall–Frenkel effect. The siliconized layer thickness of interest was adopted as the arithmetic mean of four to five measurements. The multitude of factors that contribute to the formation of a siliconized layer adhering to the metal matrix, which is, at the same time, devoid of porosity, justified the decision to use the experimental programming method to quantify the individual and collective effects of the independent parameters with a defining influence on layer growth kinetics and microstructural features. The full factorial programming method was initially adopted, and in the case that statistical checks prove that this method was not appropriate, another type of program could be adopted. The thermochemical processing was carried out in electric ovens equipped with automatic temperature regulation and control. The samples were placed in refractory steel containers and packed in solid powdery mixtures; the containers were sealed with clay latches, placed in the oven at 200 °C, and heated until the holding time temperature. After holding the temperature, the containers were taken out and air cooled, the samples being extracted when the temperature of the containers dropped below 300 °C.
The characteristics of the solid powdery mixture’s components used in the research were as follows:
- -
- Ferrosilicon (FeSi75C) with 72 ÷ 75%Si, less than 0.1%C, and about 2%Al produced by the Norwegian company FINNFJORD; fragments with an average equivalent diameter of 40 ÷ 50 mm, subsequently ground in ball mills to average diameters equivalent to 3 ÷ 4 mm.
- -
- Silica (SiO2) with a purity greater than 99.8% and an average particle diameter of about 23 μm, produced by Luoyang Tongrun Info Technology Co., Ltd., Luoyang, China.
- -
- Aluminum powder, produced by air spraying in Zlatna, Romania, via the Sherritt hydrometallurgical process, of 99.2% purity (containing 0.15%Fe; 15%O2; 0.5%N2) with an average particle diameter of about 50 μm.
- -
- Alumina powder (Al2O3) with a purity greater than 98.5% was produced at Alum S.A. Romania; a fraction greater than 150 μm (max. 10%) and a fraction less than 45 μm (max. 12%).
- -
- Ammonium chloride (NH4Cl) of analytical purity produced by Silver Chemicals Romania.
The samples used in the experiments were made of P265GH grade steel (ASTM/ASME A516 Gr.60/SR EN ISO 10028-2:2017) [6,7] with the chemical composition given in Table 1 (determined with the SPECTROLAB M10/76004135 spectrometer).

Table 1.
Chemical composition of the metallic materials.
The first series of data is related to the material that was used, and the second corresponds to the information in respect of the current standard.
The investigation of the results obtained involved the following:
- -
- Vickers HV0.05 (50 gf) hardness tests were performed on the NEOPHOT 21 N1096 series microscope.
- -
- Optical Microscopy (OM) using a Zeiss Z1m Observer microscope—Axio Vision 4.8/038-12837 (Carl Zeiss GmbH, Gottingen, Germany).
- -
- XRF analyses performed on the SPECTRO xSORT device (SPECTRO Analytical Instruments GmbH, Kleve, Germany) used to verify silicon concentration in the superficial siliconized coating.
- -
- Energy-dispersive spectrometry (EDS Sapphire-type energy-dispersive spectrometer with a resolution of 128 kV) using a Phenom ProX SEM (Eindhoven, The Netherlands).
The sequence of operations in the experimental research was as follows:
- -
- Cutting of P265GH grade steel samples with dimensions of 15 × 15 × 20 mm.
- -
- Packing in powdery solid mixtures with the composition FeSi75C or (SiO2 + Al) + NH4Cl + Al2O3 in refractory stainless-steel boxes sealed with refractory stainless-steel lids and clay latches; simultaneously, pure technical iron foils were introduced for the determination of silicon and aluminum content.
- -
- Thermal processing itself under the conditions imposed by the experimental program adopted.
- -
- Sample preparation.
- -
- Optical and electronic SEM/EDAX microscopy); XRF analyses.
- -
- Statistical processing of the experimental data obtained (the processing modality specific to the type of program adopted).
- -
- Conclusions.
3. Results and Discussion
To determine and quantify the effects of the variation of the thermal, temporal, and chemical parameters of the silicon cementation process in solid powdery media applied to P265GH steel grade on the growth kinetics of the siliconized layer (the adherent part of the layer that extends from the interface metal matrix layer to the area where porosity appears), first-order programming was used, comprising a full factorial experiment.
The reason why the first-order programming variant was adopted is related to the information from the literature [2], suggesting the limiting role of the flow of silicon atom penetration to the diffusion zone as well as the similarity between the temperature dependence of the growth kinetics layer and that of the silicon diffusion coefficient with temperature.
We can explain the correlations between the independent parameters used in the analysis (X1…X4) and the dependent ones (Y) in the form of equations (mathematical models of the interactions) of the type:
where:
- bi; bij—the real coefficients of Equation (1).
- xi; xj—the coded values of the factors (independent parameters used in analysis).
- Y—the real value of the dependent parameter. Thus, in the presented situation, Y represents the thickness of the compact and adherent siliconized layer, devoid of porosity, and formed on the surface of the product subjected to thermochemical processing or the value of the ratio of the Si mass proportions and Al in the superficial areas of the obtained layer.
For a more accurate understanding of how the independent parameters considered (processing temperature; holding time; the proportion of the active component—FeSi75C or (SiO2 + Al) powdery mixture; the proportion of NH4Cl) influence the growth kinetics of the siliconized layer area of interest, or the ratio of the Si mass proportions and Al in the superficial areas of the layer, both the singular effects of the variation of these parameters (bixi; bjxj…) and their cumulative effects (bij.xi. xj; bijk.xi.xj.xk…) were calculated.
There is a correlation between the real and coded values of the independent parameters:
where:
- xi—the coded value of the independent parameter i (or j, k … ij, ik … ijk).
 Table 2. Full factorial experiment (FFE) 2n = 24; experimental conditions and obtained results.
Table 2. Full factorial experiment (FFE) 2n = 24; experimental conditions and obtained results.- Xi0—the natural value of the independent parameter i (or j, k … ij, ik … ijk) corresponding to the chosen base level (Table 2).
To clarify the regression shown in Equation (1), the experimental conditions related to the two situations covered in the analysis (active component FeSi75C; thermic mixture—SiO2 + Al) and the results obtained are presented in Table 2.
The statistical processing of the experimental results [8,9] (contained in Table 2) allowed the determination of the coefficients of the independent parameters used in the analysis (see Table 3), whose values, after comparison with their related confidence intervals, Δbi; Δbij (see Table 4), enabled elaborating the forms of the regression Equations (5)–(7).

Table 3.
Calculated values of the coefficients of the regression equations, using the experimental values mentioned in Table 2.

Table 4.
Statistical verification of coefficients of regression equations.
Where:
- S02—the dispersion of the reproducibility of the experimental results obtained by performing three experiments in identical conditions, corresponding to the upper level of the independent parameters.
- S2bi; ij—the dispersion with which the bij; bijk coefficients are calculated.
- tα; N—the Student criterion for the significance threshold α = 0.05 and the number N of degrees of freedom (total number of experiences; N = 16) tα; N = 2.12 (tabulated value) [6,7].
The mathematical expression of the statistical verification condition of the coefficients is as follows:
Comparing the calculated values of the coefficients of the regression equations (Table 3) with their corresponding confidence intervals (Table 4), it was determined that some of them are statistically negligible (they do not manifest in their range of variation a significant influence on the dependent parameter of interest). Therefore, the forms of the regression equations for the two averages used in the analysis become:
*The active component of the solid powdery media FeSi75C
Y = δ(μm) = 83.67 + 73.99X1 + 32.98X2 − 32.25X4 + 27.58X1X2 − 22.57X1X4 − 36.01X2X4 − 12.59X3X4 + 6.40X1X2X3 − 30.58X1X2X4 − 20.47X1X3X4
**The active component of the solid powdery media (SiO2 + Al)
Y = δ(μm) = 225.81 + 158.38X1 + 42.78X2 + 24.78X2X3 + 21.44X1X2X3 + 39.42X2X3X4 − 34.16X1X2X4 + 46.16X1X2X3X4
Y′ = (%Si/%Al)mass × 100 = 2.12 + 1.109X1 − 0.881X4 − 0.392X1X2 + 0.552X1X3 − 0.511X1X4 − 0.853X3X4 − 0.619X1X2X3 − 0.535X1X2X4 − 0.785X1X3X4 + 1.076X1X2X3X4
Verification of the concordance hypothesis of the adopted mathematical model (with the help of the Fischer-F criterion), as shown in Table 5, led to the conclusion that the experimental programming variant adopted is correct, and the calculated regression Equations (5) ÷ (7) express with a maximum probability of 95% the effects of the singular and collective variation of the independent parameters considered. These are the size of the area without porosity when using the thermite mixture in thermochemical processing and the ratio of the mass proportions of Si and Al in the superficial areas of the layer.

Table 5.
Statistical verification of the compatibility of the adopted mathematical models.
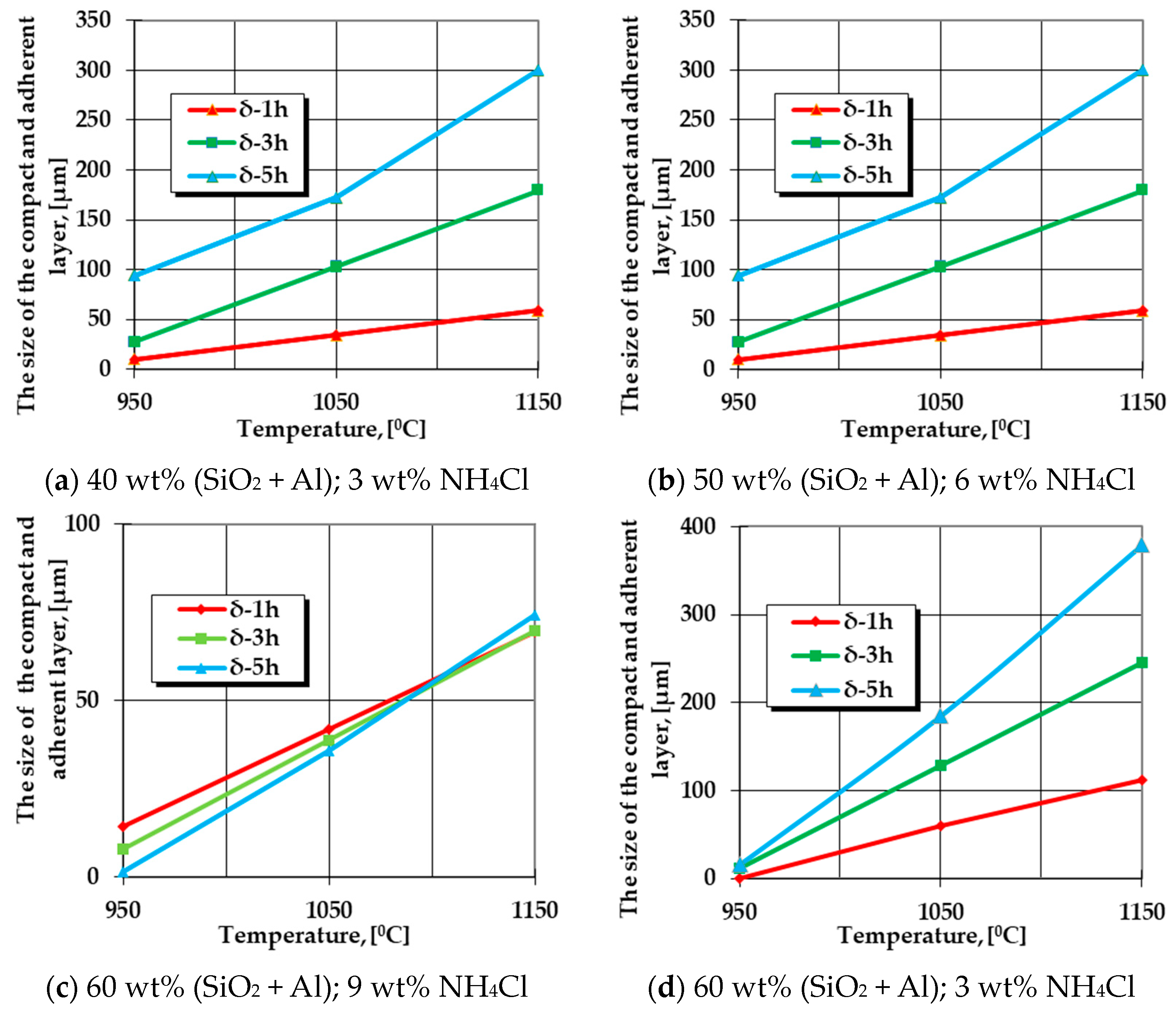
The graphical expression of regression Equation (5) is presented in Figure 1.
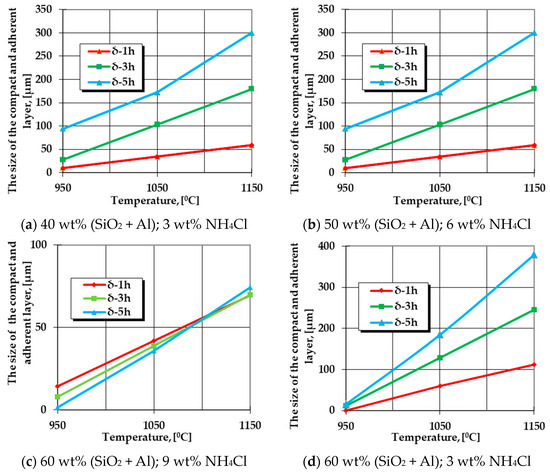
Figure 1.
Change in the growth kinetics of the silicon cemented layer (the area without porosity in direct contact with the matrix) generated by the variation of the temperature, the holding time at a specific temperature, and the proportions of FeSi75C (between 40 wt% and 60 wt%) and NH4Cl (between 3 wt% and 9 wt%).
The graphical dependencies presented in Figure 1 represent the graphical expressions of regression Equation (5) for the different processing parameters (different proportions of FeSi75C and NH4Cl).
The analysis of the information provided by the graphical expressions of regression Equation (5), as shown in Figure 1, for the different specific situations, leads to the conclusion that an increase in the holding time at the processing temperature implies an increase in the porosity-free thickness layer found in contact with the metal matrix subjected to thermochemical processing. The maximum thickness in the case of silicon cementation in the solid powdery mixture containing FeSi75 as the active component is reached for maximum values of the proportion of this component in the environment (that is, 60 wt%) and at a minimum concentration of the component with the role of activator of the reactions in the environment, which is also a cleaner/activator of the surface subjected to processing (that is, 3 wt%, the value imposed in the adopted experimental program).
To understand the results obtained, it is necessary to determine the sequence of reactions between the components of the environment used for silicon cementation: reactions in the environment, at the interface of the environment and the metal surface, and in the adsorbed state.
Active component: Activator Ballast
Dispersion component
FeSi75C/(SiO2 + Al) NH4Cl Al2O3
NH4Cl→NH3 + HCl
2NH3→2N + 3H2
↓
N2
(FeSi) + 7HCl = SiCl4 + 3.5H2 + FeCl3
SiCl4 + Fe = FeCl2 + SiCl2
or: SiCl4 + 2Fe = Si↓ + 2FeCl2
SiCl4 + Si = 2SiCl2
SiCl2 + Fe = Si↓ + FeCl2
2SiCl2 = Si + SiCl4
SiCl4 + 2H2 = Si + 4HCl
Si + 4HCl = SiCl4 + 2H2
3SiO2 + 4Al = 3Si + 2Al2O3
SiCl4 + H2 = SiCl2 + 2HCl
Some important aspects that emerge from the analysis of the chemistry of the reactions between the components of the solid powdery media used for silicon cementation (Equation (8) ÷ Equation (19)), corroborated with the thermodynamic information regarding their thermal effects and the probability of the reactions unfolding in the desired direction (predominantly exergonic reactions), are presented as follows:
- -
- Si appears in the superficial areas of thermochemically processed metal parts because of a sequence of chemical reactions, frequently between the chlorides adsorbed in the superficial layers and iron atoms (Equation (14)), or in the vicinity of the surface, because of the reactions between chlorides and hydrogen resulting from the decomposition of ammonium chloride (Equation (16)) or ammonia (Equation (9)).
- -
- Si can also appear because of the aluminothermic reaction (Equation (18)), in which case, being dependent on the position of the micro-volume in which the reaction takes place in relation to the thermochemically processed surface, it can either be adsorbed (if it is in the vicinity of the surface) or it can react with hydrochloric acid, for example, generating chlorides.
- -
- Most reactions that take place between the components of the solid powdery media used for silicon cementation take place with the release of heat. The aluminothermic reaction (Equation (18)) obviously has the greatest thermal effect, which determines much higher kinetics of the process than those recorded in the case of using ferrosilicon as the active component and ensuring the possibility of carrying out the process at much lower temperatures without affecting the kinetics.
- -
- The appearance of silicon chlorides is dependent on the presence of ammonium chloride among the components of the powdery solid media and its decomposition at the processing temperature (Equation (8)). Increasing the proportion of this component also has negative consequences in the sense that there is continuous cleaning/corrosion of the surface, which is an aspect that must be considered when choosing its proportion.
- -
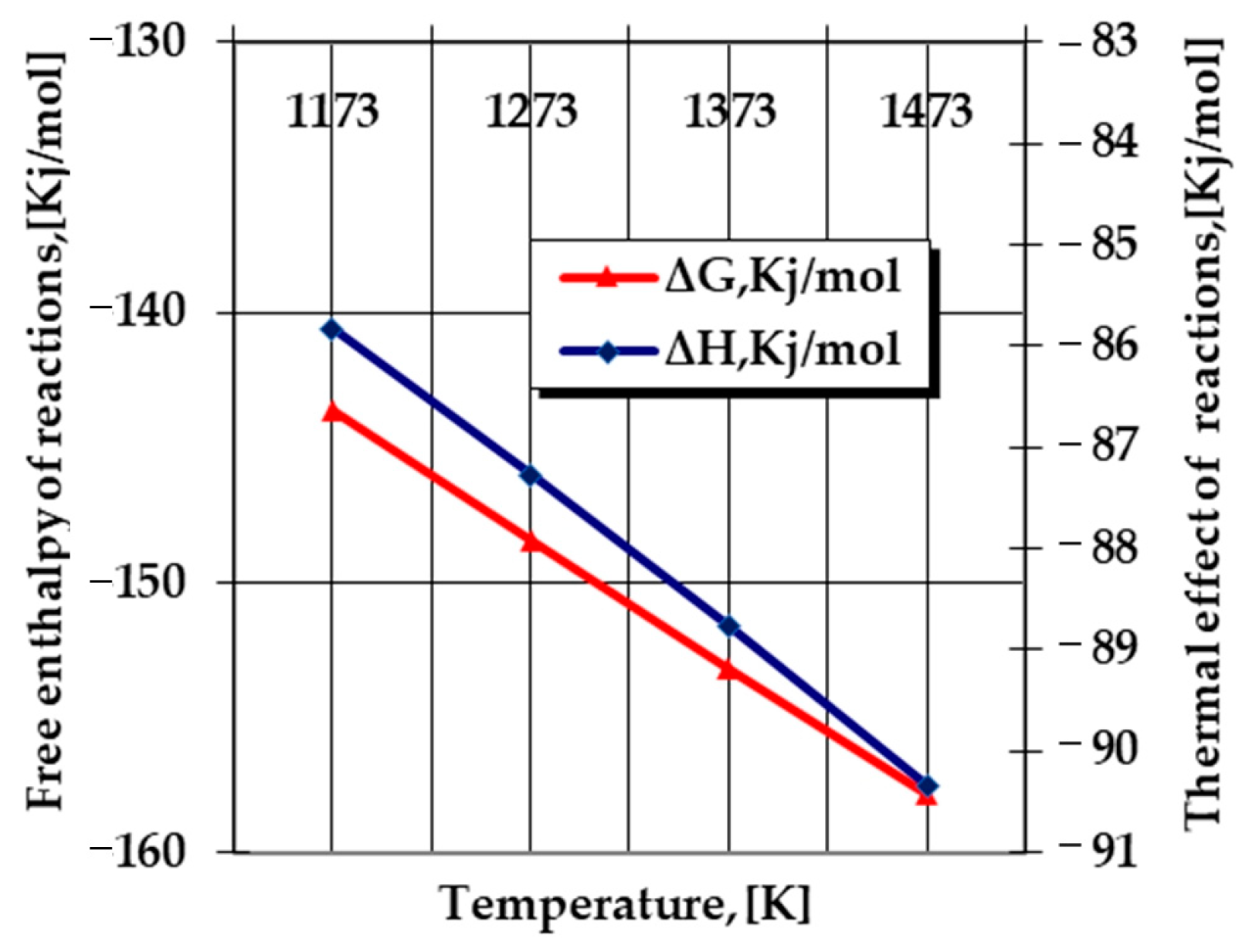
- The appearance of Si in a free state in the powdery solid media (Equations (12), (16) and (18)) can be determined as extremely likely from a thermodynamic point of view, as shown in Figure 2, in the formation of a nitride type (Si2N), according to the reaction:where nitrogen in the atomic state results from the thermo-catalytic decomposition reaction of ammonia (Equation (9)).2Si + N = Si2N
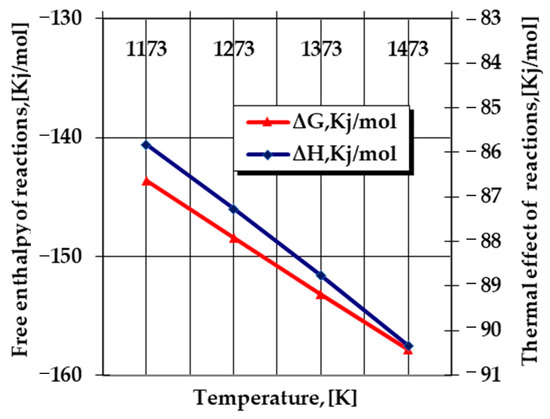 Figure 2. Evolution according to the temperature of the main thermodynamic quantities specific to Equation (20) [10].
Figure 2. Evolution according to the temperature of the main thermodynamic quantities specific to Equation (20) [10].
Silicon nitride Si2N needs to ionize and form a Si2N+-type cation with an energy in the order of 6.4 eV (~617.5 KJ/mol)—according to Paukstis and Gole [11], this is the energy that can be provided under the conditions in which the processing takes place in thermochemistry—and temperatures above 1000 °C, to which are added the special thermal effects of the aluminothermic reaction, for example.
The cations thus formed, entering the force field of the surface, in the presence of the electric double layer, are adsorbed and subsequently decompose with the release of silicon and nitrogen. It can be concluded that the saturation of the surface with silicon is based on both an atomic and an ionic mechanism of adsorption.
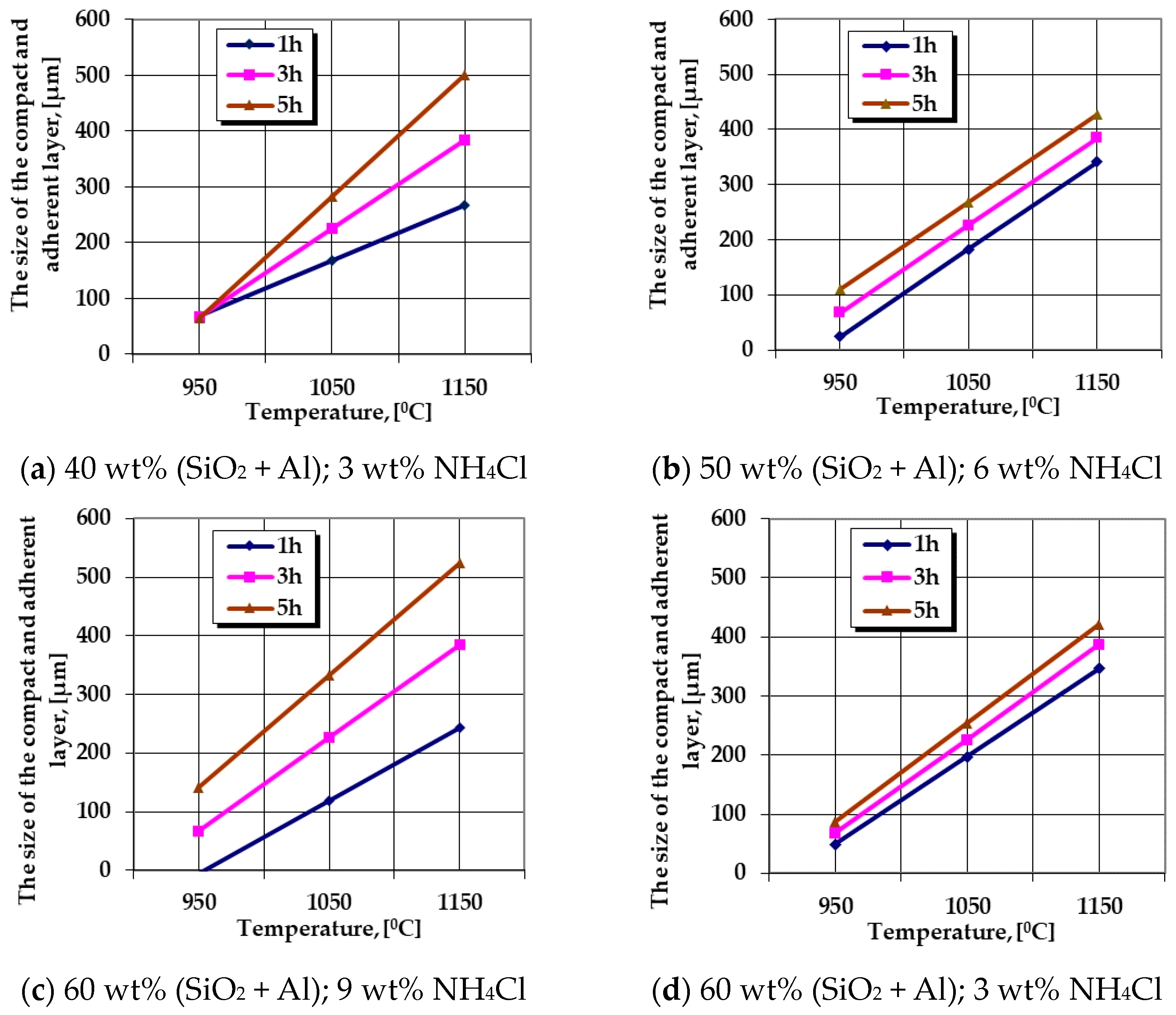
The graphical expressions of regression Equations (6) and (7) are presented in Figure 3 and Figure 4.
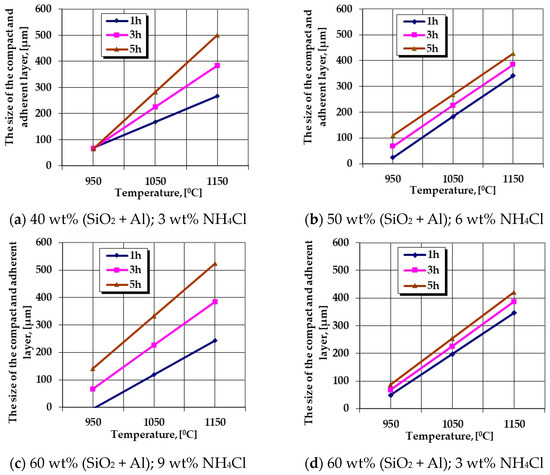
Figure 3.
The change in the growth kinetics of the thermochemically processed layer (the thickness without porosity) generated by variation of the temperature, the holding time, the proportion of the active component (SiO2 + Al), and the proportion of NH4Cl in the solid powdery mixture. Note—the graphical dependencies represent the graphical expressions of regression Equation (6) for different situations of the processing parameters.
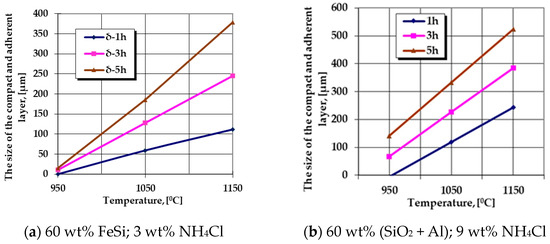
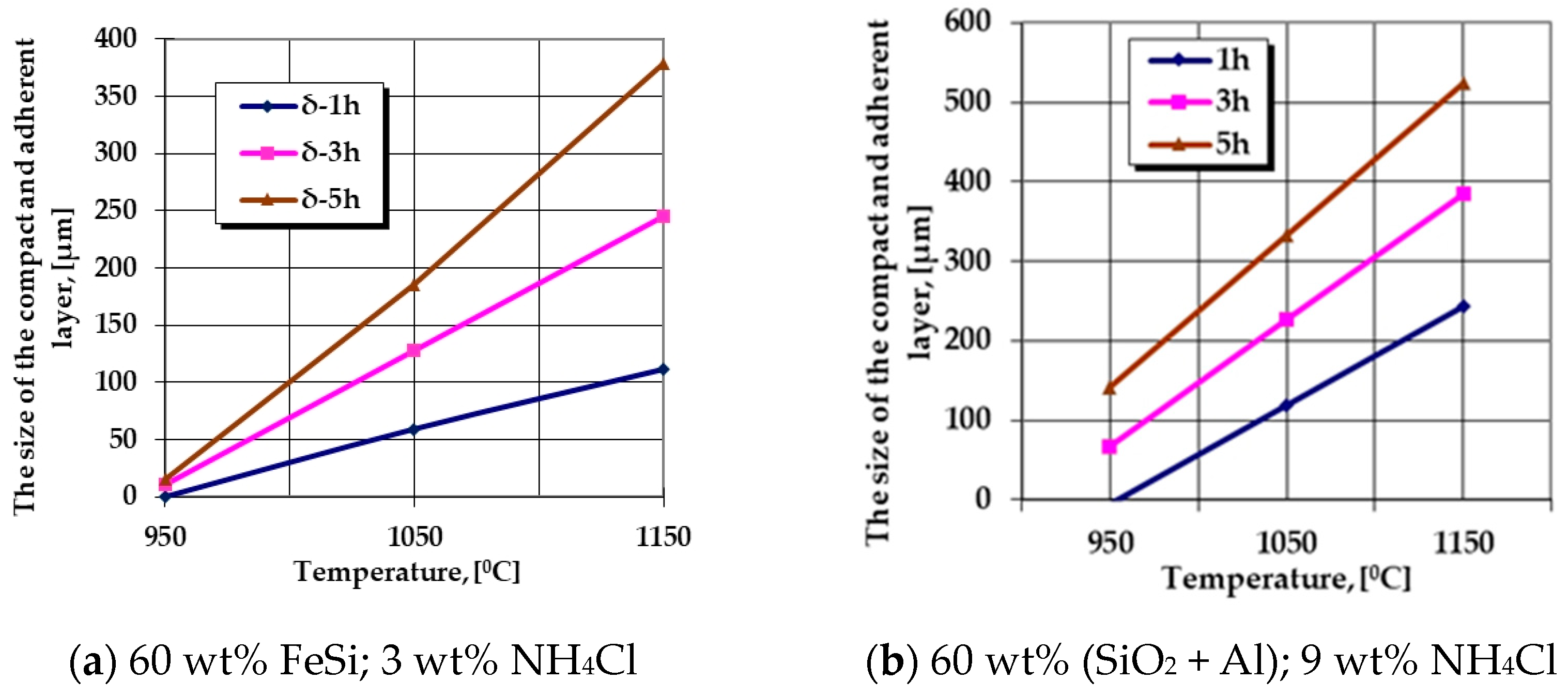
Figure 4.
Processing conditions in which the most intense growth kinetics of the no porosity thickness of the layers results (mainly Al cemented), obtained in the case of P265GH steel grade.
In the case of the use of powdery thermite mixtures (SiO2 + Al), it is possible that there will be chemical interactions between the aluminum particles isolated in the powdery solid media by the dispersing agent (Al2O3) and the gaseous products resulting from the decomposition of the halide used as an activator of the reactions (8,9,21 ÷ 26).
6HCl +2Al = Al2Cl6 + 3H2
(ΔG950°C = −376.1 kJ/mol, and ΔH950°C = −742.1 kJ/mol)
(ΔG950°C = −376.1 kJ/mol, and ΔH950°C = −742.1 kJ/mol)
Al2Cl6 = 2AlCl3
(ΔG950°C = −48.3 kJ/mol, and ΔH950°C = 111.6 kJ/mol)
(ΔG950°C = −48.3 kJ/mol, and ΔH950°C = 111.6 kJ/mol)
From a thermodynamic point of view, the reactions between hydrochloric acid vapors and Al particles lead to the formation of Al subchlorides (Equations (23) and (24)) AlCl2 and AlCl, with their subsequent disproportionation and the generation of Al in an active state that will determine the saturation of the metal surface (Equations (25) and (26)); the subchlorides are in an adsorbed state.
2HCl + Al = AlCl2 + H2
(ΔG950°C = −112.4 kJ/mol, and ΔH950°C = −110.7 kJ/mol)
(ΔG950°C = −112.4 kJ/mol, and ΔH950°C = −110.7 kJ/mol)
2HCl + 2Al = 2AlCl + H2
(ΔG950°C = −92 kJ/mol, and ΔH950°C = 48.9 kJ/mol)
(ΔG950°C = −92 kJ/mol, and ΔH950°C = 48.9 kJ/mol)
The probability of aluminum dichloride formation is higher than that of monochloride formation, but both can form under the specific conditions of the thermochemical process.
3AlCl = AlCl3 + 2Al
(ΔG950°C = −74.1 kJ/mol, and ΔH950°C = −384.1 kJ/mol)
(ΔG950°C = −74.1 kJ/mol, and ΔH950°C = −384.1 kJ/mol)
3AlCl2 = 2AlCl3 + Al
(ΔG950°C = 87.3 kJ/mol, and ΔH950°C = −298.6 kJ/mol)
(ΔG950°C = 87.3 kJ/mol, and ΔH950°C = −298.6 kJ/mol)
It can therefore be anticipated that under the specific conditions of the Si cementation process, the presence of the thermite mixture (SiO2 + Al) will not be sufficient to ensure the Si necessary for the superficial saturation of the metal product made of P265GH steel, and therefore, for the initiation and development of cementation with Si, because the probability of consuming Al in the process of forming its chlorides is certainly higher than that of initiating the metallothermic reduction reaction; other sources of Si are required.
The analysis of the information provided by the regression Equations (5)–(7) are suggestively expressed in the customized graphic representations in Figure 1, Figure 3 and Figure 4 (trends in the evolution of the growth kinetics of these areas, i.e., the evolution of the ratio of the mass proportions of Si and Al in the thermochemically processed coating), highlighting the fact that variation of the temperature and the holding time at the processing temperature strongly influences the thickness of the silicon-cemented coating and the value of the ratio of the mass proportions of Si and Al in the superficial zones of the coating. It is thus observed that systematically, regardless of the nature of the active component of the media, the increase in temperature and holding time implies an increase in the siliconized coating thickness of interest. However, the change in the thickness of this zone is strictly dependent on the way in which the two main components of the mixture are associated—the active component and the component with the role of activator of the reactions.
The analysis of the main reactions between the components of the solid powdery media used for silicon cementation highlights the major role of the components as activators of the reactions in the medium especially if the active component of the mixture is ferrosilicon.
From the analysis of the regression equations, it can be seen that in the vast majority of cases, from a statistical point of view, the effect of the singular variation of the proportion of the activator component is insignificant (the exceptions are Equations (5) and (7) for the evolution of the ratio of mass proportions), but the effect of the cumulative variation of this parameter with that related to the proportion of the active component, FeSi75C or (SiO2 + Al), or with that of temperature, is particularly significant.
It was found that depending on the type of active component, the best results regarding the kinetics of the increase in the interest area of the siliconized coating are obtained by associating a high proportion of the active component, FeSi75C, with a low proportion of the component with the role of initiation and activation of reactions in the environment, NH4Cl (at the lower limit accepted in the adopted mathematical model), and a high proportion (at the upper limit accepted in the adopted mathematical model) for the thermite mixture (SiO2 + Al).
The differences in the proportion of the activator component in the solid powdery media, recorded by the growth kinetics of the coating in the case of the two types of active components, prove once more the role of the decomposition products of the halide; they isolate the Al particles and interact with them, forming aluminum chlorides (Equations (21)–(24)) to the detriment of the aluminothermic reaction (Equation (18)). If a longer holding time is added to this association of parameters, the maximum performance is reached (maximum thickness of the analyzed coating).
The regression Equations (5)–(7) allow us, with a probability of 95%, to predict by calculation the thickness of the nonporous coating that is in direct contact with the substrate. They allow the estimation of how the thermal, temporal, and chemical parameters of the processing can be correlated to obtain a certain coating thickness, or if and how the presence of a certain parameter restricted by the imposed technological conditions can be compensated, by modification of the other parameters.
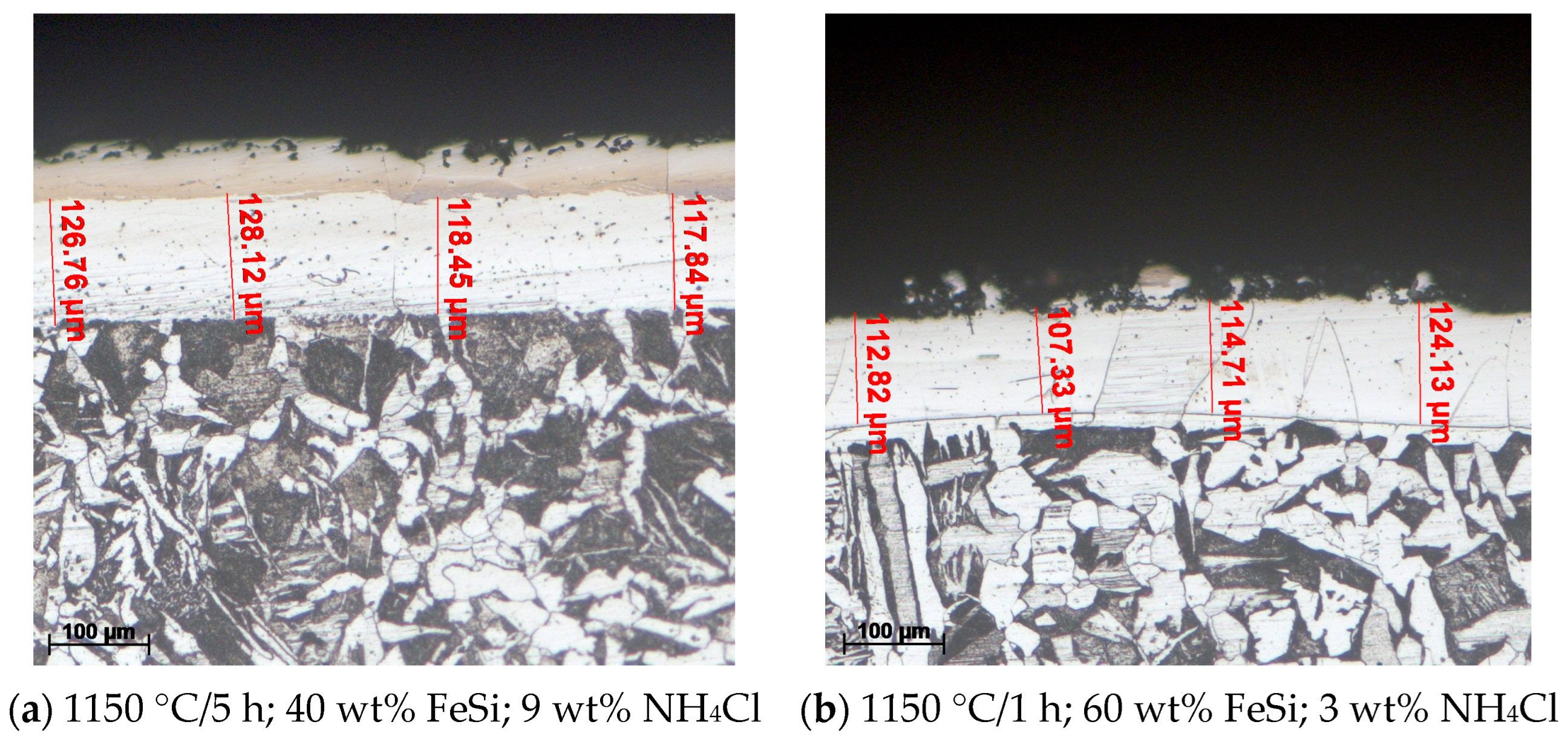
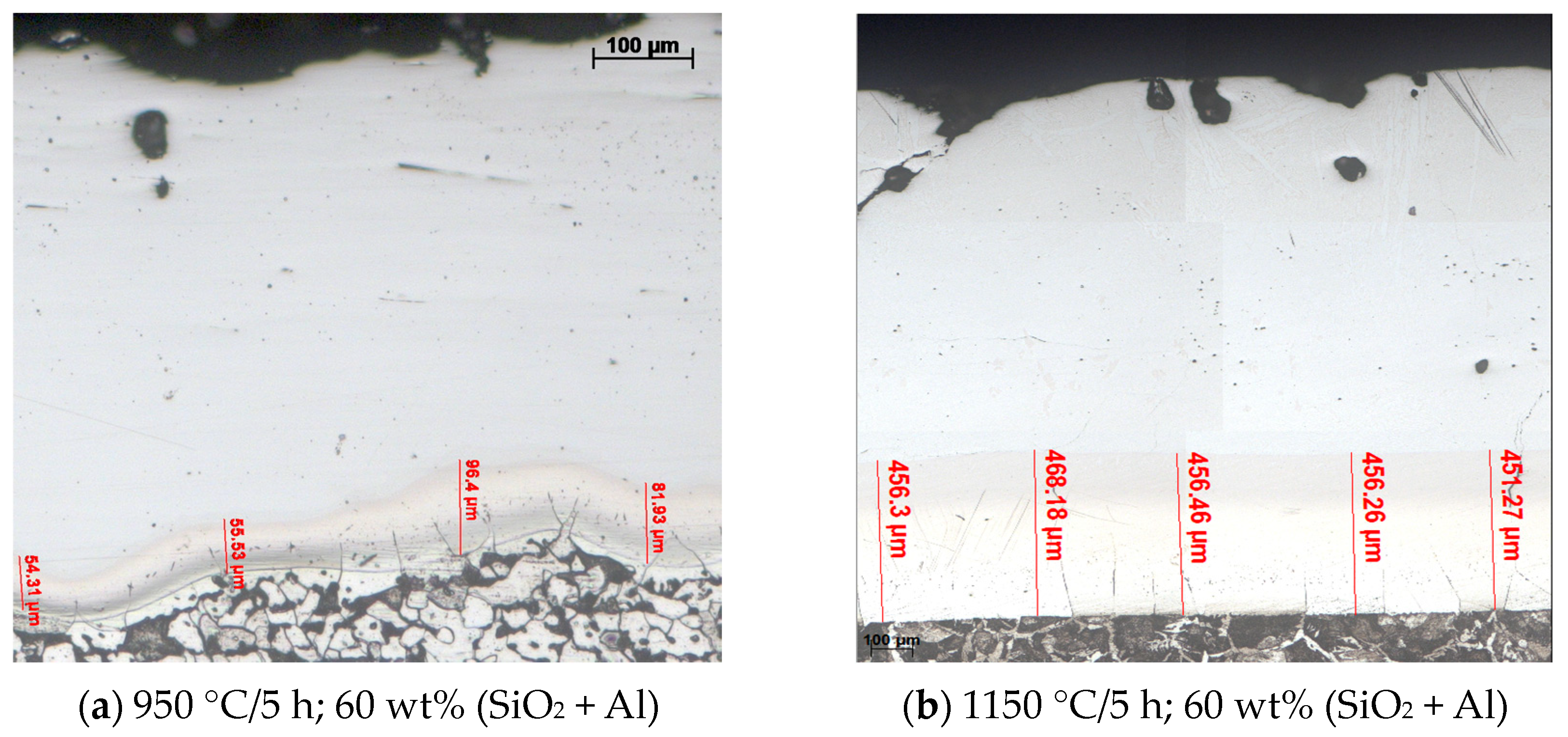
Figure 5 and Figure 6 show the optical microscopy (OM) analysis performed on the siliconized coating obtained under different conditions, highlighting the typical aspects of the obtained coating on P265GH grade steel.
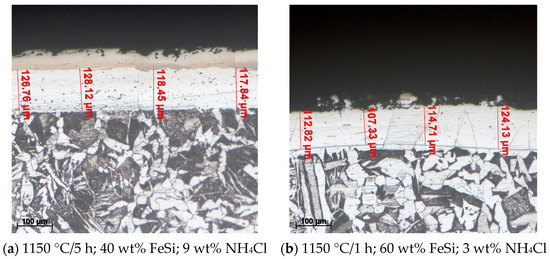
Figure 5.
The effects of changing the growth kinetics of the compact and adherent coating on the variation of the holding time at 1150 °C, the proportion of the active component (FeSi75C), and the proportion of the activator (NH4Cl) in the solid powdery mixture; etchant: Nital 2%.
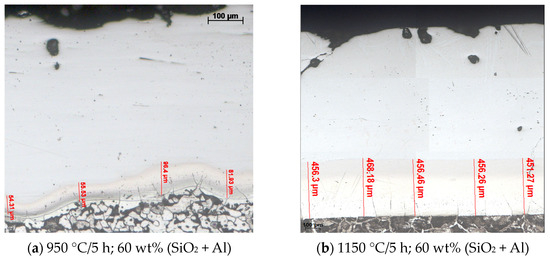
Figure 6.
The effect of changing the temperature on the thickness of the non-porosity zone of the layer; 3 wt% NH4Cl; etchant: Nital 2%.
The coating structures with a columnar morphology are made up mainly of solid solutions of silicon and predominantly aluminum in Feα in the case of use as the active component of the thermite mixture (SiO2 + Al). In the substrate (P265GH grade steel), the proportion of pearlite is considerably higher compared to steel of the same grade in the annealed state (Figure 5).
The explanation of the phenomenon is related to the dislocation of carbon in the areas adjacent to the surface along with the diffusion of silicon. This explains the unfolding of the γ→α transformation at the temperature at which a siliconized coating takes place and carbon is pushed to the deeper areas.
An increase in the concentration of carbon in the superficial areas of the products subjected to silicon cementation very likely determines a decrease in the differences between the partial diffusion coefficients of silicon in iron and vice versa, of iron in silicon, and thereby a decrease in the probability of the appearance of diffusion porosity (the decrease is the extent of manifestation of the Kirkendall–Frenkel phenomenon).
It is known that the size of the Si diffusion coefficient in the metal matrix is strictly dependent on the activity of the media used for thermochemical processing, on the processing temperature, and finally on the chemical composition of the metal matrix. The presence of C determines the braking of the formation process of the siliconized coating and an increase in the coating thickness devoid of porosity (this decreases the value of the diffusion coefficient of Si in Fe and implicitly the tendency of the porosity formation due to the decrease in the intensity of manifestation of the Kirkendall–Frenkel effect). The strongest influence manifested by the variation of the C proportion on the growth kinetics of the coating in general, and of the nonporous zone in particular, is manifested in the range of concentrations 0.05 ÷ 0.2 wt% [2].
Mn (an element with a concentration of 1.05% in the analyzed steel) has a similar tendency to C, widening the stability range of the γ phase and slightly reducing the size of the siliconized coating.
The use of the thermite mixture (SiO2 + Al) as the active component of the solid powdery media intended for the silicon cementation of steels generates a paradigm shift in the definition of siliconizing. In the superficial coatings, the ratio of the mass proportions of Si and Al changes within wide limits depending on the thermal, temporal, and chemical processing parameters, but it remains permanently strongly sub-unitary, emphasizing the fact that superficial saturation in the case of using such media is mainly achieved with Al (the coating has the characteristics of an aluminum-cemented coating).
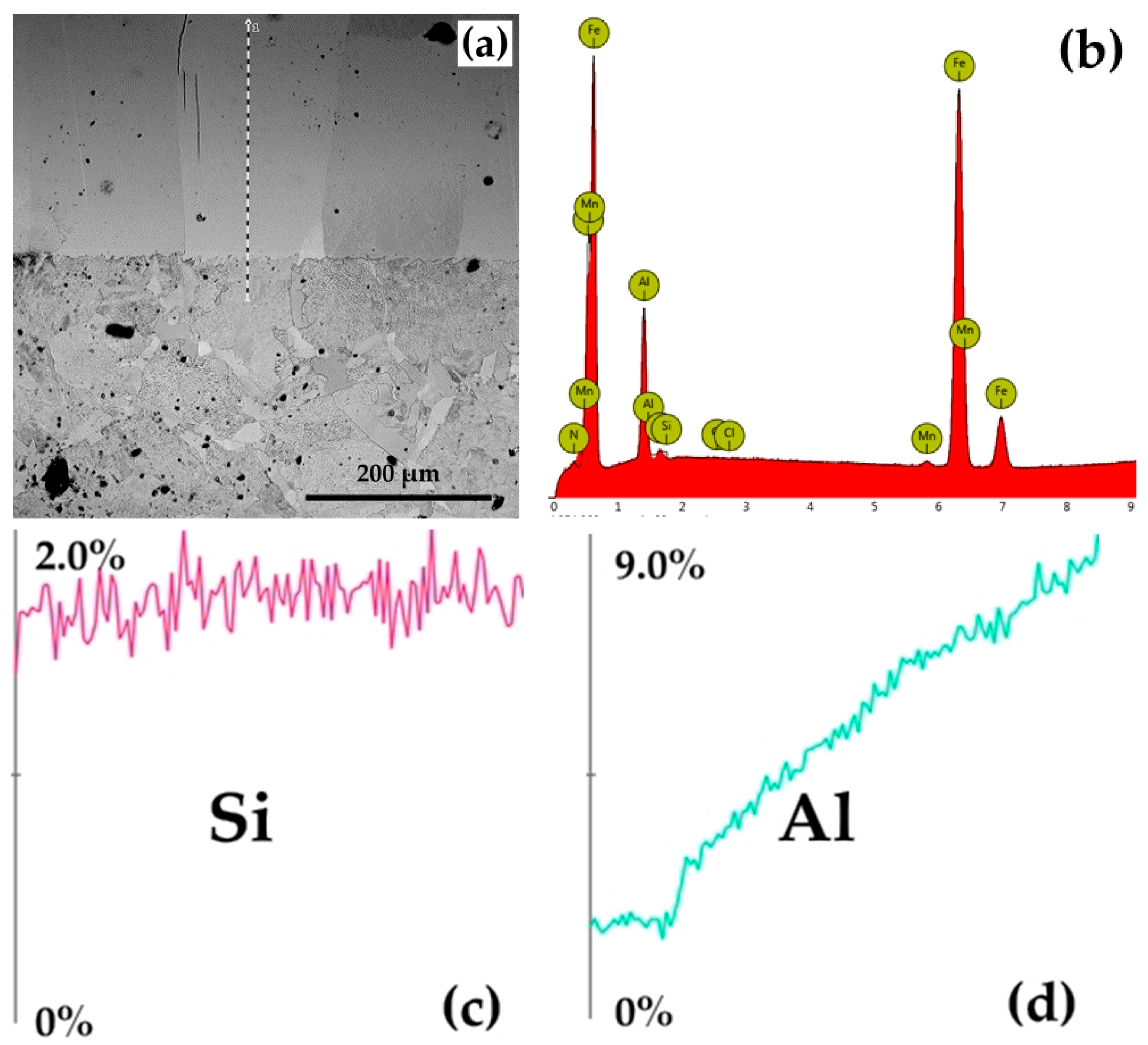
The presence of Al in high concentrations in the superficial areas of the surface of metal products subjected to silicon cementation in solid powdery media, associated with low (or zero) silicon concentration values (low %Si/%Al wt ratio), was detected via XRF analyses (X-ray fluorescence spectrometry) and quantitative microanalyses made by energy dispersion (EDS), as shown in Figure 7.
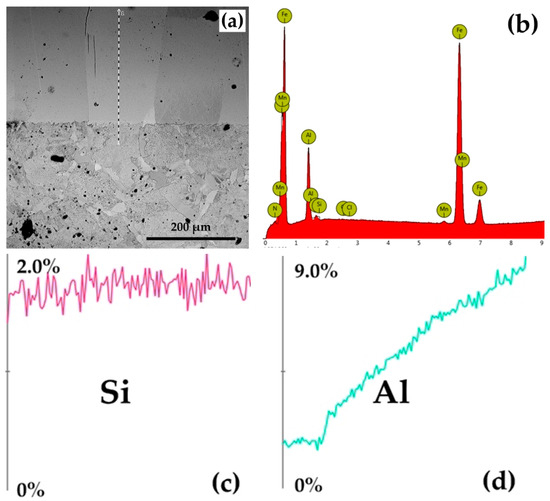
Figure 7.
The results of the EDS microanalyses: (a) linescan on the SEM image; (b) quantitative microanalyses performed by energy-dispersive spectrometry (EDS) on the P265GH samples thermochemically processed at 1150 °C/5 h in a solid powdery medium containing 60 wt% thermite mixture (SiO2 + Al) and 3 wt% NH4Cl; (c) Si distribution in the section of the thermochemically processed layer; (d) Al distribution in the section of the thermochemically processed coating. Note—The mass concentration of nitrogen in the area investigated is 1.55%, its presence being dictated by the appearance of the compound Si2N (Equation (20)).
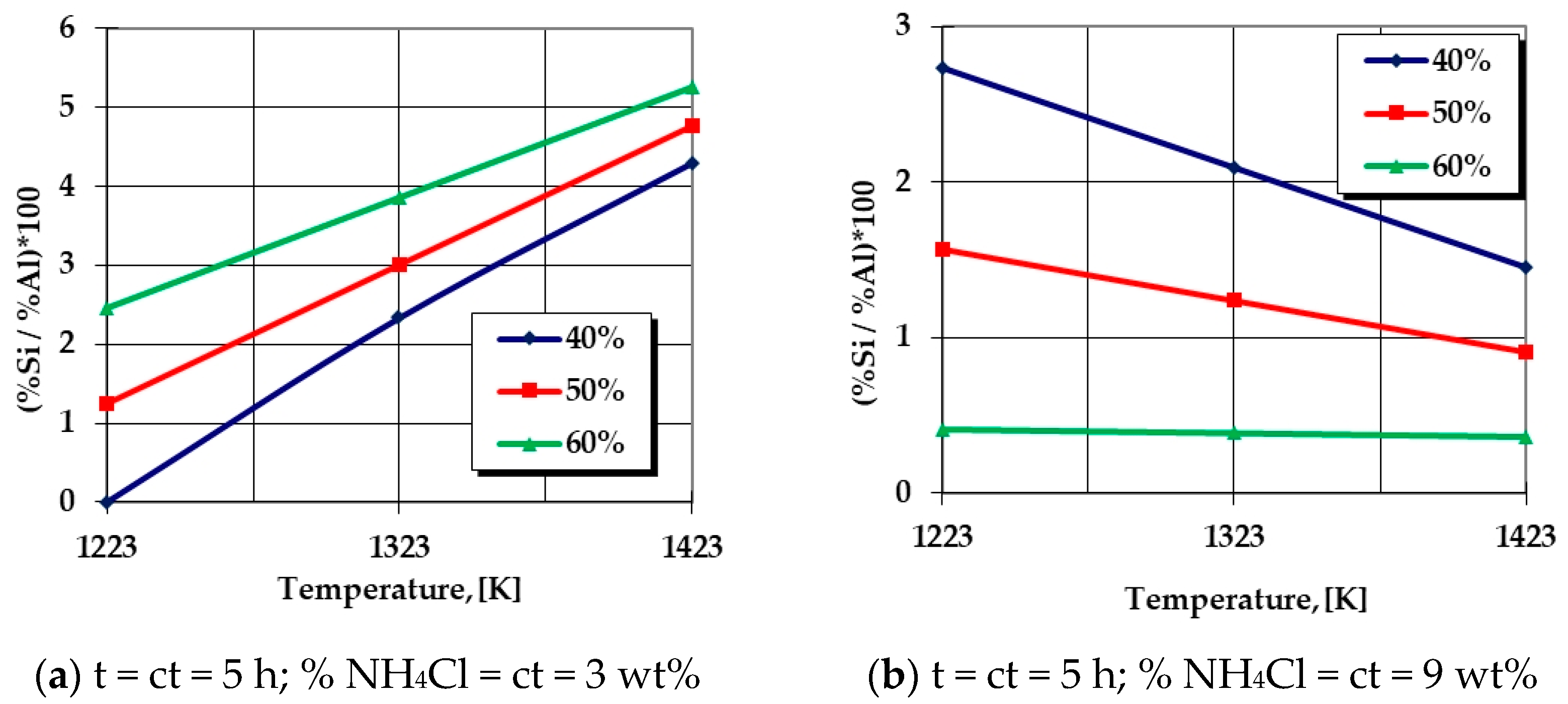
The Si percentage in the silicon-cemented coating, in the areas adjacent to the surface, is in strict correlation with the processing temperature. In addition, reduced values of the ratio of the %Si and %Al in the thermochemically processed coatings are obtained at temperatures located at the lower limit of the analyzed range in the case of experimental research (950 °C) as well as reduced proportions of the activating component (NH4Cl↓) and also the active component (SiO2 + Al)↓, as shown in Figure 8a. Similar results are reached in the case of an overdose of the activating component (NH4Cl↑), simultaneously with an increase in the proportion of the active component (SiO2 + Al)↑ of the powdery mixture, which is associated with an increase in processing temperature, as shown in Figure 8b. In all these situations, Al is predominant in the thermochemically processed coating; the process having the characteristics of aliting (aluminum cementation).
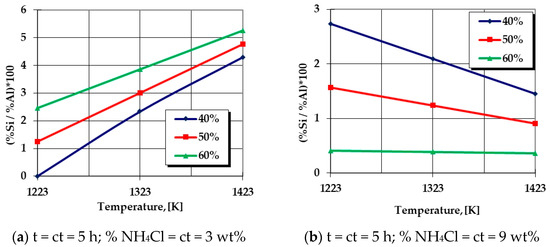
Figure 8.
Dependence of the ratio of mass proportions of Si and Al on the thermochemical processing parameters. Note—The graphical dependencies represent the graphical expressions of regression Equation (7) for different situations of the processing parameters and variable proportions of the thermite mixture (SiO2 + Al) within the limits of 40 ÷ 60 wt%.
The explanation of the phenomena is related to the fact that the Al powder is found in a dissipation medium (Al2O3) in the presence of gaseous decomposition products of NH4Cl, so the probability of Al particles interacting with them and forming aluminum chlorides is considerably higher than that of initiating the aluminothermic reaction of SiO2.
Increases in the proportion of the activating component (NH4Cl) imply an increase in the proportion of gaseous products dictated by the decomposition of the halide and thereby the probability of the formation of aluminum chlorides at the expense of Si formed by the aluminothermic reaction.
From a thermodynamic point of view, Equation (18) can also take place in the presence of an excess of Al (the reaction is strongly exergonic), like in Equation (27), the thermal effect of the latter being comparable to that of the reaction without an excess of reactants (if the reaction takes place stoichiometrically).
3SiO2 + 5Al = 3Si + Alexc + 2Al2O3
The two Equations (18) and (27) have a similar considerable thermal effect:
(ΔH = [−770.8KJ/mol @ 1223 K ÷ −780.5KJ/mol @ 1473 K])
The analysis of the results obtained (see Table 2) and the information provided by regression Equation (7) confirm the conclusion that regardless of the method of combining the independent parameters of the process (within the limits of variation imposed by the adopted research program, the ratio of %Si and %Al in the superficial areas of the coating is strongly sub-unitary, the formed layer having the predominant characteristics of an Al cemented coating.
Regression Equation (7) and its graphical expressions for some situations lead to some interesting conclusions:
- -
- An increase in the proportion of NH4Cl in the solid powdery mixture will impose an increase in the Al percentage (a decrease in the ratio of mass proportions related to Si and Al) in the superficial layer, which is more intense as the proportion of the thermite mixture (active component) is higher and the temperature is higher, as shown in Figure 8b.
- -
- For low proportions of the halide in the solid powdery mixture, as shown in Figure 8a, the probability of developing the aluminothermic reaction (18) in volume is higher because the proportion of hydrochloric acid vapors resulting from the decomposition of the halide is lower, so the development of Equations (21), (23) and (24) will be favored by a reduction in the proportion of the thermite mixture in the general powdery mixture.
The predominance of Si or Al atom flows in the iron matrix at a certain temperature can also be related to the change in the ratio of the diffusion coefficients of aluminum and silicon, depending on the temperature [5,10,12,13,14,15,16,17,18,19,20,21] and the availability of one of the two elements in the reaction medium under certain conditions.
Thus, if at a temperature of 1223 K (the lower limit of the temperature variation range in the adopted program) the diffusion coefficient of Al in Fe is an order of magnitude higher than that of Si in Fe (1.32 × 10−6 m2/s [10,17] compared to 5.71 × 10−7 m2/s, according to Ghertzriken [22]), at 1423 K, the two values are close (1.18 × 10−6 m2/s compared to 2.42 × 10−6 m2/s, according to the same bibliographic sources), so it can be concluded that regardless of the temperature, an important role is played in the saturation process of the surface by the reactions in the siliconized media; i.e., the available Si and Al generated by the reactions in the environment. However, the values of the diffusion coefficients of Fe in the solid solution of Si in Fe, or of Al in the solid solution of Al in Fe, are extremely close at low temperatures (a difference of an order of magnitude). At high temperatures, this difference increases considerably in favor of the diffusion coefficient of Fe in Al (approximately six orders of magnitude). This justifies a change in the intensity of the manifestation of the Kirkendall–Frenkel phenomenon, also affecting the size of the zone without porosity.
4. Conclusions
- (a)
- FeSi75C with high concentrations of silicon (over 70 wt%) represents a redoubtable active component especially in the range of temperatures above 1100 °C. The most intense kinetics of the formation of the area of interest of the silicon-cemented coating is obtained for high values of the proportion of FeSi75C in the medium and similarly for the processing temperature, as well as for the holding time, which is associated with low values of the activating component (about 3 wt%).
- (b)
- The mechanism by which the superficial saturation with Si takes place is very likely both atomic and ionic; during holding at the silicon cementation temperature, all the conditions are created for the synthesis reaction of silicon nitride Si2N and its subsequent ionization with the formation of cationic complexes of the Si2N+ type.
- (c)
- The use of the powdery thermite mixture (SiO2 + Al) as the active component of the solid medium certainly ensures an intensification of the coating growth kinetics with a chemical and phase composition different from that of the matrix, but it will have the predominant characteristics of an alited coating (aluminum cemented), so it is not a solution to be considered for pack silicon cementation.
- (d)
- The calculated and statistically verified mathematical models allow the anticipation by calculation of how we can associate the parameters with significant influence on the growth kinetics of the thickness in the silicon-cemented coating devoid of porosity in order to maximize this thickness or how we can combine parameters (in the case of using the thermite mixture) in such a way that we obtain a value of practical interest of the ratio between the proportions of the two elements in the coating. In this way, the control of the silicon cementation process becomes more effective and its efficiency increases.
Author Contributions
Conceptualization, M.B. and M.O.C.; methodology, M.B., M.O.C., M.D.M. and L.N.D.; software, M.O.C.; validation, M.O.C., M.D.M. and M.B.; formal analysis, M.D.M. and L.N.D.; investigation, M.O.C., M.B. and M.D.M.; resources, M.B., M.O.C. and L.N.D.; data curation, M.D.M., M.B. and M.O.C.; writing—original draft preparation, M.O.C. and M.B.; writing—review and editing, M.O.C., M.B. and M.D.M.; visualization, M.O.C., M.B. and M.D.M.; supervision, M.B., M.O.C. and L.N.D.; project administration, M.B., M.O.C., M.D.M. and L.N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garverick, L.; Scott, D.H.; Scott, W.W., Jr. Corrosion in the Petrochemical Industry, 3rd printing; ASM International: Novelty, OH, USA, 1999; pp. 11–17. ISBN 0-87170-505-2. [Google Scholar]
- Leahovici, L.S.; Voroşnin, L.G.; Şcerbakov, E.R.D.; Panin, G.G. Siliconization of Metals and Alloys; Tehnica, N.I., Ed.; Scientific and Technical Publishing House: Minsk, Russia, 1972; pp. 149, 167, 188–193, 194–210. (In Russian) [Google Scholar]
- Lahtin, I.M.; Arzamasov, B.N. Thermochemical Treatments of Steels; Metallurghia (Metallurgy Publishing House): Moscow, Russia, 1985; pp. 221–229. (In Russian) [Google Scholar]
- Kubachewski von Goldbeck, O. Fe-Si Iron-Silicon. In IRON-Binary Phase Diagrams; Springer: Berlin/Heidelberg, Germany, 1982; pp. 136–139. [Google Scholar] [CrossRef]
- Sen, U.; Ozdemir, O.; Yilmaz, S.; Sen, S. Kinetics of Iron Silicide Deposited on AISI D2 Steel by Pack Method. In Proceedings of the 22nd International Conference on Metallurgy and Materials (METAL 2013), Brno, Czech Republic, 15–17 May 2013; p. 965, ISBN 978-80-87294-39-0, ISSN 2694-9296. [Google Scholar]
- SA 516 Steel Plate. Overview of SA 516 60, SA 516 65 & SA 516 70. Available online: https://www.aasteel.com/sa-516/?print=pdf (accessed on 17 September 2023).
- EN 10028-2. Available online: http://www.boilersteel.com/alloy-steel/en-10028-2-steel-plate.html (accessed on 17 September 2023).
- Taloi, D.; Florian, E.; Bratu, C.; Berceanu, E. Optimization of Metallurgical Processes; Didactic and Pedagogical Publishing House: Bucharest, Romania, 1983; pp. 79–96, ISBN/COD:240IPCLUOP. (In Romanian) [Google Scholar]
- Dimitriu, S.; Taloi, D. Mathematical Modeling Methods of Technological Processes; Printech, Printech Publishing House: Bucuharest, Romania, 2014; pp. 181–206. (In Romanian) [Google Scholar]
- Stechauner, S. Diffusion of aluminum in ferrite and austenite—MatCalc example D30; MatCalc Version:5.44.1002; Austria. 2012. Available online: https://www.matcalc.at/wiki/doku.php?id=examples:diffusion:d30 (accessed on 17 September 2023).
- Paukstis, S.J.; Gole, J.L. The Ionization Potential of Si2N and Si2O. J. Phys. Chem. A 2002, 106, 8435–8441. [Google Scholar] [CrossRef]
- Balandin, Y.A.; Kolpakov, A.S. Diffusion Siliconizing in a Fluidized Bed. Met. Sci. Heat Treatmen 2006, 48, 127–130. [Google Scholar] [CrossRef]
- Popoola, A.P.I.; Aigbodion, V.S.; Fayomi, O.S.I.; Abdulwahab, M. Experimental Study of the Effect of Siliconizing Parameters of Thermochemical Treatment of Low Carbon Steel. Silicon 2016, 8, 201–210. [Google Scholar] [CrossRef]
- Hsu, H.W.; Tsai, W.T. High temperature corrosion behavior of siliconized 310 stainless steel. Mater. Chem. Phys. 2000, 64, 147–155. [Google Scholar] [CrossRef]
- Soderhjelm, C. Multi-Material Metal Casting: Metallurgically Bonding Aluminum to Ferrous Inserts. Ph.D. Thesis, Philosophy in Materials Science and Engineering, Worcester Polytechnic Institute, Worcester, MA, USA, 2017. [Google Scholar]
- Fujikawa, S.; Hirano, K.; Fukushima, Y. Diffusion of silicon in aluminum. Metall. Trans. A 1978, 9, 1811–1815. [Google Scholar] [CrossRef]
- Mehrer, H.; Eggersmann, M.; Gude, A.; Salamon, M.; Sepiol, B. Diffusion in intermetallic phases of the Fe-Al and Fe-Si systems. Mater. Sci. Eng. A 1997, 239–240, 889–898. [Google Scholar] [CrossRef]
- Batz, W.; Mead, H.V.; Birchenall, C.E. Diffusion of Silicon in Iron. J. Met. 1952, 10, 1070. [Google Scholar] [CrossRef][Green Version]
- Mirani, H.V.M.; Maaskant, P. Diffusion of Si in Fe-Si Containing 8 to 11 at% Si. Phys. Status Solidi A 1972, 14, 521–525. [Google Scholar] [CrossRef]
- Isobe, T.; Nakashima, H.; Hashimoto, K. Diffusion Coefficient of Interstitial Iron in Silicon. Jpn. J. Appl. Phys. 1989, 28, 1282–1284. [Google Scholar] [CrossRef]
- Hirano, K.; Agarwala, R.P.; Cohen, M. Diffusion of iron, nickel and cobalt in aluminum. Acta Metall. 1962, 10, 857–863. [Google Scholar] [CrossRef]
- Ghertzriken, S.D.; Dehtear, I.I. Diffusion in Solid State Metals and Alloys; State Publishing House of Physics and Mathematics Literature: Moskva, Russia, 1960; pp. 522–526, (Romanian Translation). [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).