Investigation of Polyelectrolyte Multilayers Deposited on Biodegradable Corona-Charged Substrates Used as Drug Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Film Creation
2.3. Corona Charging
2.4. Surface Charge Distribution Measurement
2.5. Layer-by-Layer Deposition
- Dipping in first polyelectrolyte solution for 15 min.
- Washing in deionized water for 5 min.
- Dipping in second polyelectrolyte solution for 15 min.
- Washing in deionized water for 5 min.
2.6. Differential Scanning Calorimetry (DSC)
- Cooling down from 25 °C to −70 °C with a cooling rate of 2 K/min;
- Isothermal step at −70 °C for 15 min;
- Heating from −70 °C up to 300 °C with a heating rate of 10 K/min.
2.7. Scanning Electron Microscopy (SEM)
2.8. Water Contact Angle Measurement
2.9. Benzydamine Hydrochloride (BH) Drug Release
2.10. Benzydamine Hydrochloride (BH) Drug Content
3. Results and Discussion
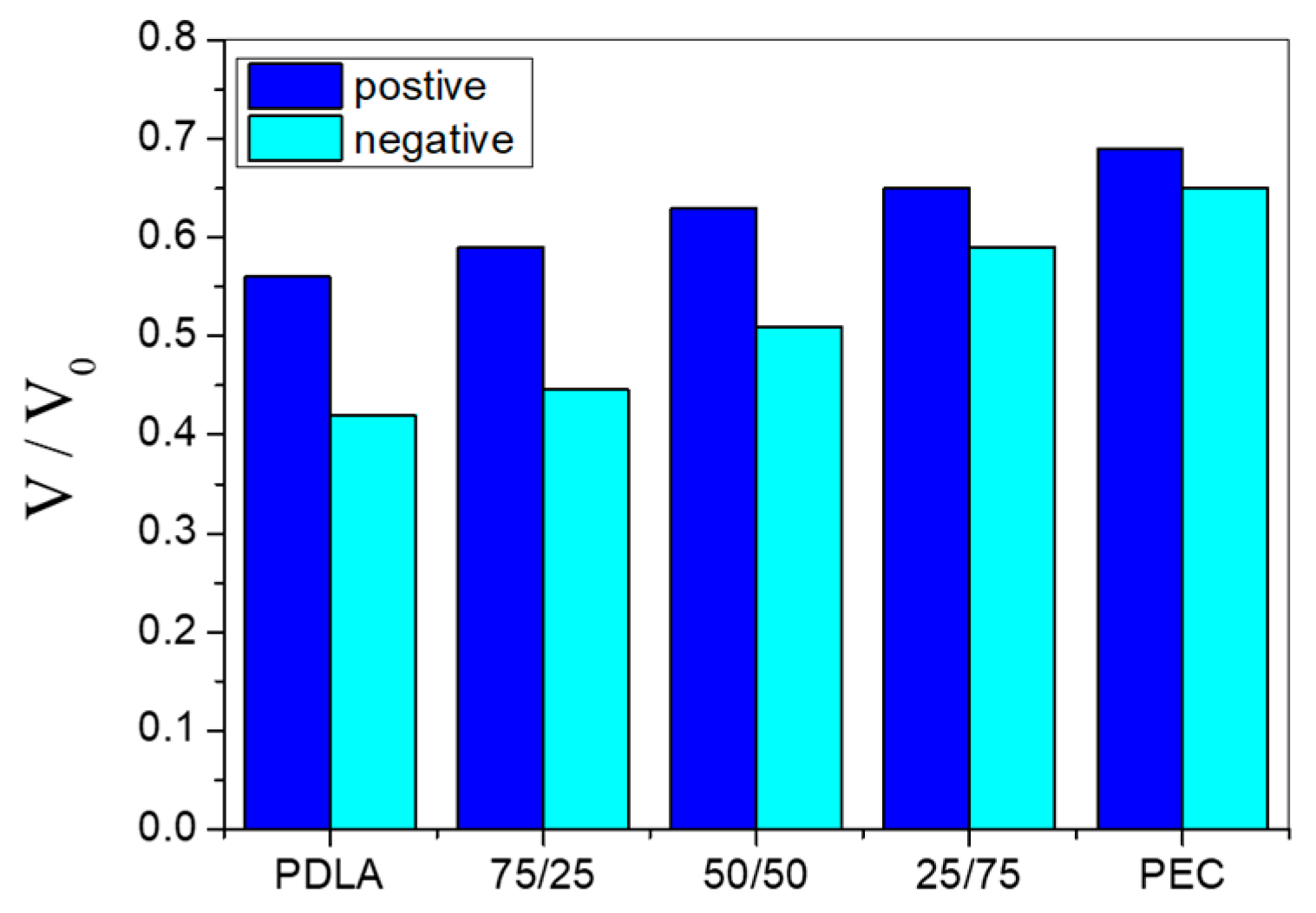
3.1. Electret Properties—Time Storage Influence
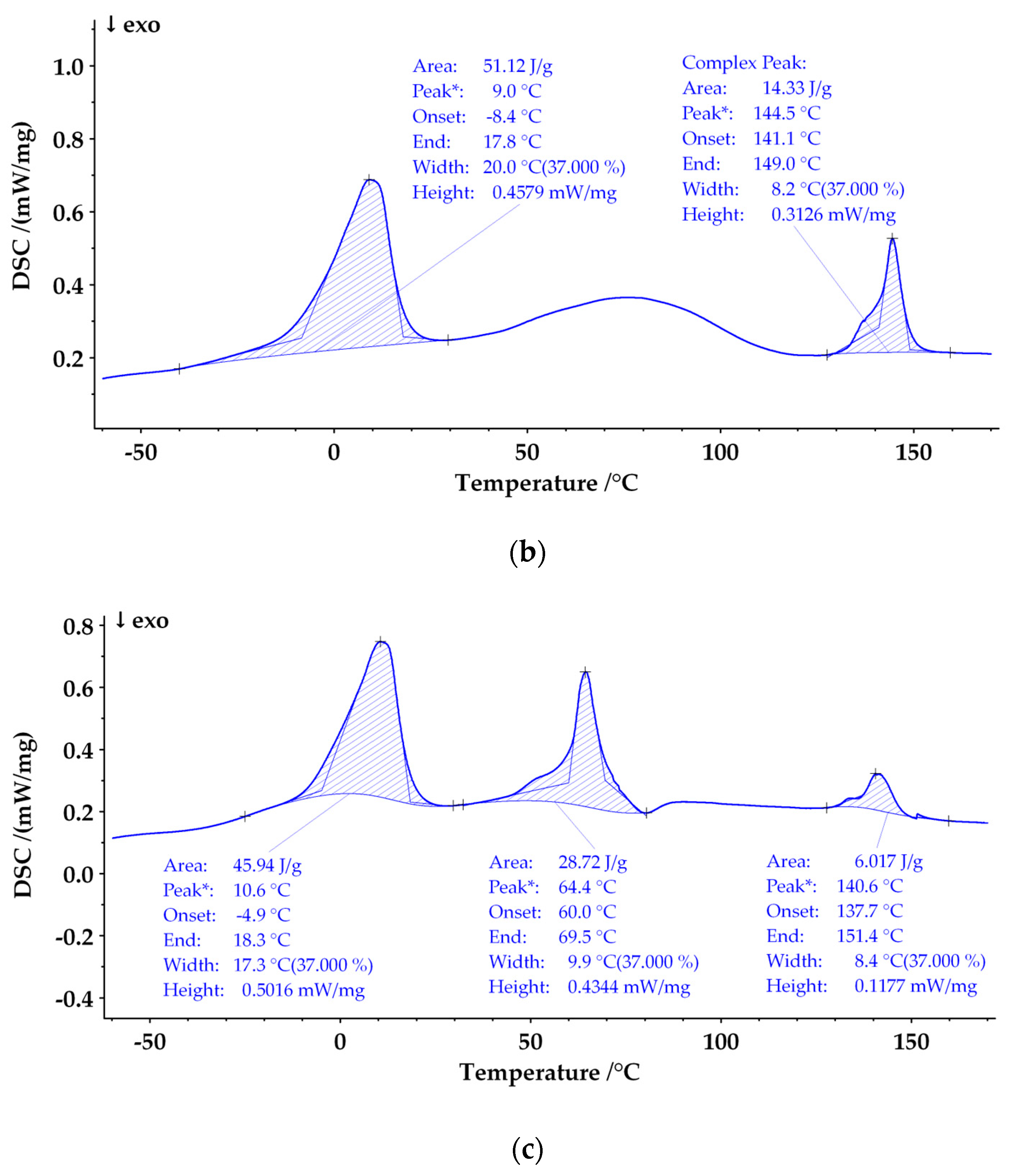
3.2. Phase State of Polylactic Acid/Poly(ε-Caprolactone) Films
3.3. PEMs’ Morphology
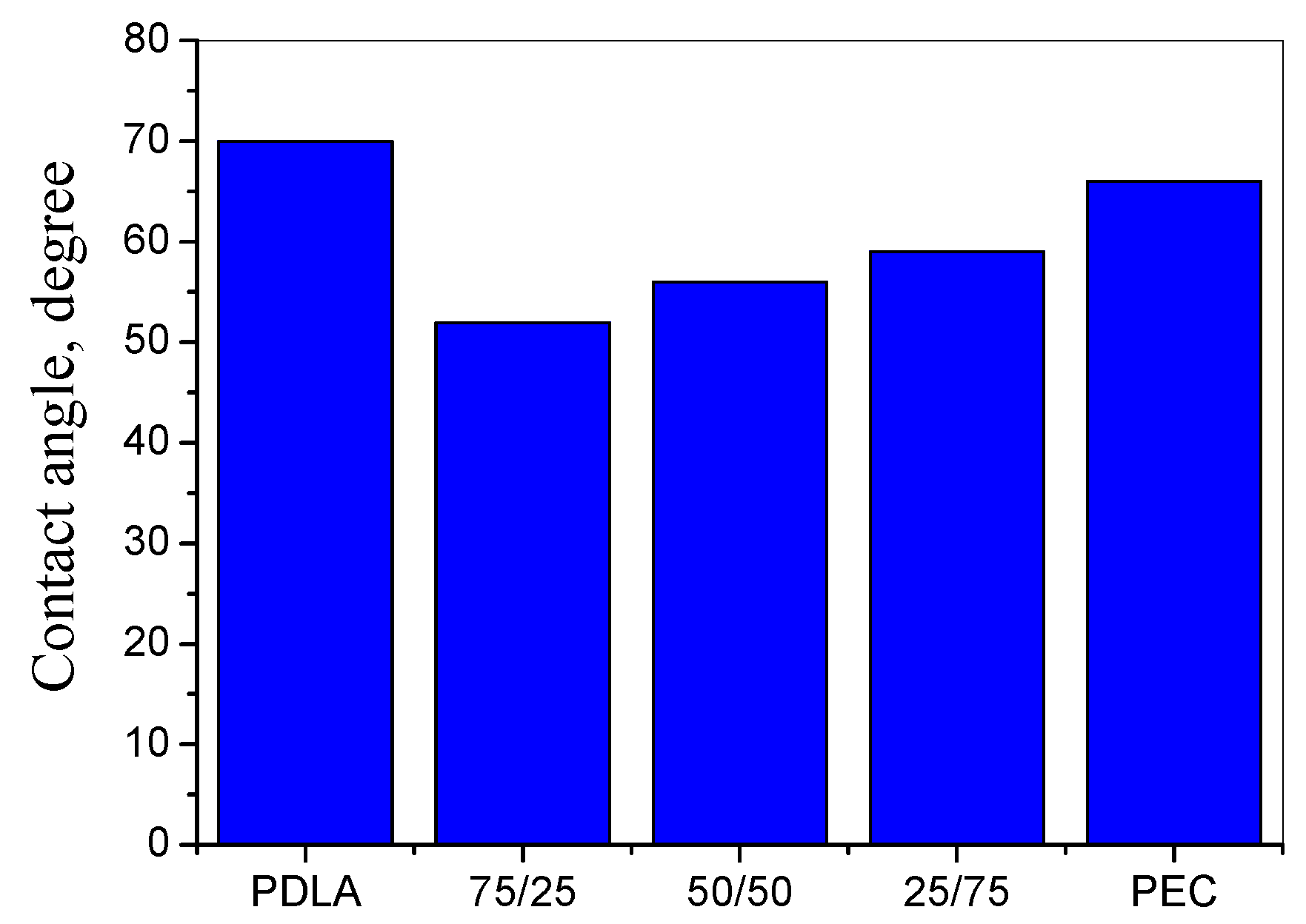
3.4. Water Contact Angle Measurements
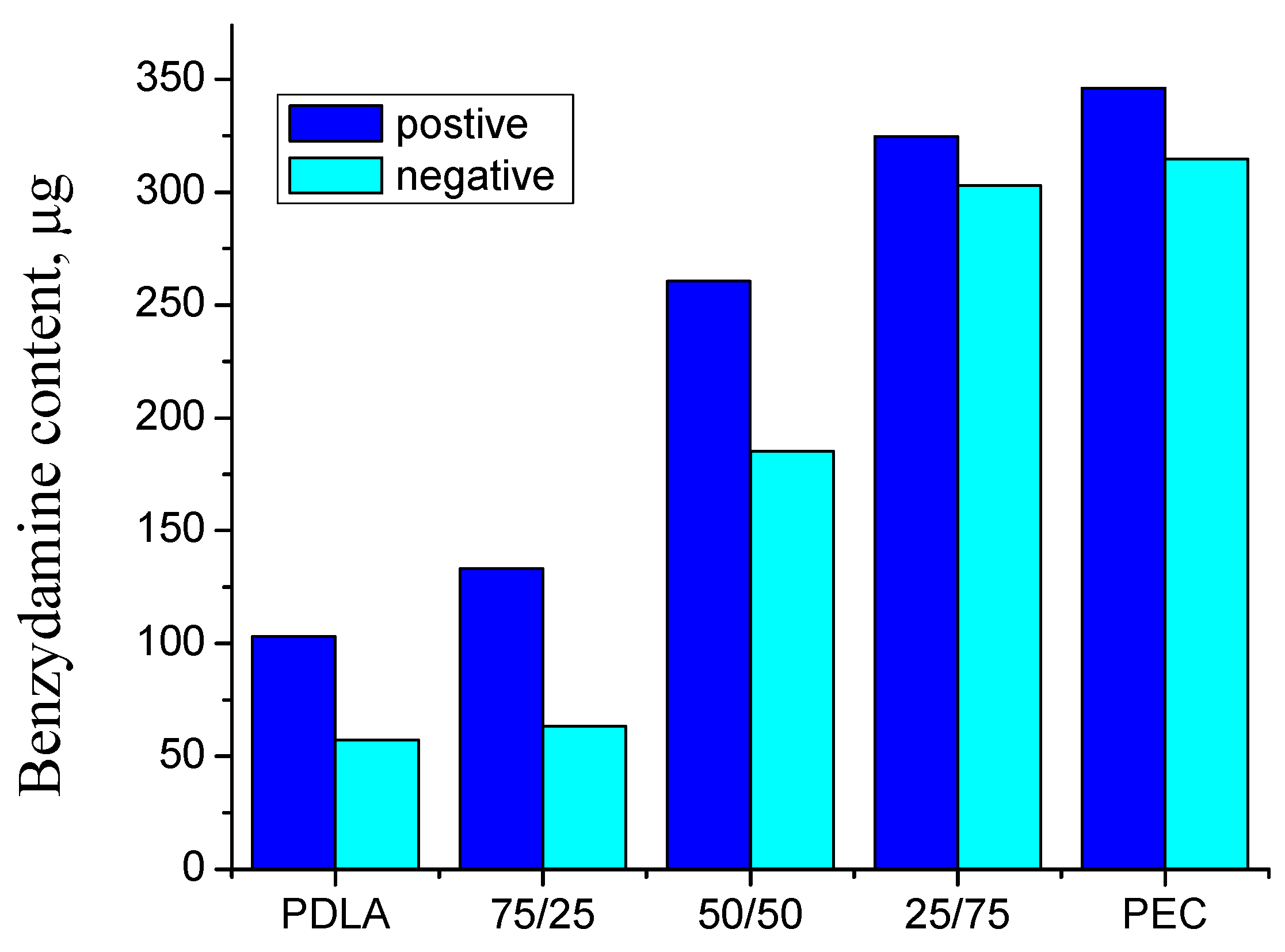
3.5. Benzydamine Hydrochloride (BH) Drug Content
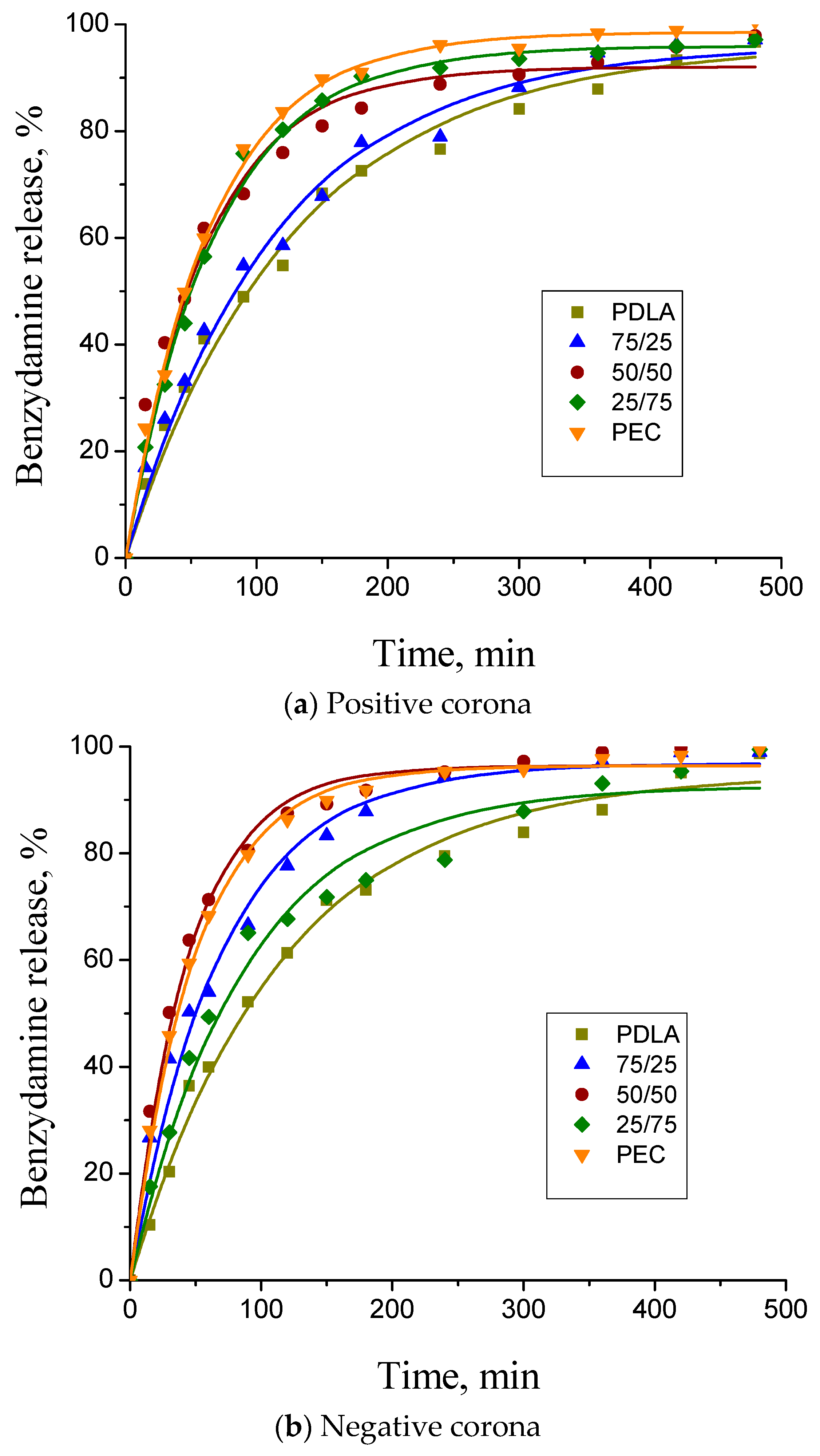
3.6. Benzydamine Hydrochloride (BH) Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers—A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Sukumaran, N.P.; Haponiuk, J.T.; Thomas, S. Biopolymers and Their Composites for Drug Delivery: A Brief Review. Macromol. Symp. 2018, 380, 1800114. [Google Scholar] [CrossRef]
- Cini, N.; Calisir, F. Layer-by-layer self-assembled emerging systems for nanosized drug delivery. Nanomedicine 2022, 17, 1961–1980. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.C.; Nandi, N.B.; Tiwari, A.; Das, J.; Roy, B. Nanotechnology Applications for Food Safety and Quality Monitoring; Sharma, A., Vijayakumar, P.S., Prabhakar, E.P.K., Kumar, R., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 17; pp. 321–340. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Coudane, J.; Nottelet, B.; Mouton, J.; Garric, X.; Van Den Berghe, H. Poly(ε-caprolactone)-Based Graft Copolymers: Synthesis Methods and Applications in the Biomedical Field: A Review. Molecules 2022, 27, 7339. [Google Scholar] [CrossRef]
- Cohn, D.; Hotovely, S.A. Designing biodegradable multiblock PCL/PLA thermoplastic elastomers. Biomaterials 2005, 26, 2297–2305. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Hutanu, D.; Frishberg, M.D.; Guo, L.; Darie, C.C. Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod. Chem. Appl. 2014, 2, 2. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol–coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.M.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene glycol (PEG): The nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J. Contr. Rel. 2022, 351, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, M.H. Synthesis and characterization of block copolymers of e-caprolactone and DL-lactide initiated by ethylene glycol or poly(ethylene glycol). Macromol. Chem. Phys. 2003, 204, 1994–2001. [Google Scholar]
- D’Antone, S.; Bignotti, F.; Sartore, L.; D’Amore, A.; Spagnoli, G.; Penco, M. Thermogravimetric investigation of two classes of block copolymers based on poly(lactic-glycolic acid) and poly(ε-caprolactone) or poly(ethylene glycol). Polym. Degrad. Stab. 2001, 74, 119–124. [Google Scholar] [CrossRef]
- Matumba, K.I.; Motloung, M.P.; Ojijo, V.; Ray, S.S.; Sadiku, E.R. Investigation of the Effects of Chain Extender on Material Properties of PLA/PCL and PLA/PEG Blends: Comparative Study between Polycaprolactone and Polyethylene Glycol. Polymers 2023, 15, 2230. [Google Scholar] [CrossRef]
- Sharifisamani, E.; Mousazadegan, F.; Bagherzadeh, R.; Latifi, M. PEG-PLA-PCL based electrospun yarns with curcumin control release property as suture. Polym. Eng. Sci. 2020, 60, 1520–1529. [Google Scholar] [CrossRef]
- Douglas, P.; Albadarin, A.B.; Al-Muhtaseb, A.H.; Mangwandi, C.; Walker, G.M. Thermo-mechanical properties of poly ε-caprolactone/poly l-lactic acid blends: Addition of nalidixic acid and polyethylene glycol additives. J. Mech. Behav. Biomed. Mater. 2015, 45, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Haq, R.H.; Marwah, O.M.; Rahman, M.N.; Ho, F.H.; Abdullah, H.; Ahmad, S.; Arifin, A.M.; Hassan, M.F.; Yunos, M.Z. Mechanical Properties of PCL/PLA/PEG composite blended with different molecular weight (MW) of PEG for Fused Deposition Modelling (FDM) filament wire. Int. J. Integr. Eng. 2018, 10, 187–192. [Google Scholar]
- Hoque, M.; McDonagh, C.; Tiwari, B.K.; Kerry, J.P.; Pathania, S. Effect of Cold Plasma Treatment on the Packaging Properties of Biopolymer-Based Films: A Review. Appl. Sci. 2022, 12, 1346. [Google Scholar] [CrossRef]
- Potaś, J.; Winnicka, K. The Potential of Polyelectrolyte Multilayer Films as Drug Delivery Materials. Int. J. Mol. Sci. 2022, 23, 3496. [Google Scholar] [CrossRef]
- Bodurov, I.; Marudova, M.; Viraneva, A.; Yovcheva, T.T.; Grigorov, A. Investigation of polyelectrolyte multilayers deposited on corona charged composite polylactic acid/poly(ε-caprolactone) substrates. J. Phys. Conf. Ser. 2021, 1762, 012006. [Google Scholar] [CrossRef]
- Grigorov, A.; Yovcheva, T.; Iliev, I.; Vlaeva, I.; Viraneva, A. Impact of physical and chemical modification on the immobilization of βgalactosidase in poly-lactic acid multilayer structures. Bulg. Chem. Comm. 2022, 54, 47–52. [Google Scholar] [CrossRef]
- Passali, D.; Arezzo, M.F.; De Rose, A.; De Simone, G.; Forte, G.; Jablko-Musial, M.; Mösges, R. Benzydamine hydrochloride for the treatment of sore throat and irritative/inflammatory conditions of the oropharynx: A cross-national survey among pharmacists and general practitioners. BMC Prim. Care 2022, 23, 154–166. [Google Scholar] [CrossRef]
- Pilicheva, B.; Uzunova, Y.; Marudova, M. Polyelectrolyte Multilayer Films as a Potential Buccal Platform for Drug Delivery. Polymers 2022, 14, 734. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar]
- Chin-San, W. Physical Properties and Biodegradability of Maleated-Polycaprolactone/Starch Composite. Polym. Degrad. Stab. 2003, 80, 127–134. [Google Scholar]
- Hutmacher, D.; Hürzeler, M.; Schliephake, H. A Review of Material Properties of Biodegradable and Bioresorbable Polymers and Devices for GTR and GBR Applications. Int. J. Oral Maxillofac. Implant. 1996, 11, 667–678. [Google Scholar]
- Giacometti, J.; Fedosov, S.; Costa, M. Corona charging of polymers: Recent advances on constant current charging. Braz. J. Phys. 1999, 29, 269–279. [Google Scholar] [CrossRef]
- Giacometti, J.A.; Oliveira, O.N. Corona charging of polymers. IEEE Trans. Electr. Insul. 1992, 27, 924–943. [Google Scholar] [CrossRef]
- Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.; Peponi, L. Design of Biodegradable Blends Based on PLA and PCL: From Morphological, Thermal and Mechanical Studies to Shape Memory Behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Priselac, D.; Poljaček, S.; Tomašegović, T.; Leskovac, M. Blends Based on Poly(ε-Caprolactone) with Addition of Poly(Lactic Acid) and Coconut Fibers: Thermal Analysis, Ageing Behavior and Application for Embossing Process. Polymers 2022, 14, 1792. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.M.Z.; Felfel, R.M.; Ogbilikana, P.S.; Thakker, D.; Grant, D.M.; Scotchford, C.A.; Ahmed, I. Single Solvent-Based Film Casting Method for the Production of Porous Polymer Films. Macromol. Mater. Eng. 2018, 303, 1700628. [Google Scholar] [CrossRef]
- Neri, F.; Scala, A.; Grimato, S.; Santoro, M.; Spadaro, S.; Barreca, F.; Cimino, F.; Speciale, A.; Saija, A.; Grassi, G.; et al. Biocompatible silver nanoparticles embedded in a PEG–PLA polymeric matrix for stimulated laser light drug release. J. Nanoparticle Res. 2016, 18, 1–14. [Google Scholar] [CrossRef]
- Park, S.B.; Sung, M.H.; Uyama, H.; Han, D.K. Poly (glutamic acid): Production, composites, and medical applications of the next-generation biopolymer. Prog. Pol. Sci. 2021, 113, 101341. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Wang, Z.; Wu, M.; Ma, J.; Gao, C. Preparation and characterization of PSf/clay nanocomposite membranes with PEG 400 as a pore forming additive. Desalination 2012, 286, 131–137. [Google Scholar] [CrossRef]
- Phaechamud, T.; Chitrattha, S. Pore formation mechanism of porous poly (dl-lactic acid) matrix membrane. Mat. Sci. Eng. C 2016, 61, 744–752. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Z.; Yin, J.; Xu, L. Preparation, Characterization and Properties of Porous PLA/PEG/Curcumin Composite Nanofibers for Antibacterial Application. Nanomaterials 2019, 9, 508. [Google Scholar] [CrossRef]
- Tsuji, H.; Horikawa, G. Porous biodegradable polyester blends of poly (L-lactic acid) and poly (ε-caprolactone): Physical properties, morphology, and biodegradation. Polym. Internat. 2007, 56, 258–266. [Google Scholar] [CrossRef]
- Lin, W.J.; Lu, C.H. Characterization and permeation of microporous poly (ε-caprolactone) films. J. Membr. Sci. 2002, 198, 109–118. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Y.C.; Zhang, P.H. Preparation and Characterisation of Polylactic Acid (PLA)/Polycaprolactone (PCL) Composite Microfibre Membranes. Fibres Text. East. Eur. 2016, 24, 17–25. [Google Scholar] [CrossRef]
- Alam, F.; Verma, P.; Mohammad, W.; Teo, J.; Varadarajan, K.M.; Kumar, S. Architected poly(lactic acid)/poly(e-caprolactone)/ halloysite nanotube composite scaffolds enabled by 3D printing for biomedical applications. J. Mater. Sci. 2021, 56, 14070–14083. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Khandwekar, A.; Patil, D.; Shouche, Y.; Doble, M. Surface Engineering of Polycaprolactone by Biomacromolecules and their Blood Compatibility. J. Biomat. Appl. 2011, 26, 227–252. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L.; Hubert, B.B.; Rhodes, C.T. The Development of USP, Dissolution and Drug Release Standards. Pharm. Res. 1990, 7, 983–987. [Google Scholar] [CrossRef]
- Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Fazio, E. Weibull Modeling of Controlled Drug Release from Ag-PMA Nanosystems. Polymers 2021, 13, 2897. [Google Scholar] [CrossRef]
- Kobry´n, J.; Sowa, S.; Gasztych, M.; Dry´s, A.; Musiał, W. Influence of Hydrophilic Polymers on the β Factor in Weibull Equation Applied to the Release Kinetics of a Biologically Active Complex of Aesculus hippocastanum. Int. J. Pol. Sci. 2017, 2017, 3486384. [Google Scholar]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Intern. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]

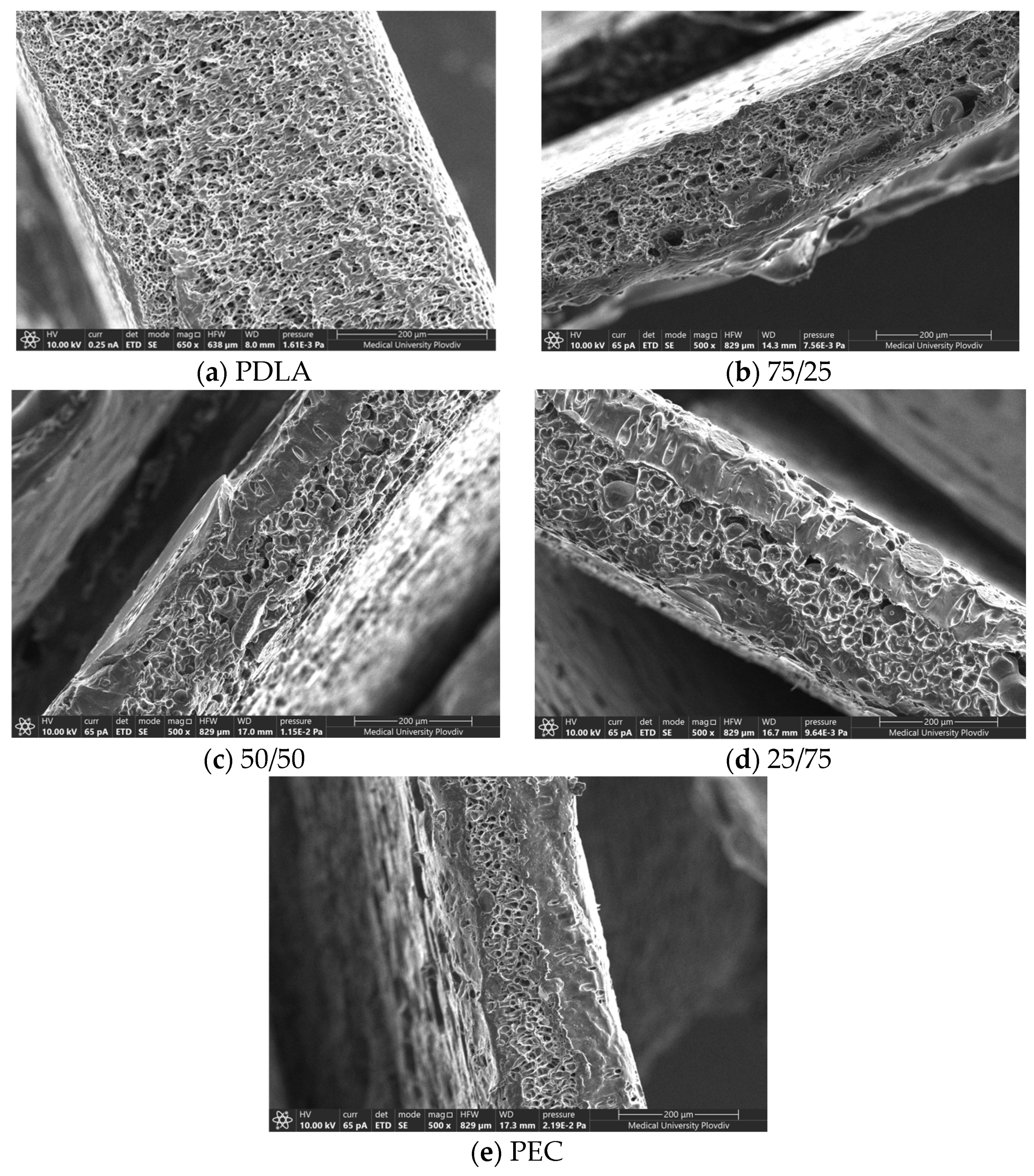
| Sample | Pore Size Diameter, µm |
|---|---|
| PDLA | 5.74 ± 1.42 |
| 75/25 | 18.76 ± 1.32 |
| 50/50 | 23.07 ± 1.87 |
| 25/75 | 24.59 ± 3.30 |
| PEC | 31.93 ± 2.87 |
| Sample | Surface Energy, mJ/m2 |
|---|---|
| PDLA | 46.39 |
| 75/25 | 55.65 |
| 50/50 | 54.79 |
| 25/75 | 51.83 |
| PEC | 49.87 |
| Sample | Positive Corona | Negative Corona | ||||
|---|---|---|---|---|---|---|
| τ | β | R2 | τ | β | R2 | |
| PDLA | 153.1 | 1.10 | 0.991 | 163.4 | 1.42 | 0.991 |
| 75:25 | 120.3 | 1.07 | 0.998 | 150.1 | 1.74 | 0.979 |
| 50:50 | 99.1 | 1.08 | 0.981 | 89.5 | 1.32 | 0.986 |
| 25:75 | 68.95 | 1.03 | 0.996 | 66.0 | 1.37 | 0.996 |
| PEC | 68.0 | 1.07 | 0.997 | 57.2 | 1.32 | 0.991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viraneva, A.; Marudova, M.; Milenkova, S.; Grigorov, A.; Yovcheva, T. Investigation of Polyelectrolyte Multilayers Deposited on Biodegradable Corona-Charged Substrates Used as Drug Delivery Systems. Coatings 2024, 14, 85. https://doi.org/10.3390/coatings14010085
Viraneva A, Marudova M, Milenkova S, Grigorov A, Yovcheva T. Investigation of Polyelectrolyte Multilayers Deposited on Biodegradable Corona-Charged Substrates Used as Drug Delivery Systems. Coatings. 2024; 14(1):85. https://doi.org/10.3390/coatings14010085
Chicago/Turabian StyleViraneva, Asya, Maria Marudova, Sofia Milenkova, Aleksandar Grigorov, and Temenuzhka Yovcheva. 2024. "Investigation of Polyelectrolyte Multilayers Deposited on Biodegradable Corona-Charged Substrates Used as Drug Delivery Systems" Coatings 14, no. 1: 85. https://doi.org/10.3390/coatings14010085
APA StyleViraneva, A., Marudova, M., Milenkova, S., Grigorov, A., & Yovcheva, T. (2024). Investigation of Polyelectrolyte Multilayers Deposited on Biodegradable Corona-Charged Substrates Used as Drug Delivery Systems. Coatings, 14(1), 85. https://doi.org/10.3390/coatings14010085







